Abstract
This work shows that the recently described Escherichia coli BtuE peroxidase protects the bacterium against oxidative stress that is generated by tellurite and by other reactive oxygen species elicitors (ROS). Cells lacking btuE (ΔbtuE) displayed higher sensitivity to K2TeO3 and other oxidative stress-generating agents than did the isogenic, parental, wild-type strain. They also exhibited increased levels of cytoplasmic reactive oxygen species, oxidized proteins, thiobarbituric acid reactive substances, and lipoperoxides. E. coli ΔbtuE that was exposed to tellurite or H2O2 did not show growth changes relative to wild type cells either in aerobic or anaerobic conditions. Nevertheless, the elimination of btuE from cells deficient in catalases/peroxidases (Hpx−) resulted in impaired growth and resistance to these toxicants only in aerobic conditions, suggesting that BtuE is involved in the defense against oxidative damage. Genetic complementation of E. coli ΔbtuE restored toxicant resistance to levels exhibited by the wild type strain. As expected, btuE overexpression resulted in decreased amounts of oxidative damage products as well as in lower transcriptional levels of the oxidative stress-induced genes ibpA, soxS and katG.
Introduction
Although the tellurium oxyanion, tellurite (TeO3 2−), is toxic to most microorganisms, the ultimate basis of its toxicity has remained elusive. Available evidence from Escherichia coli [1], Pseudomonas pseudoalcaligenes KF707 [2] and Rhodobacter capsulatus [3] supports the idea that bacterial tellurite toxicity is related to oxidative stress. In particular, E. coli exposed to K2TeO3 exhibits increased levels of cytoplasmic reactive oxygen species (ROS), mainly superoxide (O2 −) [4]. In turn, increased O2 − levels can trigger a number of metabolic effects, including protein and membrane oxidation, induction of antioxidant enzymes and inactivation of [4Fe-4S] clusters from certain dehydratases [5]–[9].
Aerobic organisms protect themselves from ROS by synthesizing antioxidant enzymes as well as low molecular weight molecules such as ascorbate and glutathione [7], [10]. E. coli contains several antioxidant enzymes, including catalases (katG and katE) [11]–[13], superoxide dismutases (MnSOD, FeSOD, and CuZnSOD) [13]–[15], alkylhydroperoxidase [13] and thiol peroxidase [16]. To cope with oxidative stress, the genes encoding these enzymes are often induced by ROS, whether it is produced in different compartments of the bacterial cell or at different growth stages [13].
Glutathione peroxidases (GPXs) are another kind of antioxidant enzyme that in eukaryotes plays an important role in defending the cell against hydroperoxides and lipid peroxides [17], [18]. Conversely, in prokaryotes the available information about GPXs is still very limited. However, a recent report identified and characterized the Se-independent GPX BtuE from E. coli, which in vitro can catalyze the decomposition of a variety of peroxides, mainly lipid peroxides, using thioredoxins A or C as the reducing agent. It was also shown that, like other E. coli antioxidant genes, btuE is induced under oxidative stress conditions [19].
Tellurite toxicity is due at least in part to the generation of oxidative stress that alters different cellular processes [9]; therefore, the role of the E. coli btuE gene product was examined in vivo. The btuE gene was cloned and its effects were analyzed in cells exposed to various ROS elicitors. Results were compared to those obtained with mutants lacking btuE and to genetically complemented ΔbtuE cells. Taken together, the emerging picture is that BtuE is involved in protecting the cell from the deleterious effects caused by exposure to tellurite as well as to other ROS elicitors.
Results
BtuE mediates resistance to ROS elicitors in E. coli
To assess whether BtuE plays a role in the resistance of E. coli to oxidative stress, growth inhibition zones were determined for wild-type, btuE-overexpressing (pBAD/btuE), btuE-deficient (ΔbtuE) and genetically complemented btuE mutant (ΔbtuE pBAD/btuE) cells (Table 1). Tested ROS elicitors included the superoxide-generating potassium tellurite [4], the hydroxyl radical elicitor chromate [20], [21], and hydrogen peroxide [7]. Cadmium chloride, whose toxicity seems not to involve ROS generation, was used as control [22].
Table 1. BtuE mediates resistance to ROS elicitors in E. coli.
| Growth inhibition zone (cm2) | ||||
| Strain | K2TeO3 | H2O2 | K2CrO4 | CdCl2 |
| BW25113 pBAD | 6.7±0.3 | 5.7±0.1 | 6.44 | 4.2±0.2 |
| BW25113 pBAD/btuE | 5.0±0.3 | 3.3±0.1 | 3.4±0.1 | 4.0±0.1 |
| ΔbtuE pBAD | 8.1±0.1 | 6.7±0.1 | 7.4±0.1 | 4.2±0.1 |
| ΔbtuE pBAD/btuE | 4.6±0.2 | 3.5±0.2 | 4.1±0.1 | 3.8±0.2 |
Growth inhibition zones for wild type, btuE-overexpressing (pBAD/btuE), btuE-deficient (ΔbtuE), and genetically complemented btuE mutant (ΔbtuE pBAD/btuE) cells were determined as described in Methods. Cells growing in the presence of 0.2% arabinose were exposed to K2TeO3 (10 µl, 1 mg ml−1), H2O2 (10 µl, 3% v/v), K2CrO4 (10 µl, 1 M) and CdCl2 (10 µl, 1 M). Parentheses indicate the amount and concentration of each toxin that was applied to the disks. Values are the mean of 4 to 6 independent experiments ± SD.
Cells overexpressing btuE exhibited increased resistance to compounds whose toxicity involves ROS generation. Conversely, the ΔbtuE strain showed increased sensitivity to all these compounds relative to wild type controls. Genetically complemented ΔbtuE cells exhibited resistance levels to K2TeO3, K2CrO4 and H2O2 that were nearly identical to those observed for the btuE-overexpressing wild type strain. In contrast, all tested strains showed similar sensitivity to the non-ROS-producer, thiol oxidizer, CdCl2 (Table 1).
Interestingly, when minimal inhibitory concentrations (MIC) were determined in liquid medium, the H2O2 MIC for pBAD/btuE cells was ten-fold higher than that of the parental, isogenic, control strain (Table S2). This result supports the previous observation that BtuE can function as a glutathione peroxidase in vitro [19].
BtuE protects E. coli from intracellular ROS
Cytoplasmic ROS levels were assessed using the probe 2′,7′-dihydrodichlorofluorescein diacetate, as described in Methods. All strains exposed to K2TeO3, paraquat or K2CrO4 exhibited significant probe activation; the slight probe activation observed in untreated cells is presumed to be related to metabolic ROS generation. In the absence of exogenous oxidants, mutants lacking btuE showed higher ROS content than did wild type cells. The E. coli pBAD/btuE strain and the complemented ΔbtuE mutants showed decreased levels of probe activation relative to non-overproducing strains (Table 2).
Table 2. btuE expression results in decreased intracellular ROS.
| Fluorescence (AU/mg protein×103) | ||||
| E. coli strain | Control | K2TeO3 | Paraquat | K2CrO4 |
| BW25113 pBAD | 14.6±0.8 | 26.5±0.8 | 22.0±1.7 | 96.1±3.0 |
| BW25113 pBAD/btuE | 10.0±1.2 | 20.0±1.2 | 12.7±2.2 | 47.2±2.8 |
| ΔbtuE pBAD | 19.1±2.2 | 26.5±0.8 | 23.1±0.9 | 95.0±3.1 |
| ΔbtuE pBAD/btuE | 10.1±1.0 | 20.8±0.7 | 13.4±2.4 | 48.3±1.8 |
Cytoplasmic ROS content was assessed by measuring the activation of 2′,7′-dihydrodichlorofluorescein diacetate in wild type, pBAD/btuE, ΔbtuE and ΔbtuE pBAD/btuE cells as described in Methods. Cells were induced with 0.2% arabinose and exposed to K2TeO3 (0.5 µg ml−1), paraquat (50 µg ml−1) or K2CrO4 (1 mM) for 15 min at 37°C. Fluorescence (AU, arbitrary units) was determined and normalized per mg of protein. Values represent the mean of three independent trials ± SD.
To further analyze the protective role of BtuE against ROS generated during the normal metabolism, we studied the effect of overexpressing btuE in strains lacking superoxide dismutases (ΔsodAB) or catalases/peroxidases (Hpx−). These strains suffer increased levels of O2 − and H2O2, respectively [23], [24]. Superoxide as well as peroxide levels were assessed by flow cytometry as described in Methods. BtuE production resulted in decreased ROS levels, showing a protective effect both in basal metabolic conditions as well as during oxidative stress caused by ROS elicitors (Fig. S1).
BtuE production results in decreased protein oxidation and damage to membrane lipids
The formation of carbonyl groups in some amino acid side chains is a conventional marker of ROS-mediated protein oxidation [25]. Spectrophotometric determination of derivatized carbonyl groups with 2,4-dinitrophenylhydrazine showed that E. coli ΔbtuE exhibited increased protein oxidation -even in the absence of toxicants- as compared to wild type cells. Genetic complementation of E. coli ΔbtuE, as well as overexpression of btuE, resulted in decreased protein oxidation, regardless of the ROS elicitor (Table 3).
Table 3. btuE expression alleviates oxidation of cytoplasmic proteins.
| Carbonyl groups (µmol/mg protein) | |||
| E. coli strain | Control | K2TeO3 | H2O2 |
| BW25113 pBAD | 8.1±5.2 | 25.0±3.1 | 16.7±1.2 |
| BW25113 pBAD/btuE | 9.6±2.9 | 10.3±0.9 | 12.3±2.2 |
| ΔbtuE pBAD | 17.1±6.0 | 33.0±10.1 | 39.6±5.0 |
| ΔbtuE pBAD/btuE | 8.3±0.9 | 9.6±1.4 | 13.2±4.6 |
Protein oxidation was determined in wild type, pBAD/btuE, ΔbtuE and ΔbtuE pBAD/btuE cells by the chemical protein carbonyl assay described in Methods. Total protein present in extracts of cells grown in the presence of 0.2% arabinose and exposed for 30 min to K2TeO3 (0.5 µg ml−1) or H2O2 (100 µM) were reacted with 2,4-dinitrophenylhydrazine, and the specific carbonyl absorbance was read at 370 nm. Values represent the mean of three independent experiments ± SD.
Thiobarbituric acid responsive substances (TBARS) are routinely used to assess oxidative stress damage to membrane lipids in diverse organisms [26], [27]. TBAR content increased ∼3- and ∼5-fold when E. coli was exposed to K2TeO3 or H2O2, respectively (Table 4). Even in the absence of toxicant, E. coli ΔbtuE showed increased (∼6-fold) levels of these substances relative to wild-type controls, suggesting that BtuE may function in controlling the level of membrane peroxidation products that are generated during the normal, basal metabolism. Interestingly, thiobarbituric acid responsive substances levels did not increase further when ΔbtuE cells were exposed to K2TeO3 or H2O2 (Table 4).
Table 4. Elimination of btuE results in increased thiobarbituric acid-reactive substances in E. coli.
| pmol TBARS/mg protein | |||
| Strain | Control | K2TeO3 | H2O2 |
| BW25113 | 24.6±5.7 | 81.20±8.0 | 131.9±18.6 |
| ΔbtuE | 162.0±9.0 | 147.0±27.1 | 144.0±2.5 |
Membrane lipid peroxidation products were determined as thiobarbituric acid-reactive substances (TBARS) in wild type (BW25113) and ΔbtuE strains in the absence (control) or presence of K2TeO3 (0.5 µg ml−1) or H2O2 (100 µM) for 30 min. Values represent the mean of three independent experiments ± SD.
Given the above results the level of lipid peroxides was determined in all studied strains, using the method described by Cha et al. [16]. BtuE overproduction resulted in decreased levels of lipid peroxides. Conversely, btuE-lacking cells showed increased levels of lipid peroxides regardless of the presence or absence of tellurite or hydrogen peroxide, suggesting that BtuE might participate in preventing membrane damage. As expected, upon genetic complementation E. coli ΔbtuE exhibited decreased levels of lipid peroxides (Table S3).
btuE expression results in decreased induction of ibpA, soxS and katG genes
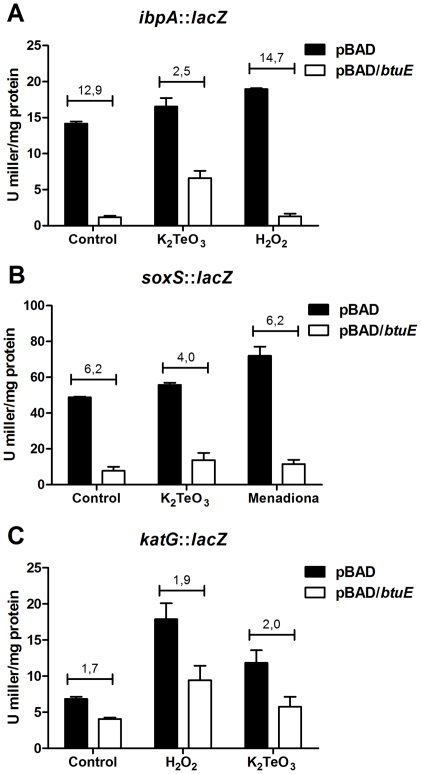
The E. coli reporter strains ADA110 [4], [28], SP11 and GS022 were used to assess the protective effect that BtuE confers against ROS elicitors. These strains harbor chromosomal insertions of the lacZ gene under the control of ibpA, soxS and katG promoters, respectively, which are induced under different stress conditions such as misfolding of cytoplasmic proteins and oxidative stress (ibpA), the presence of superoxide-generating compounds (soxS), and peroxides (katG). The effect of btuE overexpression was assessed by transforming them with pBAD/btuE or pBAD (control) plasmids and monitoring β-galactosidase activity after exposure to K2TeO3, menadione or H2O2. As expected, increased β-galactosidase activity was observed after toxicant exposure for all tested strains under control conditions. In turn, btuE overexpression resulted in a considerable decrease of enzyme activity, even in the absence of toxicants (Fig. 1). In fact, by hampering the activation of the ibpA promoter in E. coli ADA110, btuE overexpression resulted in a ∼13- (control), 2.5- (tellurite) and 15-fold (peroxide) diminution of β-galactosidase activity compared to strains harboring the pBAD vector. This result suggests that BtuE might protect the cell by decreasing oxidative stress and cytoplasmic protein misfolding, whether these are generated by basal metabolism or by ROS elicitors (Fig. 1A).
Figure 1. Effect of btuE expression on the transcriptional level of ibpA, soxS, and katG.
β-galactosidase activity was assayed as described [39] in E. coli ADA110 (ibpA::lacZ) (A), SP11 (soxS::lacZ) (B), and GS022 (katG::lacZ) (C) strains carrying pBAD or pBAD/btuE. Data are normalized to the concentration of protein. Cells were exposed for 3 h (ADA110), 30 min (SP11) or 25 min (GS022) in the absence (control) or presence of K2TeO3 (0.5 µg/ml), menadione (100 µM) or H2O2 (100 µM). Assays were carried out in the presence of 0.2% L-arabinose. Values represent the average of three independent trials ± SD. Numbers above each condition represent the pBAD/pBAD/btuE ratio.
In addition, Figs. 1B and C show that btuE overexpression results in >4- (soxS) and ∼2-fold (katG) decrease in β-galactosidase activity relative to strains harboring pBAD vector only. By diminishing the response of the ROS defense regulons soxRS and oxyR, these results suggest that BtuE might help to alleviate oxidative stress in the E. coli cytoplasm.
BtuE protects E. coli lacking catalases and peroxidases from oxidative stress
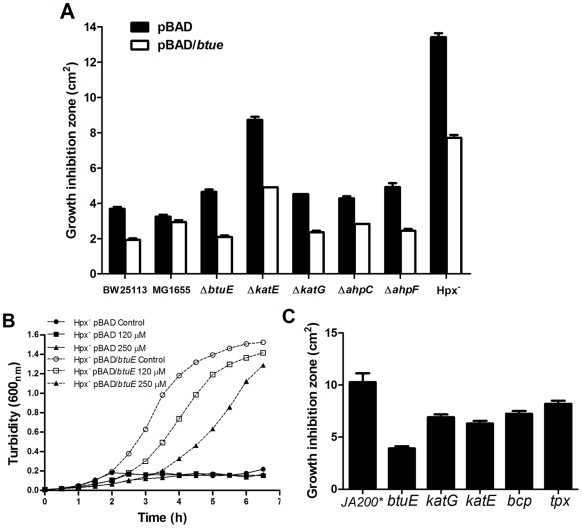
The btuE gene was expressed in different E. coli genetic backgrounds, and growth inhibition zones were determined. Fig. 2A shows that all strains exhibited greater H2O2 tolerance when btuE was overexpressed. Similar results were obtained for potassium tellurite (not shown). The same trend was observed when growth curves of the Hpx− strain overexpressing btuE were analyzed for both H2O2 (Fig. 2B) or K2TeO3 (not shown). These data support the idea that the GPX activity of BtuE protects E. coli from H2O2 exposure.
Figure 2. BtuE protects E. coli from peroxide damage.
A, wild-type (BW25113 and MG1655), ΔbtuE, catalase-deficient (ΔkatG, ΔkatE), alkyl hydroperoxidase-deficient (ΔahpC, ΔahpF), and Hpx− cells carrying pBAD or pBAD/btuE were grown aerobically in the presence of 0.2% arabinose and exposed to H2O2 (10 µl, 1 M). Growth inhibition zones represent the mean of three independent experiments ± SD. B, growth curves of Hpx− cells carrying the indicated plasmids exposed to 120 or 250 µM hydrogen peroxide. C, E. coli expressing the indicated peroxidases were grown in the presence of 1 mM IPTG and exposed to H2O2 (10 µl, 1 M). Parentheses indicates the amount and concentration of H2O2 that was applied to the disks. Bars represent the average of three independent experiments ± SD. JA200*, parental, isogenic strain that does not overexpress the analyzed peroxidases.
Given the protective effect of BtuE in E. coli Hpx− against the tested ROS elicitors, it was of interest to analyze the effect of overexpressing other peroxidase genes in this bacterium. Fig. 2C shows that BtuE generates higher H2O2 resistance than KatG and KatE catalases or BCP and Tpx peroxidases.
BtuE production results in increased resistance of E. coli to potassium tellurite and hydrogen peroxide only in aerobic conditions
Since BtuE exhibits peroxidase activity in vitro, it was reasoned that transferring the ΔbtuE mutation to an Hpx− genetic background could help in analyzing the net effect of BtuE when other H2O2 scavenging enzymes are missing. As seen in Fig. S2, the absence of btuE rendered Hpx− cells even more sensitive to TeO3 2− in the presence of oxygen. When growth curves were analyzed, the effect of the btuE mutation was more evident in aerobic conditions (Fig. S2A–B). The oxygen requirement was confirmed by determining growth inhibition zones (Fig. S2C).
Finally, the effect of the btuE mutation upon H2O2 tolerance in an Hpx− genetic background was evaluated. Fig. S2D–E shows that growth of Hpx− ΔbtuE cells is more sensitive to hydrogen peroxide than that of the parental Hpx− strain only in aerobic conditions. Again, these results were confirmed by determining growth inhibition zones (Fig. S2F).
Discussion
Since heavy metal pollution is a serious problem worldwide, there is a growing need to elucidate its toxic effects in sensitive microorganisms. It is also of interest as to unveil the resistance mechanisms that protect resistant bacteria [29]–[32].
The toxicity of some metals is mediated by the generation of oxidative stress, so the cell must invoke a number of antioxidant defences–both enzymatic and non-enzymatic–to cope with this situation. In this regard, tellurite toxicity was initially thought to arise mainly from its ability to oxidize several cellular components [5], [6], [33]. Later it was recognized that the tellurium oxyanion triggers a series of events that leads to the generation of ROS, particularly superoxide [2], [4], [8], [9], [32], [34].
The dearth of knowledge about prokaryotic glutathione peroxidases prompted us to analyze the role of the E. coli btuE gene product in cellular resistance to ROS. To assess if BtuE displays a general antioxidant function in vivo, the effect of btuE in wild-type, pBAD/btuE, ΔbtuE and ΔbtuE pBAD/btuE cells exposed to potassium tellurite and other ROS elicitors was evaluated. While in general terms ΔbtuE mutants were more sensitive to ROS elicitors, btuE overexpression resulted in enhanced cellular resistance to tellurite (∼4-fold) and hydrogen peroxide (∼10-fold) as compared to parental, wild type cells. Similar results were observed when cells were exposed to chromium, a generator of hydroxyl radicals [20], [21]. In contrast, BtuE did not influence E. coli resistance to CdCl2 (Table 1, Table S2).
To test whether BtuE might affect the level of intracellular ROS, the fluorescent, oxidation-sensitive probe 2′,7′-dihydrodichlorofluorescein diacetate was used. Tellurite, paraquat or chromate exposure resulted in increased ROS levels, above those observed in unexposed cells. E. coli ΔbtuE always exhibited higher basal ROS levels than wild type cells; conversely, E. coli pBAD/btuE showed ROS levels far below those observed in controls (Table 2). Similar results were observed when protein carbonylation was assessed (Table 3), suggesting that BtuE could participate in the response to oxidative stress by lowering cytoplasmic ROS levels.
Since thiobarbituric acid responsive substances have been used routinely to assess oxidative stress damage to lipids [4], [26], [27], the effect of BtuE on membrane lipid damage was studied. A high increase (∼6-fold) in the levels of these compounds was observed in E. coli ΔbtuE in the absence of any toxicant, suggesting that BtuE may function in preventing damage to membrane lipids or controlling the level of membrane peroxidation products (Table 4). Given that BtuE exhibits higher peroxidase activity with lipid peroxides in vitro [19], the in vivo situation was analyzed. Table S3 shows that BtuE is involved specifically in lowering lipid peroxide levels in E. coli, again indicating the importance of BtuE in membrane damage. In this context, it is interesting that Se-independent glutathione peroxidases preferentially degrade lipid peroxides [18], [35], [36].
Since tellurite toxicity is highly dependent on the presence of oxygen [1], [37] and GPXs are involved in oxidative stress, the role of BtuE in E. coli exposed to K2TeO3 both in aerobic and anaerobic conditions was analyzed. It was observed that in aerobic conditions the introduction of the btuE mutation into an Hpx− background resulted in impaired growth and in increased tellurite sensitivity (Fig. S2). Similar results were observed with hydrogen peroxide, except that in anaerobic conditions E. coli wild type strains (BW25113 and MG1655) as well as the ΔbtuE strain showed higher H2O2 sensitivity. This may be due to the fact that in aerobic conditions cells display fully induced antioxidant mechanisms to cope with peroxide [7]. In addition, no difference in peroxide resistance was observed between Hpx− and Hpx− ΔbtuE strains, suggesting that BtuE is important only in aerobic conditions (Fig. S2D–E–F). In support of this, when btuE was expressed in E. coli defective in H2O2-scavenging, increased H2O2 tolerance was observed in all btuE-complemented mutants (Fig. 2A–B).
Given that BtuE also efficiently decomposes lipid peroxides in vitro [19], we speculate that although adding hydrogen peroxide in anaerobic conditions can trigger a number of oxidative events, lipid peroxidation will not occur since it requires molecular oxygen. In this context, the toxic substrates of BtuE will be missing so that the enzyme will have no effect. Further experiments to unveil the global role of BtuE in the E. coli oxidative metabolism are under way in our laboratory.
Materials and Methods
Bacterial strains and culture conditions
Bacterial strains used in this study are listed in Table S1. Cells were grown routinely in LB medium [38] at 37°C with shaking. Growth was initiated by inoculating fresh LB medium with 1∶100 dilutions of overnight cultures. Solid media contained 2% (w/v) agar, and plates were incubated overnight at 37°C.
Anaerobic growth (liquid and solid media) was carried out in a Coy chamber (Coy Laboratory Products, Inc.) under 85% N2, 10% H2, and 5% CO2. Anaerobic buffers and media were moved into the chamber immediately after being autoclaved and allowed to equilibrate with the anaerobic atmosphere for at least 24 h prior to use.
E. coli harboring pBAD or pBAD/btuE plasmids (see below) were grown in LB containing ampicillin (100 µg ml−1) at 37°C with continuous agitation. When the cultures reached an OD600 ∼0.4, L-arabinose (0.2% final concentration) was added. Induction was for 4 h at 37°C with shaking. Strains lacking btuE (ΔbtuE) and all other mutants were grown in LB medium containing kanamycin (100 µg ml−1).
Growth curves
To ensure that all studies were being conducted with exponentially growing cells, aerobic or anaerobic overnight cultures were diluted in fresh LB medium to an OD600 ∼0.005 and grown at 37°C until they achieved an OD600 of ∼0.1–0.2. Cultures were then diluted 10-fold into fresh medium containing K2TeO3 or H2O2, and they were grown at 37°C. Absorbance at 600 nm was monitored at 30 min intervals. Cell blackening due to tellurite reduction was negligible at tellurite concentrations up to 0.1 µg ml−1. In determining anaerobic growth, absorbance measurements were carried out at 1 h intervals.
Cloning the E. coli btuE gene and strain construction
In order to amplify the btuE gene from the E. coli genome, specific primers (Table S1) were designed using the VECTOR 9 NTI (Invitrogen®) software. The PCR product was inserted into pBAD/TOPO (Invitrogen®) vector, according to manufacturer's instructions, resulting in plasmid pBAD/btuE. Identity/integrity of btuE was checked by DNA sequencing.
Strain Hpx− ΔbtuE was constructed by P1 transduction [39] between JEM216 x ΔbtuE (Table S1), selecting for kanamicyn resistance. The btuE mutation in the resulting strain was confirmed by PCR using primers listed in Table S1.
Determination of growth inhibition zones
Growth inhibition zones were determined in LB-agar plates as described [40]. In brief, overnight cultures were diluted with LB and grown at 37°C for 4 h. After dilution to an OD600 ∼0.1, 100 µl of each culture was evenly spread on the plates. Plates were air dried, and toxins to be tested (10 µl) were deposited on sterile 6 mm filter disks placed on the centres of the plates. Growth inhibition areas were determined after overnight incubation at 37°C. Determination of growth inhibition zones in anaerobic conditions followed an identical protocol, but all manipulations were carried out inside a Coy anaerobic chamber.
Determination of the minimal inhibitory concentration
Sterile stock solutions of appropriate concentrations of K2TeO3, K2CrO4, CdCl2 or H2O2 were serially diluted in a 96-well ELISA plate containing 200 µl of LB medium (plus the appropriate antibiotic) per well. Five µl of cultures grown at 37°C in LB medium supplemented with the required antibiotic(s) to an OD600 ∼0.4 were added to each well, and the plate was incubated at 37°C. Turbidity was observed visually after 24 h. MIC determinations in anaerobic conditions followed the same protocol in a Coy chamber.
Determination of intracellular reactive oxygen species
In general, cellular oxidants, including ROS, were assessed using the oxidation-sensitive probe 2′,7′-dichlorofluorescein diacetate. As demonstrated by Royall and Ischiropoulos [41], once inside the cell this esterified probe is deacetylated by intracellular esterases and the resulting compound, dichlorofluorescin, is susceptible to oxidation by ROS. Briefly, cells grown aerobically in LB medium to an OD600 ∼0.4 were exposed for 30 min to K2TeO3 (0.5 µg ml−1), paraquat (50 µg ml−1) or K2CrO4 (1 mM). They were then centrifuged, washed with 10 mM potassium phosphate buffer, pH 7.0, and incubated for 30 min in the same buffer containing the probe (10 mM final concentration). Cells were subsequently washed and disrupted by sonication. One hundred µl of the resulting cell extracts were mixed with 1 ml of the same buffer, and fluorescence intensity was determined using an Applied Biosystems Citofluor 4000 Fluorescence Multi-well plate reader (excitation 490 nm, emission 519 nm). Emission values were standardized by protein concentration [4], [42].
E. coli ΔsodAB and Hpx− strains transformed with the indicated plasmids (Table S1) were used to determine intracellular ROS by flow cytometry. Cells were grown to an OD600 ∼0.5 in the presence of arabinose at 37°C, and they were then exposed to K2TeO3 (0.5 µg ml−1) for 30 min. After centrifugation at 5,000 g for 10 min, cells were washed with saline phosphate buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.3) and diluted 1∶10 with the same buffer. Cells were incubated with 10 mM 2′,7′-dihydrodichlorofluorescein diacetate (or 127 µM dihydroethidine) for 30 min, centrifuged at 5,000 g for 10 min, and washed with the same buffer [43]. Fluorescence intensity was determined using a Becton Dickinson apparatus equipped with an argon laser.
Determination of cytoplasmic protein oxidation
Oxidized cytoplasmic proteins were assessed as described by Semchyshyn et al. [27]. Briefly, nucleic acids-free cell extracts (100 µl) were prepared from cells exposed to K2TeO3 (0.5 µg ml−1) or H2O2 (100 µM) for 30 min. The extracts were mixed with 4 volumes of 10 mM 2,4-dinitrophenylhydrazine and incubated at room temperature for 1 h with occasional vortexing. Proteins were subsequently precipitated by the addition of 500 µl of 20% trichloroacetic acid, and precipitate was pelleted by centrifugation at 14,000 g for 5 min. After three washes with a 1∶1 solution of ethanol:ethyl acetate, the sediment was dissolved in 450 µl of 50 mM dithiothreitol in 6 M guanidine HCl at 37°C. Carbonyl content was determined spectrophotometrically at 370 nm using a molar absorption coefficient of 22,000 M−1cm−1 [4], [27].
Determination of thiobarbituric acid-reactive substances
Cultures (4 ml) exposed or not exposed to K2TeO3 (0.5 µg ml−1) or H2O2 (100 µM) were centrifuged, washed twice, and suspended in 1 ml of 50 mM potassium phosphate buffer, pH 7.4, containing 0.1 mM butylated hydroxytoluene and 1 mM PMSF (phenylmethanesulfonyl fluoride). Cells were subjected to sonic disruption and centrifuged to discard the debris. The soluble fraction was mixed with 1 ml of 20% trichloroacetic acid and centrifuged at 10,000 g for 5 min. Supernatants were mixed with 2 ml of a saturated solution of thiobarbituric acid in 0.1 M HCl and 10 mM butylated hydroxytoluene. Samples were heated at 100°C for 1 h, and 1.5 ml aliquots were removed, cooled, mixed with 1.5 ml of butanol, and centrifuged at 4,000 g for 10 min. The organic fraction was removed, and the OD535 was determined. Thiobarbituric acid-reactive substances content was determined using an ε = 156 mM−1cm−1 [4], [27].
Determination of membrane lipid peroxidation
The concentration of membrane lipid peroxides was determined as described by Cha et al. [16]. Briefly, 45 mg of cell sediment were suspended in Tris-HCl (pH 7.4) buffer containing 1% sodium dodecyl sulfate. After sonicating and washing with distilled water to remove the detergent, the sediment was air dried and dissolved in 1 ml of ethanol:chloroform (2∶1 v/v). After vigorous shaking for 1 h, FOXII reagent (ferrous oxidation in the presence of xylenol orange) was added, and the mixture was shaked again for 1 h at room temperature. After centrifuging at 13,000 g for 10 min, the clear supernatant was used to determine the content of membrane lipid peroxides at 560 nm [16].
Supporting Information
Effect of BtuE in the generation of intracellular ROS. Cytoplasmic superoxide (A) or ROS (B) were determined by flow cytometry using dihydroethidine or 2′,7′-dihydrodichlorofluorescein diacetate in E. coli ΔsodAB or Hpx− strains, respectively, exposed or not to K2TeO3 (0,5 µg/ml) for 30 min in the presence of 0.2% L-arabinose. Representative profiles of fluorescence intensity regarding the cell number (above) for the analyzed strains and histograms representing % of fluorescence intensity of control (pBAD) and pBAD/btuE cells (below) are shown. 100% of fluorescence intensity corresponds to the strain carrying pBAD only. Bars represent the average of three independent experiments ± SD. Numbers above each condition represent the pBAD/pBAD/btuE ratio.
(TIF)
BtuE protects E. coli from potassium tellurite and hydrogen peroxide in aerobic conditions. E. coli Hpx− and Hpx−ΔbtuE strains were grown aerobically (A) or anaerobically (B) in LB medium to an OD600 ∼0.01, and K2TeO3 was added to a final concentration of 0 (control, ○, •), 0.001 (□, ▪) and 0.005 µg ml−1 (Δ, ▴). Data are representative of three independent experiments. (C), Growth inhibition zones were assessed for Hpx− and Hpx− ΔbtuE cells grown aerobically (+O2) or anaerobically (−O2) and exposed to K2TeO3 (10 µl, 1 µg/µl). Values represent the mean of three independent experiments ± SD. E. coli Hpx− and Hpx−ΔbtuE were grown aerobically (D) or anaerobically (E) in LB medium to an OD600 ∼0.01, and H2O2 was added to a final concentration of 0 (control, ○, •), 15 (□, ▪) and 30 µM (Δ, ▴). Data are representative of three independent experiments. (F), Growth inhibition zones were assessed for Hpx− and Hpx− ΔbtuE cells grown aerobically (+O2) or anaerobically (−O2) and exposed to H2O2 (10 µl, 1 M). Values represent the mean of three independent experiments ± SD.
(TIF)
Bacterial strains, plasmids and primers used in this study.
(DOCX)
BtuE mediates resistance to potassium tellurite and other ROS elicitors in E. coli .
(DOCX)
Elimination of btuE results in decreased lipid peroxide levels in E. coli .
(DOCX)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: F.A.A. received doctoral fellowships from Conicyt and MECESUP UCH407, Chile. J.M.P. was sponsored by a postdoctoral fellowship from Dirección de Investigación, Universidad de Santiago de Chile. J.M.S. received a doctoral fellowship from MECESUP, Chile. This work was supported by grants # 1090097 from Fondecyt and Dicyt-USACH, to C.C.V, and from National Institutes of Health grant GM049640 to J.A.I. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tantaleán JC, Araya MA, Saavedra CP, Fuentes DE, Pérez JM, et al. The Geobacillus stearothermophilus V iscS gene, encoding cysteine desulfurase, confers resistance to potassium tellurite in Escherichia coli K-12. J Bacteriol. 2003;19:5831–5837. doi: 10.1128/JB.185.19.5831-5837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borsetti F, Tremaroli V, Michelacci F, Borghese R, Winterstein C, et al. Tellurite affects in Rhodobacter capsulatus cell viability and superoxide dismutase activity under oxidative stress conditions. Res Microbiol. 2005;156:807–813. doi: 10.1016/j.resmic.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Tremaroli V, Fedi S, Zannoni D. Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress in cells of Pseudomonas pseudoalcaligenes KF707. Arch Microbiol. 2007;187:127–135. doi: 10.1007/s00203-006-0179-4. [DOI] [PubMed] [Google Scholar]
- 4.Pérez JM, Calderón IL, Arenas FA, Fuentes DE, Pradenas GA, et al. Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS ONE. 2007;2:e211. doi: 10.1371/journal.pone.0000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor DE. Bacterial tellurite resistance. Trends Microbiol. 1999;7:111–115. doi: 10.1016/s0966-842x(99)01454-7. [DOI] [PubMed] [Google Scholar]
- 6.Turner RJ, Weiner JH, Taylor DE. Tellurite-mediated thiol oxidation in Escherichia coli. Microbiology. 1999;145:2549–2557. doi: 10.1099/00221287-145-9-2549. [DOI] [PubMed] [Google Scholar]
- 7.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 8.Calderón IL, Elías AO, Fuentes EL, Pradenas GA, Castro ME, et al. Tellurite-mediated disabling of [4Fe-4S] clusters of Escherichia coli dehydratases. Microbiology. 2009;155:1840–1846. doi: 10.1099/mic.0.026260-0. [DOI] [PubMed] [Google Scholar]
- 9.Chasteen TG, Fuentes DE, Tantaleán JC, Vásquez CC. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol Rev. 2009;33:820–832. doi: 10.1111/j.1574-6976.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- 10.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Åslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci USA. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gort AS, Ferbe DM, Imlay JA. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol Microbiol. 1999;32:179–191. doi: 10.1046/j.1365-2958.1999.01343.x. [DOI] [PubMed] [Google Scholar]
- 13.Storz G, Imlay JA. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 14.Liochev SI, Benov L, Touati D, Fridovich I. Induction of the soxRS regulon of Escherichia coli by superoxide. J Biol Chem. 1999;274:9479–9481. doi: 10.1074/jbc.274.14.9479. [DOI] [PubMed] [Google Scholar]
- 15.Schellhorn HE. Regulation of hydroperroxidase (catalase) expression in Escherichia coli. FEMS Microbiol Lett. 1994;131:113–119. doi: 10.1111/j.1574-6968.1995.tb07764.x. [DOI] [PubMed] [Google Scholar]
- 16.Cha MK, Kim WC, Lim CJ, Kim K, Kim IH. Escherichia coli periplasmic thiol peroxidase acts as lipid hydroperoxide peroxidase and the principal antioxidative function during anaerobic growth. J Biol Chem. 2004;279:8769–8778. doi: 10.1074/jbc.M312388200. [DOI] [PubMed] [Google Scholar]
- 17.Herbette S, Lenne C, Leblanc N, Julien JL, Drevet JR, et al. Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem. 2002;269:2414–2420. doi: 10.1046/j.1432-1033.2002.02905.x. [DOI] [PubMed] [Google Scholar]
- 18.Herbette S, Roeckel-Drevet P, Roeckel-Drevet J. Seleno-independent glutathione peroxidases: more than simple antioxidant scavengers. FEBS J. 2007;274:2163–2180. doi: 10.1111/j.1742-4658.2007.05774.x. [DOI] [PubMed] [Google Scholar]
- 19.Arenas FA, Díaz WA, Leal CA, Pérez-Donoso JM, Imlay JA, et al. The Escherichia coli btuE gene, encodes a glutathione peroxidase that is induced under oxidative stress conditions. Biochem Biophys Res Commun. 2010;398:690–694. doi: 10.1016/j.bbrc.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh M, Nakamura M, Suzuki T, Hawai K, Horitsu H, et al. Mechanism of chromium(VI) toxicity in Escherichia coli: is hydrogen peroxide essential in Cr(VI) toxicity? J Biochem. 1995;117:780–786. doi: 10.1093/oxfordjournals.jbchem.a124776. [DOI] [PubMed] [Google Scholar]
- 21.Ackerley DF, Barak Y, Lynch SV, Curtin J, Matin A. Effect of chromate stress on Escherichia coli K-12. J Bacteriol. 2006;188:3371–3381. doi: 10.1128/JB.188.9.3371-3381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang A, Crowley D. Global gene expression responses to cadmium toxicity in Escherichia coli. J Bacteriol. 2005;187:3259–3266. doi: 10.1128/JB.187.9.3259-3266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seaver LC, Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx− mutants of Escherichia coli. Proc Natl Acad Sci USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 26.Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, et al. Bactericidal activity of photocatalytic TiO(2) reaction: towards an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65:4094–4098. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semchyshyn H, Bagnyukova T, Storey K, Lushchak V. Hydrogen peroxide increases the activities of soxRS regulon enzymes and the levels of oxidized proteins and lipids in Escherichia coli. Cell Biol Intern. 2005;29:898–902. doi: 10.1016/j.cellbi.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro E, Baneyx F. Stress-based identification and classification of antibacterial agents: second generation Escherichia coli reporter strains and optimization of detection. Antimicrob Agents Chemother. 2002;46:2490–2497. doi: 10.1128/AAC.46.8.2490-2497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver S, Phung L. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 30.Nies DH. Microbial heavy-metal resistance. Appl Environ Microbiol. 1999;51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 31.Silver S, Phung L. A bacterial view of the periodic table: genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol. 2005;32:587–605. doi: 10.1007/s10295-005-0019-6. [DOI] [PubMed] [Google Scholar]
- 32.Tremaroli V, Workentine ML, Weljie AM, Vogel HJ, Ceri H, et al. Metabolomic investigation of the bacterial response to a metal challenge. Appl Environ Microbiol. 2009;75:719–728. doi: 10.1128/AEM.01771-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Summers A, Jacoby G. Plasmid determined resistance to tellurium compounds. J Bacteriol. 1977;129:276–281. doi: 10.1128/jb.129.1.276-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez JM, Arenas FA, Pradenas GA, Sandoval JM, Vásquez CC. YqhD is an aldehyde reductase that protects Escherichia coli from harmful lipid peroxidation-derived aldehydes. J Biol Chem. 2008;283:7346–7353. doi: 10.1074/jbc.M708846200. [DOI] [PubMed] [Google Scholar]
- 35.Avery A, Willetts S, Avery S. Genetic dissection of the phospholipid hydroperoxidase activity of yeast gpx3 reveals its functional importance. J Biol Chem. 2004;279:46652–46658. doi: 10.1074/jbc.M408340200. [DOI] [PubMed] [Google Scholar]
- 36.Gaber A, Yoshimura K, Tamoi M, Takeda T, Nakano Y, et al. Induction and functional analysis of two reduced nicotinamide adenine dinucleotide phosphate-dependent glutathione peroxidase-like proteins in Synechocystis PCC 6803 during the progression of oxidative stress. Plant Physiol. 2004;136:2855–2861. doi: 10.1104/pp.104.044842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borghese R, Borsetti F, Foladori P, Ziglio G, Zannoni D. Effects of the metalloid oxyanion tellurite (TeO3 2−) on growth characteristics of the photorophic bacterium Rhodobacter capsulatus. Appl Environ Microbiol. 2004;70:6595–6602. doi: 10.1128/AEM.70.11.6595-6602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch EF, Maniatis T. Cold Spring Harbor, N.Y: 2nd Ed. Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: a laboratory manual. [Google Scholar]
- 39.Miller JH. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. Experiments in Molecular Genetics, 201–205, 352–355,and 431–433. [Google Scholar]
- 40.Fuentes DE, Fuentes EL, Castro ME, Pérez JM, Araya MA, et al. Cysteine metabolism-related genes and bacterial resistance to potassium tellurite. J Bacteriol. 2007;189:8953–8960. doi: 10.1128/JB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Royall JA, Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 42.Echave P, Tamarit J, Cabiscol E, Ros J. Novel antioxidant role of alcohol dehydrogenase E from Escherichia coli. J Biol Chem. 2003;32:30193–30198. doi: 10.1074/jbc.M304351200. [DOI] [PubMed] [Google Scholar]
- 43.Herrera G, Martínez A, Blanco M, O'Connor J. Functional assays of oxidative stress using genetically engineered Escherichia coli strains. Curr Protocols Cyt. 2003:11.16.1–11.16.9. doi: 10.1002/0471142956.cy1116s24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of BtuE in the generation of intracellular ROS. Cytoplasmic superoxide (A) or ROS (B) were determined by flow cytometry using dihydroethidine or 2′,7′-dihydrodichlorofluorescein diacetate in E. coli ΔsodAB or Hpx− strains, respectively, exposed or not to K2TeO3 (0,5 µg/ml) for 30 min in the presence of 0.2% L-arabinose. Representative profiles of fluorescence intensity regarding the cell number (above) for the analyzed strains and histograms representing % of fluorescence intensity of control (pBAD) and pBAD/btuE cells (below) are shown. 100% of fluorescence intensity corresponds to the strain carrying pBAD only. Bars represent the average of three independent experiments ± SD. Numbers above each condition represent the pBAD/pBAD/btuE ratio.
(TIF)
BtuE protects E. coli from potassium tellurite and hydrogen peroxide in aerobic conditions. E. coli Hpx− and Hpx−ΔbtuE strains were grown aerobically (A) or anaerobically (B) in LB medium to an OD600 ∼0.01, and K2TeO3 was added to a final concentration of 0 (control, ○, •), 0.001 (□, ▪) and 0.005 µg ml−1 (Δ, ▴). Data are representative of three independent experiments. (C), Growth inhibition zones were assessed for Hpx− and Hpx− ΔbtuE cells grown aerobically (+O2) or anaerobically (−O2) and exposed to K2TeO3 (10 µl, 1 µg/µl). Values represent the mean of three independent experiments ± SD. E. coli Hpx− and Hpx−ΔbtuE were grown aerobically (D) or anaerobically (E) in LB medium to an OD600 ∼0.01, and H2O2 was added to a final concentration of 0 (control, ○, •), 15 (□, ▪) and 30 µM (Δ, ▴). Data are representative of three independent experiments. (F), Growth inhibition zones were assessed for Hpx− and Hpx− ΔbtuE cells grown aerobically (+O2) or anaerobically (−O2) and exposed to H2O2 (10 µl, 1 M). Values represent the mean of three independent experiments ± SD.
(TIF)
Bacterial strains, plasmids and primers used in this study.
(DOCX)
BtuE mediates resistance to potassium tellurite and other ROS elicitors in E. coli .
(DOCX)
Elimination of btuE results in decreased lipid peroxide levels in E. coli .
(DOCX)