Abstract
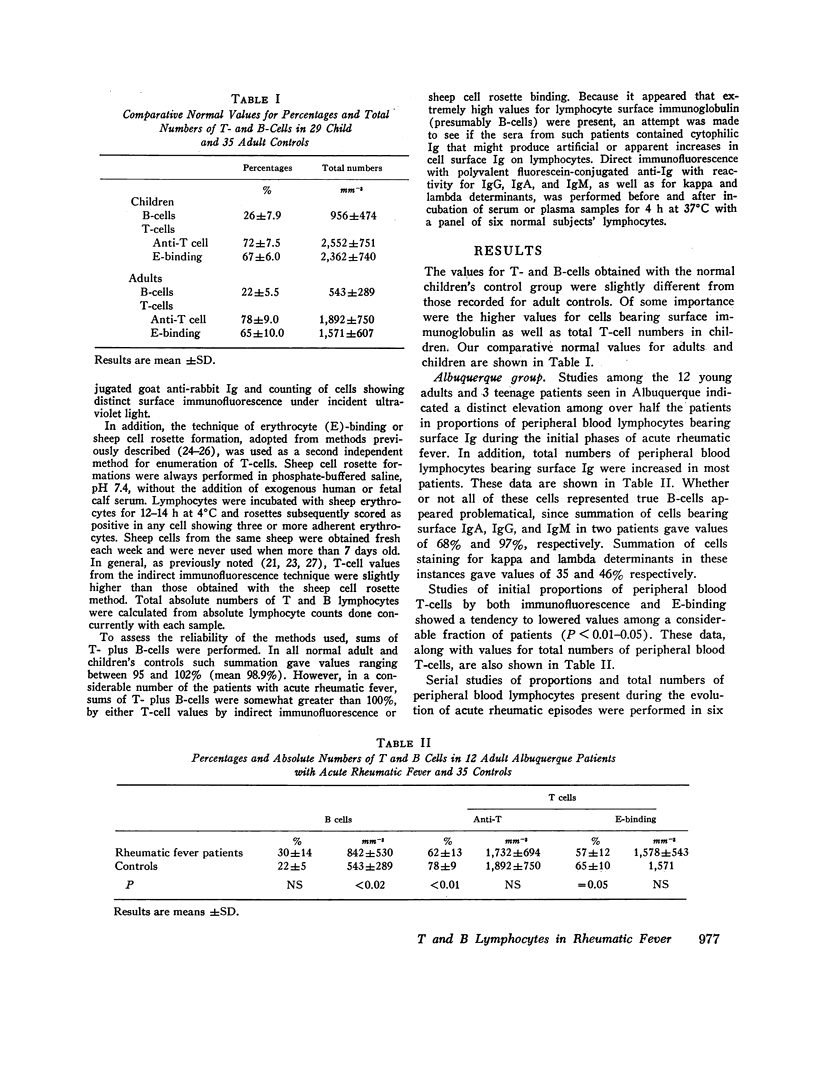
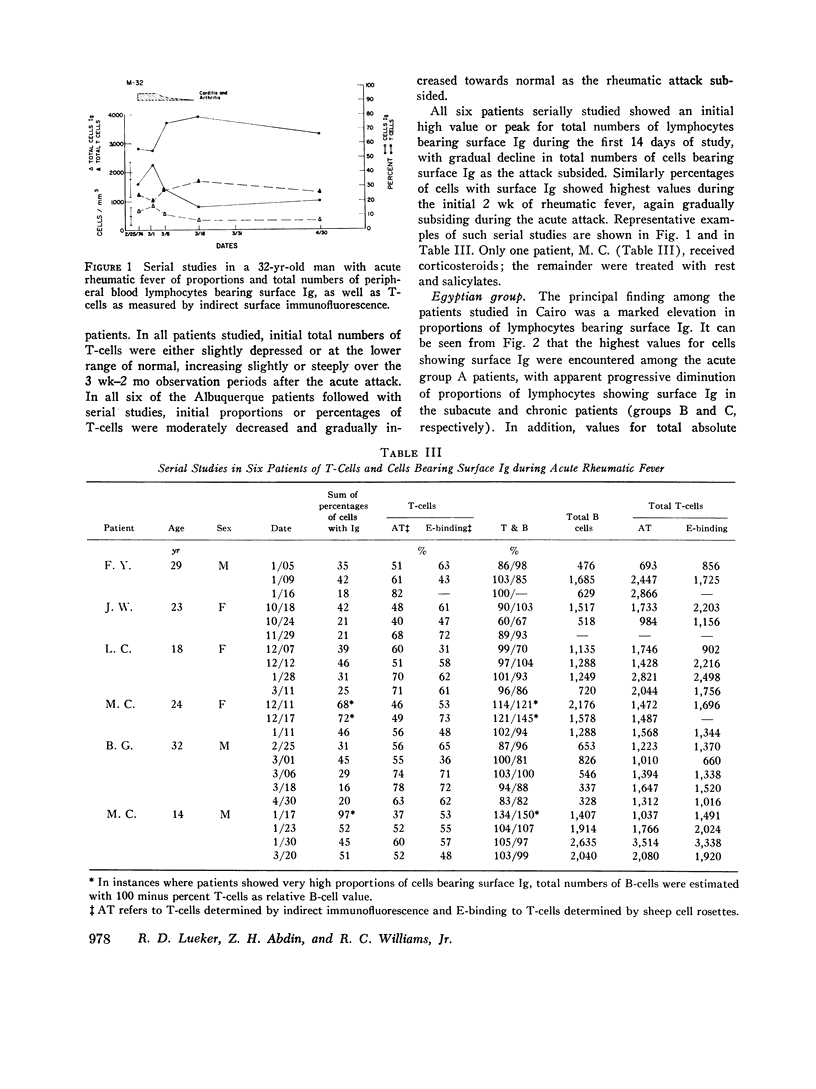
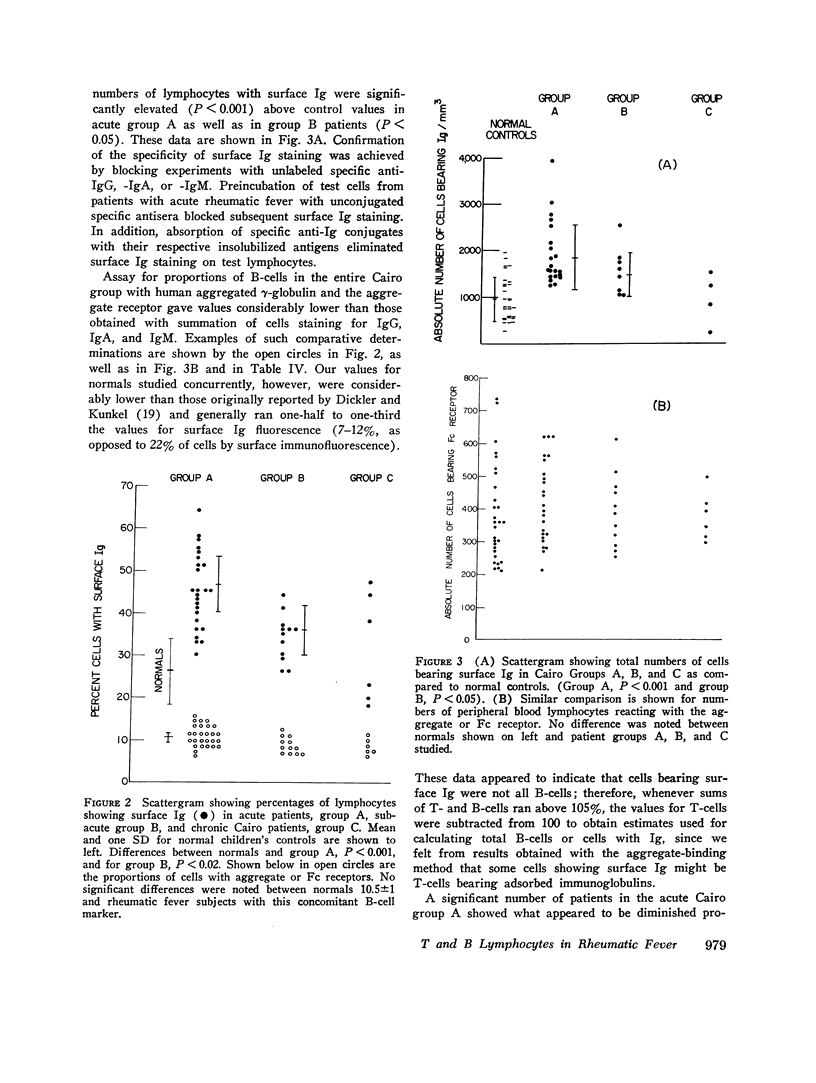
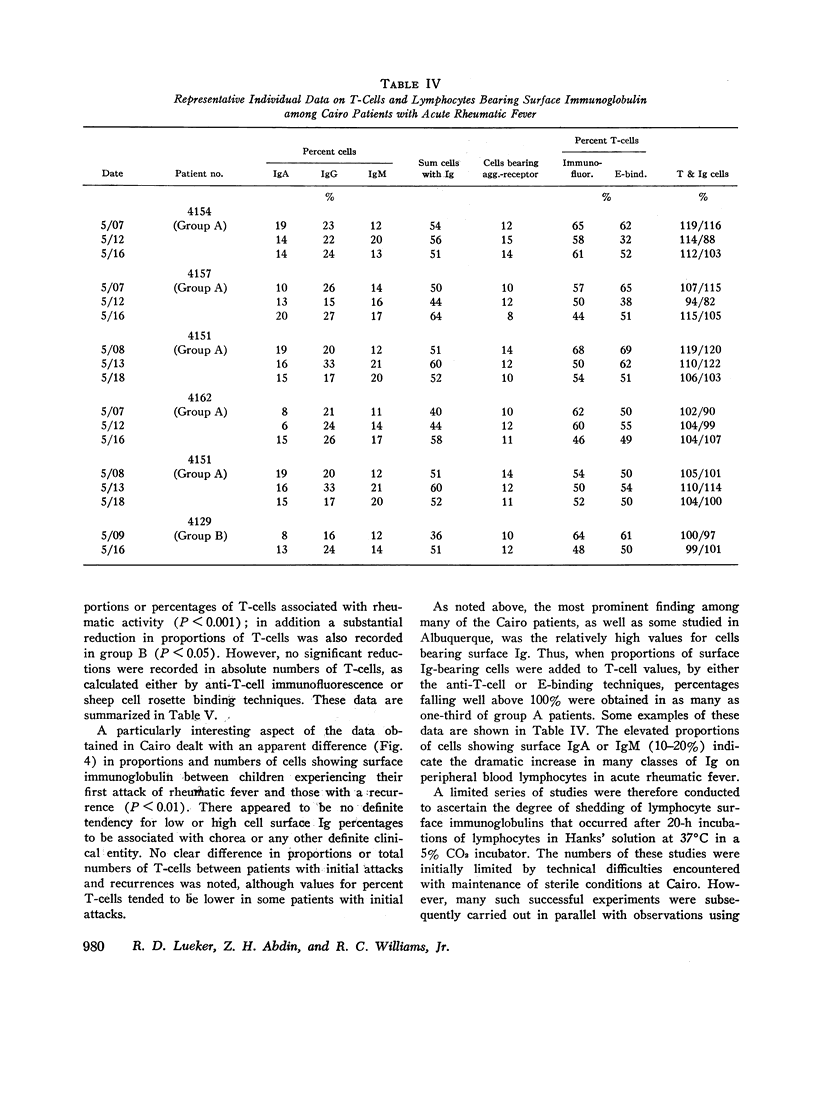
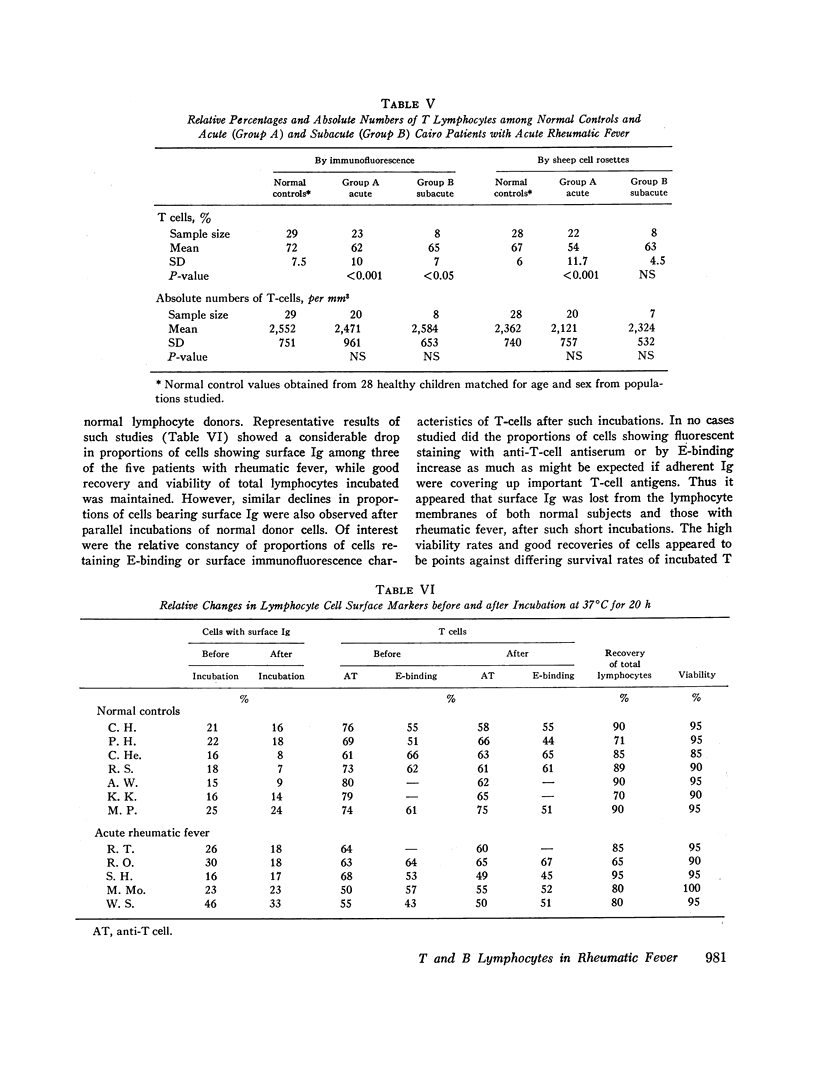
Proportions and total numbers of thymus-derived (T) and bone marrow-derived (B) peripheral blood lymphocytes were studied in 53 patients with acute rheumatic fever, diagnosed on the basis of modifified Jones criteria. An elevation in both proportions and absolute numbers of cells bearing surface Ig was found in most patients, particularly during the first 7 days after onset. Conversely, T-cell proportions and numbers were often found to be depressed early in the acue phases of rheumatic fever. Proportions of cells bearing surface Ig did not correlate with another B-cell marker, the aggregated gamma globulin receptor, suggesting that such cells bearing surface Ig were not all B lymphocytes. Incuvation for 20 h at 37 per cent C of cells showing high proportions of surface Ig-bearing surface Ig in both normal and rheumatic fever subjects, although there was no appreciable increment in proportions of lymphocytes expressing T-cell markers. Patients with initial attacks showed higher percentages and total numbers of Ig-bearing lymphocytes (P smaller than 0.01) than did those with rneumatic fever recurrences. Elevations in numbers and proportions of peripheral blood lymphocytes bearing Ig appeared to correlate with the relative acute nature of the rheumatic fever attack.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Bankhurst A. D., Torrigiani G., Allison A. C. Lymphocytes binding human thyroglobulin in healthy people and its relevance to tolerance for autoantigens. Lancet. 1973 Feb 3;1(7797):226–230. doi: 10.1016/s0140-6736(73)90066-4. [DOI] [PubMed] [Google Scholar]
- Bentwich Z., Kunkel H. G. Specific properties of human B and T lymphocytes and alterations in disease. Transplant Rev. 1973;16:29–50. doi: 10.1111/j.1600-065x.1973.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Bernstein I. M., Webster K. H., Williams R. C., Jr, Strickland R. G. Reduction in circulating T lymphocytes in alcoholic liver disease. Lancet. 1974 Aug 31;2(7879):488–490. doi: 10.1016/s0140-6736(74)92015-7. [DOI] [PubMed] [Google Scholar]
- Brown G., Greaves M. F., Lister T. A., Rapson N., Papamichael M. Expression of human T and B lymphocyte cell-surface markers on leukaemic cells. Lancet. 1974 Sep 28;2(7883):753–755. doi: 10.1016/s0140-6736(74)90945-3. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- Crone M., Koch C., Simonsen M. The elusive T cell receptor. Transplant Rev. 1972;10:36–56. doi: 10.1111/j.1600-065x.1972.tb01538.x. [DOI] [PubMed] [Google Scholar]
- DeHoratius R. J., Strickland R. G., Williams R. C., Jr T and B lymphocytes in acute and chronic hepatitis. Clin Immunol Immunopathol. 1974 Apr;2(3):353–360. doi: 10.1016/0090-1229(74)90053-1. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B. Studies of the human lymphocyte receptor for heat-aggregated or antigen-complexed immunoglobulin. J Exp Med. 1974 Aug 1;140(2):508–522. doi: 10.1084/jem.140.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröland S. S. Binding of sheep erythrocytes to human lymphocytes. A probable marker of T lymphocytes. Scand J Immunol. 1972;1(3):269–280. doi: 10.1111/j.1365-3083.1972.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Fröland S. S., Natvig J. B. Class, subclass, and allelic exclusion of membrane-bound Ig of human B lymphocytes. J Exp Med. 1972 Aug 1;136(2):409–414. doi: 10.1084/jem.136.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröland S., Natvig J. B., Berdal P. Surface-bound immunoglobulin as a marker of B lymphocytes in man. Nat New Biol. 1971 Dec 22;234(51):251–252. doi: 10.1038/newbio234251a0. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Green C. A. Haemolytic Streptococcal Infections and Acute Rheumatism. Ann Rheum Dis. 1942 May;3(1):4–41. doi: 10.1136/ard.3.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A. Selective depletion of Ig-bearing lymphocytes by cyclophosphamide in rheumatoid arthritis and systemic lupus erythematosus. Guidelines for dosage. Arthritis Rheum. 1974 Jul-Aug;17(4):363–374. doi: 10.1002/art.1780170405. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN M. H., SVEC K. H. IMMUNOLOGIC RELATION OF STREPTOCOCCAL AND TISSUE ANTIGENS. III. PRESENCE IN HUMAN SERA OF STREPTOCOCCAL ANTIBODY CROSS-REACTIVE WITH HEART TISSUE. ASSOCIATION WITH STREPTOCOCCAL INFECTION, RHEUMATIC FEVER, AND GLOMERULONEPHRITIS. J Exp Med. 1964 Apr 1;119:651–666. doi: 10.1084/jem.119.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. F., Paterson P. Y., Hartz R. S., Embury S. H. Rheumatic carditis: in vitro responses of peripheral blood leukocytes to heart and streptococcal antigens. Arthritis Rheum. 1972 Nov-Dec;15(6):600–608. doi: 10.1002/art.1780150606. [DOI] [PubMed] [Google Scholar]
- Mellbye O. J., Messner R. P., DeBord J. R., Williams R. C., Jr Immunoglobulin and receptors for C3 on lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1972 Jul-Aug;15(4):371–380. doi: 10.1002/art.1780150408. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Lindström F. D., Williams R. C., Jr Peripheral blood lymphocyte cell surface markers during the course of systemic lupus erythematosus. J Clin Invest. 1973 Dec;52(12):3046–3056. doi: 10.1172/JCI107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Orr K. B., Paraskevas F. Cell surface associated gamma globulin in lymphocytes. V. Detection of early cytophilic complexes reacting with T- and B-lymphocytes. J Immunol. 1973 Feb;110(2):456–464. [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Read S. E., Fischetti V. A., Utermohlen V., Falk R. E., Zabriskie J. B. Cellular reactivity studies to streptococcal antigens. Migration inhibition studies in patients with streptococcal infections and rheumatic fever. J Clin Invest. 1974 Aug;54(2):439–450. doi: 10.1172/JCI107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman S. T., Ayoub E. M. Qualitative and quantitative aspects of the human antibody response to streptococcal group A carbohydrate. J Clin Invest. 1974 Oct;54(4):990–996. doi: 10.1172/JCI107840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talal N., Sylvester R. A., Daniels T. E., Greenspan J. S., Williams R. C., Jr T and B lymphocytes in peripheral blood and tissue lesions in Sjögren's syndrome. J Clin Invest. 1974 Jan;53(1):180–189. doi: 10.1172/JCI107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANNAMAKER L. W., RAMMELKAMP C. H., Jr, DENNY F. W., BRINK W. R., HOUSER H. B., HAHN E. O., DINGLE J. H. Prophylaxis of acute rheumatic fever by treatment of the preceding streptococcal infection with various amounts of depot penicillin. Am J Med. 1951 Jun;10(6):673–695. doi: 10.1016/0002-9343(51)90336-1. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Winfield J. B., Siegal F., Wernet P., Bentwich Z., Kunkel H. G. Analyses of lymphocytes from patients with rheumatoid arthritis and systemic lupus erythematosus. Occurrence of interfering cold-reactive antilymphocyte antibodies. J Clin Invest. 1974 Nov;54(5):1082–1092. doi: 10.1172/JCI107852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]
- Yu D. T., Clements P. J., Paulus H. E., Peter J. B., Levy J., Barnett E. V. Human lymphocyte subpopulations. Effect of corticosteroids. J Clin Invest. 1974 Feb;53(2):565–571. doi: 10.1172/JCI107591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B. Mimetic relationships between group A streptococci and mammalian tissues. Adv Immunol. 1967;7:147–188. doi: 10.1016/s0065-2776(08)60128-5. [DOI] [PubMed] [Google Scholar]
- Zabriskie J. B. The relationship of streptococcal cross-reactive antigens to rheumatic fever. Transplant Proc. 1969 Dec;1(4):968–975. [PubMed] [Google Scholar]


