Abstract
Circulating immune complexes were identified in cryoproteins isolated from serial samples of serum from six patients with acute viral hepatitis with and without arthritic symptoms. Cryoprecipitates were analyzed for the presence of hepatitis-B surface antigen (HBsAg) and hepatitis-B surface antibody (anti-HBs) by hemagglutination inhibition and hemagglutination. Complement components were detected by counter electrophoresis, and immunoglobulins were detected by gel diffusion. HBsAg, IgG, and IgM were identified in cryoprecipitates from all hepatitis patients, but were higher in concentration in patients with arthritis. Only cryoprecipitates from hepatitis patients with arthritis contained IgA and complement components C3, C4, and C5 as well as IgG and IgM, which disappear with resolution of the arthritis. The subtypes of IgG in these cryoprecipitates were predominantly the complement-fixing IgG1 and IgG3, HBsAg and anti-HBs were concentrated several-fold in the cryoprecipitates when compared to the serum concentration. Sequential studies in two patients demonstrated that the initial appearance of anti-HBs in the cryoprotein complex was associated with the detection in the complex of IgM suggesting a primary immune response to HBsAg. The C3 activator fragment (C3A) of the properdin complex was found in fresh serum obtained from three hepatitis patients with arthritis and not in uncomplicated hepatitis. The cryoprecipitable immune complexes from patients with arthritis converted C3PA in fresh normal sera to C3A in vitro whereas cryoprotein isolated from patients with uncomplicated hepatitis had no such effect. Thus, the transient appearance of circulating complement-fixing immune complexes in patients with the arthritis of acute hepatitis is associated with activation of both classical and alternate complement pathways and suggests that they play an important role in the pathogenesis of these serum sickness-like extrahepatic symptoms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert E., Isselbacher K. J., Schur P. H. The pathogenesis of arthritis associated with viral hepatitis. Complement-component studies. N Engl J Med. 1971 Jul 22;285(4):185–189. doi: 10.1056/NEJM197107222850401. [DOI] [PubMed] [Google Scholar]
- Alpert E., Schur P. H., Isselbacher K. J. Sequential changes of serum complement in HAA related arthritis. N Engl J Med. 1972 Jul 13;287(2):103–103. doi: 10.1056/NEJM197207132870215. [DOI] [PubMed] [Google Scholar]
- Combes B., Shorey J., Barrera A., Stastny P., Eigenbrodt E. H., Hull A. R., Carter N. W. Glomerulonephritis with deposition of Australia antigen-antibody complexes in glomerular basement membrane. Lancet. 1971 Jul 31;2(7718):234–237. doi: 10.1016/s0140-6736(71)92572-4. [DOI] [PubMed] [Google Scholar]
- DIXON F. J., VAZQUEZ J. J., WEIGLE W. O., COCHRANE C. G. Pathogenesis of serum sickness. AMA Arch Pathol. 1958 Jan;65(1):18–28. [PubMed] [Google Scholar]
- Gocke D. J., Howe C. Rapid detection of Australia antigen by counterimmunoelectrophoresis. J Immunol. 1970 Apr;104(4):1031–1034. [PubMed] [Google Scholar]
- Gocke D. J., Hsu K., Morgan C., Bombardieri S., Lockshin M., Christian C. L. Vasculitis in association with Australia antigen. J Exp Med. 1971 Sep 1;134(3 Pt 2):330s–336s. [PubMed] [Google Scholar]
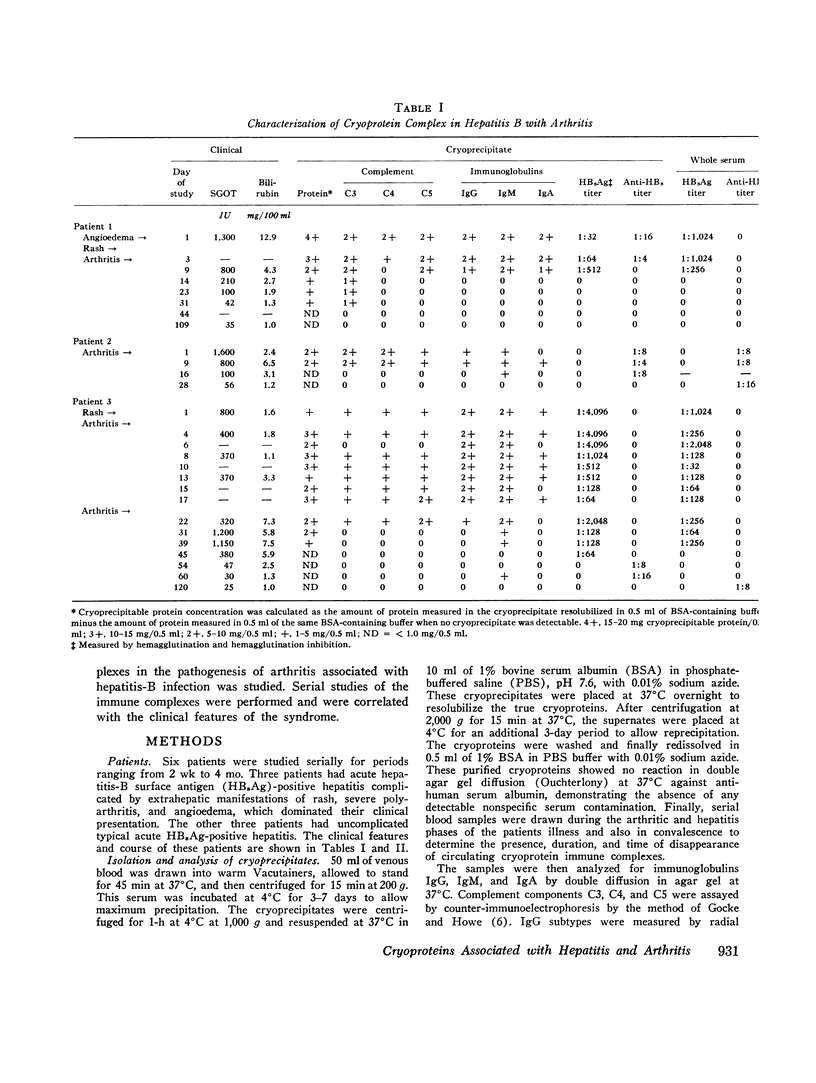
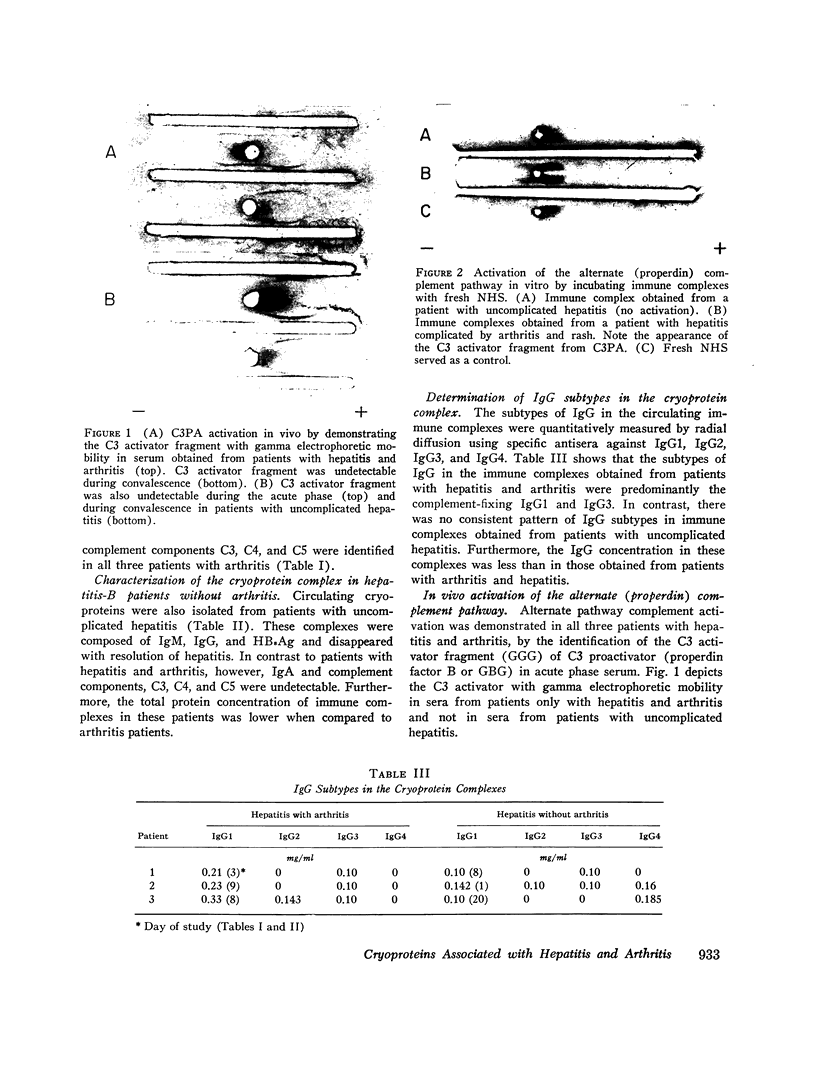
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Newman S. L., Struth A. G. An abnormality of the alternate pathway of complement activation in sickle-cell disease. N Engl J Med. 1973 Apr 19;288(16):803–808. doi: 10.1056/NEJM197304192881601. [DOI] [PubMed] [Google Scholar]
- Jordan R. E., Day N. K., Luckasen J. R., Good R. A. Complement activation in pemphigus vulgaris blister fluid. Clin Exp Immunol. 1973 Sep;15(1):53–63. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lander H. J., Holland P. V., Alter H. J., Chanock R. M., Purcell R. H. Antibody to hepatitis-associated antigen. Frequency and pattern of response as detected by radioimmunoprecipitation. JAMA. 1972 May 22;220(8):1079–1082. doi: 10.1001/jama.220.8.1079. [DOI] [PubMed] [Google Scholar]
- Lander J. J., Giles J. P., Purcell R. H., Krugman S. Viral hepatitis, type B (MS-2 strain). Detection of antibody after primary infection. N Engl J Med. 1971 Aug 5;285(6):303–307. doi: 10.1056/NEJM197108052850601. [DOI] [PubMed] [Google Scholar]
- MIRICK G. S., SHANK R. E. An epidemic of serum hepatits studied under controlled conditions. Trans Am Clin Climatol Assoc. 1959;71:176–190. [PMC free article] [PubMed] [Google Scholar]
- Madaliński K., Sztachelska-Budkowska A., Brzosko W. J. DEAE-cellulose chromatography: a method for dissociation of soluble immune complexes of hepatitis B antigen. J Infect Dis. 1974 Apr;129(4):371–375. doi: 10.1093/infdis/129.4.371. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natvig J. B., Kunkel H. G. Human immunoglobulins: classes, subclasses, genetic variants, and idiotypes. Adv Immunol. 1973;16:1–59. doi: 10.1016/s0065-2776(08)60295-3. [DOI] [PubMed] [Google Scholar]
- Onion D. K., Crumpacker C. S., Gilliland B. C. Arthritis of hepatitis associated with Australia antigen. Ann Intern Med. 1971 Jul;75(1):29–33. doi: 10.7326/0003-4819-75-1-29. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Trepo C. Role of immune complexes involving SH antigen in pathogenesis of chronic active hepatitis and polyarteritis nodosa. Lancet. 1971 Jun 26;1(7713):1309–1312. doi: 10.1016/s0140-6736(71)91883-6. [DOI] [PubMed] [Google Scholar]
- Reed W. D., Eddleston A. L., Cullens H., Williams R., Zuckerman A. J., Peters D. K., Williams D. G., Maycock W. A. Infusion of hepatitis-B antibody in antigen-positive active chronic hepatitis. Lancet. 1973 Dec 15;2(7842):1347–1351. doi: 10.1016/s0140-6736(73)93321-7. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Gigli I., Austen K. F. The complement system of man. 3. N Engl J Med. 1972 Sep 21;287(12):592–596. doi: 10.1056/NEJM197209212871206. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Sandberg A. L., Götze O., Müller-Eberhard H. J., Osler A. G. Complement utilization by guinea pig 1 and 2-immunoglobulins through the C3 activator system. J Immunol. 1971 Sep;107(3):920–923. [PubMed] [Google Scholar]
- Shumaker J. B., Goldfinger S. E., Alpert E., Isselbacher K. J. Arthritis and rash. Clues to anicteric viral hepatitis. Arch Intern Med. 1974 Mar;133(3):483–485. doi: 10.1001/archinte.133.3.483. [DOI] [PubMed] [Google Scholar]
- Teisberg P., Grottum K. A., Myhre E., Flatmark A. In-vivo activation of complement in hereditary nephropathy. Lancet. 1973 Aug 18;2(7825):356–358. doi: 10.1016/s0140-6736(73)93194-2. [DOI] [PubMed] [Google Scholar]
- Vyas G. N., Shulman N. R. Hemagglutination assay for antigen and antibody associated with viral hepatitis. Science. 1970 Oct 16;170(3955):332–333. doi: 10.1126/science.170.3955.332. [DOI] [PubMed] [Google Scholar]
- Westberg N. G., Naff G. B., Boyer J. T., Michael A. F. Glomerular deposition of properdin in acute and chronic glomerulonephritis with hypocomplementemia. J Clin Invest. 1971 Mar;50(3):642–649. doi: 10.1172/JCI106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. G., Lachmann P. J., Charlesworth J. A., Peters D. K. Role of C3b in the breakdown of C3 in hypocomplementaemic mesangiocapillary glomerulonephritis. Lancet. 1973 Mar 3;1(7801):447–449. doi: 10.1016/s0140-6736(73)91877-1. [DOI] [PubMed] [Google Scholar]