Abstract
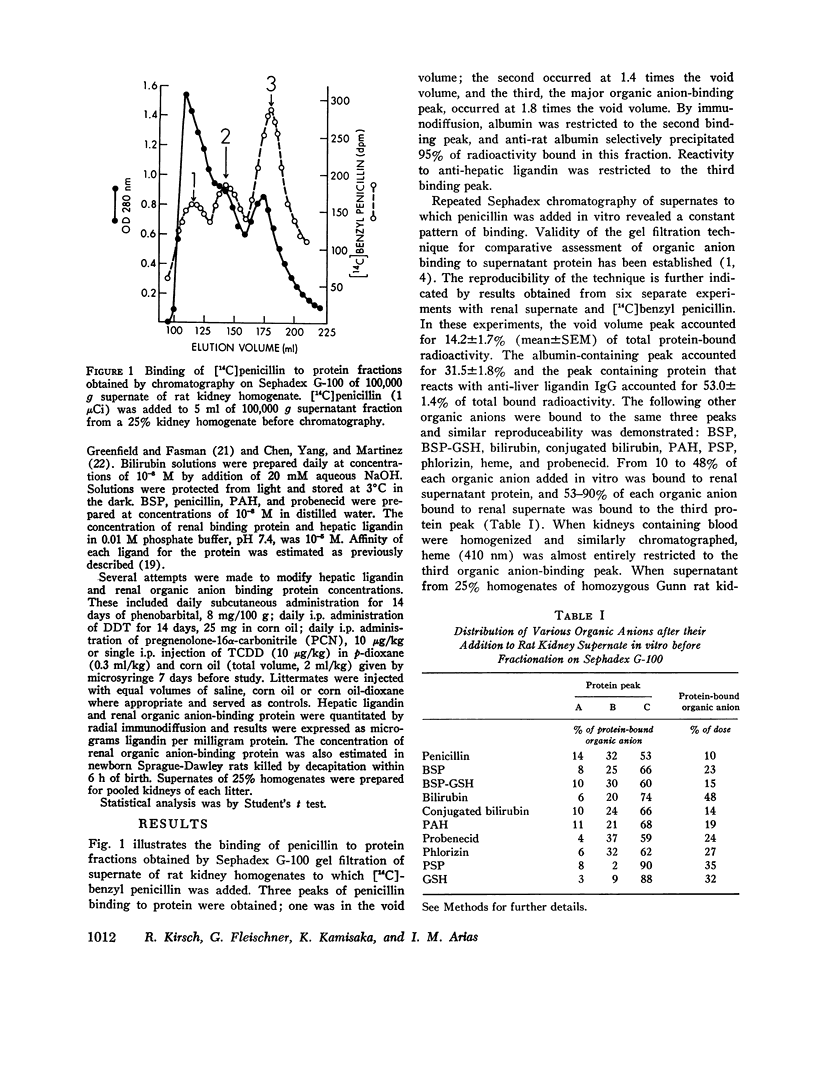
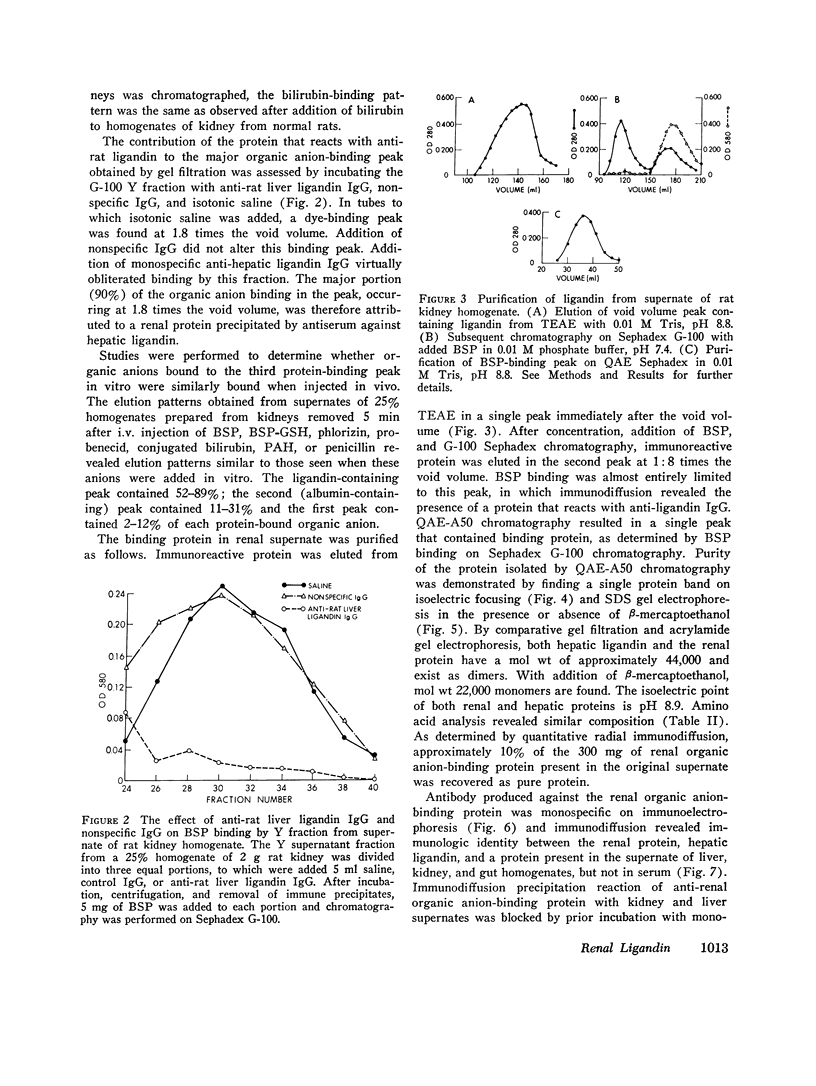
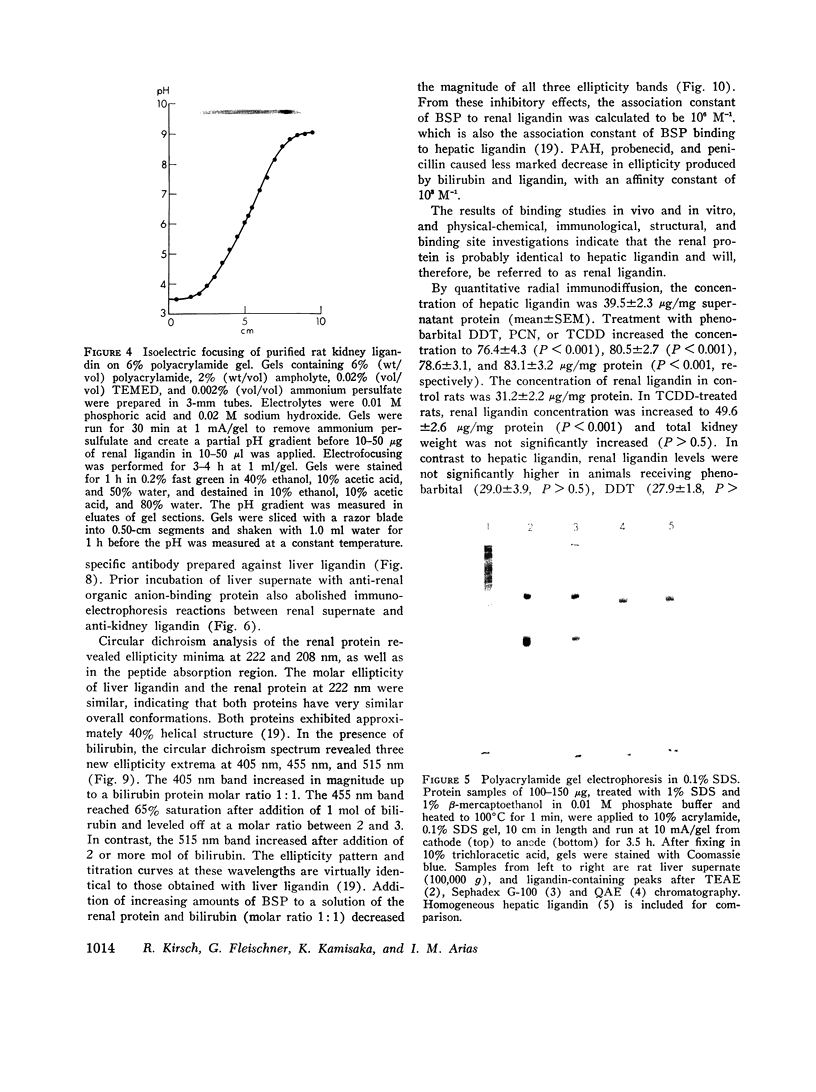
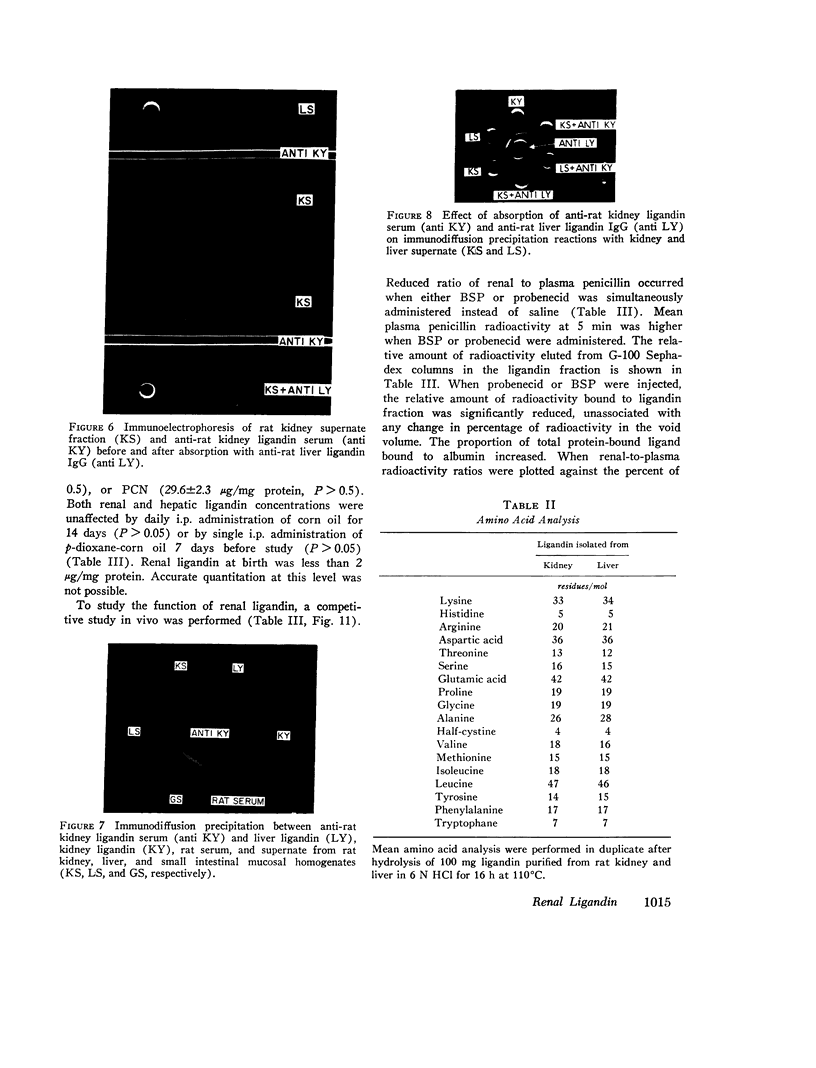
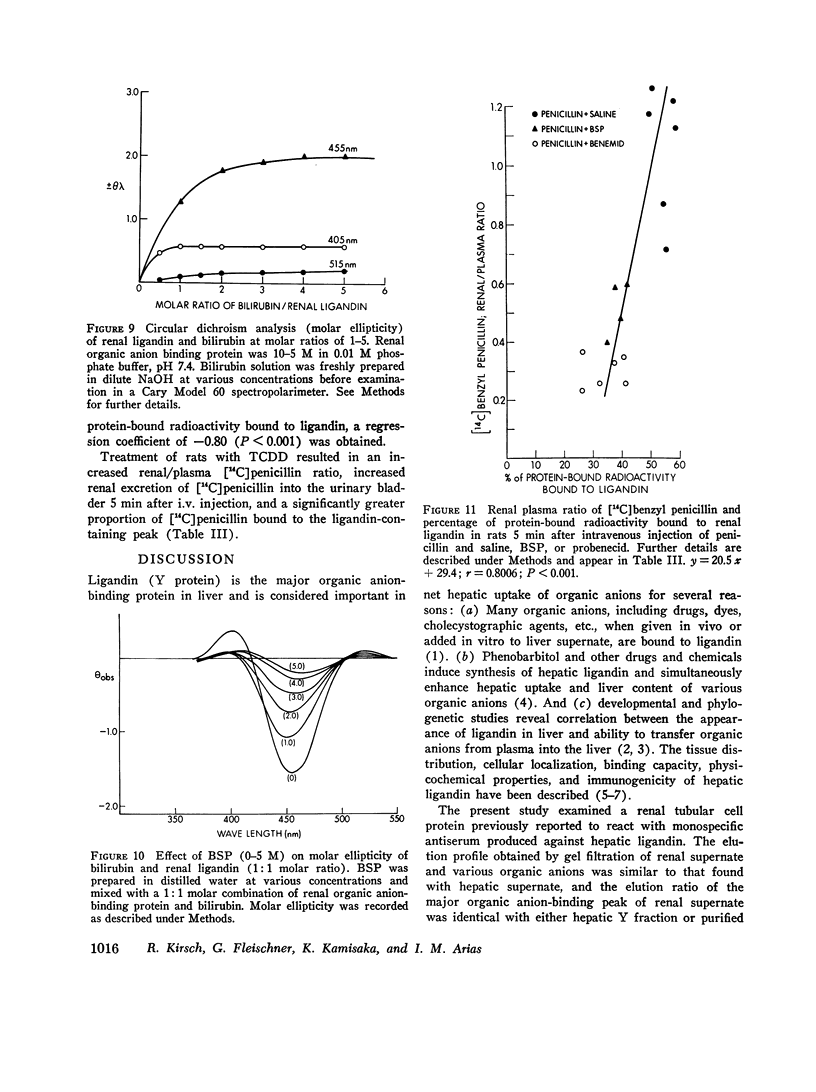
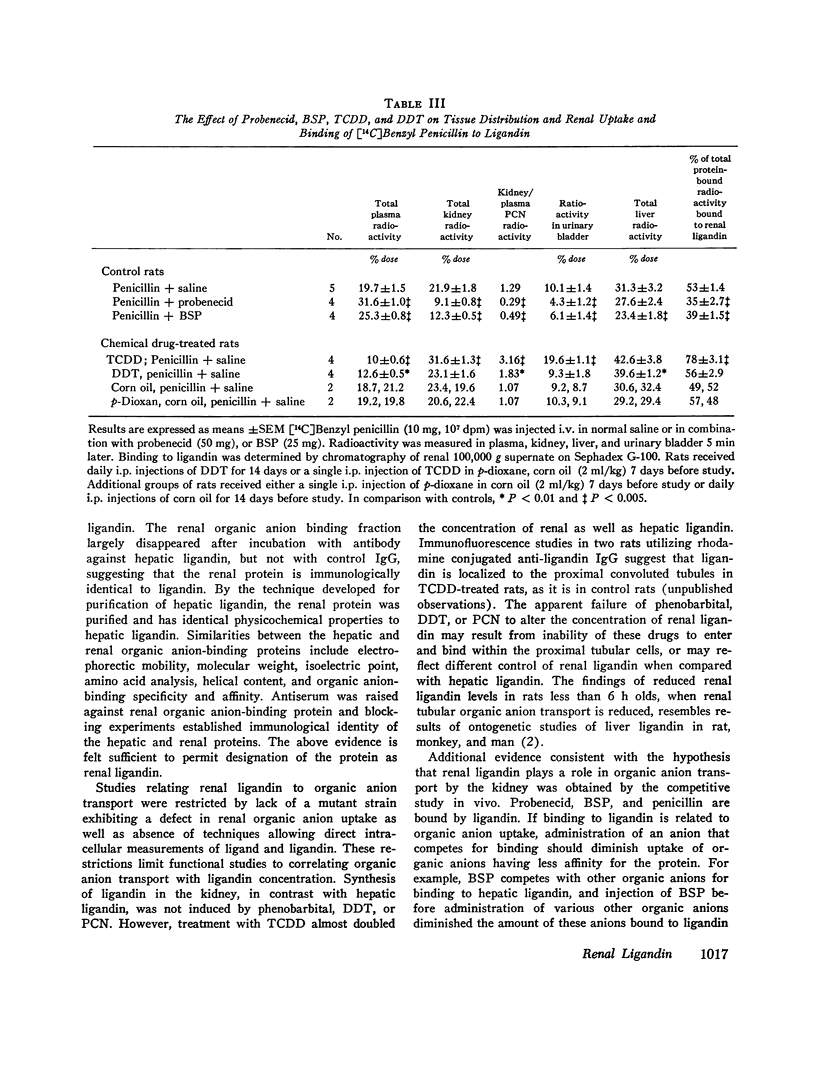
Sephadex gel filtration of the 1000,000 g supernate of homogenates of rat kidney revealed binding of various organic anions (penicillin, Bromsulphalein [BSP], bilirubin, phenolsulfonphthalein [PSP], phlorizin, glutathione [GSH], p-amino hippurate (PAH), probenecid, conjugated bilirubin, and BSP-GSH) to a nonalbumin-containing protein fraction (Y), which precipated on addition of monospecific anti-rat liver ligandin (Y protein)-IgG, but not control IgG. Quantitatively similar organic anion binding was observed in vivo after injection of BSP, BSP-GSH, phlorizin, probenecid, conjugated bilirubin, PAH, or penicillin. The binding protein was purified to apparent homogeneity and is a basic protein (pI 8.9) of 44,000 daltons with two apparently identical subunits of 22,000 daltons. Monospecific antibody was produced against the renal protein. The results of binding studies in vivo and in vitro and phsicochemical, immunologic, structural, and binding site investigations indicate that the renal protein is identical to hepatic ligandin. Immunofluorescent studies utilizing anti-ligandin IgG previously localized ligandin in the kidney to all proximal tubular cells. By quantitative radial immunodiffusion, the concentration of renal ligandin was 31.2 plus or minus 2.2 mug/mg supernatant protein and was increased 160% above basal values by pretreatment of rats with tetrachloro-dibenzo-p-dioxin. Pretreatment with phenobarbital, DDT, or pregnene-16alpha-carbonitrile did not increase renal ligandin concentration but doubled hepatic ligandin concentration. Circular dichroism studies of renal ligandin revealed percent helical structure similar to hepatic ligandin and primary association contrasts were derived for BSP (10-6 M-1) and PAH, probenecid, and penicillin (10-3 M-1). Administration of BSP or probenecid simultaneously with [C14] penicillin resulted in increased plasma retention and reduced kidney and urinary bladder content of [14C] penicillin and a correlation coefficient of -0.8 between total kidney/plasma radioactivity and percent of protein-bound radioactivity bound to ligandin in the kidney. These studies indicate that renal and hepatic ligandin are identical. Their response to drugs and chemicals varies. Competitive binding between several organic anions for ligandin correlated with their renal uptake from plasma, which suggests that ligandin may function in the proximal tubular cell as a component of the renal organic anion transport system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blauer G., King T. E. Interactions of bilirubin with bovine serum albumin in aqueous solution. J Biol Chem. 1970 Jan 25;245(2):372–381. [PubMed] [Google Scholar]
- Bárány E. H. The liver-like anion transport system in rabbit kidney, uvea and choroid plexus. II. Efficiency of acidic drugs and other anions as inhibitors. Acta Physiol Scand. 1973 Aug;88(4):491–504. doi: 10.1111/j.1748-1716.1973.tb05478.x. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Fleischner G., Robbins J., Arias I. M. Immunological studies of Y protein. A major cytoplasmic organic anion-binding protein in rat liver. J Clin Invest. 1972 Mar;51(3):677–684. doi: 10.1172/JCI106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Fleischner G., Gatmaitan Z., Arias I. M., Jakoby W. B. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Arias I. M. Circular dichroism studies of Y protein (ligandin), a major organic anion binding protein in liver, kidney, and small intestine. Ann N Y Acad Sci. 1973 Nov 26;226:148–153. doi: 10.1111/j.1749-6632.1973.tb20477.x. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Betheil J. J., Arias I. M. Competitive binding of bilirubin, sulfobromophthalein, indocyanine green and other organic anions to human and bovine serum albumin. Biochim Biophys Acta. 1974 Sep 13;365(1):169–180. doi: 10.1016/0005-2795(74)90261-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Deficiency of hepatic organic anion-binding protein, impaired organic amnion uptake by liver and "physiologic" jaundice in newborn monkeys. N Engl J Med. 1970 Nov 19;283(21):1136–1139. doi: 10.1056/NEJM197011192832104. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. I., Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Phylogenetic study of organic anion transfer from plasma into the liver. Nat New Biol. 1971 Jun 30;231(26):277–279. doi: 10.1038/newbio231277a0. [DOI] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Ostrow J. D., Murphy N. H. Isolation and properties of conjugated bilirubin from bile. Biochem J. 1970 Nov;120(2):311–327. doi: 10.1042/bj1200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Habig W. H., Jakoby W. B. Mercapturic acid formation: the several glutathione transferases of rat liver. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1123–1128. doi: 10.1016/0006-291x(73)90616-5. [DOI] [PubMed] [Google Scholar]
- Poland A., Glover E. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin, a potent inducer of aryl hydrocarbon hydroxylase, with 3-methylcholanthrene. Mol Pharmacol. 1974 Mar;10(2):349–359. [PubMed] [Google Scholar]
- Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Studies of Y and Z, two hepatic cytoplasmic organic anion-binding proteins: effect of drugs, chemicals, hormones, and cholestasis. J Clin Invest. 1971 Nov;50(11):2242–2252. doi: 10.1172/JCI106721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P., Drysdale J. W. Isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Apr 27;236(1):17–28. doi: 10.1016/0005-2795(71)90144-9. [DOI] [PubMed] [Google Scholar]
- WANG L. Plasma volume, cell volume, total blood volume and F cells factor in the normal and splenectomized Sherman rat. Am J Physiol. 1959 Jan;196(1):188–192. doi: 10.1152/ajplegacy.1958.196.1.188. [DOI] [PubMed] [Google Scholar]
- WEINER I. M., WASHINGTON J. A., 2nd, MUDGE G. H. On the mechanism of action of probenecid on renal tubular secretion. Bull Johns Hopkins Hosp. 1960 Jun;106:333–346. [PubMed] [Google Scholar]
- Whelan G., Hoch J., Combes B. A direct assessment of the importance of conjugation for biliary transport of sulfobromophthalein sodium. J Lab Clin Med. 1970 Apr;75(4):542–557. [PubMed] [Google Scholar]