Abstract
Congenital muscular dystrophies (CMD) such as muscle-eye-brain disease caused by defective glycosylation of α-dystroglycan (α-DG) exhibit defective photoreceptor synaptic function. Mouse knockouts of dystroglycan and its extracellular matrix binding partner pikachurin recapitulate this phenotype. In this study, pikachurin-α-dystroglycan interactions in several mouse models of CMD were examined by pikachurin overlay experiments. The results show that hypoglycosylation of α-dystroglycan resulted in markedly reduced pikachurin-α-dystroglycan interactions. Expression of pikachurin is abolished at the outer plexiform layer of two mouse models, protein O-mannose N-acetylglucosaminyl transferase 1 (POMGnT1) knockout and Largemyd mice. Overexpressing LARGE restored this interaction in POMGnT1 knockout cells. These results indicate that pikachurin interactions with α-dystroglycan and its localization at the photoreceptor ribbon synapse require normal glycosylation of α-dystroglycan.
Introduction
The extracellular matrix receptor α-dystroglycan (α-DG) binds to the transmembrane β-DG, which in turn interacts with dystrophin and utrophin via its intracellular domain. Dystroglycan and dystrophin are highly expressed by photoreceptors at presynaptic terminal in the outer plexiform layer [1;2]. Electroretinography recording of dark adapted Duchenne muscular dystrophy patients with defective dystrophin and the mouse model mdxCv3, a splicing mutation that disrupts expression of the 427 kDa and 70 kDa isoforms of dystrophin [3], show reduced b-wave amplitude [4–6] affecting the photoreceptor/bipolar cell transmission [7]. Dystroglycan knockout mice showed similar b-wave abnormalities [8]. These results indicate an essential role of dystroglycan in photoreceptor synaptic function.
α-DG is heavily substituted by O-linked mannosyl glycans, such as Siaα2,3Galβ1,4GlcNAcβ1,2Man-Ser/Thr [9]. Many CMD patients with brain and eye defects have mutations in genes encoding glycosyltransferases (or putative glycosyltransferases) [10] including POMT1 (encoding protein O-mannosyltransferase 1), POMT2, POMGnT1 (encoding protein O-mannose N-acetylglucosaminyl transferase 1), LARGE (encoding like-glycosyltransferase), FKTN (encoding fukutin), and FKRP (encoding fukutin-related protein). POMT1 and POMT2 transfer mannose to serine or threonine residues [11]. POMGnT1 then transfers N-acetylglucosamine to O-linked mannose [12;13]. LARGE is involved in extending an unidentified phosphoryl glycosylation branch off O-linked mannose [14] and complex N- and mucin-type O-glycosylations of α-DG [15;16]. α-DG interacts with high affinity to several extracellular matrix components, including laminin [17], agrin [18], perlecan [19], neurexin [20], and pikachurin [21]. A common molecular phenotype in CMD is the hypoglycosylation of α-DG, which leads to significant loss of its binding activity to laminin and perlecan [22]. Functionally, POMGnT1 knockout mice [23], the natural LARGE mutant Largemyd mice [24;25], and chimeric fukutin knockout mice [26], exhibit reduced b-waves in their electroretinograms, indicating defective synaptic functions at the photoreceptor synapse when α-DG is hypoglycosylated.
It is not known which extracellular matrix molecule(s) mediates the effect of α-DG hypoglycosylation at the photoreceptor synapse. Pikachurin is a secreted extracellular matrix protein that is expressed at the photoreceptor synapse [21]. Pikachurin knockout in the mouse results in defective ribbon synapses in the outer plexiform layer and a delayed b-wave with reduced amplitude. In this study, we examined pikachurin-dystroglycan interactions in several CMD models. Our results show that pikachurin-dystroglycan binding is dependent on proper glycosylation of α-DG, suggesting that defective synaptic functions in CMD may be caused by defective pikachurin-dystroglycan interactions.
Materials and Methods
Animals
Protocols for animal usage were approved by the Institutional Animal Care and Use Committee of Upstate Medical University and adhered to National Institutes of Health guidelines.
POMGnT1 knockout mice were generated by Lexicon Genetics Incorporated (The Woodlands, TX) [23]. POMT2-floxed mice were generated at the Gene Targeting and Transgenic Facility at the University of Connecticut Health Science Center (unpublished). Dag1-floxed [27], Largemyd, Nestin-Cre transgenic [28], and Emx1-Cre knock-in mice [29] were from the Jackson Laboratories (Bar Harbor, MI). Dag1-floxed mice were crossed with Nestin-Cre transgenic mice to yield dystroglycan knockout mice, Dag1f/f;Nestin-Cre(+). POMT2-floxed mice were crossed with Emx1-Cre knock-in mice to obtain POMT2 knockout. Emx1-Cre knock-in mice express Cre recombinase in Emx1-expressing cells of the forebrain and mediate recombination in radial glial cells [29]. POMT2-deficient neural stem cells were isolated from POMT2f/f;Emx1-Cre(+) animals.
Antibodies
Antibodies were obtained as follows: Monoclonal anti-Bassoon from Assay Designs (Ann Arbor, MI); Monoclonal anti-c-Myc from Sigma-Aldrich (St. Louis, MO); Monoclonal IIH6C4 from Millipore Corporation (Billerica, MA); Monoclonal anti-β-dystroglycan (clone 7D11) from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA); Polyclonal anti-pikachurin from Waco Chemicals USA, Inc. (Richmond, VA).
Neural stem cell culture
Neural stem cells were isolated from POMGnT1 knockout, POMT2f/f;Emx1-Cre(+) (POMT2 knockout), and wildtype mice. Briefly, neocortical wall was dissected from embryonic day 13.5 fetuses, trypsinized, and triturated. The dissociated cells were cultured as neural spheres in neural basal medium (Invitrogen, Carlsbad, CA) supplemented with B27 (minus vitamin A), FGF-2 (20 ng/ml), EGF (20 ng/ml), and heparin (2 ng/ml).
To overexpress LARGE in neural stem cells, an adenoviral vector for c-Myc tagged human LARGE (Ad-LARGE) was constructed at Vector Biolabs (Philadelphia, PA). Twenty μl (5 ×1012 viral particles/ml) of Ad-LARGE was added to each 150 mm culture dish. The cells were harvested 2 days after infection and homogenized in a Dounce homogenizer in cold lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% TritonX-100,) supplemented with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), and centrifuged at 16,100 g for 20 minutes at 4 °C. The supernatant was used for Western blot analysis.
Western blot
Wheat germ agglutinin (WGA)-agarose gel (EY Laboratories, San Mateo, CA) was used to isolate glycoproteins. For 4 mg of total protein lysates, 50 μl of WGA-gel were added. After binding overnight, WGA-gel was washed 3 times with lysis buffer. Bound glycoproteins were eluted with gel-loading dye, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 8%), and electrotransferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 3% BSA in Tris-buffered saline (TBS, 50 mM Tris-HCl, pH 7.4, 150 mM NaCl) and incubated with primary antibody overnight. After washing with TBST (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.05% Tween 20), the membranes were incubated with appropriate secondary antibodies conjugated with horseradish peroxidase and washed. Immunoreactive signal was visualized with SuperSignal west pico chemiluminescent substrate (Thermo Scientific, Rockford, IL).
Pikachurin overlay
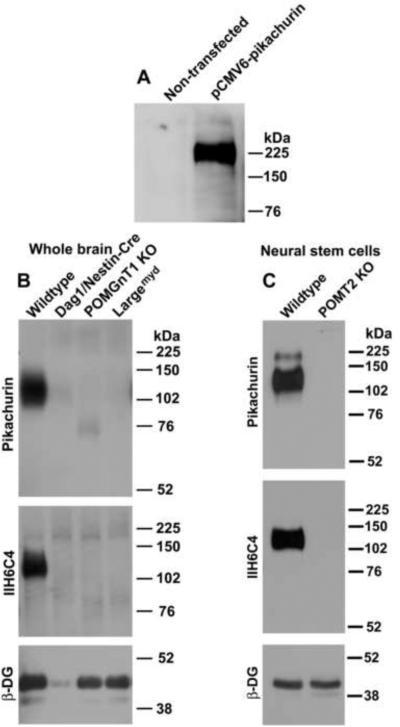
A pCMV6 Entry expression plasmid containing c-Myc-tagged cDNA encoding the human EGF-like, fibronectin type III and laminin G domains (EGFLAM), transcript variant 1, also known as pikachurin, was obtained from Origene (Rockville, MD). Human embryonic kidney (HEK) 293 cells were transfected with the pikachurin expression plasmid using FuGENE®HD reagent (Roche Diagnostics, Indianapolis, IN). Twenty-four hours after transfection, the cells were washed and conditioned medium was collected with serum-free DMEM after an additional 48 hours. Western blot analysis with anti-c-Myc antibody revealed a 225 kDa pikachurin band only in the conditioned medium from transfected cells but not in that from non-transfected cells (Figure 1A).
Figure 1.
Hypoglycosylation of α-DG led to dramatically reduced laminin binding.
Glycoproteins were isolated from the whole brains and cultured neural stem cells by WGA-agarose, separated on SDS-PAGE, and transferred onto PVDF membranes. Pikachurin overlay and Western blot then were carried out. (A) Recombinant pikachurin (detected with anti-c-Myc) was detected in conditioned medium from HEK293 cells transfected with pikachurin expression plasmid. (B) Whole brain lysates. (C) Neural stem cells. Pikachurin binding was readily detected in the wildtype but dramatically reduced or abolished in Dag1f/f;Nestin-Cre(+), POMGnT1 knockout, and Largemyd mice and in POMT2-deficient neural stem cells. Abbreviations: β-DG, β-dystroglycan; KO, knockout.
To carry out pikachurin overlay, the PVDF membranes were blocked with 3% BSA in TBS for an hour. The membranes were then incubated with pikachurin-conditioned medium overnight at 4 °C. After washing with TBST containing 1 mM CaCl2 and 1 mM MgCl2, bound pikachurin was detected with rabbit anti-pikachurin or anti-c-Myc by standard immunoblot procedures with all buffers containing 1 mM CaCl2 and 1 mM MgCl2.
Immunofluorescence staining
Animals were deaply anesthetized by intraperitoneal injection of pentobarbital (400 mg/kg body weight) and perfused with 4% paraformaldehyde. The eyes were excised and the lenses removed. The retinas were cut into 10 μm sections with a Cryostat and mounted onto slides. After blocking with 3% bovine serum albumin (BSA) in phosphate buffer (pH7.4), the sections were incubated with anti-β-dystroglycan, anti-Bassoon, and anti-pikachurin and washed with phosphate buffer. Then, the sections were incubated with biotinylated goat anti-mouse IgG or goat anti-rabbit IgG. After washing, the sections were incubated with Avidin-FITC. All sections were counterstained with 4', 6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO) to show nuclei. Images were taken on a Nikon Elclipse TE200 fluorescence microscope with DeltaVision Core Restoration Microscopy software (Applied Precision, LLC, Issaquah, WA).
Results
Pikachurin binding to α-DG is dependent on proper glycosylation
To determine whether α-DG interactions with pikachurin require proper glycosylation, we isolated glycoproteins from whole brain lysates by WGA-affinity from several mouse models, Dag1f/f:Nestin-Cre(+), POMGnT1 knockout, and Largemyd mice. Pikachurin overlay and Western blot analysis with IIH6C4 and β-DG antibodies were carried out. In the wildtype, strong pikachurin-binding to α-DG was detected at 125 kDa (Figure 1B), while in dystroglycan knockout, pikachurin bound at the 125 kDa location was diminished as expected. A residual signal was detected due to the presence of meninges and blood vessels not expected to express the Cre recombinase in Nestin-Cre transgenic mice. Indeed, remnant amounts of β-DG were detected in the brain lysate of brain-specific knockout mice of dystroglycan. Interestingly, in both POMGnT1 knockout and Largemyd mice models, pikachurin signals at 125 kDa were abolished. In POMGnT1 knockout mouse, weak pikachurin binding at 75 kDa was consistently detected, likely due to the presence of hypoglycosylated α-DG. As expected, immunoblot with IIH6C4, an antibody that recognizes functionally glycosylated form of α-DG, confirmed hypoglycosylation of α-DG in POMGnT1 knockout and Largemyd mice as evidenced by loss of immunoreactivity.
POMGnT1 and LARGE are involved in further extension of O-linked mannose into branched oligosaccharides. Mouse knockout of POMT2 is expected to result in complete absence of all O-mannosyl glycosylation [11], we therefore analyzed POMT2 knockout neural stem cells (Figure 1C). We found no pikachurin binding to glycoproteins isolated from POMT2 knockout cells. Wildtype neural stem cells used as a control showed strong pikachurin binding. IIH6C4 immunoblot confirmed hypoglycosylation of α-DG in POMT2 knockout cells as expected. Taken together, these results indicate that pikachurin-α-DG interactions require O-mannosyl glycosylation of α-DG.
LARGE overexpression promotes pikachurin binding in POMGnT1 knockout cells
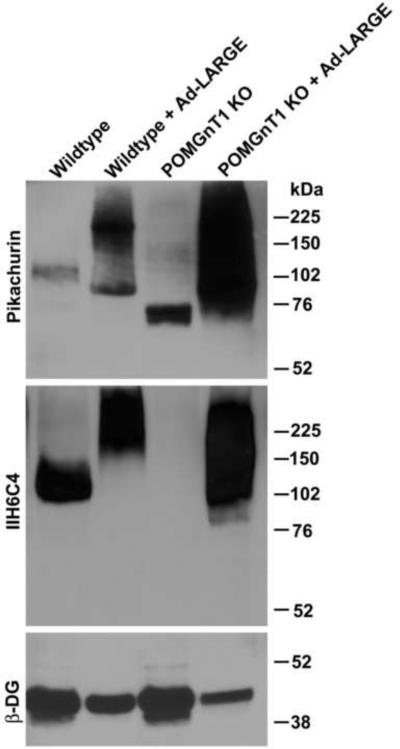
LARGE overexpression hyperglycosylates α-DG and increases laminin binding in not only wildtype cells but also cells isolated from CMD patients with mutations in (presumed) glycosyltransferases. We examined whether overexpressing LARGE had a similar effect on pikachurin interactions with α-DG (Figure 2). In wildtype neural stem cells, overexpression of LARGE increased glycosylation of α-DG as revealed by increased IIH6C4 immunoreactivity with increased molecular weight. Interestingly, pikachurin binding was also increased by LARGE overexpression. In POMGnT1 knockout neural stem cells, we observed only residual pikachurin binding at about 75 kDa. Overexpression of LARGE in POMGnT1 knockout neural stem cells increased not only IIH6C4 immunoreactivity but also pikachurin binding. These results indicate that LARGE overexpression restores pikachurin binding in POMGnT1 knockout cells as well.
Figure 2.
LARGE overexpression enhances pikachurin binding in POMGnT1 knockout cells.
Wildtype and POMGnT1-deficient neural stem cells were infected with Ad-LARGE. Pikachurin overlay and IIH6C4 immunoblot analysis were then carried out on glycoproteins isolated from cell lysates. Pikachurin binding was dramatically increased by LARGE overexpression in both wildtype and POMGnT1-deficient neural stem cells.
Pikachurin expression is reduced at the outer plexiform layer of CMD models
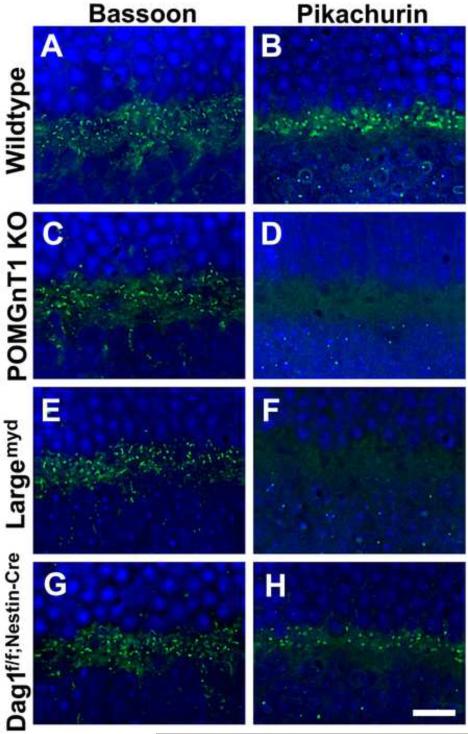
In a previous study, we showed that β-DG was expressed in the outer plexiform layer of wildtype and POMGnT1 knockout mice [30]. To evaluate the expression of pikachurin protein in the outer plexiform layer of mouse models of CMD, immunofluorescence staining with antibodies against Bassoon (a ribbon synapse marker) and pikachurin was carried out. POMT2f/f;Emx1-Cre(+) retinas were not included in this analysis because there was no Cre activity in the retina. In the wildtype retinas, Bassoon-positive ribbon synapses (Figure 3A) and pikachurin-positive punctas (Figure 3B) were detected in the outer plexiform layer. In the retinas of POMGnT1 knockout (Figure 3D) and Largemyd (Figure 3F) mice, pikachurin immunoreactivity was abolished in the outer plexiform layer while Bassoon immunoreactivity was similar to that of the wildtype (Figure 3C and E). Pikachurin immunofluorescence in other retinal layers was at the background level. These results indicate that pikachurin localization to the photoreceptor synapse in the outer plexiform layer is lost in the retinas of POMGnT1 knockout and Largemyd mice.
Figure 3.
Diminished pikachurin expression in the outer plexiform layer of the mutant mouse retinas.
Retinal sections were immunofluorescence stained with antibodies against Bassoon (A, C, E, and G) and pikachurin (B, D, F, and H). (A and B) Wildtype retina. Bassoon and pikachurin immunofluorescence were observed at the ribbon synapses in the outer plexiform layer. (C and D) POMGnT1 knockout retina. While ribbon synapses were immunoreactive to anti-Bassoon, immunoreactivity to anti-pikachurin was lost. (E and F) Largemyd retina. Pikachurin immunoreactivity at the ribbon synapse was lost. (G and H) Dag1f/f;Nestin-Cre(+) retina. While no noticeable changes were observed for the density of Bassoon immunoreactive ribbon synapses, the density of pikachurin-positive puncta was reduced. Each pikachurin-positive puncta showed similar fluorescence intensity to the wildtype. Scale bar in H: 10 μm.
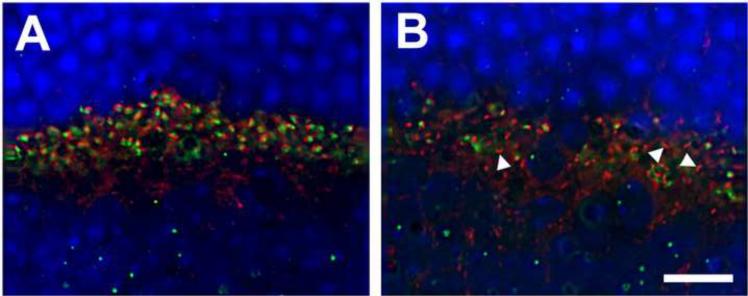
In the retinas of Dag1f/f;Nestin-Cre(+) mice, Bassoon immunofluorescence staining was also similar to that of the wildtype (Figure 3G). However, pikachurin-positive punctas showed reduced density although the fluorescence intensity of each puncta was similar to that of the wildtype (Figure 3H). We counted pikachurin-positive punctas from the micrographs (representing a span of 52 μm of outer plexiform layer). In the wildtype retinas, there were 67.82 pikachurin-positive punctas per image. By contrast, there were 32.50 pikachurin-positive punctas per image for the retinas of Dag1f/f;Nestin-Cre(+) mice (p<0.001, Chi-square analysis), indicating a reduction in pikachurin-positive ribbon synapses. We next carried out double immunofluorescence staining with antibodies against Bassoon (red fluorescence) and pikachurin (green fluorescence). While virtually all Bassoon-positive punctas were adjacent to pikachurin-positive punctas in the outer plexiform layer of wildtype retinas (Figure 4A), many Bassoon-positive punctas in the retinas of Dag1f/f;Nestin-Cre(+) mice were devoid of adjacent pikachurin-positive punctas (arrowheads in Figure 4B), indicating the presence of pikachurin-negative ribbon synapses.
Figure 4.
Ribbon synapses devoid of pikachurin were present in Dag1f/f;Nestin-Cre(+) retinas.
Retinal sections were immunostained with antibodies against Bassoon (red fluorescence) and pikachurin (green fluorescence). (A) Wildtype retina. Virtually all Basson-positive ribbon synapses were accompanied by immunoreactivity to anti-pikachurin. (B) Dag1f/f;Nestin-Cre(+) retina. Basson-positive ribbon synapses devoid of pikachurin immunoreactivity were frequently observed (arrowheads). Scale bar: 10 μm.
Reduced number but normal immunofluorescence intensity in pikachurin-positive puncta suggested that dystroglycan deletion in the retina may be mosaic. Indeed, when Nestin-cre transgenic mice were crossed with a global double-fluorescent Cre reporter mouse line [31], both red and green fluorescent cells were widely distributed in double transgenic retinas, indicating the mosaic nature of Nestin-Cre-mediated deletion in the retina (data not shown). Presence of dystroglycan in the outer plexiform layer of Dag1f/f;Nestin-Cre(+) mouse retinas supports this conclusion [8]. Taken together, these results suggest dystroglycan and its functional glycosylation are required for localization of pikachurin to the photoreceptor synapse.
Discussion
In this study, we show that proper glycosylation of α-DG is required for its interaction with pikachurin. Markedly reduced or abolished α-DG binding to pikachurin is observed in POMGnT1 knockout, POMT2 knockout, and Largemyd mouse brains or cells. Furthermore, pikachurin localization at the ribbon synapses was not detected in POMGnT1 and Largemyd mouse retinas. In Dag1f/f;Nestin-Cre(+) retinas, ribbon synapses without pikachurin were frequently observed. While manuscript was in preparation, in a very recent study, Kanagawa, et al [32] show that pikachurin interaction with α-DG and its localization at the outer plexiform layer were affected in POMGnT1 knockout and Largemyd mouse retinas. Together, these findings suggest that the synaptic functional defect found in CMD models may be attributable to the diminished pikachurin-α-DG interactions.
Hyperglycosylation by LARGE bypasses deficiency in O-mannosyl glycosylation to rescue laminin binding of α-DG [33]. This unique property has been proposed as a possible therapy for CMD caused by defective glycosylation of α-DG. Our results show that LARGE overexpression also rescues pikachurin-α-DG interactions in POMGnT1-deficient cells, posing the possibility that LARGE may also be used to restore defective retinal functions caused by diminished pikachurin-α-DG binding in CMD. In this regard, it will be necessary to determine which cell type is required to express functionally glycosylated α-DG for maintaining pikachurin expression at the ribbon synapse. The Emx1-Cre knock-in mice used in this study does not drive expression of Cre in the retina precluding cell type specific study with this Cre-driver line. Crossing of POMT2- and Dag1-floxed mice with retinal cell type specific Cre transgenic mice will enable the determination of cell type specific requirement of functionally glycosylated α-DG in pikachurin localization to the ribbon synapses.
POMGnT1 knockout [30], Largemyd [25], and a FKRP hypomorphic [34] mice exhibit disruptions in the inner limiting membrane. Because pikachurin is not expressed at this location, it probably does not play a role in causing the inner limiting membrane disruptions. Other ligands of α-DG such as laminin-111 may be involved.
Of the extracellular matrix molecules that bind to α-DG, only pikachurin is known to be expressed at the photoreceptor ribbon synapses. No evidence exists that laminin-111, laminin-211, perlecan, and neurexins are expressed at the photoreceptor synapse. However, it is likely some other isoforms of laminins are present in the outer plexiform layer because α3, α4, α5, β2, and γ3 laminin chains are detected by immunofluorescence in the outer plexiform layer [35]. Furthermore, β2 laminin deficient mice exhibit a reduced b-wave amplitude [36]. Thus, it will be interesting to determine the isoform(s) of laminin expressed in the outer plexiform layer, whether it binds to dystroglycan in a glycosylation dependent manner, and how it cooperates with pikachurin to regulate synaptic function.
Research highlights.
O-mannosyl glycosylation of α-dystroglycan is required for its binding to pikachurin. LARGE overexpression restores pikachurin-dystroglycan interactions.
Pikachurin expression at the ribbon synapses requires dystroglycan and its glycosylation.
Acknowledgements
We thank Dr. Brian Howell for reading the manuscript and Ms. Bonnie Lee for editing. This research was supported by NIH grants NS066582 and HD060458 (to H.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ueda H, Gohdo T, Ohno S. Beta-dystroglycan localization in the photoreceptor and Muller cells in the rat retina revealed by immunoelectron microscopy. J. Histochem. Cytochem. 1998;46:185–191. doi: 10.1177/002215549804600207. [DOI] [PubMed] [Google Scholar]
- [2].Schmitz F, Holbach M, Drenckhahn D. Colocalization of retinal dystrophin and actin in postsynaptic dendrites of rod and cone photoreceptor synapses. Histochemistry. 1993;100:473–479. doi: 10.1007/BF00267828. [DOI] [PubMed] [Google Scholar]
- [3].Cox GA, Phelps SF, Chapman VM, Chamberlain JS. New mdx mutation disrupts expression of muscle and nonmuscle isoforms of dystrophin. Nat. Genet. 1993;4:87–93. doi: 10.1038/ng0593-87. [DOI] [PubMed] [Google Scholar]
- [4].Cibis GW, Fitzgerald KM, Harris DJ, Rothberg PG, Rupani M. The effects of dystrophin gene mutations on the ERG in mice and humans. Invest Ophthalmol. Vis. Sci. 1993;34:3646–3652. [PubMed] [Google Scholar]
- [5].Pillers DA, Weleber RG, Woodward WR, Green DG, Chapman VM, Ray PN. mdxCv3 mouse is a model for electroretinography of Duchenne/Becker muscular dystrophy. Invest Ophthalmol. Vis. Sci. 1995;36:462–466. [PubMed] [Google Scholar]
- [6].Pillers DA, Bulman DE, Weleber RG, Sigesmund DA, Musarella MA, Powell BR, Murphey WH, Westall C, Panton C, Becker LE. Dystrophin expression in the human retina is required for normal function as defined by electroretinography. Nat. Genet. 1993;4:82–86. doi: 10.1038/ng0593-82. [DOI] [PubMed] [Google Scholar]
- [7].Fitzgerald KM, Cibis GW, Giambrone SA, Harris DJ. Retinal signal transmission in Duchenne muscular dystrophy: evidence for dysfunction in the photoreceptor/depolarizing bipolar cell pathway. J. Clin. Invest. 1994;93:2425–2430. doi: 10.1172/JCI117250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Satz JS, Philp AR, Nguyen H, Kusano H, Lee J, Turk R, Riker MJ, Hernandez J, Weiss RM, Anderson MG, Mullins RF, Moore SA, Stone EM, Campbell KP. Visual impairment in the absence of dystroglycan. J. Neurosci. 2009;29:13136–13146. doi: 10.1523/JNEUROSCI.0474-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J. Biol. Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- [10].Muntoni F, Torelli S, Brockington M. Muscular dystrophies due to glycosylation defects. Neurotherapeutics. 2008;5:627–632. doi: 10.1016/j.nurt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, Herrmann R, Straub V, Talim B, Voit T, Topaloglu H, Toda T, Endo T. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase. POMGnT1, Dev. Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- [13].Zhang W, Betel D, Schachter H. Cloning and expression of a novel UDP-GlcNAc:alpha-D-mannoside beta1,2-N-acetylglucosaminyltransferase homologous to UDP-GlcNAc:alpha-3-D-mannoside beta1,2-N-acetylglucosaminyltransferase I. Biochem. J. 2002;361:153–162. doi: 10.1042/0264-6021:3610153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 2010;327:88–92. doi: 10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patnaik SK, Stanley P. Mouse large can modify complex N- and mucin O-glycans on alpha-dystroglycan to induce laminin binding. J. Biol. Chem. 2005;280:20851–20859. doi: 10.1074/jbc.M500069200. [DOI] [PubMed] [Google Scholar]
- [16].Aguilan JT, Sundaram S, Nieves E, Stanley P. Mutational and functional analysis of Large in a novel CHO glycosylation mutant. Glycobiology. 2009;19:971–986. doi: 10.1093/glycob/cwp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- [19].Peng HB, Ali AA, Daggett DF, Rauvala H, Hassell JR, Smalheiser NR. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes. Commun. 1998;5:475–489. doi: 10.3109/15419069809005605. [DOI] [PubMed] [Google Scholar]
- [20].Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J. Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sato S, Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura N, Miyoshi T, Sawai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat. Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- [22].Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- [23].Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H. A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose beta1,2-N-acetylglucosaminyltransferase (POMGnT1) Mech. Dev. 2006;123:228–240. doi: 10.1016/j.mod.2005.12.003. [DOI] [PubMed] [Google Scholar]
- [24].Holzfeind PJ, Grewal PK, Reitsamer HA, Kechvar J, Lassmann H, Hoeger H, Hewitt JE, Bittner RE. Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Large(myd) mouse defines a natural model for glycosylation-deficient muscle - eye - brain disorders. Hum. Mol. Genet. 2002;11:2673–2687. doi: 10.1093/hmg/11.21.2673. [DOI] [PubMed] [Google Scholar]
- [25].Lee Y, Kameya S, Cox GA, Hsu J, Hicks W, Maddatu TP, Smith RS, Naggert JK, Peachey NS, Nishina PM. Ocular abnormalities in Large(myd) and Large(vls) mice, spontaneous models for muscle, eye, and brain diseases. Mol. Cell Neurosci. 2005;30:160–172. doi: 10.1016/j.mcn.2005.07.009. [DOI] [PubMed] [Google Scholar]
- [26].Takeda S, Kondo M, Sasaki J, Kurahashi H, Kano H, Arai K, Misaki K, Fukui T, Kobayashi K, Tachikawa M, Imamura M, Nakamura Y, Shimizu T, Murakami T, Sunada Y, Fujikado T, Matsumura K, Terashima T, Toda T. Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum. Mol. Genet. 2003;12:1449–1459. doi: 10.1093/hmg/ddg153. [DOI] [PubMed] [Google Scholar]
- [27].Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- [28].Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- [29].Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hu H, Candiello J, Zhang P, Ball SL, Cameron DA, Halfter W. Retinal ectopias and mechanically weakened basement membrane in a mouse model of muscle-eye-brain (MEB) disease congenital muscular dystrophy. Mol. Vis. 2010;16:1415–1428. [PMC free article] [PubMed] [Google Scholar]
- [31].Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- [32].Kanagawa M, Omori Y, Sato S, Kobayashi K, Miyagoe-Suzuki Y, Takeda S, Endo T. T.Furukawa, T.Toda, Post-translational maturation of dystroglycan is necessary for pikachurin binding and ribbon synaptic localization. J. Biol. Chem. 2010 doi: 10.1074/jbc.M110.116343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang W, Schachter H, Dumanski JP, Cohn RD, Nishino I, Campbell KP. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat. Med. 2004;10:696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- [34].Ackroyd MR, Skordis L, Kaluarachchi M, Godwin J, Prior S, Fidanboylu M, Piercy RJ, Muntoni F, Brown SC. Reduced expression of fukutin related protein in mice results in a model for fukutin related protein associated muscular dystrophies. Brain. 2009;132:439–451. doi: 10.1093/brain/awn335. [DOI] [PubMed] [Google Scholar]
- [35].Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J. Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J. Neurosci. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]