Abstract
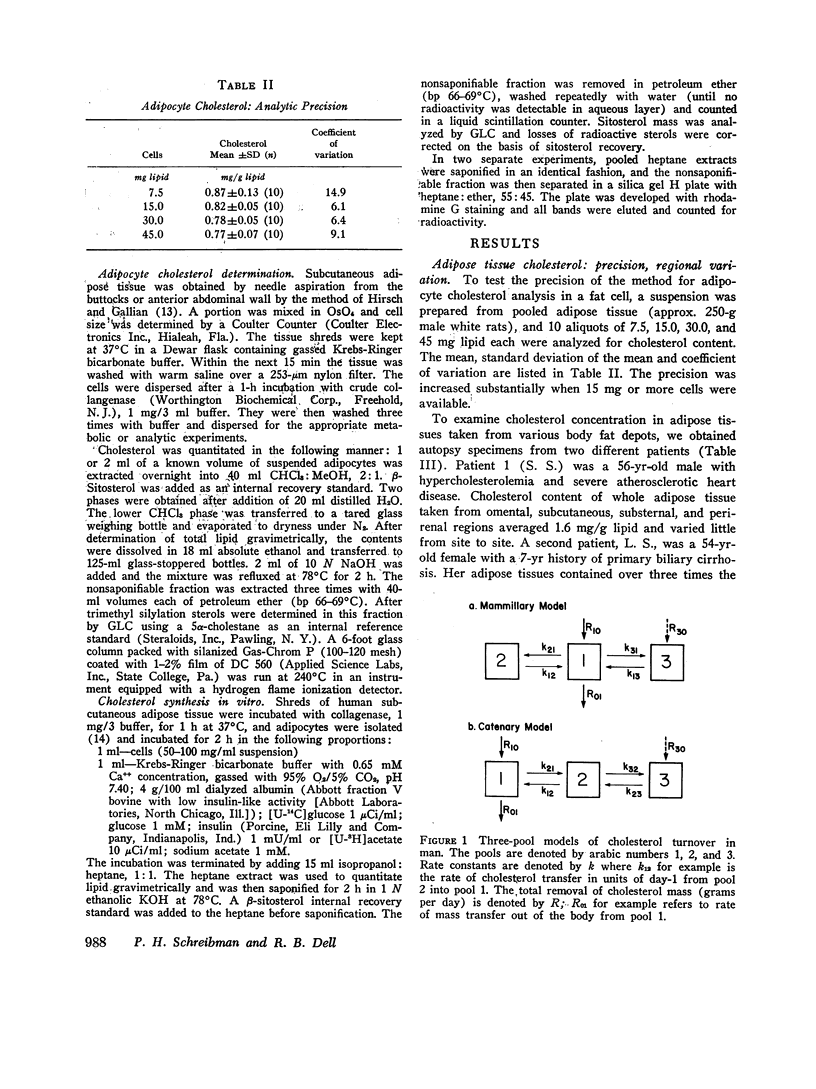
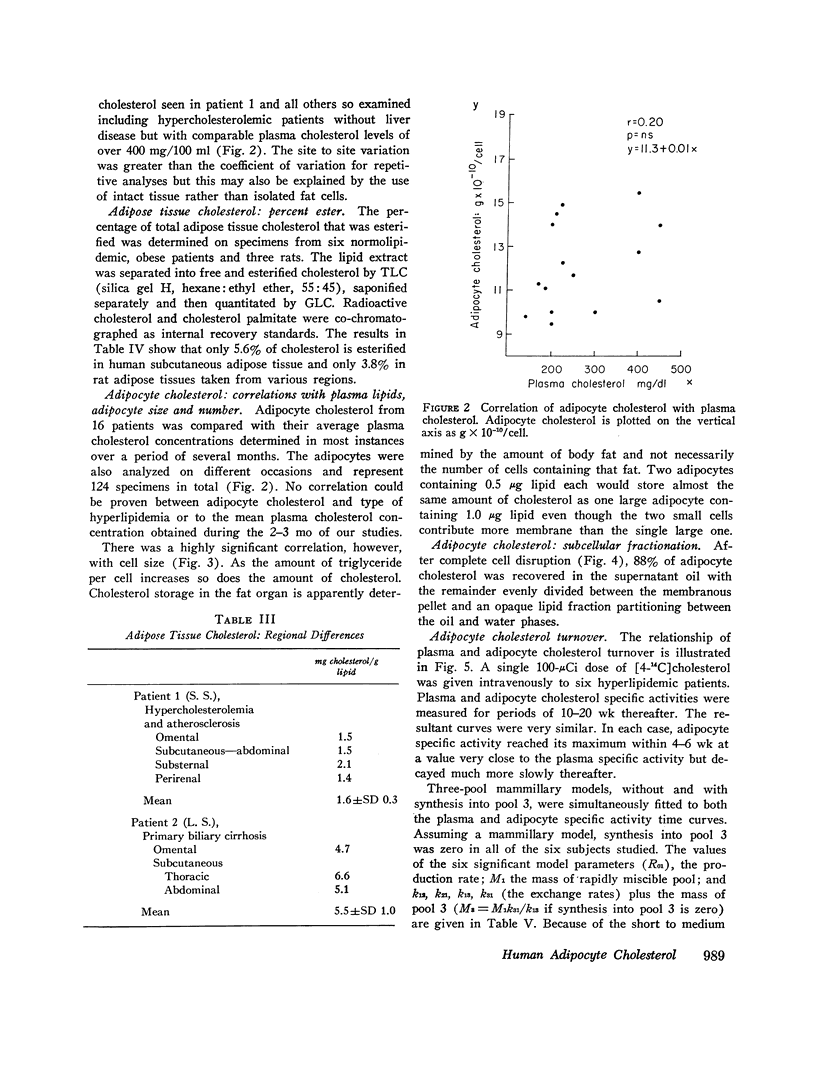
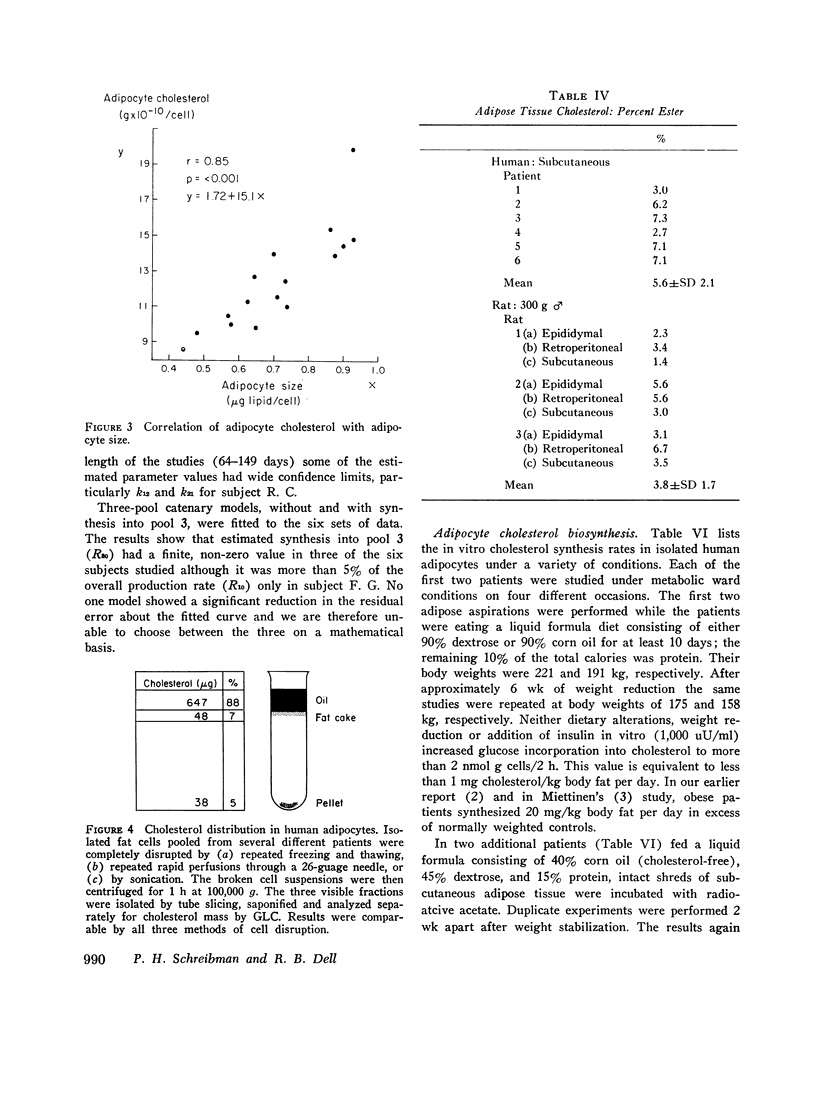
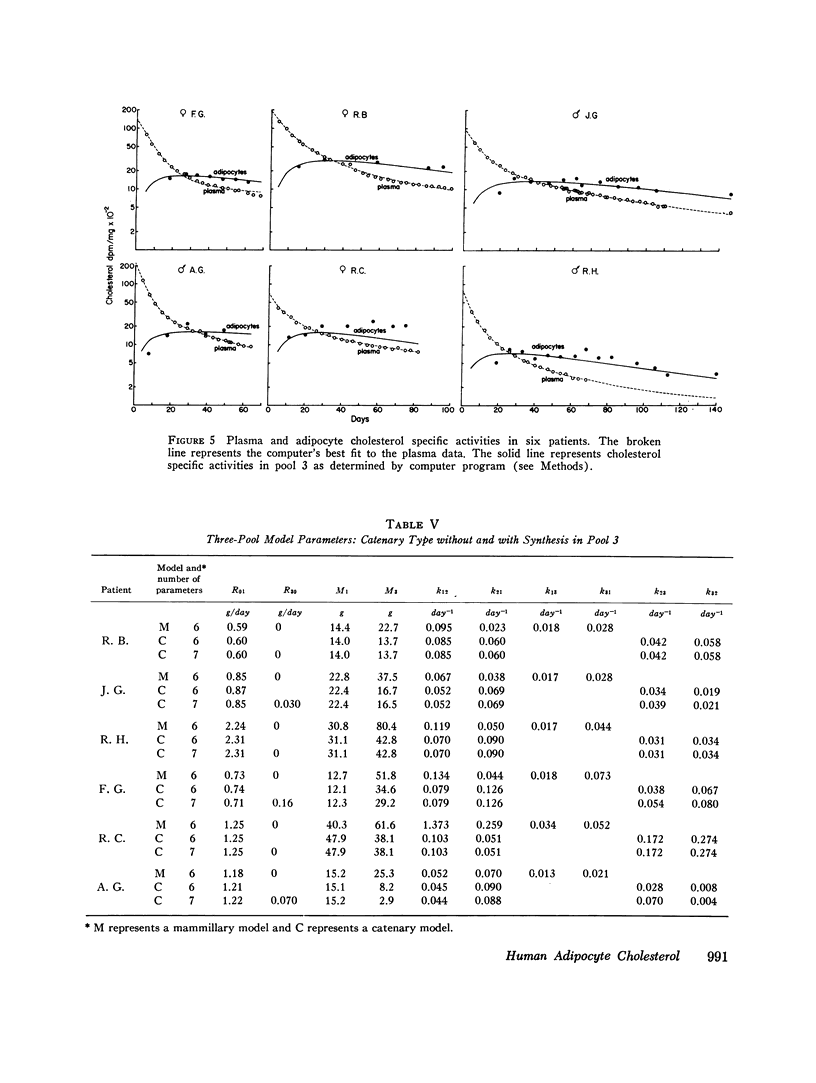
By analysis of 124 specimens in 16 different patients, isolated human adipocyte cholesterol concentration is highly correlated with fat cell size but not with plasma cholesterol concentration. Less than 6 percent of total cholesterol is esterified; after subcellular fractionation, 88 percent of the cholesterol is recovered in the triglyceride-rich supernatant oil. This latter finding supports the observation that fat cell cholesterol is determined by triglyceride content, and hence by fat cell size. After intravenous administrtion of radioactive cholesterol, the sum of a three-exponential equation was fit simultaneously to both the plasma and adipocyte specific activity time curves in six patients. In five of the six, a slowly turning over pool (pool 3) closely fit the adipocyte data. Two model structures, mammillary and catenary, were fitted to the data. There was no synthesis in pool 3 using a mammillary model but a mean 5.3 percent of the total body production rate was found in compartment 3 if a catenary model was assumed. Although a catenary model is biologically unlikely, it could not be excluded. Obesity is associated with an increased cholesterol synthetic rate equal to 20 mg/day for each kilogram of body fat. To test (by an independent method) if this synthesis might be occurring in adipose tissue, human fat cells were obtained under a wide variety of dietary conditions and incubated in vitro with radioactive glucose or acetate. Incorportation of these precursors into sterol could account for no more than 1 mg cholesterol synthesis/kg fat per day. These in vitro data taken together with the in vivo mammillary compartmental analysis data are compatible with the possiblity that the excess cholesterol synthesis of obesity occurs in pool 1, most likely from hepatic or intestinal sites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crouse J. R., Grundy S. M., Ahrens E. H., Jr Cholesterol distribution in the bulk tissues of man: variation with age. J Clin Invest. 1972 May;51(5):1292–1296. doi: 10.1172/JCI106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell R. B., Sciacca R., Lieberman K., Case D. B., Cannon P. J. A weighted least-squares technique for the analysis of kinetic data and its application to the study of renal xenon washout in dogs and man. Circ Res. 1973 Jan;32(1):71–84. doi: 10.1161/01.res.32.1.71. [DOI] [PubMed] [Google Scholar]
- Farkas J., Angel A., Avigan M. I. Studies on the compartmentation of lipid in adipose cells. II. Cholesterol accumulation and distribution in adipose tissue components. J Lipid Res. 1973 May;14(3):344–356. [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P., Dell R. B. Three-pool model of the long-term turnover of plasma cholesterol in man. J Lipid Res. 1973 Mar;14(2):178–188. [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Lacroix D. E., Mattingly W. A., Wong N. P., Alford J. A. Cholesterol, fat, and protein in dairy products. J Am Diet Assoc. 1973 Mar;62(3):275–279. [PubMed] [Google Scholar]
- Miettinen T. A. Cholesterol production in obesity. Circulation. 1971 Nov;44(5):842–850. doi: 10.1161/01.cir.44.5.842. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Schreibman P. H., Ahrens E. H., Jr Cholesterol metabolism in human obesity. J Clin Invest. 1973 Oct;52(10):2389–2397. doi: 10.1172/JCI107428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Samuel P., Perl W. Long-term decay of serum cholesterol radioactivity: body cholesterol metabolism in normals and in patients with hyperlipoproteinemia and atherosclerosis. J Clin Invest. 1970 Feb;49(2):346–357. doi: 10.1172/JCI106243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shean F. C., Austen W. G., Buckley M. J., Mundth E. D., Scannell J. G., Daggett W. M. Survival after Starr-Edwards aortic valve replacement. Circulation. 1971 Jul;44(1):1–8. doi: 10.1161/01.cir.44.1.1. [DOI] [PubMed] [Google Scholar]
- WILKENS J. A., DE WIT H., BRONTE-STEWART B. A proposed mechanism for the effect of different dietary fats on some aspects of cholesterol metabolism. Can J Biochem Physiol. 1962 Aug;40:1091–1100. [PubMed] [Google Scholar]



