Abstract
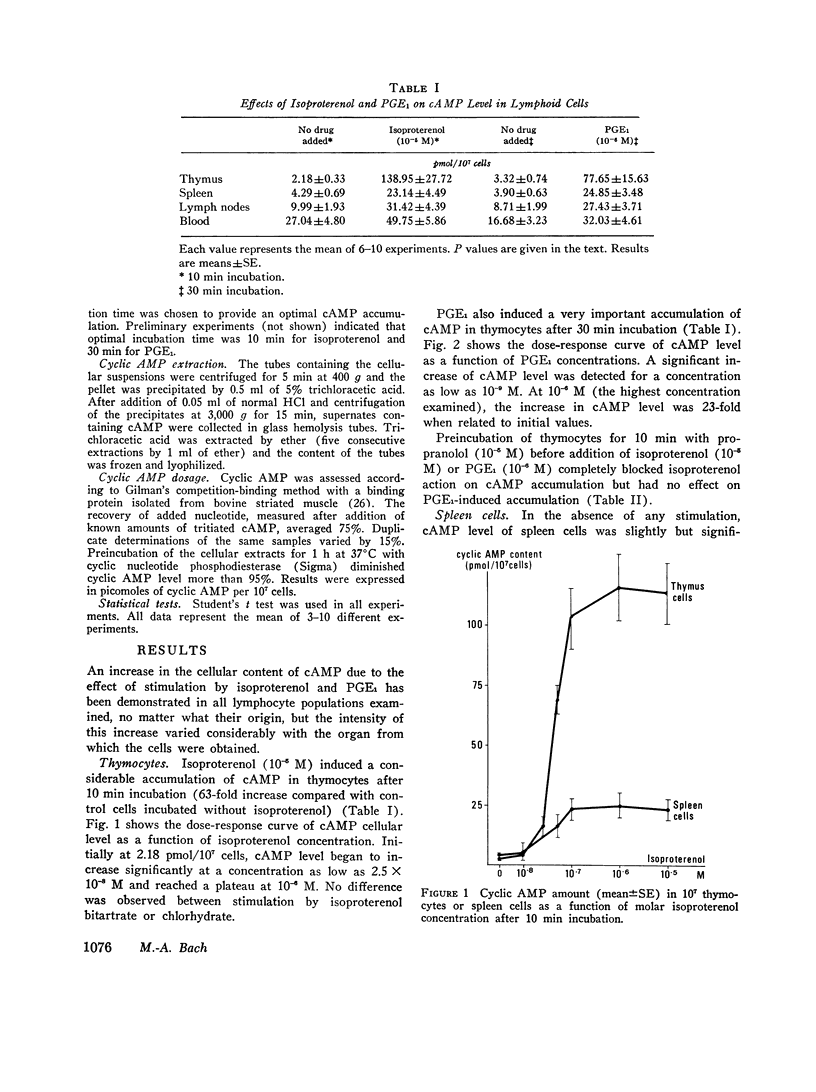
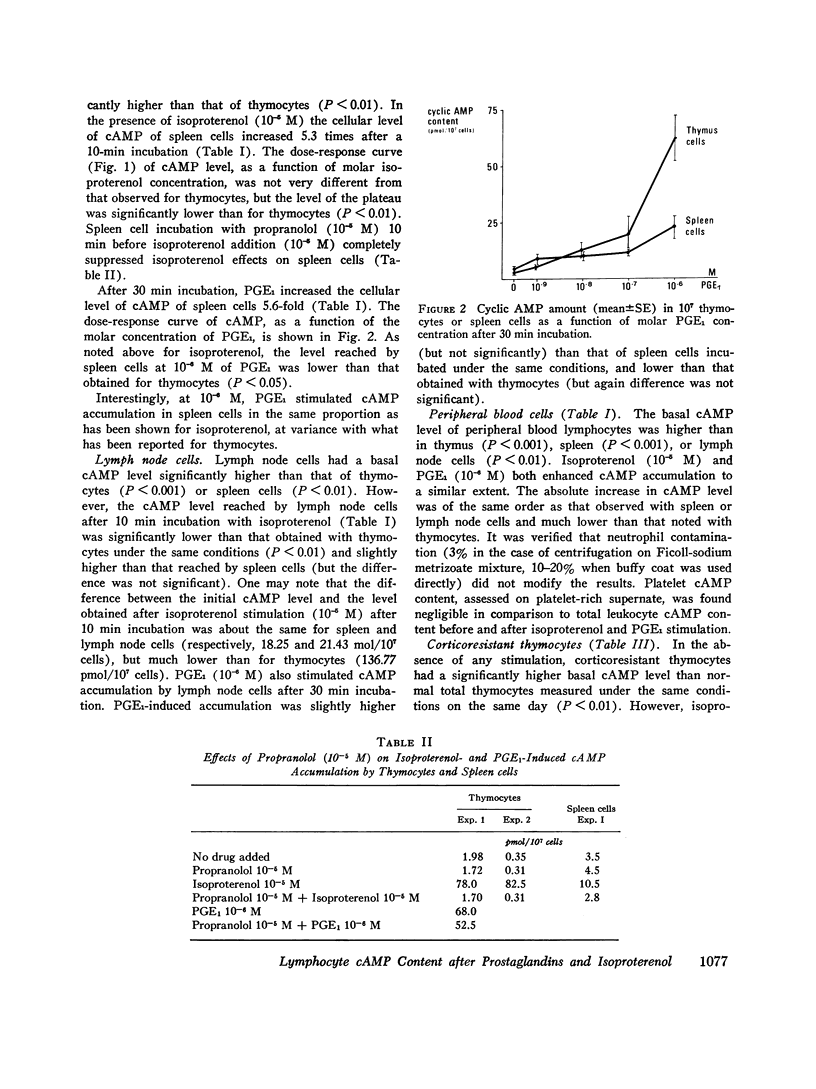
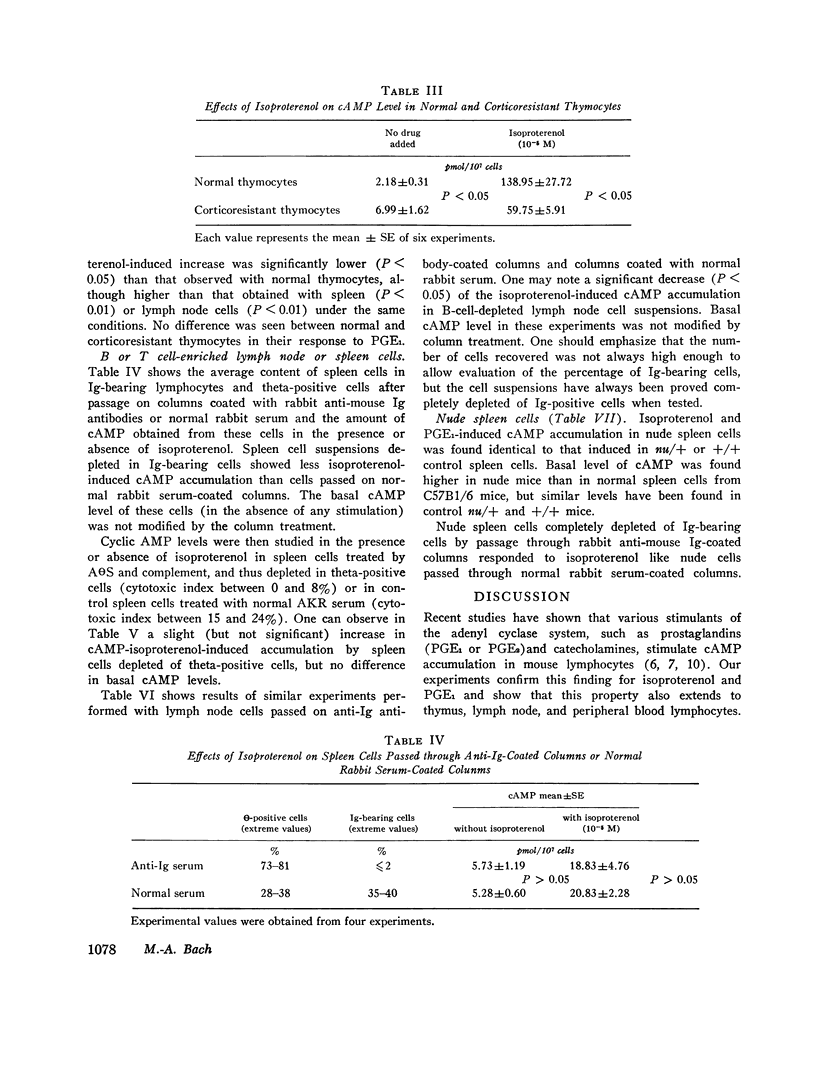
Various lymphocyte populations have been studied for their content in cyclic adenosine 3′,5′-monophosphate (cAMP) before and after stimulation by isoproterenol and prostaglandin E1 (PGE1). Basal cAMP levels vary among lymphocytes according to their origin: peripheral blood lymphocytes show high cAMP level while spleen and lymph node cells and thymocytes show lower levels. Thymocytes are extremely sensitive to the stimulating effects of isoproterenol and PGE1, much more than spleen and lymph node or peripheral blood cells. Corticoresistant thymocytes are less sensitive to isoproterenol stimulation than normal thymocytes, but are significantly more sensitive than peripheral thymus-derived (T)-cells. Studies using bone-marrow-derived (B) or T cell depletion with anti-immunoglobulin-coated columns and antitheta serum (AθS) indicate that lymph node B cells synthesize more cAMP in the presence of isoproterenol than T cells. However, this difference between T and B cells has not been found in spleen cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A., Bach J. F. Effets de l'AMP cyclique sur les cellules formant les rosettes spontanées. C R Acad Sci Hebd Seances Acad Sci D. 1972 Dec 6;275(23):2783–2786. [PubMed] [Google Scholar]
- Bach M. A., Bach J. F. Studies on thymus products. VI. The effects of cyclic nucleotides and prostaglandins on rosette-forming cells. Interactions with thymic factor. Eur J Immunol. 1973 Dec;3(12):778–783. doi: 10.1002/eji.1830031208. [DOI] [PubMed] [Google Scholar]
- Bosing-Schneider R., Kolb M. Influence of cyclic AMP on early events of the immune induction. Nature. 1973 Jul 27;244(5413):224–225. doi: 10.1038/244224a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Lichtenstein L. M., Weissmann G., Zurier R. Effects of cholera enterotoxin on adenosine 3',5'-monophosphate and neutrophil function. Comparison with other compounds which stimulate leukocyte adenyl cyclase. J Clin Invest. 1973 Mar;52(3):698–708. doi: 10.1172/JCI107231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun W., Ishizuka M. Antibody formation: reduced responses after administration of excessive amounts of nonspecific stimulators. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1114–1116. doi: 10.1073/pnas.68.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks D. J., MacManus J. P., Whitfield J. F. The effect of prostaglandins on cyclic AMP production and cell proliferation in thymic lymphocytes. Biochem Biophys Res Commun. 1971 Sep;44(5):1177–1183. doi: 10.1016/s0006-291x(71)80210-3. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Middleton E., Jr Lymphocyte blast transformation. I. Demonstration of adrenergic receptors in human peripheral lymphocytes. Cell Immunol. 1970 Dec;1(6):583–595. doi: 10.1016/0008-8749(70)90024-9. [DOI] [PubMed] [Google Scholar]
- Harris R., Ukaejiofo E. O. Rapid preparation of lymphocytes for tissue-typing. Lancet. 1969 Aug 9;2(7615):327–327. doi: 10.1016/s0140-6736(69)90096-8. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Hirschhorn R., Grossman J., Weissmann G. Effect of cyclic 3',5'-adenosine monophosphate and theophylline on lymphocyte transformation. Proc Soc Exp Biol Med. 1970 Apr;133(4):1361–1365. doi: 10.3181/00379727-133-34690. [DOI] [PubMed] [Google Scholar]
- Kook A. I., Trainin N. Hormone-like activity of a thymus humoral factor on the induction of immune competence in lymphoid cells. J Exp Med. 1974 Jan 1;139(1):193–207. doi: 10.1084/jem.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Henney C. S., Bourne H. R., Greenough W. B., 3rd Effects of cholera toxin on in vitro models of immediate and delayed hypersensitivity. Further evidence for the role of cyclic adenosine 3',5'-monophosphate. J Clin Invest. 1973 Mar;52(3):691–697. doi: 10.1172/JCI107230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus J. P., Whitfield J. F., Youdale T. Stimulation by epinephrine of adenyl cyclase activity, cyclic AMP formation, DNA synthesis and cell proliferation in populations of rat thymic lymphocytes. J Cell Physiol. 1971 Feb;77(1):103–116. doi: 10.1002/jcp.1040770112. [DOI] [PubMed] [Google Scholar]
- Makman M. H. Properties of adenylate cyclase of lymphoid cells. Proc Natl Acad Sci U S A. 1971 May;68(5):885–889. doi: 10.1073/pnas.68.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmon K. L., Weinstein Y., Shearer G. M., Bourne H. R., Bauminger S. Separation of specific antibody-forming mouse cells by their adherence to insolubilized endogenous hormones. J Clin Invest. 1974 Jan;53(1):22–30. doi: 10.1172/JCI107542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Multer M. M., Boone R. F. Enhanced effects of prostaglandin E1 and dibutyryl cyclic AMP upon human lymphocytes in the presence of cortisol. J Clin Invest. 1973 Sep;52(9):2129–2137. doi: 10.1172/JCI107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Evidence for subpopulation of mature lymphocytes within mouse thymus. Nat New Biol. 1971 Feb 10;229(6):182–184. doi: 10.1038/newbio229182a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. G., Ryan W. L. The effect of cyclic AMP and related compounds on human lymphocyte transformation (HLT) stimulated by phytohemagglutinin (PHA). Rev Eur Etud Clin Biol. 1970 Aug-Sep;15(7):774–777. [PubMed] [Google Scholar]
- Singhal S. K., Wigzell H. In vitro induction of specific unresponsiveness of immunologically reactive, normal bone marrow cells. J Exp Med. 1970 Jan 1;131(1):149–164. doi: 10.1084/jem.131.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Strom T. B., Carpenter C. B., Garovoy M. R., Austen K. F., Merrill J. P., Kaliner M. The modulating influence of cyclic nucleotides upon lymphocyte-mediated cytotoxicity. J Exp Med. 1973 Aug 1;138(2):381–393. doi: 10.1084/jem.138.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Braceland B. M., Gillan D. J. The influence of calcium on the cyclic AMP-mediated stimulation of DNA synthesis and cell proliferation by prostaglandin E 1 . J Cell Physiol. 1972 Jun;79(3):353–362. doi: 10.1002/jcp.1040790305. [DOI] [PubMed] [Google Scholar]


