Abstract
Major progress has been made in developing infectious HCV cell culture systems and these systems have been useful in identifying novel HCV antivirals. However, more rapid and sensitive assays using infectious cell based HCV systems would facilitate the development of additional antivirals, including small molecules directed at unique targets such as the HCV RNA internal ribosomal entry site (IRES). We have found that the V3 region (28 aa) of NS5A of HCV JFH1 can be deleted from the genome with only modest effects on the titer of infectious virus produced in cell culture. Moreover, the V3 region can be replaced with the Renilla reniformis luciferase (Rluc) gene resulting in an infectious virus that stably expresses an NS5A-Rluc fusion protein. Infected cells cultured in 96-well plates provided a robust luciferase signal that accurately reflected the production of infectious virus. This infectious HCV reporter system was used to test the activity of three benzimidazole compounds that bind the HCV RNA IRES. Compounds in this chemical class of small molecules bind and alter the IRES RNA structure at low to sub-micromolar concentrations and interfere with viral replication. The current study shows that these compounds inhibit HCV replication in an infectious HCV cell culture system, defines their IC50 in this system, and provides a platform for the rapid testing of next generation inhibitors.
Keywords: Hepatitis C, HCV, IRES, Benzimidazole, Renilla luciferase, NS5A
1. Introduction
Chronic infection with the hepatitis C virus (HCV) is a major risk factor for developing cirrhosis and hepatocellular carcinoma. Approximately 3% of the worldwide population is chronically infected with HCV (Alter and Seeff, 2000; Bialek and Terrault, 2006). A preventive vaccine has not been developed and limits of current therapeutics include serious side effects and therapy usually lasting 48 weeks with only a 50% sustained virological response rate (Bowen and Walker, 2005; De Francesco and Migliaccio, 2005; Fried et al., 2002; Houghton and Abrignani, 2005).
HCV has a single-stranded 9.6-kb RNA genome of positive polarity containing a 5' internal ribosomal entry site (IRES) element (Penin et al., 2004; Reed and Rice, 2000). IRES driven HCV RNA translation produces a polyprotein of approximately 3,000 amino acids (aa). The polyprotein precursor is co- and post-translationally processed by cellular and viral proteases to yield the mature structural and nonstructural proteins (Yi et al., 2007). The structural proteins include the core protein, which forms the viral nucleocapsid, and the envelope glycoproteins E1 and E2. The nonstructural proteins, NS2 through NS5B, include the NS2-3 autoprotease, the NS3 serine protease, an RNA helicase located in the C-terminal region of NS3, the NS4A polypeptide, the NS4B and NS5A proteins, and the NS5B RNA-dependent RNA polymerase (Jones et al., 2007; Moradpour et al., 2007; Reed and Rice, 2000).
A recent major advance was the development of an infectious virus system based on the transfection of human hepatoma cells with genomic HCV RNA (JFH1) isolated from a patient with fulminant hepatitis (Kato et al., 2001; Lindenbach et al., 2005; Wakita et al., 2005; Zhong et al., 2005). This cell culture model allows all stages of the HCV life cycle to be studied. However, using viral protein immunostaining for detecting and measuring virus replication places limits on the rapidity of replication assays and their quantitation. To address this problem, a wide variety of infectious chimeric HCV cell culture systems have been engineered. A J6/JFH-1 chimera with Renilla luciferase (Rluc) followed by the foot-and-mouth disease virus (FMDV) 2A sequence and a Ubi sequence placed at the 5' end of the core gene resulted in a infectious clone producing Rluc and infectious progeny virus (Tscherne et al., 2006). The larger firefly luciferase (Fluc) gene was used successfully to develop infectious JFH-1 derived HCV bicistronic reporter systems using the HCV IRES and EMC IRES to drive translation of Fluc and the HCV polyprotein, respectively (Koutsoudakis et al., 2006). However, bicistronic constructs have some limits which include loss of the reporter gene with prolonged passage. Other approaches have inserted the Gaussia luciferase (Gluc) flanked by the FMDV 2A protease between the p7 and NS2 region of the HCV polyprotein (Phan et al., 2009). This approach results in the secretion of Gluc into the media of Jc1/Gluc2A HCV infected hepatoma cells where supernatants can be assayed for Gluc to measure viral replication. A chimeric JFH1 virus that had the Rluc gene inserted into the NS5A C-terminal region produced infectious chimeric HCV particles, but at a relatively low titer that makes 96-well assays impractical (Kim et al., 2007). Despite these advances, insertion of reporter genes into the HCV genome has been challenging and the development of new approaches to this problem may have multiple applications.
The NS5A protein of HCV is multifunctional. It contains an interferon sensitivity-determining region (ISDR) spanning aa 237–276, which may confer relative resistance to interferon alpha therapy (Polyak et al., 1999). The NS5A protein has an amphipathic α-helix and a zinc-binding domain at the amino terminus, both of which are required for viral replication (Brass et al., 2002; Tellinghuisen et al., 2004, 2005). In addition, the phosphorylation of the NS5A protein appears to play a role in HCV RNA replication (Appel et al., 2005). The NS5A protein also interacts with a number of cellular proteins, including double-stranded RNA-dependent protein kinase (PKR), and alters the host cell antiviral response to HCV (Evans et al., 2004). NS5A of HCV-1a and 1b has a highly variable region of twenty-four amino acids, which has been designated the V3 region (Inchauspe et al., 1991). This region is located in domain III of NS5A (aa2356 to 2379 of the polyprotein). However, the requirement and biological role of the V3 region in the HCV life cycle remains unknown.
The goal of this study was to determine if the V3 region of NS5A of HCV JFH1 was essential for viral replication and if it could be replaced with Rluc to measure the antiviral effect of benzimidazole compounds in an infectious HCV cell culture system. Previous studies of subgenomic replicons and HCV JC1 have shown that part of V3 or V3 plus adjacent regions could be deleted without major deleterious effects on replication (Moradpour et al., 2004; Appel et al., 2005; McCormick et al., 2006; Liu et al., 2006; Appel et al., 2008). However, V3 has not been specifically deleted or replaced in replicon or infectious HCV cell culture systems. We report that the V3 region is not essential for HCV JFH1 replication and that it can be replaced with the Renilla luciferase gene resulting in an infectious virus producing easily measured Renilla activity. This system enables rapid assays of virus replication in 96-well plates and was used to test the ability of a benzimidazole class of small molecules, which are known to bind to the HCV IRES RNA at low to sub-micromolar concentrations, to inhibit HCV replication in cultured hepatoma cells (Seth et al., 2005).
2. Materials and Methods
2.1. Cell culture
A human hepatoma cell line, Huh 7.5, was generously provided by Dr. Charles M. Rice (Blight et al., 2002) and was maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 100 U/ml of penicillin, 100 µg/ml of streptomycin, nonessential amino acids, and 10% fetal bovine serum (FBS) (Invitrogen) at 37°C in 5% CO2. All experiments described in this study were performed using these cells.
2.2. Antibodies
The monoclonal antibody to the NS5A protein was a gift from Dr. Chen Liu (University of Florida, Gainesville, FL). The monoclonal antibody against the NS3 protein (Abcam, MA), anti-Renilla luciferase monoclonal antibody (Chemicon, CA) and the secondary goat anti-mouse IgG conjugated with Alexa Fluor 594 (Invitrogen) were commercially obtained.
2.3. Plasmids
Plasmid constructs were based on the consensus sequence of HCV pJFH1 which was kindly provided by Dr. T. Wakita (Kato et al., 2001). The V3 region of NS5A was deleted in frame by overlapping extension PCR using the following two distinct pairs of primers: NSi-5298-For 5'-ATCGCCACATGCATGCAAGCTGACCTTGAG-3'; BsrG -7782-Rev 5'-GTGCGTTGTACAGTACACCTTGTTATGGTA-3' and ΔV3-7490-For 5'AAGACCTTTGGCCAGTCAGAGACAGGTTCCGCC-3'; Δv3-7403-rev 5'-GGAACCTGTCTCTGACTGGCCAAAGGTCTTGAT-3'. This construct was designated JFH-ΔV3. To facilitate subcloning of Rluc, a MluI site was engineered by replacing V3 region of NS5A using the following primers: NSi-5298-For 5'-ATCGCCACATGCATGCAAGCTGACCTTGAG-3';BsrG -7782-Rev 5'-GTGCGTTGTACAGTACACCTTGTTA TGGTA-3' and Mlu-V3-For 5'-TTTGGCCAGACGCGTTCAGAGACAGGTTCC-3'; Mlu-V3-Rev 5'-TGTCTCTGAACGCGTCTGGCCAAAGGTCTT-3'. This construct was named as JFH-ΔV3-MluI. The entire Rluc gene was amplified by PCR from the pGL4.75 vector (Promega) using the primers 5'-TTATCCTACGCGTGCTTCCAAGGTGTACGAC-3' and 5'-ATCTTAACGCGTCTGCTCGTTCTTCAGCAC-3' and subcloned into MluI site in construct JFH-ΔV3-MluI. This construct was named as JFH-ΔV3-Rluc. For the negative control, the GDD motif was altered to GND by mutagenesis of the encoding nucleotides and was introduced into JFH, a mutation that has been previously shown to abolish the RNA polymerase activity of NS5B (Wakita et al., 2005). This amino acid substitution was done by overlap extension PCR and this construct was named as JFH-GND. All new clones were sequenced and the correct full length clones were chosen for the subsequent experiments.
2.4. HCV RNA Transfection
To generate the full-length genomic RNA, pJFH-1, pJFH-GND, pJFH-1-ΔV3, and pJFH-ΔV3-Rluc were linearized at the 3' end of the HCV cDNA with XbaI. The linearized plasmid DNA was purified and used as a template for T7 in vitro transcription (MEGAscript; Ambion, Austin, TX). In vitro transcribed RNAs above were transfected into cells by electroporation as described by Krieger et al. (2001). Briefly, trypsinized cells were washed twice and resuspended with serum-free Opti-MEM (Invitrogen) at 1 × 107 cells per ml. Ten micrograms of RNA were mixed with 0.4 ml of the cells in a 4-mm cuvette. A Bio-Rad Gene Pulser system was used to deliver a single pulse at 0.27 kV and 960 µF, and the cells were plated in T75 Costar flasks (Corning). Transfected cells were cultured in complete DMEM for the times indicated in figure legends. Cells were passaged every 3–4 days; the presence of HCV in the corresponding supernatants was determined by immunofluorescence assays (IFA) for the NS5A proteins.
2.5. Titration of Infectious HCV
The titer of infectious HCV was determined by immunofluorescence, where the number of cell foci stained for the NS5A protein was directly visualized microscopically as described previously (Zhong et al., 2005). Briefly, cell supernatants were serially diluted 10-fold in complete DMEM. The supernatant was used to infect 1×104 naïve Huh 7.5 cells in 96-well plates. The inocula were incubated with cells for 2h at 37°C and then supplemented with fresh complete DMEM. The level of HCV infection was determined three days postinfection by immunofluorescence staining for HCV NS5A. The viral titer is expressed as focus-forming units per milliliter of supernatant (ffu/ml).
2.6. Immunofluorescence assay (IFA)
Cells infected by HCV were washed with PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100. Fixed cells were blocked with 1% bovine serum albumin and 1% normal goat serum in PBS. HCV NS5A protein was detected in cells by incubation with an NS5A-specific monoclonal antibody and visualized with the secondary goat anti-mouse IgG conjugated with Alexa Fluor 594 fluorescein (Invitrogen, 1:1,000 dilutions). Cover slips were mounted onto slides with DAPI (Vector labs), and the HCV proteins were visualized by fluorescence microscopy (Nikon E400).
2.7. Western blot analysis
The HCV-infected Huh 7.5 cells were lysed in a radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, 1% Nonidet P40, 0.5% sodium deoxycholate) containing a cocktail of proteinase inhibitors (Roche). The total protein for each sample was measured with a standard protein assay (Bio-Rad). Twenty-five micrograms of total protein for each sample was analyzed by 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked by incubating them with 5% skim milk. HCV proteins were detected with monoclonal antibodies specific to NS3, NS5A and Rluc proteins, horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Bio-Rad) and a chemiluminescence substrate (Pierce). β-actin was used as a control and was detected with an anti-β-actin monoclonal antibody (Sigma). The protein levels were quantified using Quantity One software (Bio-Rad).
2.8. Renilla Luciferase Reporter Assay for Viral Replication and Infectivity
For viral replication assays, the HCV RNA transfected cells were plated in triplicate in 24-well plates. Cells were lysed with a passive lysis buffer (Promega) at 72 hours. The culture plates were gently rocked at room temperature for 15 minutes and stored at −80°C. To determine the infectivity of culture medium containing reporter virus JFH-ΔV3-Rluc, cell-free supernatant (centrifuged at 1500 g for 10 minutes) obtained from HCV RNA transfected cells was inoculated onto naïve Huh 7.5 cells in 96-well plates (in triplicate). At 72 hours post-inoculation the cells were lysed and stored at −80°C. Renilla luciferase activity was measured in cell lysates (20 µl) using a Renilla Luciferase Assay System kit (Promega). Rluc activity was normalized by protein concentration which was measured with the RCDC assay (Bio-Rad).
2.9. Inhibition of HCV replication following infection
Huh 7.5 cells were grown overnight in 24 (JFH1-wt) or 96-well (JFH-ΔV3-Rluc) plates under standard conditions. Cells were infected for 2 hours with JFH-wt or JFH-ΔV3-Rluc at a multiplicity of infection (moi) of 0.1. Interferon-α (IFN-α) (Fitzgerald Industries Int., Acton, MA), 2'-C-methyladenosine nucleoside analogue and the benzimidazole small molecule antiviral compounds were serially diluted in complete DMEM medium and then added to cells rinsed with PBS. After two days of incubation at 37°C, HCV replication was measured by Renilla luciferase assays or Western blotting of NS3 protein. Normalized luciferase activity and NS3 protein levels were plotted as a function of inhibitor concentration and (IC50) values were calculated from a linear fit of the inhibitor response.
2.10. RT-PCR
HCV virion RNA (vRNA) of JFH1-wt, JFH-ΔV3 and JFH-ΔV3-Rluc in the culture medium was extracted with LS Trizol reagent (Invitrogen). The vRNA was reverse transcribed by using Superscript III Reverse Transcriptase (Invitrogen) and random primers. The resulting cDNA was used as a template for subsequent PCR with Platinum® Pfx DNA polymerase (Invitrogen) and the following primers: JFH-6815-FOR, 5'-TTAATTCCTATGCTGTCGGGT CCCAGCT-3'; JFH-7765-Rev, 5'-GTGCGTTGTACAGTACACCTTGTTATGG-3'. The PCR amplicon was analyzed by 1% agarose gel electrophoresis and sequenced using standard methods.
2.11. Small molecule inhibitors
The benzimidazole inhibitors developed at Isis Pharmaceuticals are a class of potent small molecule compounds that target the HCV IRES RNA (Seth et al., 2005). Three HCV replication inhibitors belonging to the benzimidazole chemical class were dissolved in water and added to cell culture media as described above and in the figure legends (Seth et al., 2005). A well studied 2'-C-methyladenosine nucleoside analogue which is an HCV NS5B polymerase inhibitor was used as a control in these studies (Eldrup et al., 2004).
An IC50 of 5.4 µM was reported for Isis-13 in an HCV replicon assay, an IRES RNA KD=0.72 µM was measured by a mass spectrometry assay (Seth et al., 2005), and an IRES RNA Kd=0.6 µM was measured by FRET assay (Parsons et al., 2009). The reported RNA KD for Isis-04 was 8 µM; however, no measurements of antiviral activity were reported. For Isis-22, an RNA KD=2.2 µM for HCV IRES RNA binding was measured as part of the current study using 2-aminopurine labeled RNA (Paulsen et al., 2010). It should be noted that the entire region of domain IIa of the HCV IRES where the inhibitors bind is extremely conserved in most HCV genotypes. For example, in 1a, 1b and 2a there is only a single nucleotide variation found at position 107. In JFH1 (2a) this is a C giving a CG pair, while in 1a and 1b this is U giving a UG pair. To address this question we examined the effect of this variation on the binding of a compound to domain IIa. We deleted this base pair (Paulsen et al. 2010) and showed there is no effect on Isis-11 binding. The benzimidazole class of HCV inhibitors have low cellular toxicity: data for 6 benzimidazole analogs in Huh7 cells have been reported (CC50> 100 µM, including Isis-04 and Isis-13 (Seth et al., 2005). Isis-13 was also shown by Hermann and coworkers to be non-toxic up to the 25 µM maximum concentration tested (Parsons et al., 2009). In addition, we have performed cytotoxicity assays in Huh 7.5 cells for all compounds used in this study with a standard cell viability assay which are reported in Results (Promega CellTiter 96 Aqueous One Solution Proliferation Assay) (Mosmann, 1983).
3. Results
3.1. Location of the V3 region in HCV JFH1, and the effect of deletion and replacement of V3 with Renilla luciferase (Rluc) on viral replication
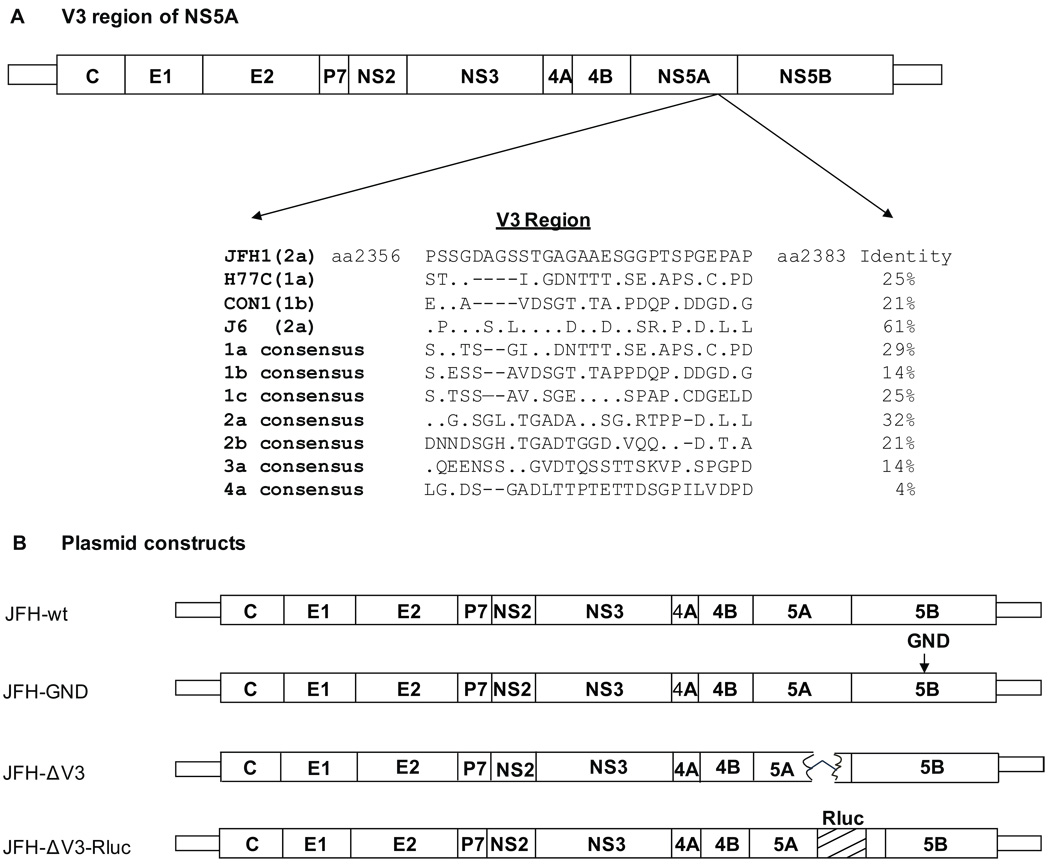
The 24 amino acid V3 region of NS5A is located between aa2356 and 2379 in the polyprotein of HCV-H (genotype 1a), HCV-J (1b) and HCV-BK (1b) strains (Inchauspe et al., 1991). Using DNASTAR and ClustalW2 software we compared the V3 sequences of HCV1a (H77C) and HCV1b (Con1) with the JFH1 strain (2a) and identified the V3 region of JFH1 which was 28 amino acids in length (aa2356 to 2383 of the polyprotein; or aa380 to 407 of NS5A). The amino acid identity of JFH1 to H77C (1a), Con1 (1b) and J6 (2a) was 25%, 21% and 61%, respectively. The V3 region of the HCV genotype 2a consensus sequence shows only 32% identify with the V3 region of JFH1, while the V3 consensus sequence of other genotypes have a 4–29% identity with JFH1 (Fig.1A). The V3 region was replaced with the gene encoding Rluc and plasmid JFH-GND was prepared by site directed mutagenesis of the GDD motif of the NS5B polymerase (Fig.1B) as a negative control for viral replication (Wakita et al., 2005). The integrity of all plasmids was confirmed by sequencing.
Figure 1. Schematic representation of the V3 region of HCV NS5A and constructs used in this study.
Panel A, location of the V3 region in HCV strains JFH1, H77C, Con1, J6 and selected consensus sequences. The V3 region of HCV 2a includes 28aa, whereas HCV 1a and 1b has 24-26aa. Panel B, Diagrams of the JFH1 (JFH) based HCV constructs. V3 was deleted in JFH-ΔV3. The V3 region was replaced with the Renilla luciferase (Rluc) gene in JFH-ΔV3-Rluc. The GDD motif of NS5B was changed to GND by site directed mutagenesis in JFH-GND to provide a negative control.
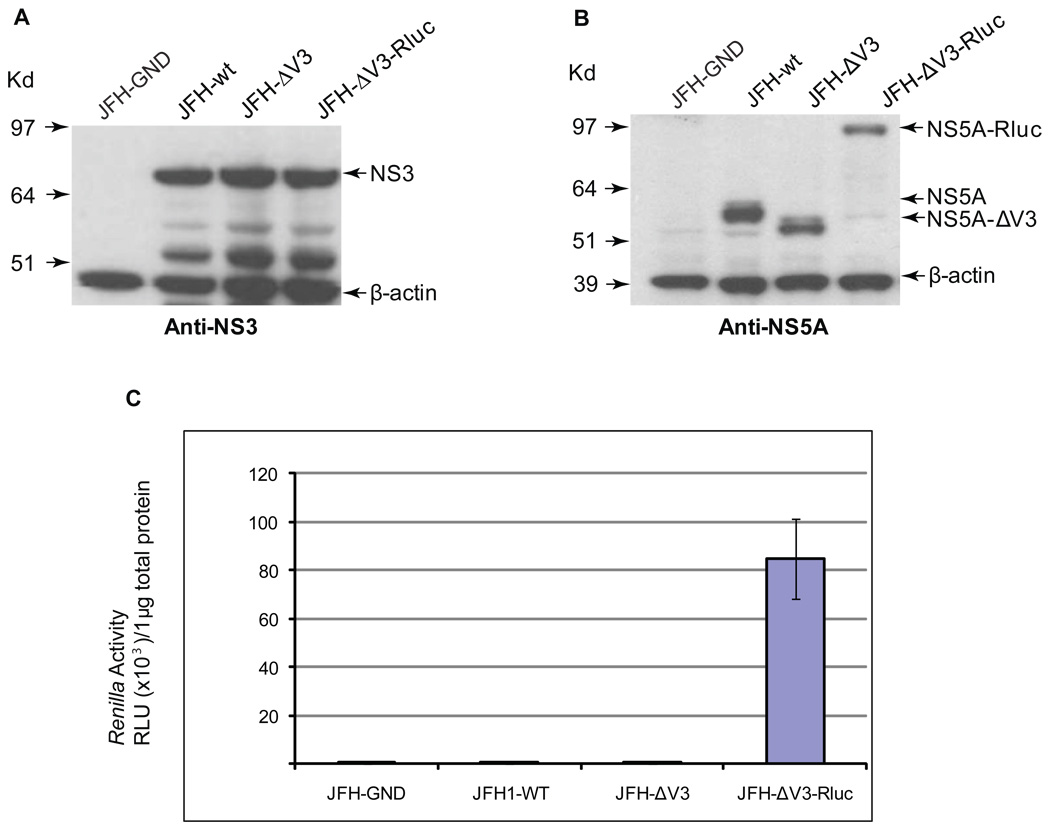
We compared the viral protein expression resulting from transfection by electroporation of Huh 7.5 cells with RNA transcribed in vitro from the JFH-GND, JFH-wt, JFH-ΔV3, and JFH-ΔV3-Rluc plasmids. Three days after transfection, cell lysates were prepared and the levels of the HCV NS5A and NS3 protein were evaluated by Western blot analysis using anti-NS5A and anti-NS3 antibodies. Similar levels of NS3 protein were expressed in the cells transfected with JFH-wt, JFH-ΔV3, or JFH-ΔV3-Rluc RNA (Fig.2A). The NS5A-ΔV3-Rluc fusion protein with the predicted molecular mass was easily detected in cells (Fig.2B). Three days after transfection of cells with JFH-ΔV3-Rluc RNA, luciferase activity was approximately 12,000-fold higher than control cells transfected with JFH1-wt RNA (Fig.2C). These results provide evidence that the V3 region of NS5A of HCV JFH-1 can be replaced with a relatively large insert encoding Rluc without major adverse effects on virus replication.
Figure 2. Analysis of HCV replication following RNA transfection.
Panels A and B, Western blot analyses of Huh 7.5 cells transfected with JFH-GND, JFH-wt, JFH ΔV3, or JFH ΔV3-Rluc RNA were done at least twice and representative examples are shown (see Materials and Methods). Protein levels of NS5A, NS3, β-actin were determined by Western blotting. Panel C, the expression of NS5A-ΔV3-Rluc, was monitored by measuring luciferase activity in the cells transfected with JFH-GND, JFH-wt, JFH-ΔV3, or JFH-ΔV3-Rluc. Luciferase activity was normalized by protein concentration of cell lysates. Assays were done in triplicate and experiments done three times. Data presented as mean ± standard deviation (n=9) of relative light units (RLU).
3.2. Effect of replacing the V3 region with Rluc on the production of infectious HCV viral particles
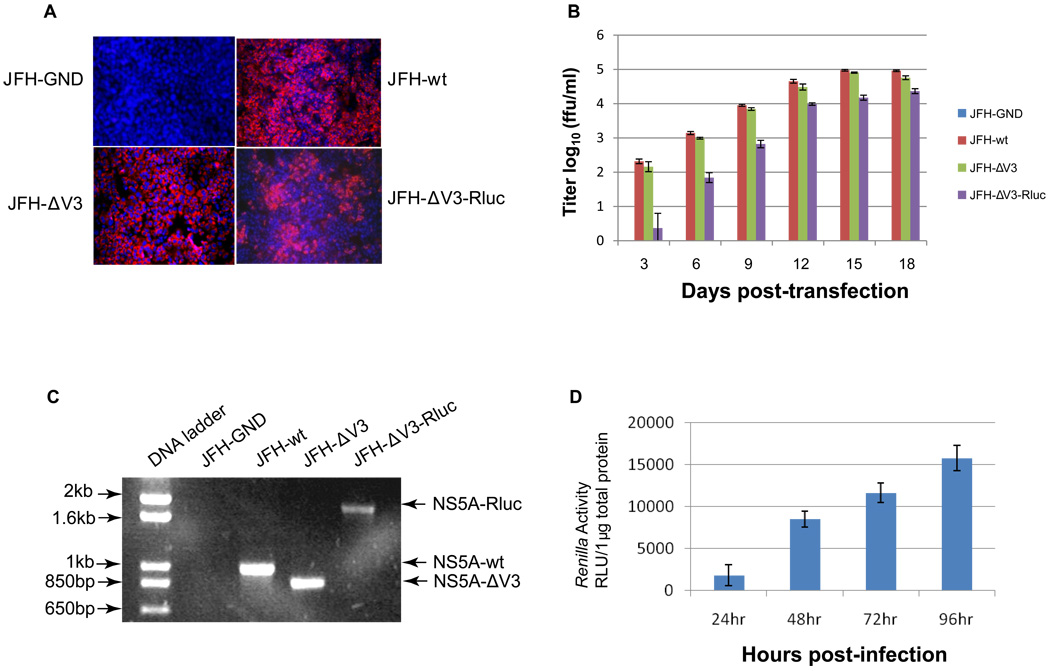
To determine if Huh 7.5 cells transfected with JFH-ΔV3 and JFH-ΔV3-Rluc RNA released infectious viral particles, we inoculated naïve Huh 7.5 cells with supernatants collected three days post-transfection from the appropriate cells as described above. Immunofluorescence (IFA) staining for NS5A detected positive Huh 7.5 cells three days post-transfection (not shown). The transfected cells were subcultured at three day intervals (approximately 90–95% confluent) for a total of 18 days and the infectious virus titers in cell supernatants were measured by inoculation of naïve Huh 7.5 cells. Naïve Huh 7.5 cells that were inoculated with supernatants from RNA transfected cells and subcultured for 15 days showed marked immunofluorescence staining for NS5A for all constructs except the NS5B polymerase GND negative control (Fig. 3A). Maximum virus titers of 1.0×105 ffu/ml for JFH-wt (day 15 post-transfection), 8.0×104 ffu/ml for JFH-ΔV3 (day 15 post-transfection) and 2.0×104 ffu/ml for JFH-ΔV3-Rluc (day 18 post-transfection) were observed (Fig. 3B), demonstrating that the titers of infectious viral particles produced from JFH-ΔV3 transfected cells were similar to those of JFH-wt transfected cells. The titers of infectious viral particles produced by JFH-ΔV3-Rluc transfected cells were less than the control JFH-wt cells, but not dramatically different. Luciferase activity of HCV JFH-ΔV3-Rluc infected cells was easily measured by a standard luciferase assay and increased up to four days post-inoculation of naïve Huh 7.5 cells with infectious cell supernatants (Fig.3D). In addition, HCV virion RNA was also detected by RT-PCR in supernatants (1500×g for 10 minutes) from naïve cells inoculated with day 15 cell culture supernatants. PCR products from NS5A or NS5A-ΔV3-Rluc RNA recovered from infected cell supernatants and agarose gel analysis showed the predicted size PCR products (Fig.3C) (see Materials and Methods). These results demonstrate that the deletion of V3 or the replacement of V3 with Rluc in HCV JFH1 does not abrogate viral replication and only moderately impairs the production of infectious viral particles. This provides direct evidence that V3 region of HCV JFH1 NS5A is not essential for maintenance of the HCV life cycle, but could still have a function in virus replication, or in the production of infectious virus particles.
Figure 3. Infectivity assay of virus particles produced following RNA transfection of cells.
Panel A, detection of HCV replication by NS5A immunofluorescence assays (IFA) following infection of naïve cells (see Materials and Methods). Huh 7.5 cells were infected with the supernatant collected at 15 days after transfection with JFH-GND, JFH-wt, JFH-ΔV3, and JFH-ΔV3-Rluc RNA. Panel B, Production of infectious HCV particles in cell culture supernatants following transfection with viral RNA (see Materials and Methods). The viral titer is expressed as focus-forming units per ml of supernatant (ffu/ml) as determined by the average number of NS5A-positive foci detected by immunofluorescence for NS5A. Assays were done in triplicate in 96-well plates and performed two times. The data are presented as mean ± standard deviation (n=6). Panel C, Detection of HCV virion RNA in the culture medium by RT-PCR spanning the NS5A V3 region (see Materials and Methods). The DNA products were analyzed by 1% agarose gel electrophoresis. Experiments were performed three times and a representative experiment is shown. Panel D, Renilla luciferase activity was measured in the Huh 7.5 cells following infection with the JFH-ΔV3-Rluc virus at a multiplicity of infection (moi) of 0.1 (see Materials and Methods). Assays were done in triplicate three times and the data are shown as mean ± standard deviation (n=9).
3.3. Stability of the HCV JFH-ΔV3-Rluc reporter virus after multiple passages
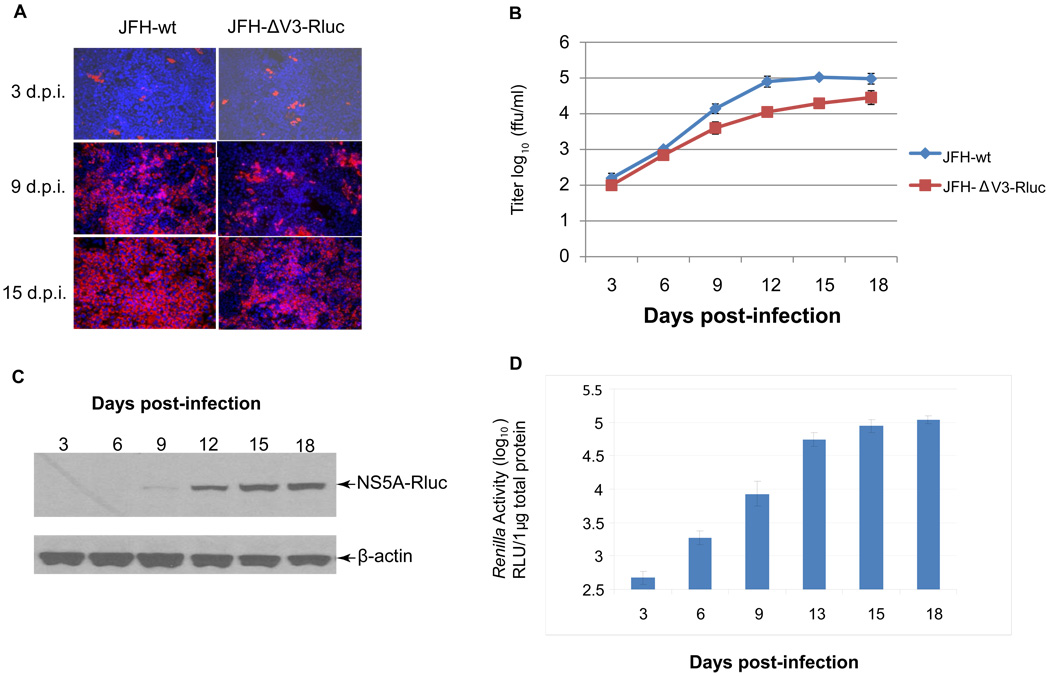
To determine if cells infected with JFH-ΔV3-Rluc would continue to produce infectious virus after serial passage, the titer of virus in supernatants prior to each passage was measured. Cells were passaged every three days and monitored for NS5A-Rluc fusion protein synthesis and Renilla luciferase (Rluc) activity after cells were infected at a low multiplicity of infection (moi 0.01) (Figs.4A, 4C, & 4D). Clarified cell culture supernatants from each passage were added to naïve Huh 7.5 cells to measure the titer of infectious virus in focus forming units (ffu) per ml of supernatant (see Materials and Methods). JFH-wt was used in parallel experiments as a control. Three days after inoculation of cells, infectious particles accumulated exponentially in the supernatants reaching a maximal titer of 1.0×105 ffu/ml for JFH1-wt on day 15 and 2.5×104 ffu/ml for JFH-ΔV3-Rluc on day 18 (Fig.4B). The rate of production of infectious viral particles with JFH-ΔV3-Rluc was slightly slower than JFH-wt. The percentage of NS5A-positive cells increased from approximately 0.1% for both JFH-ΔV3-Rluc and JFH1-wt on day three to 70% for JFH-ΔV3-Rluc and almost 100% for JFH1-wt on day 15 post infection (Fig.4A). To further access the genomic stability of serially passaged JFH-ΔV3-Rluc, the NS5A-Rluc fusion protein was monitored by Western blotting over time. Lysates of the JFH-ΔV3-Rluc virus infected Huh 7.5 cells were analyzed by Western blotting using an anti-Rluc antibody. An NS5A-Rluc fusion protein recognized by this antibody of the predicted molecular weight of 94 kDa was detected and increased over 18 days of passaging in cells (Fig.4C). At the same time the luciferase activity of JFH-ΔV3-Rluc was measured as a function of the numbers of days post-infection with viral supernatant (Fig.4D). Moreover, the intensity of the immunostained protein was consistent with the titration assays (Fig.4C) and Renilla luciferase activity assay (Fig.4D). These results provide direct evidence that the JFH-ΔV3-Rluc virus produced by RNA transfection can be passaged in Huh 7.5 cells without a major loss of infectivity over 18 days and that the virus rapidly infects a high proportion of cells.
Figure 4. Kinetics of virus production following infection of naïve Huh 7.5 cells with JFH-ΔV3-Rluc or JFH-wt virus.
Panel A, naïve Huh 7.5 cells were infected with JFH-wt and JFH-ΔV3-Rluc supernatants at an moi of 0.01. Cells were passaged every three days and analyzed at the indicated times for NS5A expression by immunofluorescence (red). Nuclei were counterstained using DAPI (blue). Panel B, virus titers in cell culture supernatants collected at the indicated times post-infection were measured by determining focus-forming units with NS5A immunofluorescence assays. Assays were done in triplicate, performed twice and data presented as mean ± standard deviation (n=6). Panel C, Detection of the NS5A-Rluc fusion protein in serially passaged cells by Western blotting with anti-Rluc. Experiments were performed twice and a representative result is shown (see Materials and Methods). Panel D, Renilla luciferase activity was measured at the indicated times post-inoculation with the JFH-ΔV3-Rluc virus. Assays were done in triplicate, performed twice and data shown as mean ± standard deviation (n=6).
3.4. Using Renilla luciferase produced by JFH-ΔV3-Rluc infected cells to more rapidly measure activity of antiviral molecules
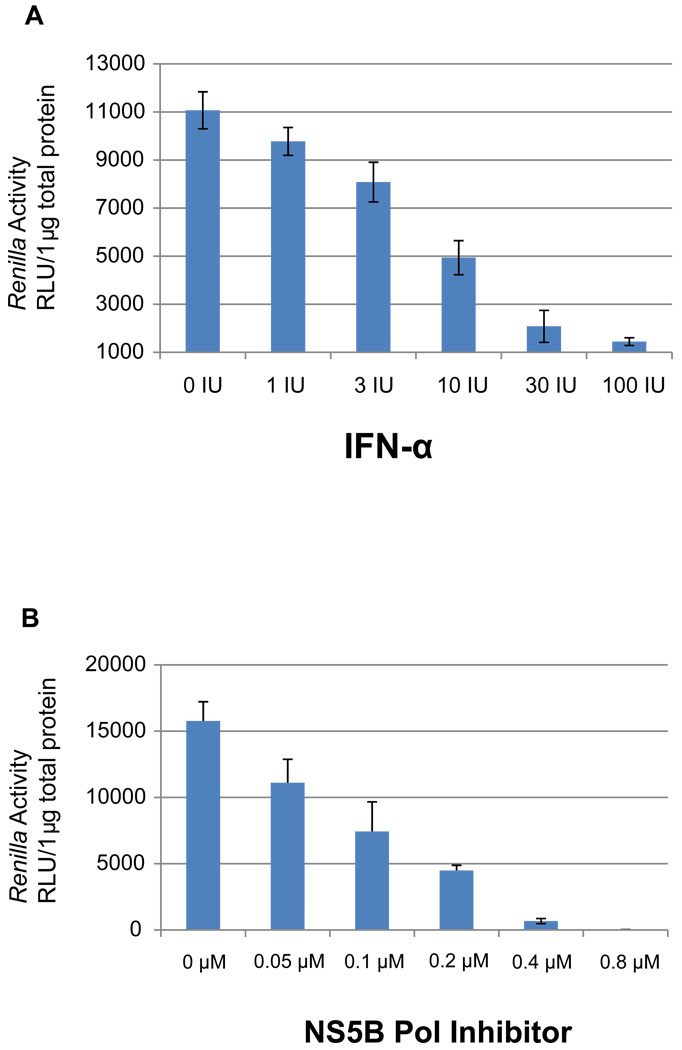
IFN-α has been well documented to inhibit HCV replication in cell culture (Cai et al., 2005; Guo et al., 2001). Increasing concentrations of IFN-α were added to naïve Huh 7.5 cells 2 hours post-inoculation with JFH-ΔV3-Rluc infectious supernatants. Two days post-inoculation, cell lysates were harvested and the level of the Renilla luciferase activity was measured, demonstrating that IFN-α markedly inhibited luciferase activity in JFH-ΔV3-Rluc infected cells (Fig.5). This provided evidence that the JFH-ΔV3-Rluc reporter virus responds to a standard antiviral agent in a similar manner to that reported for the JFH-wt virus and HCV replicon (Cai et al., 2005; Guo et al., 2001).
Figure 5. Effect of IFN-α and a 2'-C-methyladenosine nucleoside analogue NS5B polymerase inhibitor on JFH-ΔV3-Rluc reporter virus replication in cell culture.
Panel A, two hours post-infection with the JFH-ΔV3-Rluc reporter virus (moi=0.1) cells had increasing concentrations of IFN-α added followed by two days of incubation (see Materials and Methods). The levels of Renilla luciferase in cell lysates were determined as described in Figures 2–4. Cell cultures were performed in triplicate in 96-well plates, the experiment was done twice and results are reported in relative light units (RLU) as mean ± standard deviation (n=6). The figure shows a representative result of three independent experiments. Panel B, As an additional control, the effect of a well studied 2'-C-methyladenosine nucleoside analogue NS5B polymerase inhibitor was tested in the JFH-ΔV3-Rluc cell culture system. Cells were cultured and infected as in Panel A. Increasing concentrations of the nucleoside analogue were added and followed by two days of incubation (see Materials and Methods). The levels of Renilla luciferase in cell lysates were determined as in Panel A. Cell cultures were performed in triplicate in 96-well plates, the experiment was done twice and results are reported in relative light units (RLU) as mean ± standard deviation (n=6).
The utility of the JFH-ΔV3-Rluc reporter virus readout was further validated using a 2'-C-methyladenosine nucleoside analogue HCV RNA-dependent RNA polymerase (NS5B pol) inhibitor that has been demonstrated to inhibit recombinant NS5B pol in vitro and HCV replication in hepatoma cells (Carroll et al., 2003; Eldrup et al., 2004). This 2'-C-methyladenosine compound inhibited replication of JFH-ΔV3-Rluc in a concentration-dependent manner (Fig. 5).This compound inhibited replication with an IC50 of 0.28 µM (Fig. 5), comparable to that reported in cell-based assays using an HCV replicon (Eldrup et al., 2004).
3.5. Measuring the antiviral effect of small molecule benzimidazole derivatives that bind the HCV IRES
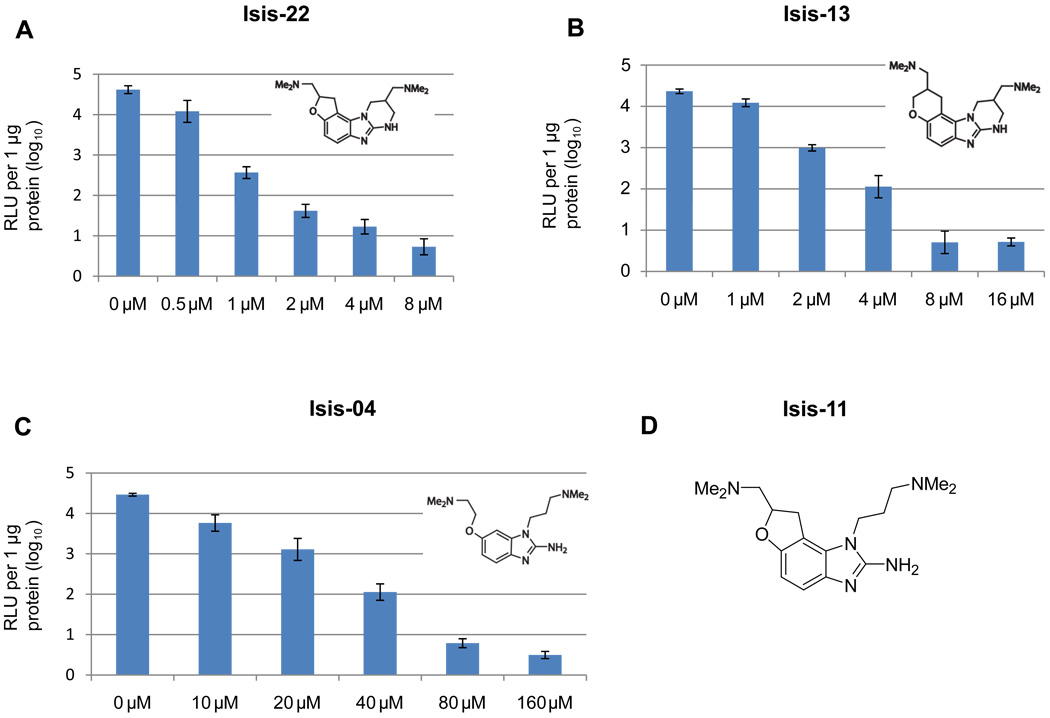
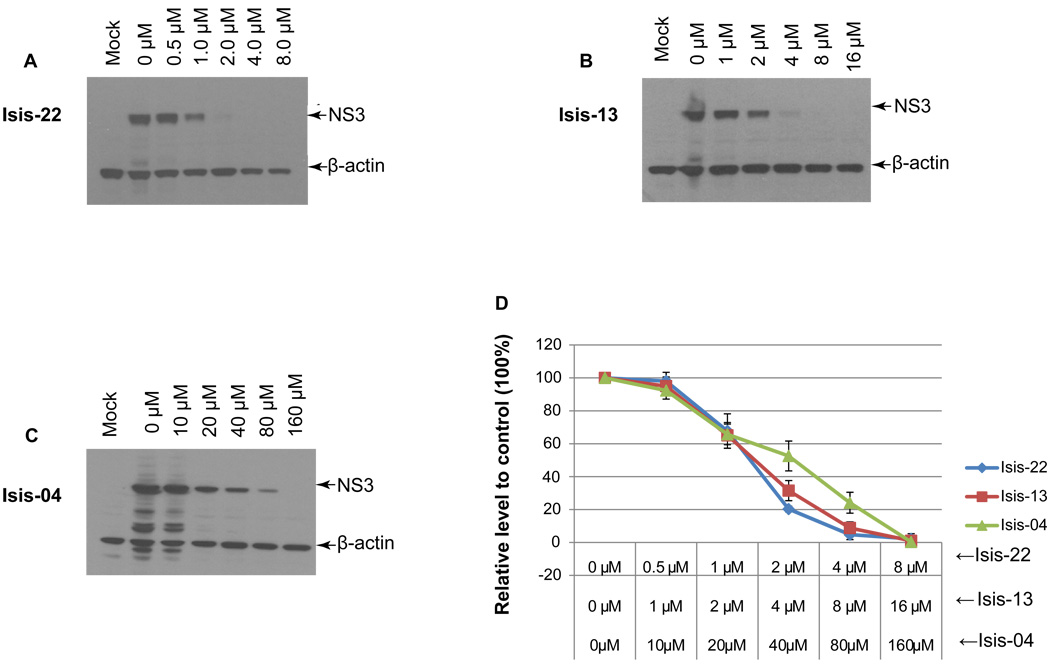
The benzimidazole compounds are a class of potent small molecules that both bind the HCV RNA IRES and have antiviral activity in the HCV replicon system (Seth et al., 2005). We selected three members of this class, Isis-22, Isis-13 and Isis-04 and tested their ability to inhibit the replication of HCV in naïve cells infected with the JFH-ΔV3-Rluc reporter virus in 96 well plates. Published cell toxicity assay data for Isis-4 and 13 are provided in Materials and Methods. We also performed cell toxicity assays and found CC50 values (mean±STD, n=3) in Huh 7.5 cells for Isis-22, -13, -4 and the 2'-C-methyladenosine nucleoside analogue of 65±5, 86±20, 665±62, and 112±19 µM, respectively. Control studies were done with JFH-wt infected cells in 24-well plates using immunoblotting of the NS3 protein as an end point. At 2 hours post-inoculation with JFH-ΔV3-Rluc infectious supernatants, varying concentrations of the benzimidazole compounds were added to the cell culture media. Two days post-inoculation, cell lysates were harvested and the level of the Renilla luciferase activity was measured. All three benzimidazole compounds showed a dose response inhibition of HCV replication as measured by Renilla activity (Fig.6). The IC50 values (mean ± standard deviation) were 2.23 ± 0.21 µM, 4.83 ± 0.47 µM and 50.42 ± 8.05 µM, for Isis-22, Isis-13, and Isis-04, respectively (Table 1). These results were compared to those from cells infected with the JFH-wt virus where the NS3 protein detected by immunoblotting was used to measure HCV replication (Fig.7). The IC50 values determined by immunoblotting were 1.94 ± 0.33 µM, 3.89 ± 0.37 µM, and 48.49 ± 6.34 µM, respectively and were in the same range found by measuring Renilla activity for the JFH-ΔV3-Rluc inoculated cells (Table 1). In addition, NS3 immunoblotting assays were done to measure IC50 values with JFH-ΔV3-Rluc infected cells (in duplicate six well plates). The mean IC50 values for Isis-22, -13, -4, and 2'CMeA were 2.9, 4.6, 46.6, and 0.35 µM, respectively. The Rluc assay results of the JFH-ΔV3-Rluc virus were more easily obtained as compared to the immunoblotting studies.
Figure 6. Measuring antiviral activity of benzimidazole compounds that bind the IRES of HCV RNA with JFH-ΔV3-Rluc infected cells.
At three hours post-infection with the JFH-ΔV3-Rluc virus (moi=0.1) Huh 7.5 cell cultures had increasing concentrations of compounds added followed by two days of incubation. Renilla luciferase activity in cell lysates was determined as in Figures 2–5. Incubations were performed in triplicate in 96-well plates, experiments were done twice, data are reported as the mean ± standard deviation (n=6) of relative light units (RLU) and used to determine the IC50 for each compound. Panel A, B and C show the results for three different benzimidazole compounds (Isis-22, 13 and 04, see Materials and Methods). Panel D shows the structure of Isis-11 which is referred to in the Discussion regarding its high-resolution NMR structure in complex with domain IIa of the HCV IRES RNA (Paulsen, et al., 2010; Seth et al., 2005).
Table 1.
Antiviral activity of HCV inhibitor measured with the HCV JFH1-wt and JFH-ΔV3-Rluc viruses
| HCV Inhibitor | JFH-wta IC50 (µM) | JFH-ΔV3-Rlucb IC50 (µM) |
|---|---|---|
| ISIS-22 | 1.94 ± 0.33 | 2.23 ± 0.21 |
| ISIS-13 | 3.89 ± 0.37 | 4.83 ± 0.47 |
| ISIS-04 | 48.49 ± 6.34 | 50.42 ± 8.05 |
| 2’CMeA | 0.35 ±0.02 | 0.28±0.03 |
Mean ± standard deviation (n=3)
Mean ± standard deviation (n=6)
Figure 7. Measuring antiviral activity of benzimidazole compounds by immunoblotting of the NS3 protein produced by HCV JFH-wt.
Huh 7.5 cells were infected with the JFH-wt virus, cultured, and incubated in 24-well plates with inhibitors as in Figure 6 except that cells were cultured for a total of three days to assure that the NS3 protein levels could be detected. NS3 protein levels detected by immunoblotting were used to estimate the IC50 (see Materials and Methods). The level of NS3 was normalized to the level of β-actin and the level of NS3 protein relative to controls (no inhibitor, 100% level of NS3) was plotted against inhibitor concentrations. Cell cultures were done in three independent experiments, representative immunoblots are shown for each compound (Panel A, B and C) and the data are presented as mean ± standard deviation (n=3) of the NS3 levels normalized to β-actin (Panel D).
4. Discussion
The NS5A protein of HCV has an important but mechanistically unclear role in viral RNA replication and virus production (Appel et al., 2008; Huang et al., 2007). As an essential component of the replication complex, NS5A is a large phosphoprotein made up of three domains. The involvement of domain I and domain II of NS5A in HCV RNA replication has been well documented (Brass et al., 2002; Tellinghuisen et al., 2005, 2008b). Although the function of domain III is less understood, it has been shown to be non-essential for competence of an HCV replicon (Liu et al., 2006). Recently, domain III was reported to perform a critical role in the early phase of HCV assembly, as deletions or mutations severely reduced or abolished the production of infectious virus (Appel et al., 2008; Masaki et al., 2008; Tellinghuisen et al., 2008a,b). However, the mechanisms responsible for the role of domain III in virion assembly remain to be determined.
A highly variable 24 amino acid region, designated V3, was reported within domain III of NS5A between aa 2356 and 2379 in HCV genotype 1a and 1b isolates (Inchauspe et al., 1991). Evidence for the involvement of this region of NS5A in determining the responsiveness of patients with chronic hepatitis C to interferon therapy has been presented (Bouzgarrou et al., 2009; Cuevas et al., 2008; Nousbaum et al., 2000). However, the specific role of the V3 region in the viral life cycle is not known. In comparing V3 sequences of HCV1a (H77C), J6 (2a) and HCV1b (Con1), we determined that the V3 region of the JFH1 strain of HCV is 28 amino acids in length and located from aa 2356 to 2383 (aa 380 to 407 of NS5A). The sequence identity of the V3 region in JFH1 as compared to H77C (1a), Con1 (1b) and J6 (2a) is 25%, 21% and 61%, respectively (Fig.1A). However, the V3 region of JFH1 strain of HCV genotype 2a shows only a 32% identity with the genotype 2a consensus sequence, while the V3 region of JFH1 has a 4–29% identity with the consensus sequence of all other HCV genotypes (Fig.1A).
Our results demonstrate that 28 aa V3 region of HCV JFH1 is not essential for viral replication or the release of infectious virus particles (Figs.2 & 3). The deletion of V3 and replacement of V3 with the Rluc gene had surprisingly little effect on the quantity of infectious chimeric HCV produced following serial passage or infection of cells over 18 days. Upon passaging infected cells for 18 days, titers of infectious virus increased and reached a maximum level of 1.0 × 105 ffu/ml for JFH-wt, 8.0 × 104 ffu/ml for JFH-ΔV3, 2.0 × 104 ffu/ml for JFH-ΔV3-Rluc (Fig.3B). Previous reports deleting domain II or III of NS5A have shown that these regions are not strictly required for viral replication following RNA transfection, but are required for the production of infectious HCV particles (Appel et al., 2008; Moradpour et al., 2004; Tellinghuisen et al., 2008b). In contrast, our report is consistent with the observation that deleting a region of NS5A that includes V3 and extends 21 aa towards the C terminus had only a modest inhibition on the production of infectious HCV particles (Appel et al., 2008).
One HCV Rluc reporter virus has been previously described where the Rluc insert was located at amino acid 418 of NS5A, which is downstream from the V3 region (Kim et al., 2007). However, a limitation of this reporter system was the relatively low titer of infectious virus produced, resulting in an inability to conduct studies in 96-well plates. Our results show that the NS5A protein can tolerate a large insert the size of Rluc (310 aa) in the V3 region with only a modest effect on assembly of infectious virus particles. The high degree of variability of the V3 region is consistent with this observation, and suggests that the loop is not part of a major structural or functional domain of NS5A, allowing large insertions in this region to be well tolerated. The structure of domain I at the amino-terminus of NS5A, which includes the zinc-binding domain that is essential for RNA replication, has been reported (Tellinghuisen et al., 2005); however, there is no published structure of the complete NS5A protein. Recent studies have provided evidence that the assembly of infectious HCV particles occurs on lipid droplets (LDs) in a membranous environment and that the core protein recruits replication complexes to the surface of LDs through an interaction with the C-terminus of NS5A (Boulant et al., 2006; Masaki et al., 2008). The relative lack of an effect on infectious virus production of inserting Rluc in the V3 region of the carboxy-terminal domain III of NS5A predict that the interaction between NS5A and the core protein is not disrupted.
The development of the HCV-JFH1 cell culture system, and derivatives of this system, not only allow the entire HCV life cycle to be studied, but also permit identification of antivirals targeting all aspects of HCV infection (Cai et al., 2005; Wakita et al., 2005). In this study, the titer of JFH-ΔV3-Rluc reached 2.5 × 104 ffu/ml and easily permitted accurate assays of viral replication to be done in a 96-well-plate format. Moreover, our results demonstrated that the JFH-ΔV3-Rluc virus can produce progeny virus which can be serially passaged to naive Huh 7.5 cells at three day intervals for at least 18 days after the initial infection of cells with a low moi (0.01)(Fig.4). These results indicate that the JFH-ΔV3-Rluc virus could be used to study various aspects of the HCV life cycle and is useful for identifying new antiviral compounds as well as for studying host factors involved in HCV replication in 96-well format assays.
The benzimidazole classes of inhibitors are the only examples, to our knowledge, of potent small molecule inhibitors targeting the HCV IRES RNA. This report represents their first testing in an infectious HCV cell culture system. Isis-13 has a high affinity for domain IIa of the HCV IRES RNA and was shown to have an IC50 of 5.4 µM in an HCV replicon assay (Seth et al., 2005). Isis-04 was selected because the RNA KD was relatively weaker at 8 µM and we predicted this would correlate with a higher IC50; this was corroborated by both the JFH-ΔV3-Rluc (IC50 of 50.42 ± 8.05 µM) and control (IC50 of 48.49 ± 6.34 µM) viral assays. The result with Isis-04 shows the luciferase reporter is a sensitive system with the potential for application to high-throughput screening to identify compounds with relatively high IC50’s. Compound Isis-22 was anticipated to have activity similar to that of Isis-13, but had not been tested previously for either RNA binding or HCV antiviral activity. As expected, this compound had an IC50 similar to Isis-13 with values of 2.2 and 1.9 µM in the JFH-ΔV3-Rluc and control virus assays, respectively. We were also intrigued by compound Isis-22 because the dihydrofuran ring is a feature of Isis-11 for which we have determined a high-resolution NMR structure in complex with domain IIa of the HCV IRES RNA (Begley and Varani, 2009; Paulsen et al., 2010). The doubly constrained Isis-22 (Fig.7A) had the lowest IC50 of the three compounds, comparable to that determined previously for the singly constrained Isis-11 (Seth et al., 2005). Hermann and co-workers have shown that Isis-13 induces an RNA conformational change similar to that we have described for Isis-11, suggesting that both cyclic and acyclic functionalities on the longer dimethylamino side chain bind similarly to the HCV IRES RNA (Begley and Varani, 2009; Parsons et al., 2009). Constraining both dimethylamino side chains in cyclic structures may improve RNA binding affinity, but differences in pKa’s may affect cellular permeability, in turn affecting the IC50. pKa calculations show that the two dimethylamino groups of Isis13 are 8.50 and 9.41, while in the acyclic Isis-04 the pKa’s are calculated to be 8.32 and 8.97 (Hilal et al., 1995).The doubly constrained compounds also exist as stereoisomer mixtures – we have shown for Isis-11 that there is some stereochemical preference for RNA binding, raising the possibility that a specific isomer of either Isis-13 or Isis-22 would have a sub-micromolar IC50 in the infectious HCV reporter system described (Paulsen et al., 2010).
In summary, our results show that the deletion of V3 of JFH1 NS5A and replacing it with Rluc in JFH NS5A did not abrogate HCV replication and only moderately impaired infectious viral particle production. The sensitivity and rapidity of this infectious chimeric HCV reporter system should make it useful for identifying new HCV antiviral compounds from chemical libraries, as well as optimizing lead compounds with low micromolar activity. The V3 region of HCV JFH1 NS5A is dispensable in the HCV life cycle and suggests that the replacement of this region of the JFH1 genome with other inserts could produce additional useful chimeric viruses for studying HCV and antivirals.
Acknowledgements
We thank T. Wakita for providing the plasmid containing the HCV JFH1 cDNA and C.M. Rice for providing Huh 7.5 cells. We thank C. Liu for the anti-NS5A monoclonal antibody. We also thank S. Young for her illustrations. L. Xiao was supported in part by a fellowship from Taizhou People's Hospital, Taizhou, China. This work was supported in part by NIH Grant CA63640 to C. Hagedorn and a grant from the US-Israel BSF to D. Davis.
Abbreviations
- Rluc
Renilla reniformis luciferase
- RLU
relative light units
- IFA
immunofluorescence assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- Appel N, Pietschmann T, Bartenschlager R. Mutational analysis of hepatitis C virus nonstructural protein 5A: potential role of differential phosphorylation in RNA replication and identification of a genetically flexible domain. J. Virol. 2005;79:3187–3194. doi: 10.1128/JVI.79.5.3187-3194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel N, Zayas M, Miller S, Krijnse-Locker J, Schaller T, Friebe P, Kallis S, Engel U, Bartenschlager R. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 2008;4:e1000035. doi: 10.1371/journal.ppat.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley DW, Varani G. Locking out viral replication. Nat. Chem. Biol. 2009;5:782–783. doi: 10.1038/nchembio.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek SR, Terrault NA. The changing epidemiology and natural history of hepatitis C virus infection. Clin. Liver Dis. 2006;10:697–715. doi: 10.1016/j.cld.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant S, Montserret R, Hope RG, Ratinier M, Targett-Adams P, Lavergne JP, Penin F, McLauchlan J. Structural determinants that target the hepatitis C virus core protein to lipid droplets. J. Biol. Chem. 2006;281:22236–22247. doi: 10.1074/jbc.M601031200. [DOI] [PubMed] [Google Scholar]
- Bouzgarrou N, Hassen E, Mahfoudh W, Gabbouj S, Schvoerer E, Ben Yahia A, Ben Mami N, Triki H, Chouchane L. NS5A(ISDR-V3) region genetic variability of Tunisian HCV-1b strains: Correlation with the response to the combined interferon/ribavirin therapy. J. Med. Virol. 2009;81:2021–2028. doi: 10.1002/jmv.21641. [DOI] [PubMed] [Google Scholar]
- Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- Brass V, Bieck E, Montserret R, Wolk B, Hellings JA, Blum HE, Penin F, Moradpour D. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 2002;277:8130–8139. doi: 10.1074/jbc.M111289200. [DOI] [PubMed] [Google Scholar]
- Cai Z, Zhang C, Chang KS, Jiang J, Ahn BC, Wakita T, Liang TJ, Luo G. Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. J. Virol. 2005;79:13963–13973. doi: 10.1128/JVI.79.22.13963-13973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SS, Tomassini JE, Bosserman M, Getty K, Stahlhut MW, Eldrup AB, Bhat B, Hall D, Simcoe AL, LaFemina R, Rutkowski CA, Wolanski B, Yang Z, Migliaccio G, De Francesco R, Kuo LC, MacCoss M, Olsen DB. Inhibition of hepatitis C virus RNA replication by 2'-modified nucleoside analogs. J. Biol. Chem. 2003;278:11979–11984. doi: 10.1074/jbc.M210914200. [DOI] [PubMed] [Google Scholar]
- Cuevas JM, Torres-Puente M, Jimenez-Hernandez N, Bracho MA, Garcia-Robles I, Wrobel B, Carnicer F, del Olmo J, Ortega E, Moya A, Gonzalez-Candelas F. Genetic variability of hepatitis C virus before and after combined therapy of interferon plus ribavirin. PLoS One. 2008;3:e3058. doi: 10.1371/journal.pone.0003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- Eldrup AB, Allerson CR, Bennett CF, Bera S, Bhat B, Bhat N, Bosserman MR, Brooks J, Burlein C, Carroll SS, Cook PD, Getty KL, MacCoss M, McMasters DR, Olsen DB, Prakash TP, Prhavc M, Song Q, Tomassini JE, Xia J. Structure-activity relationship of purine ribonucleosides for inhibition of hepatitis C virus RNA-dependent RNA polymerase. J. Med. Chem. 2004;47:2283–2295. doi: 10.1021/jm030424e. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Rice CM, Goff SP. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc. Natl. Acad. Sci. USA. 2004;101:13038–13043. doi: 10.1073/pnas.0405152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Guo JT, Bichko VV, Seeger C. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 2001;75:8516–8523. doi: 10.1128/JVI.75.18.8516-8523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilal SH, Karickhoff SW, Carreira LA. A Rigorous Test for SPARC's Chemical Reactivity Models: Estimation of More Than 4300 Ionization pKa's. Quant. Struc. Act. Rel. 1995;14:348. [Google Scholar]
- Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- Huang Y, Staschke K, De Francesco R, Tan SL. Phosphorylation of hepatitis C virus NS5A nonstructural protein: a new paradigm for phosphorylation-dependent viral RNA replication? Virology. 2007;364:1–9. doi: 10.1016/j.virol.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Inchauspe G, Zebedee S, Lee DH, Sugitani M, Nasoff M, Prince AM. Genomic structure of the human prototype strain H of hepatitis C virus: comparison with American and Japanese isolates. Proc. Natl. Acad. Sci. USA. 1991;88:10292–10296. doi: 10.1073/pnas.88.22.10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT, Murray CL, Eastman DK, Tassello J, Rice CM. Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J. Virol. 2007;81:8374–8383. doi: 10.1128/JVI.00690-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Furusaka A, Miyamoto M, Date T, Yasui K, Hiramoto J, Nagayama K, Tanaka T, Wakita T. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J. Med. Virol. 2001;64:334–339. doi: 10.1002/jmv.1055. [DOI] [PubMed] [Google Scholar]
- Kim CS, Jung JH, Wakita T, Yoon SK, Jang SK. Monitoring the antiviral effect of alpha interferon on individual cells. J. Virol. 2007;81:8814–8820. doi: 10.1128/JVI.02824-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoudakis G, Kaul A, Steinmann E, Kallis S, Lohmann V, Pietschmann T, Bartenschlager R. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J. Virol. 2006;80:5308–5320. doi: 10.1128/JVI.02460-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Lohmann V, Bartenschlager R. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 2001;75:4614–4624. doi: 10.1128/JVI.75.10.4614-4624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- Liu S, Ansari IH, Das SC, Pattnaik AK. Insertion and deletion analyses identify regions of non-structural protein 5A of Hepatitis C virus that are dispensable for viral genome replication. J. Gen. Virol. 2006;87:323–327. doi: 10.1099/vir.0.81407-0. [DOI] [PubMed] [Google Scholar]
- Masaki T, Suzuki R, Murakami K, Aizaki H, Ishii K, Murayama A, Date T, Matsuura Y, Miyamura T, Wakita T, Suzuki T. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J. Virol. 2008;82:7964–7976. doi: 10.1128/JVI.00826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CJ, Maucourant S, Griffin S, Rowlands DJ, Harris M. Tagging of NS5A expressed from a functional hepatitis C virus replicon. J. Gen. Virol. 2006;87:635–640. doi: 10.1099/vir.0.81553-0. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Evans MJ, Gosert R, Yuan Z, Blum HE, Goff SP, Lindenbach BD, Rice CM. Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. J. Virol. 2004;78:7400–7409. doi: 10.1128/JVI.78.14.7400-7409.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat .Rev. Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nousbaum J, Polyak SJ, Ray SC, Sullivan DG, Larson AM, Carithers RL, Jr, Gretch DR. Prospective characterization of full-length hepatitis C virus NS5A quasispecies during induction and combination antiviral therapy. J. Virol. 2000;74:9028–9038. doi: 10.1128/jvi.74.19.9028-9038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J, Castaldi MP, Dutta S, Dibrov SM, Wyles DL, Hermann T. Conformational inhibition of the hepatitis C virus internal ribosome entry site RNA. Nat. Chem. Biol. 2009;5:823–825. doi: 10.1038/nchembio.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RB, Seth PP, Swayze EE, Griffey RH, Skalicky JJ, Cheatham TE, 3rd, Davis DR. Inhibitor-induced structural change in the HCV IRES domain IIa RNA. Proc. Natl. Acad. Sci. USA. 2010;107:7263–7268. doi: 10.1073/pnas.0911896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology. 2004;39:5–19. doi: 10.1002/hep.20032. [DOI] [PubMed] [Google Scholar]
- Phan T, Beran RK, Peters C, Lorenz IC, Lindenbach BD. Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1–E2 glycoprotein and NS3-NS4A enzyme complexes. J. Virol. 2009;83:8379–8395. doi: 10.1128/JVI.00891-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak SJ, Paschal DM, McArdle S, Gale MJ, Jr, Moradpour D, Gretch DR. Characterization of the effects of hepatitis C virus nonstructural 5A protein expression in human cell lines and on interferon-sensitive virus replication. Hepatology. 1999;29:1262–1271. doi: 10.1002/hep.510290438. [DOI] [PubMed] [Google Scholar]
- Reed KE, Rice CM. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr. Top. Microbiol. Immunol. 2000;242:55–84. doi: 10.1007/978-3-642-59605-6_4. [DOI] [PubMed] [Google Scholar]
- Seth PP, Miyaji A, Jefferson EA, Sannes-Lowery KA, Osgood SA, Propp SS, Ranken R, Massire C, Sampath R, Ecker DJ, Swayze EE, Griffey RH. SAR by MS: discovery of a new class of RNA-binding small molecules for the hepatitis C virus: internal ribosome entry site IIA subdomain. J. Med. Chem. 2005;48:7099–7102. doi: 10.1021/jm050815o. [DOI] [PubMed] [Google Scholar]
- Tellinghuisen TL, Marcotrigiano J, Gorbalenya AE, Rice CM. The NS5A protein of hepatitis C virus is a zinc metalloprotein. J. Biol. Chem. 2004;279:48576–48587. doi: 10.1074/jbc.M407787200. [DOI] [PubMed] [Google Scholar]
- Tellinghuisen TL, Marcotrigiano J, Rice CM. Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature. 2005;435:374–379. doi: 10.1038/nature03580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellinghuisen TL, Foss KL, Treadaway J. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 2008a;4:e1000032. doi: 10.1371/journal.ppat.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellinghuisen TL, Foss KL, Treadaway JC, Rice CM. Identification of residues required for RNA replication in domains II and III of the hepatitis C virus NS5A protein. J. Virol. 2008b;82:1073–1083. doi: 10.1128/JVI.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscherne DM, Jones CT, Evans MJ, Lindenbach BD, McKeating JA, Rice CM. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J. Virol. 2006;80:1734–1741. doi: 10.1128/JVI.80.4.1734-1741.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Ma Y, Yates J, Lemon SM. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J. Virol. 2007;81:629–638. doi: 10.1128/JVI.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]