Abstract
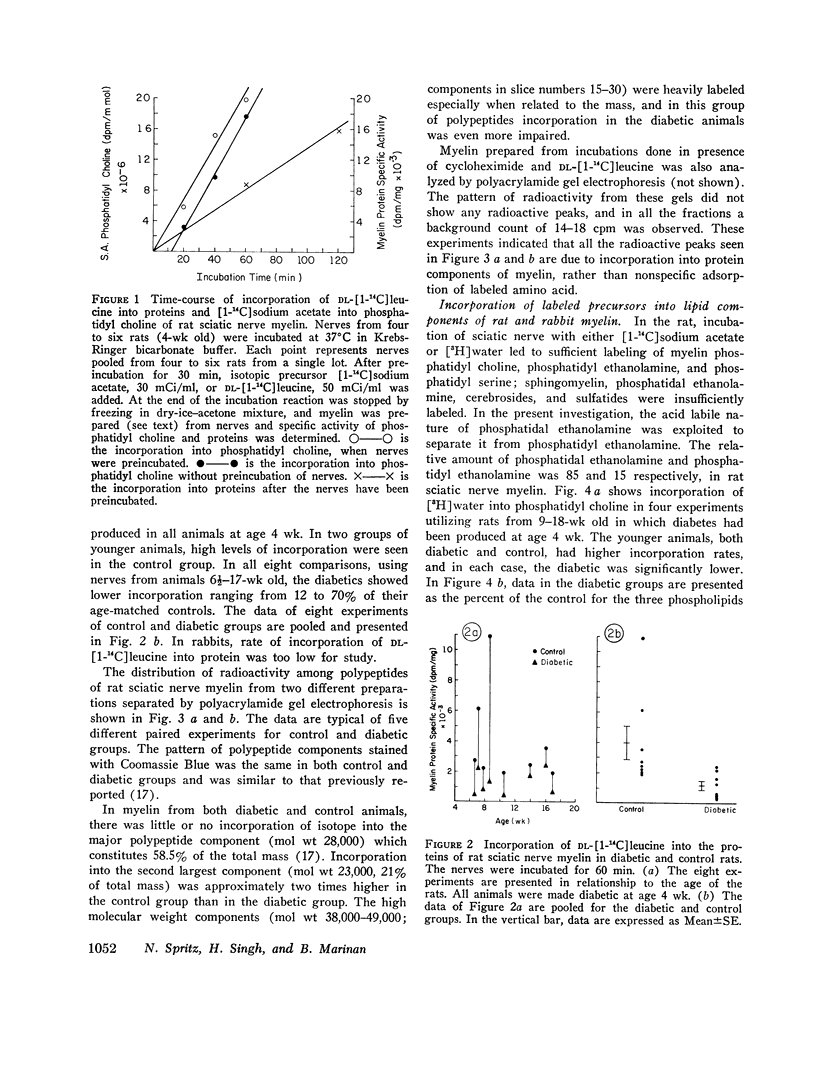
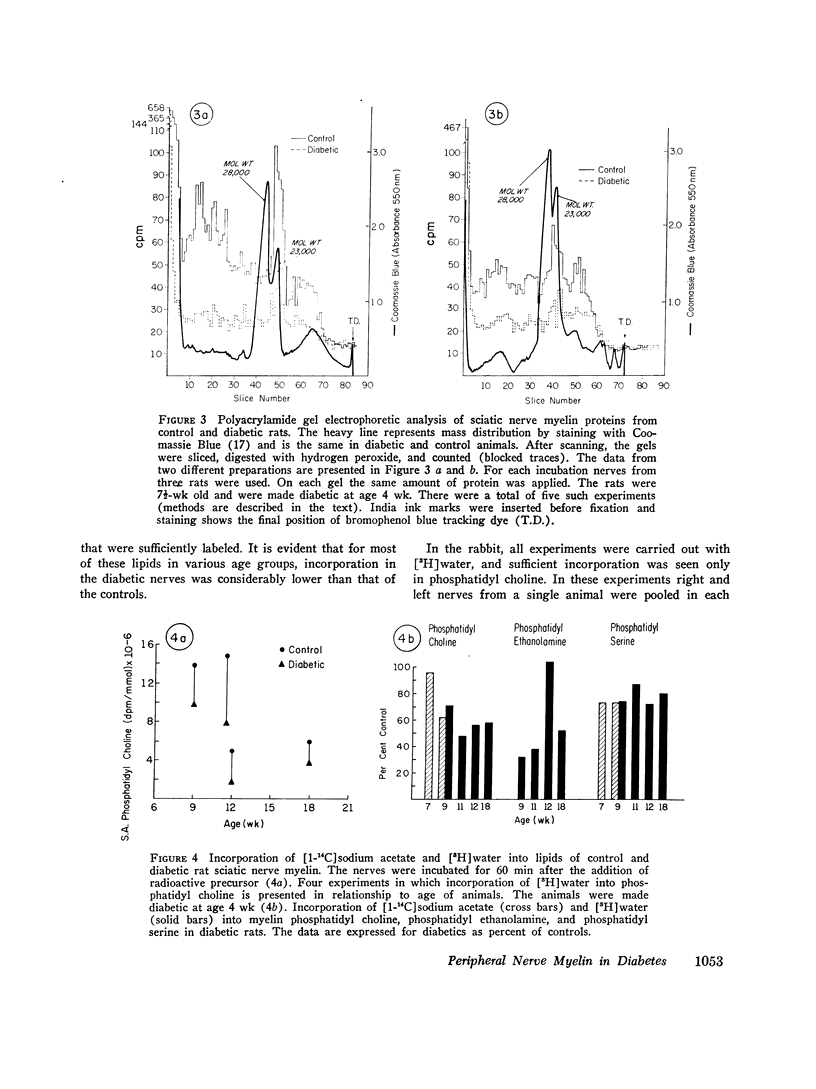
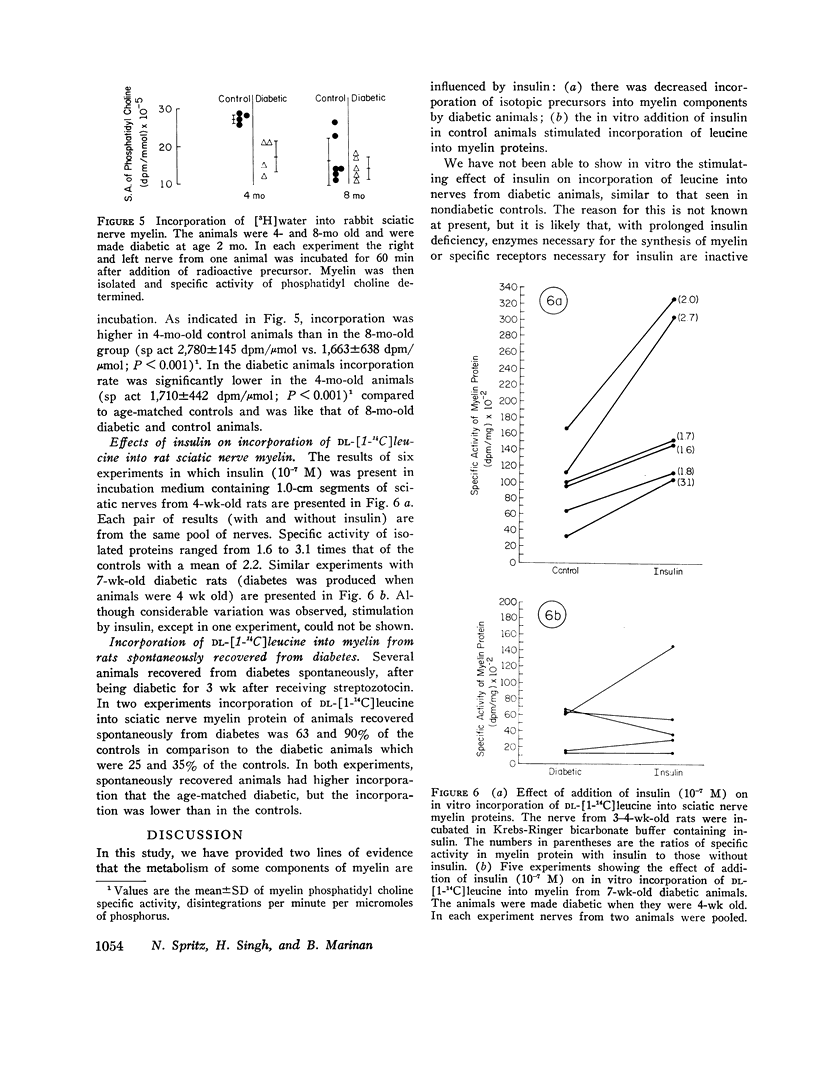
Previous in vitro studies of the metabolism of the peripheral nerve have been based on incorporation of radioactive precursor into components isolated from whole nerve. In this study we have determined incorporation secifically into myelin components of peripheral nerve by isolating myelin after incubating whole nerves with lipid or protein precursors and by determining the specific activity of the components of that membrane. The effect of diabetes on such incorporation was also studied. In the rat, in vitro incorporation of DL-[1-14C]leucine into protein components of myelin was decreased by 30-88% in diabetic animals as compared to controls. The major polypeptide constituent of rat sciatic nerve myelin (mol st 28,000; 58.5% of total mass of proteins) was not labeled in either the diabetic or the control group. In diabetes incorporation rate into a polypeptide of mol wt 23,000, which constitutes 21% of total mass, was approximately one half that of controls. In polypeptides of mol wt 38,000-49,000, which are heavily labeled in normal animals, but constitute only about 5% of total mass of proteins, depression of incorporation was e-en more marked in the diabetics. While these marked differences in incorporation between diabetic and control animals were observed, the amount of protein and its distribution among the constituent polypeptides was the same in both groups. In young rats made diabetic with streptozotocin and young rabbits made diabetic with alloxan, there was a lower rate of incorporation of the lipid precursors, [1-14C]sodium acetate or [3H]water, into myelin components. In older animals of both species incorporation in the controls was considerably lower than in the yount animals, and the effect of diabetes was no longer apparent. In nondiabetic animals, the in vitro addition of insulin (10-7 M) stimulated incorporation of DL-[1-14C]leucine into myelin proteins 1.6-3.1 times that of controls. This stimulation by insulin in vitro was not seen in diabetic animals. In animals in which diabetes had spontaneously recovered, however, incorporation rate in the in vitro experiments approached that of controls and were significantly above that in animals whose diabetes persisted. Since myelin is the palsma membrane of the Schwann cell, these studies provide evidence that the Schwann cell is affected by insulin and that some aspects of the metabolism of myelin are altered in insulin-deficient states.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUTILIO L. A., NORTON W. T., TERRY R. D. THE PREPARATION AND SOME PROPERTIES OF PURIFIED MYELIN FROM THE CENTRAL NERVOUS SYSTEM. J Neurochem. 1964 Jan;11:17–27. doi: 10.1111/j.1471-4159.1964.tb06719.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Eliasson S. G. Lipid synthesis in peripheral nerve from alloxan diabetic rats. Lipids. 1966 Jul;1(4):237–240. doi: 10.1007/BF02531608. [DOI] [PubMed] [Google Scholar]
- FIELD R. A., ADAMS L. C. INSULIN RESPONSE OF PERIPHERAL NERVE. I. EFFECTS ON GLUCOSE METABOLISM AND PERMEABILITY. Medicine (Baltimore) 1964 May;43:275–279. doi: 10.1097/00005792-196405000-00006. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Field R. A., Adams L. C. Insulin response of peripheral nerve. II. Effects on lipid metabolism. Biochim Biophys Acta. 1965 Dec 2;106(3):474–479. [PubMed] [Google Scholar]
- Guinn G. An ultrasensitive chemical test for quantitative chromatography of sugars. J Chromatogr. 1967 Sep;30(1):178–182. doi: 10.1016/s0021-9673(00)84127-8. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Cell membranes: structure and synthesis. Annu Rev Biochem. 1969;38:263–288. doi: 10.1146/annurev.bi.38.070169.001403. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pratt J. H., Berry J. F., Kaye B., Goetz F. C. Lipid class and fatty acid composition of rat brain and sciatic nerve in alloxan diabetes. Diabetes. 1969 Aug;18(8):556–561. doi: 10.2337/diab.18.8.556. [DOI] [PubMed] [Google Scholar]
- Rawlins F. A., Smith M. E. Myelin synthesis in vitro: a comparative study of central and peripheral nervous tissue. J Neurochem. 1971 Oct;18(10):1861–1870. doi: 10.1111/j.1471-4159.1971.tb09592.x. [DOI] [PubMed] [Google Scholar]
- SHICHIRI M. Biochemical studies of peripheral nerves in alloxan diabetic animals. I. C14-incorporation into lipid and C14O2-production from C14-labelled precursors. Med J Osaka Univ. 1963 Jan;13:305–312. [PubMed] [Google Scholar]
- Singh H., Spritz N., Geyer B. Studies of brain myelin in the "quaking mouse". J Lipid Res. 1971 Jul;12(4):473–481. [PubMed] [Google Scholar]
- Singh H., Spritz N. Polypeptide components of myelin from rat peripheral nerve. Biochim Biophys Acta. 1974 Jun 7;351(2):379–386. doi: 10.1016/0005-2795(74)90202-5. [DOI] [PubMed] [Google Scholar]
- Spritz N., Singh H., Geyer B. Myelin from human peripheral nerves. Quantitative and qualitative studies in two age groups. J Clin Invest. 1973 Feb;52(2):520–523. doi: 10.1172/JCI107210. [DOI] [PMC free article] [PubMed] [Google Scholar]


