Abstract
Tension development and relaxation in cardiac muscle are regulated at the thin filament via Ca2+ binding to cardiac troponin C (cTnC) and strong cross-bridge binding. However, the influence of cTnC Ca2+-binding properties on these processes in the organized structure of cardiac sarcomeres is not well-understood and likely differs from skeletal muscle. To study this we generated single amino acid variants of cTnC with altered Ca2+ dissociation rates (koff), as measured in whole troponin (cTn) complex by stopped-flow spectroscopy (I61Q cTn > WT cTn > L48Q cTn), and exchanged them into cardiac myofibrils and demebranated trabeculae. In myofibrils at saturating Ca2+, L48Q cTnC did not affect maximum tension (Tmax), thin filament activation (kACT) and tension development (kTR) rates, or the rates of relaxation, but increased duration of slow phase relaxation. In contrast, I61Q cTnC reduced Tmax, kACT and kTR by 40–65% with little change in relaxation. Interestingly, kACT was less than kTR with I61Q cTnC, and this difference increased with addition of inorganic phosphate, suggesting reduced cTnC Ca2+-affinity can limit thin filament activation kinetics. Trabeculae exchanged with I61Q cTn had reduced Tmax, Ca2+ sensitivity of tension (pCa50), and slope (nH) of tension-pCa, while L48Q cTn increased pCa50 and reduced nH. Increased cross-bridge cycling with 2-deoxy-ATP increased pCa50 with WT or L48Q cTn, but not I61Q cTn. We discuss the implications of these results for understanding the role of cTn Ca2+-binding properties on the magnitude and rate of tension development and relaxation in cardiac muscle.
Keywords: contraction, troponin C, calcium binding kinetics, cardiac muscle, cooperative activation, relaxation
INTRODUCTION
Activation and relaxation of cardiac myofilaments are controlled by Ca2+-mediated, coordinated interactions between the thin and thick filament proteins. Thin filament activation (or the process by which binding sites for myosin on actin become available) is initiated by Ca2+ binding to cardiac troponin C (cTnC).1 Subsequent interactions between troponin (Tn) subunits result in increased mobility of tropomyosin and increased probability of myosin interaction with actin [1]. Strong myosin binding to actin (i.e. cross-bridges) displaces tropomyosin [2, 3] and enhances Ca2+ binding to troponin in cardiac muscle[2]. These cooperative events are important for rapid thin filament activation and force development in cardiac muscle. Relaxation involves deactivation of the Tn complex following Ca2+ dissociation from cTnC and reduction of strong cross-bridge binding, though the mechanistic details are less well known [4], and it is not known whether this process is cooperative in nature.
The heart operates at sub-maximal levels of Ca2+ where the mechanism of cooperative activation may depend heavily upon myofilament protein isoform and structure, even in the absence of adrenergic modulation. For example, we [5–9] and others [10] have demonstrated that, while both Ca2+ binding (to Tn) and cross-bridge binding (to actin) are required for complete activation of thin filaments in both skeletal and cardiac muscle, cardiac thin filament activation is relatively more dependent on strong cross-bridge binding at sub-saturating Ca2+. It has been demonstrated in skeletal muscle that the Ca2+ binding properties of TnC can affect cooperative activation and tension development and relaxation kinetics [11–15]. Previous studies have also examined the relationships between specific point mutations in cardiac TnC (cTnC) and how they alter cTn Ca2+ binding properties [16] and Ca2+ sensitivity of tension in cardiac muscle [17]. However, it is not clear how cTnC Ca2+ binding properties contribute to the complex cooperative interactions between the thin and thick filaments during thin filament activation in the organized structure of the cardiac sarcomere. Further, it is not known how the properties of cTnC influence the kinetics of tension activation and relaxation or cooperative activation in cardiac muscle. As such, a more detailed understanding of how altered Ca2+ binding properties of cTnC can affect mechanical function of cardiac muscle is warranted.
We hypothesize that the Ca2+ binding properties of cTnC are a primary determinant of thin filament activation and contractile kinetics in cardiac muscle, as evidenced by steep cooperativity of tension generation and a narrow spread of activation along thin filaments [8]. To examine this, we introduced site-directed mutations in the N-terminus of rat cTnC that increased or decreased cTn Ca2+dissociation rate (koff) [16, 18] to study their effects on cooperative activation and the kinetics of tension development and relaxation in demembranated rat ventricular trabeculae and mouse ventricular myofibrils. Cross-bridge binding and cycling was independently augmented using 2 deoxy-ATP (dATP; [7]). The use of demembranated trabeculae allowed multiple activations at varying Ca2+ with little degradation of tension, making it possible to study both Ca2+ and cross-bridge components of cooperative thin filament activation in the same preparation [8, 9, 14, 15]. The use of myofibril preparations allowed measurements of the rate of contractile activation (kACT), rate of tension redevelopment (kTR), and the biphasic slow and fast phases of relaxation (kREL,slow and kREL,fast, respectively) during the same activation trial, because rapid changes in [Ca2+] throughout these small preparations were not limited by diffusion rates.
We found that activation kinetics (kACT) were slowed by increased cTn Ca2+ koff (I61Q cTnC), and became slower than tension redevelopment kinetics (kTR); this difference was increased with increased inorganic phosphate. The duration of slow phase relaxation was not affected by increased koff (I61Q cTnC), but was prolonged by decreased koff (L48Q cTnC). L48Q cTnC and I61Q cTnC enhanced or reduced (respectively) the Ca2+ sensitivity of steady-state tension (pCa50), consistent with the presumed changes in Ca2+ affinity of cTn with the mutations. However, both cTn complexes resulted in a large reduction in the slope (nH) of the steady-state tension-pCa relationship, implicating cTn Ca2+ binding as a major component of cooperative thin filament activation in cardiac muscle. This reduction in nH could not be rescued by strong cross-bridge augmentation. These results differ from our previous findings in fast skeletal muscle [12, 14, 15] and suggest that Tn Ca2+ binding properties are an important component in the differential regulation of tension development and relaxation in the two striated muscle types. Preliminary abstract reports of this work have been previously published [19, 20].
MATERIALS AND METHODS
Proteins, Solution Biochemistry, and Exchange
Rat cardiac TnC (cTnC) mutations were introduced by site-directed mutagenesis. Recombinant cTnC, cTnI, and cTnT were extracted and purified from E. coli [21]. Whole cTn complexes were formed using WT or mutant rat cTnC and WT rat cTnI and WT rat cTnT (see Supplementary Materials for details).
Ca2+ dissociation rate (koff) from whole cTn (containing WT or mutant cTnC) and reconstituted thin filaments was measured at 15 °C using an Applied Photophysics Ltd. (Leatherhead, U.K.) model SX-18MV stopped-flow instrument as previously described [14, 22, 23]. Ca2+ dissociation from whole cTn or reconstituted thin filaments was measured by rapidly mixing the protein with the fluorescing Ca2+ chelator Quin-2. Buffer composition, thin filament reconstitution methods, and detection details are included in the Supplementary Material.
Protein exchange into myofilaments was accomplished with passive exchange of cTnC in myofibrils or whole cTn exchange in trabeculae. Native cTnC in myofibrils was replaced with recombinant cTnC through (mass-action) passive exchange at 4 °C overnight in relaxing solution with 0.05 mg mL−1 recombinant rat cTnC. No myofibril exchange group developed any Ca2+-independent tension in relaxing solution, indicating complete regulation following the exchange protocol. Maximal tension (Tmax) was the same for untreated, treated control, and WT cTnC-exchanged myofibrils. Replacement of native cTnC in trabeculae was accomplished by passive (mass action) exchange via incubation of preparations in a high concentration of whole cTn under rigor conditions, as previously described [24]. In a subset of experiments after exchange, an additional incubation in 1 mg mL−1 cTnC (WT or mutant) was completed to ensure that all cTn complexes were complete, and this was confirmed by no increase in steady-state tension (data not shown). Reported post-exchange relative Tmax values (Table 3) are from back-to-back measurements in pCa 4.0 or 3.5 comparing just prior to exchange and immediately following exchange. Additional details are in the online Supplementary Material. Analysis of Variance (ANOVA) was used to compare between groups and when differences were significant, Student’s unpaired t tests were used with statistical significance set at P < 0.05.
Table 3.
Steady-state tension parameters after whole cTn exchange in demembranated rat trabeculae at 15 °C.
| cTn Type | Relative Tmax | pCa50 | nH | kTR,max (s−1) |
|---|---|---|---|---|
| WT cTn (8) | 0.83 ± 0.05 | 5.25 ± 0.08 | 6.5 ± 1.3 | 7.6 ± 0.8 |
| L48Q cTn (8) | 0.85 ± 0.06 | 5.63 ± 0.08* | 2.6 ± 0.4* | 7.2 ± 1.4 |
| I61Q cTn (14) | 0.19 ± 0.02* | 4.77 ± 0.05* | 2.2 ± 0.4* | 9.2 ± 0.8 |
P ≤ 0.01 vs. WT
Experimental Animals and Tissue Preparations
All animal procedures performed in Italy were conducted in accordance with the official regulations of the European Community Council on Use of Laboratory Animals (Directive 86/609/EEC) and protocols were approved by the Ethical Committee for Animal Experiments of the University of Florence. Male C57 mice were killed by rapid cervical dislocation and myofibrils were prepared as previously described [25] with minor modifications (see Supplementary Material). Myofibrils were prepared by homogenization of 4–8 strips of ventricular tissue in rigor solution on ice, washed free of glycerol, and used for up to 5 days.
All animal procedures performed in the United States were conducted in accordance with the US National Institutes of Health Policy on Humane Care and Use of Laboratory Animals and were approved by the University of Washington (UW) Animal Care Committee. Rats were housed in the Department of Comparative Medicine at UW and cared for in accordance with the UW Institutional Animal Care and Use Committee (IACUC) procedures. Male Sprague-Dawley rats were anesthetized via intraperitoneal injection of pentobarbital (50 mg kg−1). When animals had no reflexive response, the heart was rapidly excised and demembranated right ventricular trabeculae were prepared as previously described [5] (see Supplementary Material for details). Individual trabeculae were dissected from the right ventricle and prepared for mechanical measurements by wrapping the ends in aluminum foil T-clips for attachment to the mechanical apparatus as previously described [5].
Mechanical Measurements
Experimental physiological Ca2+ solutions were calculated as previously described for myofibril [26] and trabeculae [27] mechanics. Details are in the online Supplemental Data. Phosphate (Pi) concentration was reduced in all myofibril solutions to <5 μmol L−1 by a Pi scavenging enzyme system (purine-nucleoside-phosphorylase with substrate 7-methyl-guanosine) as previously described [28] except in solutions containing 0.5 mmol L−1 added Pi. A low-Pi, pCa 4.0 solution for trabeculae omitted the creatine phosphate and creatine phosphokinase, yielding ~0.1 mmol L−1 Pi by NMR analysis. Some trabeculae solutions substituted 5 mmol L−1 2-deoxy-ATP (dATP) for ATP.
For myofibril experiments, mechanical data were collected at 15 °C from small bundles of cardiac myofibrils as previously described [29]. Sarcomere length (SL) was initially set 10 – 20% above slack length and was 2.29 ± 0.02 μm (mean ± S.E.M., n = 63). At this SL, average myofibril initial length (Lo) was 52 ± 3 μm and diameter was 3.88 ± 0.05 μm (4 – 8 myofibrils). Measurement procedures for activation rate (kACT; with rapid increase in Ca2+), rate of tension redevelopment (kTR; following a rapid release-restretch transient), relaxation rates (slow phase: kREL,slow and fast phase: kREL,fast), and slow phase duration are detailed in the Supplementary Material. ANOVA was used to compare between myofibril groups after cTnC exchange and when differences were significant, Student’s unpaired t tests were used with statistical significance set at P < 0.05. All values reported in Table 2 for myofibril tension and kinetics are means ± S.E.M.
Table 2.
Tension generation and relaxation parameters after cTnC exchange in mouse ventricular myofibrils at 15 °C. Values given are mean ± S.E.M.; number in parentheses is number of myofibrils. Tmax, maximum isometric tension; kACT, rate constant of tension rise following step-wise pCa decrease (8.0 to 3.5) by fast solution switching; kTR, rate constant of tension redevelopment following release-restretch of maximally activated myofibrils; kREL, rate constant of tension relaxation during slow or fast phase.
| MYOFIBRIL BATCHES | TENSION GENERATION | RELAXATION |
||||
|---|---|---|---|---|---|---|
| Slow Phase | Fast Phase | |||||
| Tmax (mN mm−2) | kACT (s−1) | kTR (s−1) | Duration (ms) | kREL (s−1) | kREL (s−1) | |
| Sham Treated Control | 111 ± 10 (10) | 7.26 ± 0.36 (11) | 7.31 ± 0.48 (11) | 59 ± 4 (10) | 1.68 ± 0.21 (9) | 21.8 ± 3.0 (10) |
| xcTnC | 3 ± 0.7 (7)*° | - | - | - | - | - |
| WT cTnC | 112 ± 12 (10) | 7.71 ± 0.32 (10) | 7.84 ± 0.28 (10) | 47 ± 5 (10) | 1.62 ± 0.17 (10) | 24.1 ± 2.8 (10) |
| L48Q cTnC | 97 ± 9 (9) | 7.97 ± 0.30 (11) | 8.01 ± 0.37 (11) | 85 ± 6 (10)* | 1.75 ± 0.34 (10) | 14.5 ± 1.4 (10) |
| I61Q cTnC | 40 ± 4 (20)*° | 3.40±0.29 (20)*° | 4.55± 0.35 (20)*° | 48 ± 2 (19)$ | 1.71 ± 0.16 (18) | 22.6 ± 2.4 (20) |
P < 0.01 vs. Control
P < 0.01 vs. WT
P < 0.02 vs. Control
For trabeculae experiments, mechanical data were collected at 15 °C from demembranated right ventricular trabeculae mounted on a mechanical apparatus (detailed in the Supplementary Material). SL was initially set to 2.25 μm, Lo was 1.09 ± 0.06 mm, diameter was 107 ± 5 μm (mean ± S.E.M.; n=48) and SL was monitored throughout the experiment. Passive tension was determined at pCa 9.0 and was subtracted from total tension to obtain the active tension values reported. Tmax measured at pCa 4.0 just prior to cTn exchange was 60.5 ± 4.0 mN mm−2 (mean ± S.E.M., n=48). The kTR was determined in trabeculae from the half-time of tension recovery and tension-pCa and kTR-pCa data were fitted with the Hill Equation to get reported pCa50 and nH values (see Supplementary Material for equations). Reported pCa50 and nH values for tension-pCa are the average of individual fits for each experimental curve ± S.E.M. Reported pCa50 values for kTR-pCa represent fits to the average data and are reported ± S.E. of the fits. ANOVA was used to compare between cTn exchange groups after exchange and when differences were significant, Student’s unpaired t tests were used with statistical significance set at P < 0.05.
RESULTS
Ca2+ dissociation rate (koff) from cTn
Stopped-flow spectroscopy was used to measure Ca2+ dissociation rate (koff) from whole cTn complexes containing recombinant wild type (WT) cTnI, cTnT and either WT cTnC (control), L48Q cTnC, or I61Q cTnC (denoted WT cTn, L48Q cTn, or I61Q cTn, respectively). The koff was determined for each cTn by fitting fluorescence data with exponential curves, as previously described [14]. At 15°C (the temperature used for mechanics measurements) koff was ~30 s−1 for WT cTn, in agreement with previous reports [23, 30]. The mutations in cTnC altered koff, reducing it by 75% for L48Q cTn (in agreement with a recent report using a modified cTnC with attached fluorescent probe [31]) and increasing it by 2.3-fold for I61Q cTn (Table 1). Reconstitution of thin filaments with WT or mutant whole cTn, Tm, and actin resulted in a 2.2–3.4-fold increase in koff for all cTnC variants versus whole cTn koff. Rates for WT cTn and L48Q cTn in thin filaments agree with recently reported rates [31]. The relative effect of the mutations in cTnC remained the same as for whole cTn, such that koff was reduced by 63% for L48Q cTn and increased by 3.2-fold for I61Q cTn in thin filaments (Table 1).
Table 1.
Ca2+ dissociation rate (koff) from mutant cTnC in whole cTn complex or in reconstituted thin filaments by stopped-flow spectroscopy with Quin-2 fluorescence at 15 °C.
| cTn Type | cTn koff (s−1) (n) | TF koff (s−1) (n) |
|---|---|---|
| WT cTn | 29.7 ± 0.5 (10) | 75.4 ± 4.8 (3) |
| L48Q cTn | 7.3 ± 0.1* (9) | 28.0 ± 4.1* (3) |
| I61Q cTn | 67.0 ± 9.3* (8) | 237.7 ± 30.5* (4) |
P ≤ 0.01 vs. WT. TF, thin filament.
Maximal activation and relaxation in ventricular myofibrils
WT, I61Q or L48Q cTnC was exchanged into mouse ventricular myofibrils to determine effects on maximal isometric tension development and relaxation kinetics following rapid increase and decrease in bathing [Ca2+]. The effectiveness of the cTnC exchange protocol was examined in parallel for each batch of myofibrils using another mutant, D65A cTnC, which does not bind Ca2+ at the N-terminus (xcTnC). These myofibrils developed < 3% of the maximal Ca2+ activated (pCa 3.5) steady-state tension (Tmax) obtained for treated controls or those exchanged with WT cTnC (Table 2). Thus, if we assume that cTnC for cTn in myofibrils is relatively similar for the mutants in this study (an assumption based on the fact that the C-terminal portion of the protein is the same), these measurements suggest our protocol was successful in replacing the great majority of native cTnC with mutant cTnC in myofibrils.
Example tension traces of the maximal Ca2+ activation-relaxation protocol for three myofibrils exchanged with WT, L48Q or I61Q cTnC are shown in Figure 1. Tmax and the rates of tension activation (kACT) and tension redevelopment (kTR) did not differ for L48Q cTnC vs. WT cTnC, and in both of these myofibril groups kACT did not differ from kTR (Table 2). In contrast, Tmax, kACT and kTR were all reduced by ~40–65% with I61Q cTnC, and kTR was significantly faster than kact (Fig. 1C; P < 0.02). The slower kACT vs. kTR suggests that thin filament activation kinetics became rate limiting to tension activation with I61Q cTnC (see Discussion). To further test this idea, we measured kACT and kTR in the presence of 0.5 mmol L−1 inorganic phosphate (Pi), which affects cross-bridge cycling specifically (Fig. 2), without influencing thin filament activation kinetics. Addition of Pi caused a similar decrease in Tmax (~15%) for control and I61Q cTnC. Interestingly, with I61Q cTnC both kACT and kTR were increased with Pi, but the increase was greater for kTR (P < 0.01) which became similar to the rate for control myofibrils (Fig. 2B, C). In contrast, 0.5 mmol L−1 Pi increased kTR and kACT similarly in control myofibrils. Thus we conclude that I61Q cTnC limits the magnitude (Tmax) and slows the rate of tension activation (kACT) via reduction in thin filament activation kinetics. The rates of the slow (kREL,slow) and fast (kREL,fast) phases of relaxation for did not differ for myofibrils with L48Q cTnC, I61Q cTnC and WT cTnC. However, while the duration of slow phase relaxation was not affected with I61Q cTnC, it was almost doubled with L48Q cTnC (P < 0.01; Fig. 1D, Table 2). Thus, L48Q cTnC did not affect the rate of activation or relaxation, but prolonged (slow phase) relaxation by ~40 ms. In contrast, I61Q cTnC had no effect on relaxation, but dramatically slowed activation.
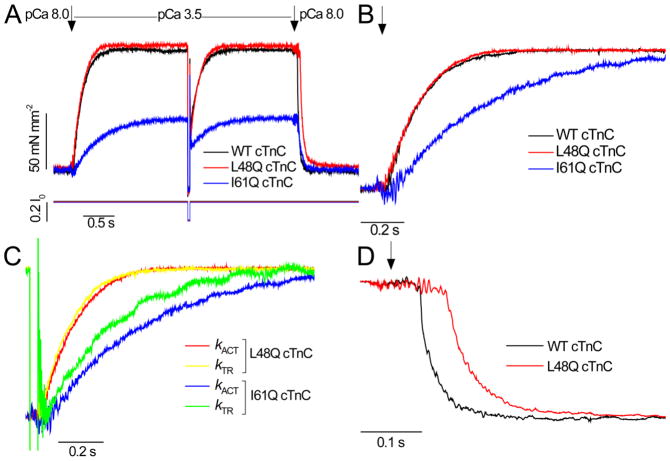
Figure 1.
Example tension traces of activation-relaxation cycle in mouse ventricular myofibrils. A, Tension traces for myofibrils exchanged with WT cTnC (black), L48Q cTnC (red), or I61Q cTnC (blue) are shown for activation from pCa 8 to pCa 3.5 by rapid solution change (first arrow), tension redevelopment resulting from a length release-restretch (see length trace below panel A), and relaxation from pCa 3.5 to pCa 8 (second arrow) at 15 °C and <5 μmol L−1 Pi (due to Pi scavenging, see Methods). Passive tension is the difference between initial tension and zero tension during the length release. B, Traces normalized to maximum tension for each condition and displayed with an expanded time scale demonstrate that activation rate (kACT) was decreased with I61Q cTnC (blue) but not with L48Q cTnC (red). C, Expanded time-scale traces of kACT and tension re-development rate (kTR) super-imposed to show both rates are the same for L48Q cTnC (red and yellow, respectively) but differ for I61Q cTnC (blue and green, respectively). D, Normalized tension traces demonstrate that during relaxation, slow phase rate (kREL,slow) was unchanged but duration is prolonged with L48Q cTnC (red) vs. WT cTnC (black), and that fast phase rate (kREL,fast) did not differ.
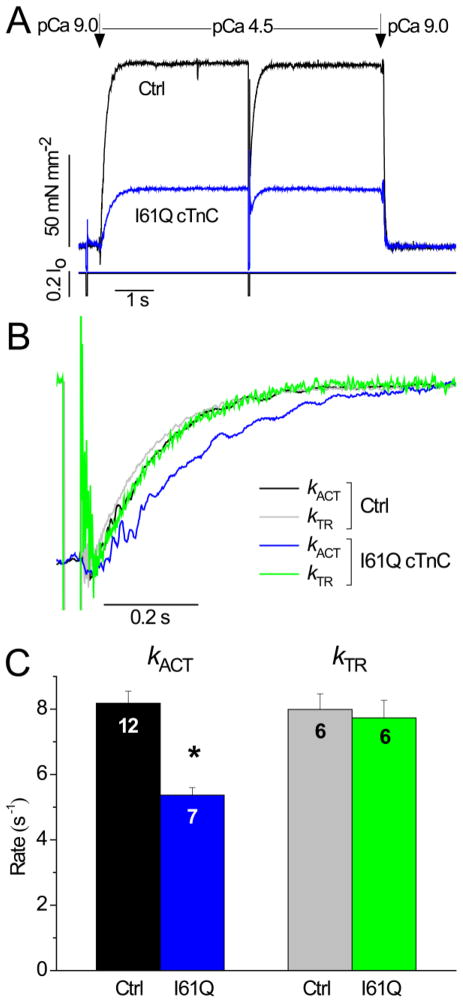
Figure 2.
Activation kinetics in the presence of 0.5 mmol L−1 inorganic phosphate (Pi) for control myofibrils vs. myofibrils with I61Q cTnC. A, Example traces of activation and relaxation at pCa 4.5 for WT cTnC (control; black) and I61Q cTnC (blue) with 0.5 mmol L−1 Pi. Length trace below tension trace shows transient for measurement of kTR. B, Normalized kACT and kTR traces demonstrating no difference between these rates for WT cTnC (control; black and grey, respectively), but slower kACT vs. kTR for I61Q cTnC (blue vs. green, respectively). C, Summary of kACT and kTR rates for 6–12 myofibrils. * P < 0.02.
Ca2+ dependence of tension development in ventricular trabeculae
To determine the influence of L48Q and I61Q cTnC on cardiac muscle contractile properties at varying Ca2+ concentrations, whole cTn containing these cTnC mutants were exchanged into demembranated rat ventricular trabeculae. As with myofibrils, exchange with D65A cTnC (in whole cTn complex) reduced Tmax to a very low level (~8% of pre-exchanged values), suggesting good protein exchange with this technique (Fig. 3A).
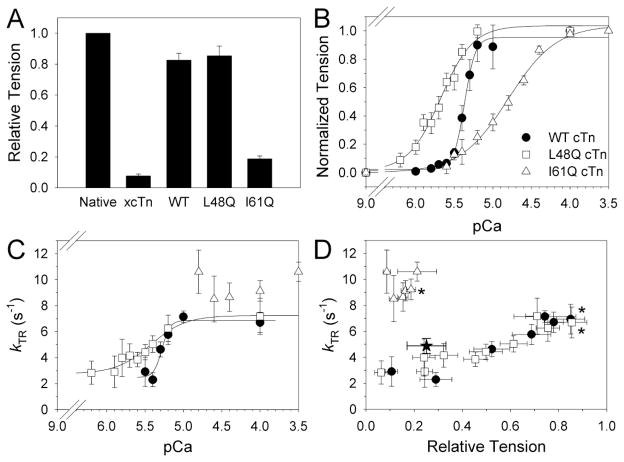
Figure 3.
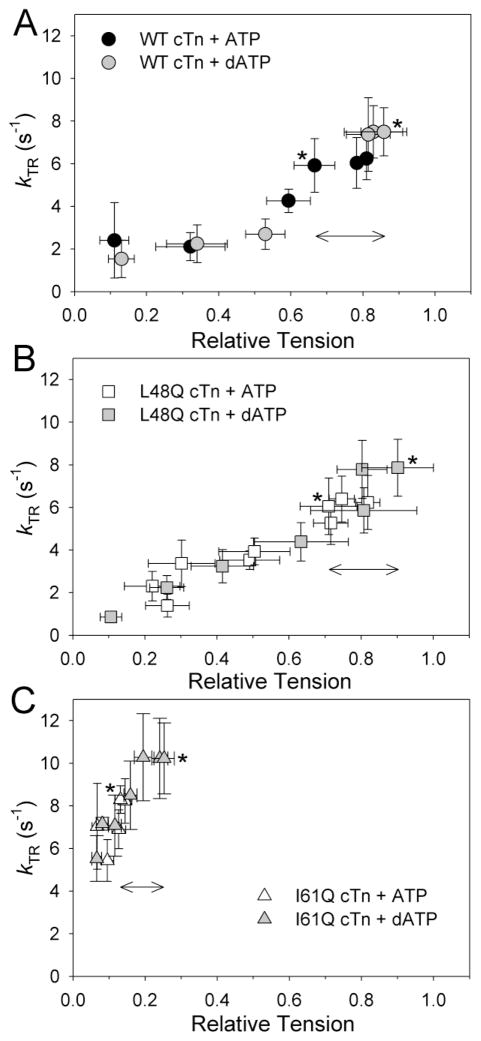
Tension and kTR as Ca2+ was varied for rat trabeculae with WT, L48Q, or I61Q cTn. A, Tmax for exchange groups is shown relative to pre-exchange native Tmax (1.0). Control measurements with xcTn (containing D65A cTnC which does not bind Ca2+ at site II) reduced Tmax to <10% of WT cTn, suggesting almost complete exchange of cTn. Exchange with WT or L48Q cTn maintained ~85% Tmax. With I61Q cTn Tmax was greatly reduced. B, Tension-pCa curves (normalized to Tmax for each condition) of summarized data for WT (black circles), L48Q (white squares), and I61Q cTn (white triangles). See Table 3 for fit parameters. C, kTR-pCa data shows increased Ca2+ sensitivity of kTR (pCa50) with L48Q cTn vs. WT cTn and a dramatically reduced Ca2+ dependence of kTR with I61Q cTn. D, kTR data binned by pCa value and plotted vs. tension to show kTR dependence on cross-bridge number (relative tension). Symbols in panels C and D are the same as panel B, and the black star (D) indicates kTR,max in I61Q cTnC-exchanged trabeculae in a low-Pi pCa 4.0 solution, which was 5.3 ± 0.6 s−1 (or ~45% reduced from kTR,max in the traditional activating solutions with ~0.5 mmol L−1 contaminating Pi). * Relative Tmax and kTR,max immediately following cTn exchange (rather than at end of pCa curve).
Similar to mouse ventricular myofibrils, Tmax (Fig. 3A) and kTR,max (Fig. 3C) were not affected by L48Q cTn (vs. WT cTn; Table 3). Example tension traces are shown in Supplementary Figure S1. For I61Q cTn, Tmax was reduced by ~75%, which was similar to the reduction for mouse ventricular myofibrils (~65%; Table 2). Interestingly, kTR,max (~9 s−1) for I61Q cTn was similar to WT and L48Q cTn (by ANOVA; Table 3). This contrasts results in myofibrils where kTR,max was reduced by ~40% in the absence of Pi (Table 2), but is similar to myofibril results in the presence of 0.5 mmol L−1 Pi (Fig. 2). Activation solutions for trabeculae contained 15 mmol L−1creatine phosphate and 5 mmol L−1 ATP, which results in a contaminating Pi level of ~0.5 mmol L−1 (by NMR analysis; see Supplementary Material) that is likely higher in the center of the preparation due to limited substrate and product diffusion and the coupled ATPase and creatine kinase reactions. This suggests that these results with rat trabeculae are in good agreement with the mouse ventricular myofibril measurements.
When the Ca2+ concentration of activation solutions was varied in trabeculae, both Ca2+ sensitivity of tension (pCa50) and apparent cooperativity of activation (slope, nH) were strongly influenced by the cTnC mutants (Fig. 3B, Table 3). L48Q cTn increased pCa50 by ~0.4 units compared with WT cTn, and I61Q cTn decreased pCa50 by ~0.5 units. These shifts in Ca2+ sensitivity were anticipated based on our earlier work with similar skeletal TnC mutants in skeletal fibers [14] and the work of others in trabeculae [17]. However, there was an unexpected large reduction in nH (~4 units) for both L48Q and I61Q cTn (Table 3), indicating an apparent loss of cooperativity of tension generation with both mutants. This contrasts our previous results in skeletal muscle, where slowing Ca2+-koff of TnC had no effect on nH [14], and will be discussed in more detail below (see Discussion).
As with steady-state tension, the pCa50 of kTR (Fig. 3C) increased with L48Q cTn (5.43 ± 0.06) compared with WT cTn (5.28 ± 0.03). Both lagged behind pCa50 of tension [5] by similar amounts (Fig. 3B). Interestingly, the Ca2+ dependence of kTR with I61Q cTn was greatly diminished (Fig. 3C). Mono-exponential fits to force traces for I61Q cTn resulted in similar values for kTR (compared with determination from t1/2 values), suggesting this loss of Ca2+ dependence was not an artifact of measurement techniques at low forces. To determine the influence of cycling cross-bridge number (i.e. steady-state tension level) on kTR, values in Figure 3C were re-plotted as a function of relative tension as Ca2+ was varied (Fig. 3D). The data demonstrate kTR did not differ with L48Q cTn vs. WT cTn for similar numbers of cycling cross-bridges (independent of [Ca2+]). The situation appears to be quite different with I61Q cTn, where kTR values (8–10 s−1) were equal to or greater than values obtained for kTR,max with WT (or L48Q) cTn, even though the number of cycling cross-bridges was greatly reduced. These data suggest that while I61Q cTnC greatly limited strong cross-bridge binding, it has no effect or slightly increases attached cross-bridge cycling kinetics.
Effect of strong cross-bridge augmentation on tension in trabeculae
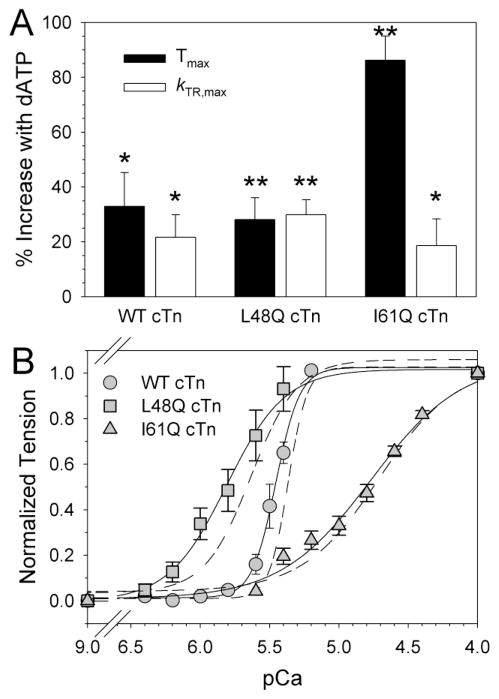
Cardiac thin filament activation is more sensitive to changes in strong cross-bridge binding than skeletal muscle [2, 8, 32–34], especially during sub-maximal Ca2+ activation. To determine how the strong cross-bridge component of thin filament activation might depend on cTnC Ca2+ binding properties and whether this (in turn) affects cross-bridge cycling kinetics, we augmented strong cross-bridge binding and cycling (independent of [Ca2+]) by replacing ATP with 2 deoxy-ATP (dATP) as the substrate for contraction. dATP increased Tmax and kTR,max by ~20–35% in trabeculae with WT cTnC (Fig. 4A; P < 0.05), as we have reported previously [5, 7, 8]. Similar increases in Tmax and kTR,max occurred with L48Q cTnC. Interestingly, with I61Q cTnC, dATP increased Tmax by 86 ± 9% (P < 0.01), to approximately half of Tmax with WT cTn, while kTR,max was increased by only 19 ± 10% (similar to WT or L48Q cTnC). This much greater effect on Tmax than on kTR,max suggests I61Q cTnC limits strong cross-bridge binding more than it affects cross-bridge cycling kinetics during isometric contraction.
Figure 4.
Effect of 5 mmol L−1 dATP on Tmax, kTR,max, and tension-pCa in trabeculae exchanged with WT, L48Q, or I61Q cTn. A, Tmax and kTR,max increased in the presence of dATP (vs. ATP) in back-to-back maximal activations (pCa 4.0). *P<0.05, **P<0.01 vs. ATP. B, Tension-pCa relationships with dATP for WT (grey circles), L48Q (grey squares), and I61Q cTn (grey triangles). Hill fit curves for ATP are shown as dashed lines for this subset of experiments, where paired comparisons were made with dATP for L48Q (left), WT (middle), and I61Q cTn (right). See text for fit values.
Measurements of steady-state tension at sub-maximal Ca2+ levels are consistent with this idea. Figure 4B shows normalized tension-pCa relationships for paired comparisons between ATP (dashed lines from fit of the data) and dATP (symbols and solid fit lines) in the same trabeculae following cTn exchange. The order of tension measurements (with ATP versus dATP) was varied for individual experiments to reduce the possibility that preparation rundown was a factor in determining values for pCa50. dATP did not affect nH with WT cTn, L48Q or I61Q cTn, suggesting little or no effect on cooperative thin filament activation. However, while dATP increased pCa50 with L48Q cTnC and WT cTnC, it had no effect with I61Q cTnC. For WT cTnC (n=4), pCa50 increased from 5.40 ± 0.01 (ATP) to 5.46 ± 0.03 (dATP; P < 0.05), in agreement with our previous conclusion that dATP enhances cross-bridge binding at sub-maximal Ca2+ [5–7]. The augmentation of cross-bridge binding was similar or greater for L48Q cTn (n=5), where pCa50 increased from 5.57 ±0.08 (ATP) to 5.74 ± 0.10 with dATP (P < 0.01). In contrast, with I61Q cTn (n=9) the Ca2+ sensitivity of tension was not altered by dATP, as pCa50 for ATP (4.70 ± 0.05) was not different than for dATP (4.74 ± 0.04; Fig. 4B). Thus, even though dATP almost doubled strong cross bridge binding at all levels of Ca2+ activation with I61Q cTn, including Tmax (Fig. 4A), it could not rescue the dramatic reduction in pCa50 or nH (Fig. 3B; Table 3) caused by the reduced Ca2+ binding (to cTnC) component of thin filament activation in cardiac muscle.
Measurements of kTR with dATP at sub-maximal Ca2+ levels were consistent with the hypothesis that I61Q cTnC has only minor effects on cross-bridge cycling rates. dATP increases the rates of cross-bridge tension generation and cross-bridge detachment, but appears to affect the former more than the latter, resulting in increased recruitment and rate of cross-bridge cycling at all levels of Ca2+ activation in cardiac muscle [5, 7]. These effects on crossbrige cycling kinetics were not affected by cTn mutants, as dATP did not affect the kTR-tension relationship with varying Ca2+ for trabeculae exchanged with L48Q, I61Q or WT cTn (Fig. 5). The data, with tension normalized to Tmax for ATP, showed no elevation of kTR at lower tensions but an extension of both tension and kTR at the highest levels of Ca2+ activation by dATP for all three cTn exchanges. Back-to-back measurements of Tmax and kTR,max are marked with an asterisk (*) in each panel of Figure 5 to illustrate increases with dATP during maximal Ca2+ activation (summarized in Fig. 4A), and magnitude increases in Tmax are indicated by a double-headed arrow. Since dATP does not increase kTR at similar tension levels (compared with ATP), it suggests cross-bridge cycling kinetics were not directly affected by the cTnC mutants used in this study.
Figure 5.
Effect of 5 mmol L−1 dATP on kTR-tension relationship in trabeculae exchanged with WT, L48Q, or I61Q cTn. In a subset of trabeculae exchanged with WT (A), L48Q (B), or I61Q cTn (C), kTR-tension relationships were little affected as pCa was varied in the presence of ATP (A, black symbols; B, C, white symbols) or dATP (grey symbols). * Values from back-to-back maximal activations in ATP and dATP (rather than at end of pCa curve).
DISCUSSION
The purpose of this study was to investigate how altering the Ca2+ binding properties of cTn influence cooperative thin filament activation and the kinetics of tension generation and relaxation when exchanged into cardiac muscle. The most significant findings of this work, where cTn complexes with altered koff were exchanged into cardiac myofibrils and demembranated trabeculae, are (1) while the Ca2+ sensitivity (pCa50) of tension was decreased by I61Q cTnC (faster koff vs. WT cTn) and increased by L48Q cTnC (slower koff vs. WT cTn) both mutants resulted in an apparent loss of cooperativity of thin filament activation (nH); (2) I61Q cTnC slowed the rate of thin filament activation, while L48Q cTnC had no effect on activation kinetics but prolonged relaxation (slow phase) duration and (3) cross-bridge binding was modulated by cTn koff but the rate of cross-bridge cycling and tension redevelopment (when Ca2+ binding was in near steady-state; kTR) were little affected. As a result, this study suggests that directly modulating the Ca2+ binding properties of cTnC using point mutations could alter cardiac contraction, because changes in Ca2+ sensitivity are large, with little effect on relaxation kinetics and no effect on myosin kinetics.
cTn Regulation of Ca2+ Sensitivity (pCa50) and Cooperative (nH) Tension Generation
Decreasing (L48Q cTnC) or increasing (I61Q cTnC) cTn Ca2+-koff in whole cTn and reconstituted thin filaments resulted in increased and decreased Ca2+ sensitivity of tension (pCa50), respectively. This study correlates Ca2+ dissociation rates measured in reconstituted thin filaments with isometric tension in the geometrically constrained lattice structure of cardiac muscle, demonstrating that changes in koff can translate to changes in Ca2+ sensitivity of tension for these particular cTnC mutants. A similar result, correlating koff from reconstituted thin filaments with solution myosin ATPase rates, was recently reported [31]. In our study, it was interesting to find that both cTnC mutants (L48Q and I61Q) greatly reduced the slope (nH) of the tension-pCa relationship (Fig. 3B). The large reduction in nH with I61Q cTnC was somewhat expected, and this may result from I61Q cTnC disrupting strong-crossbridge-induced increases in Ca2+ binding that is normally found in cardiac muscle [2, 32–34]. This idea is supported by the tension-pCa curves (Fig. 3B), which show that at ~pCa 5.5, tension begins to rise for WT and I61Q cTnC but the slope of the curve is steep for WT cTnC and more flat for I61Q cTnC, suggesting that some form of cooperativity is lost with I61Q cTnC.
The apparent loss of cooperativity (nH) with L48Q cTnC likely occurs for a different reason, as pCa50 was increased, not decreased (Fig. 3B) and Tmax is not reduced (Fig. 3A). Tension is initiated at much lower [Ca2+] (i.e. higher pCa) with L48Q cTnC versus WT cTnC (Fig. 3B), and most of the tension increases are observed at low levels of Ca2+-mediated thin filament activation. Although the affinity of L48Q cTnC for the switch peptide of cTnI (cTnI128–180) was recently shown to decrease slightly [31], its reduced koff and increased Ca2+ binding affinity likely more than compensates for this, making L48Q cTnC a more effective activator of thin filaments at lower [Ca2+] and less dependent on cooperative cross-bridge activation mechanisms. If L48Q cTnC had caused greater strong cross-bridge-induced increases in Ca2+ binding, in addition to an increased pCa50 we would also expect nH to also increase, but this did not occur. Therefore, the cooperative coupling between strong-crossbridge binding and increased Ca2+ binding to cTnC was likely reduced with L48Q cTnC.
Cardiac vs. Skeletal Muscle Differences in Cooperative Activation
An additional purpose of this study was to compare these results in cardiac muscle with similar measurements in fast skeletal muscle from previous studies. These comparisons can be quite helpful in understanding the molecular mechanisms involved in myofilament regulation of contraction and relaxation. Further, differences in contractile mechanisms may translate to important functional differences between cardiac and skeletal muscle and lead to therapeutics designed specifically for cardiac disease.
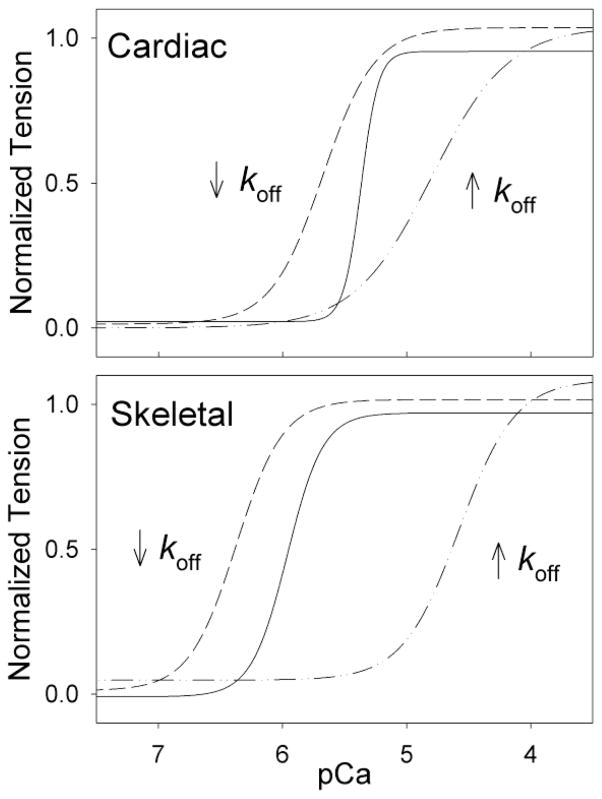
The decrease observed in nH for both L48Q cTnC and I61Q cTnC contrasts results from our similar experiments in fast skeletal muscle[14], suggesting mechanistic differences in cooperative activation of these two striated muscle types. We previously reported that skeletal TnC (sTnC) mutants with decreased (M80Q sTnCF27W) or increased (I60Q sTnCF27W) sTn Ca2+ koff also resulted in increased and decreased (respectively) pCa50 of tension in demembranated rabbit psoas fibers [15, 19]. However, while these shifts were similar in magnitude to what we report here for cardiac muscle (Table 3), the changes in nH were minimal. These differences in tension-pCa relationships are illustrated in Figure 6, which shows the fit lines of our data for cardiac and skeletal muscle. Davis et al. (2004) also reported that mutant sTnCs reconstituted into skinned skeletal fibers shifted pCa50 with little change in nH, even though these were different point mutations in sTnC than what we had used (see their Fig. 6C [35]). Further, the loss of nH in cardiac muscle may not be particular to the cTnC mutants selected for this study. Norman et al. (2007) reported a similar (but smaller) increase and decrease in pCa50 with two different cTnC mutants that also had higher and lower cTnC Ca2+ binding affinity (respectively), and these mutants also caused a loss of nH (see their Fig. 2B [17]). Interestingly, pseudo-phosphorylation of cTnI at serine 23, serine 24, and threonine 144 (which mimics phosphorylation by PKCβ) also increases koff, reduces pCa50 and reduces nH [36]. Thus, cooperative thin filament activation and tension development in cardiac muscle appears to strongly depend on the Ca2+ binding properties of cTn, in contrast to fast skeletal muscle. Indeed, cardiac muscle cooperativity appears to depend heavily on strong-binding cross-bridges increasing either Ca2+ binding to cTnC per se [2, 32–34] or interaction strength between cTnC, cTnI, and/or actin. If either are the case, L48Q cTnC and I61Q cTnC may both alter this form of cooperativity and do so within individual functional units of the thin filament (defined as the number of actins made available for myosin binding when Ca2+ binds to an individual cTn). In cardiac muscle this functional unit of activation appears to be similar or smaller than a structural regulatory unit (7 actins: 1 Tn: 1 tropomyosin) [8]. This contrasts skeletal muscle, where the functional unit is larger than the structural unit (10–12 actins [9]) and accounts for the majority of cooperative activation (nH), leaving little role for TnC Ca2+ binding kinetics in cooperative activation of skeletal muscle.
Figure 6.
Hill fits to cardiac data (Fig. 3B) and parallel previous studies with rabbit psoas skeletal fibers with sTnC mutants that decreased (M80Q sTnCF27W; [14]) or increased (I60Q sTnC; [15]) koff. The data, collected under similar solution and temperature conditions, demonstrate that cardiac muscle has a lower pCa50, that altering koff has similar effects on pCa50, but that nH is greatly reduced in cardiac but not skeletal muscle.
cTn Regulation of Tension Development and Relaxation Kinetics
Measurement of tension development and relaxation rates provides insight into the dynamic processes of Ca2+-mediated thin filament activation and cross-bridge cycling. kTR,max was not affected in myofibrils or trabeculae with L48Q cTnC and was slowed with I61Q cTnC only when Pi was ≤ 5 μM. In myofibrils with I61Q cTnC, addition of 0.5 mmol L−1 Pi increasd kTR,max to a value similar to those with WT or L48Q cTnC, suggesting I61Q cTnC only affected cross-bridge cycling at sub-physiological [Pi]. Comparisons of kACT vs. kTR indicate this occurred via thin filament activation dynamics. kACT reflects the combined rates of Ca2+ binding to cTn, thin filament activation, and tension generation, while kTR measures the rate of tension generation when Ca2+ binding (to cTn) and thin filament activation are in near steady state [5]. While some thin filament deactivation could occur during shortening length steps [37] with the kTR protocol, Ca2+ remains in equilibrium with cTnC (enabling a weak-binding myosin state, i.e. the “closed” thin filament state [3]), whereas the thin filament is inactivated (i.e. in the “blocked” state) preceding kACT. Interestingly, the difference in kACT vs. kTR in myofibrils with I61Q cTnC increased with addition of 0.5 mmol L−1 Pi (Fig. 2), which influences cross-bridge cycling specifically. We previously reported a similar difference between kACT and kTR in trabeculae activated using caged Ca2+ in the absence of a phosphate mop, and demonstrated a similar increase in both rates with dATP [5]. Together these studies demonstrate that thin filament activation kinetics can limit the rate tension develops in cardiac muscle, even under saturating Ca2+ conditions. This limitation may be even greater at more physiological [Pi], a question that we are investigating.
It has been suggested that kTR during sub-maximal Ca2+ activation is slowed by (relatively slow) cooperative events in thin filament activation [38, 39], and that loss of these interactions may elevate sub-maximal kTR. Our trabeculae data with I61Q cTnC support this idea (Fig. 3D), but the data with L48Q cTnC do not since pCa50 is higher but nH is lower and kTR is unchanged. In previous studies using sTnC mutants in skeletal muscle we suggested that elevated sub-maximal kTR may result from an effect on cross-bridge attachment and detachment rates (fapp and gapp;[40]) when koff is greatly increased [13, 15]. This could occur via reduced TnC-TnI interactions or by indirect effect to increase cross-bridge detachment kinetics, via greater tendency for tropomyosin to move towards an inhibiting position. Because skeletal data and models suggest that crossbridge kinetics are altered when sTnC kinetics are altered [12, 13], we test this hypothesis using a four-state mass action kinetic model that incorporates cardiac kinetics and strong-cross-bridge binding feedback (see Appendix and Scheme 1 in Supplementary Material).
Little is known about the influence of thin filament properties on relaxation, especially in cardiac muscle. In fast skeletal muscle, cross-bridge detachment kinetics (gapp) are thought to dominate the isometric (slow) phase relaxation rate [4, 15, 41–43]. The fast phase of relaxation occurs when some sarcomeres lose activation while others rapidly shorten under reduced load, resulting in sarcomere inhomogeneity, deactivation, and exponential tension decline [4, 42, 43]. In agreement with this, we found no effect of L48Q or I61Q cTnC on the fast (kREL,fast) or slow (kREL,slow) relaxation phase rates (Table 2). Interestingly, however, the duration of kREL,slow was prolonged with L48Q cTnC, but not I61Q cTnC. This could occur if slower koff allows detaching cross-bridges to re-attach, thus retarding thin filament deactivation without affecting gapp.
Conclusion
Regulation of activation and tension development in cardiac muscle must be controlled at the cellular (sarcomere) level because all cardiomyocytes are activated during systole. Our current results suggest the Ca2+ binding properties of cTn may allow for optimal cooperativity in activation of contraction, providing rapid tension development that is very sensitive to [Ca2+]. The consequences of a rapid koff of cTnC in cardiac (vs. skeletal) muscle may also be a greater dependence on strong cross-bridge binding for a given level of activation. Thus factors that alter the probability of strong cross-bridge binding, such as myofilament protein phosphorylation or changes in myofilament lattice spacing can have a profound effect in cardiac muscle, steepening the sarcomere length-tension relationship under conditions that affect pre-load and the Frank-Starling relationship. Interestingly, since increasing cTn koff had no effect on relaxation duration or kinetics in this study, it suggests that koff may also be optimized for rapid relaxation, which is advantageous for diastolic function (especially during adrenergic stimulation). Better understanding of these regulatory properties is also important for understanding the genetic basis of familial cardiomyopathies, as disease-linked mutations in several myofilament proteins including cTnC have recently been reported which either increase or decrease the Ca2+ sensitivity of steady-state tension in demembranated muscle preparations [44–47] or interfere with TnC-TnI interactions [48]. Ultimately, therapies targeting the Ca2+ sensitivity of myofilaments must be specifically designed to account for the kinetics and activation principles of the cardiac system.
Research Highlights.
Cooperative cardiac thin filament activation depends on cTnC Ca2+ binding kinetics
cTnC Ca2+ dissociation rate (koff) affects acto-myosin binding but not cycling.
Altering koff affects Ca2+ sensitivity of isometric tension development
Increasing koff can decrease the rate of contractile activation
Decreasing koff can increase the duration of isometric relaxation
Supplementary Material
Acknowledgments
We greatly acknowledge Drs. James Sellers (NIH) and Earl Homsher (UCLA) for use of facilities and assistance with the thin filament koff measurements; Dr. Martin Kushmerick for NMR analysis of activation solutions; and Zhaoxiong Luo and An-Yue Tu for construction of cTnC mutants and preparation of cTn complexes. Additional thanks go to Drs. Svetlana Tikunova and Jonathan Davis, who assisted in initial selection of mutations and in methods of analysis for whole cTn koff stopped-flow experiments. We thank Dr. Albert Gordon for comments on the manuscript. This work was supported by USA NIH grants HL61683 and HL65497 to M.R. and Ministero dell Universitá e della Ricera, Italy grants PRIN2006 & 2008 to C.T. and C.P. Financial support by Telethon-Italy (grant # GGP06007) is also gratefully acknowledged. K.L. Kreutziger was a recipient of a Graduate Fellowship in Biomedical Engineering from The Whitaker Foundation. M. Regnier is an Established Investigator of the American Heart Association.
Biographies
Kareen L. Kreutziger earned a B.S. in Biomedical Engineering at the University of Rochester (Rochester, NY, USA) in 2001 and her Ph.D. in Bioengineering at the University of Washington (Seattle, WA, USA) in 2007 with Dr. Michael Regnier. She was a Whitaker Foundation Pre-doctoral Fellow in Biomedical Engineering (2001–6). She is currently a Post-doctoral Fellow studying cardiac tissue engineering at the Center for Cardiovascular Biology with Dr. Charles E. Murry in the Department of Pathology at the University of Washington.
Nicoletta Piroddi Born in 1967. B.Sc. in Biology 1993. Ph.D. in Physiology 1998 (University of Florence, Italy) with Professor Corrado Poggesi. Full time Researcher in Physiology at Medical Faculty, University of Florence, since 2004. Teaching Physiology to students at Medical, Science and Pharmacy Faculties, University of Florence, since 2003. Present activity: lab research on molecular mechanisms underlying the force generation process in striated muscle by the use of skeletal and cardiac myofibrils from human and animal muscles.
Chiara Tesi Born 1960. B.Sc. 1985 (University of Florence, Italy). Research Fellowship, Biochemistry Unit Inserm U128 Montpellier-France (1986–1988). Ph.D. 2000 in Physiology (University of Florence). 1992-present: Associated Researcher, Associated Professor and Full Professor of Physiology, Medical Faculty, University of Florence, Italy. Member of the Biomedical PhD School of the University of Florence. Scientific Interests: study of the mechanism of chemomechanical coupling and its regulation in muscle contraction using biochemical and mechanical approaches.
Corrado Poggesi Born 1950. M.D. 1975 (University of Pavia, Italy). Initial academic career, Medical Faculty, University of Pavia. Senior Scientist, Department of Pharmacology, Mayo Clinic, Rochester, MN, USA (1986–87). Since 1994 Professor of Physiology, Medical Faculty, University of Florence, Italy. Visiting Scientist at the Department of Physiology and Biophysics, D. Geffen School of Medicine, UCLA, CA, USA (2003). Chair, Department of Physiological Sciences, University of Florence (2006–08). Present: Head of the Biomedical PhD School of the University of Florence; member of the Academic Senate of the University of Florence.
Michael Regnier is Professor and Vice-Chair of the Department of Bioengineering, and adjunct Professor of Physiology and Biophysics at the University of Washington (Seattle, WA, USA). He is also an Affiliate Investigator of the Benaroya Research Institute at Virginia Mason Hospital (Seattle, WA, USA) and an Established Investigator of the American Heart Association.
Footnotes
Abbreviations: ANOVA, analysis of variance; BDM, 2,3-butanedione monoxime; cTn, cardiac troponin; cTnC, cardiac troponin C; cTnI, cardiac troponin I; cTnT, cardiac troponin T; dATP, 2-deoxy-ATP; DTT, dithiothreitol; fapp, rate of cross-bridges transitioning into tension-generating states; gapp, rate of cross-bridges transitioning out of tension-generating states; kACT, tension activation rate; Ki, inhibition constant; koff, Ca2+ dissociation rate; kon, Ca2+ association rate; kREL,fast, fast phase relaxation rate; kREL,slow, slow phase relaxation rate; kTR, tension redevelopment rate; L0, initial length; nH, Hill coefficient; MOPS, 3-(N-mopholino) propanesulfonic acid; pCa, log[Ca2+]; pCa50, pCa at half-maximal activation; Pi, inorganic phosphate; SL, sarcomere length; sTnC, skeletal troponin C; Tmax, maximal tension; Tn, troponin; xcTnC, a cardiac troponin C mutant that does not bind Ca2+ at site II; WT, wild type.
DISCLOSURES
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kareen L. Kreutziger, Email: klk3@u.washington.edu.
Nicoletta Piroddi, Email: nicoletta.piroddi@unifi.it.
Chiara Tesi, Email: chiara.tesi@unifi.it.
Corrado Poggesi, Email: corrado.poggesi@unifi.it.
Michael Regnier, Email: mregnier@u.washington.edu.
References
- 1.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000 Apr;80(2):853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann PA, Fuchs F. Evidence for a force-dependent component of calcium binding to cardiac troponin C. Am J Physiol. 1987 Oct;253(4 Pt 1):C541–6. doi: 10.1152/ajpcell.1987.253.4.C541. [DOI] [PubMed] [Google Scholar]
- 3.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993 Aug;65(2):693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poggesi C, Tesi C, Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Arch. 2005 Mar;449(6):505–17. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- 5.Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys J. 2004 Sep;87(3):1815–24. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regnier M, Martyn DA, Chase PB. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys J. 1998 Apr;74(4):2005–15. doi: 10.1016/S0006-3495(98)77907-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regnier M, Rivera AJ, Chen Y, Chase PB. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ Res. 2000 Jun 23;86(12):1211–7. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- 8.Gillis TE, Martyn DA, Rivera AJ, Regnier M. Investigation of thin filament near-neighbor regulatory unit interactions during skinned rat cardiac muscle force development. J Physiol. 2007 Feb 22;580(Pt 2):561–76. doi: 10.1113/jphysiol.2007.128975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regnier M, Rivera AJ, Wang CK, Bates MA, Chase PB, Gordon AM. Thin filament near-neighbour regulatory unit interactions affect rabbit skeletal muscle steady-state force-Ca(2+) relations. J Physiol. 2002 Apr 15;540(Pt 2):485–97. doi: 10.1113/jphysiol.2001.013179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MX, Spyracopoulos L, Sykes BD. Binding of cardiac troponin-I147–163 induces a structural opening in human cardiac troponin-C. Biochemistry. 1999 Jun 29;38(26):8289–98. doi: 10.1021/bi9901679. [DOI] [PubMed] [Google Scholar]
- 11.Piroddi N, Tesi C, Pellegrino MA, Tobacman LS, Homsher E, Poggesi C. Contractile effects of the exchange of cardiac troponin for fast skeletal troponin in rabbit psoas single myofibrils. J Physiol. 2003 Nov 1;552(Pt 3):917–31. doi: 10.1113/jphysiol.2003.051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Gonzalez A, Fredlund J, Regnier M. Cardiac troponin C (TnC) and a site I skeletal TnC mutant alter Ca2+ versus crossbridge contribution to force in rabbit skeletal fibres. J Physiol. 2005 Feb 1;562(Pt 3):873–84. doi: 10.1113/jphysiol.2004.077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Gonzalez A, Gillis TE, Rivera AJ, Chase PB, Martyn DA, Regnier M. Thin-filament regulation of force redevelopment kinetics in rabbit skeletal muscle fibres. J Physiol. 2007 Mar 1;579(Pt 2):313–26. doi: 10.1113/jphysiol.2006.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreutziger KL, Gillis TE, Davis JP, Tikunova SB, Regnier M. Influence of enhanced troponin C Ca2+ binding affinity on cooperative thin filament activation in rabbit skeletal muscle. J Physiol. 2007 Jun 21;583(1):337–50. doi: 10.1113/jphysiol.2007.135426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreutziger KL, Piroddi N, Scellini B, Tesi C, Poggesi C, Regnier M. Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle. J Physiol. 2008 Aug 1;586(Pt 15):3683–700. doi: 10.1113/jphysiol.2008.152181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikunova SB, Davis JP. Designing calcium-sensitizing mutations in the regulatory domain of cardiac troponin C. J Biol Chem. 2004 Aug 20;279(34):35341–52. doi: 10.1074/jbc.M405413200. [DOI] [PubMed] [Google Scholar]
- 17.Norman C, Rall JA, Tikunova SB, Davis JP. Modulation of the rate of cardiac muscle contraction by troponin C constructs with various calcium binding affinities. Am J Physiol Heart Circ Physiol. 2007 Oct;293(4):H2580–7. doi: 10.1152/ajpheart.00039.2007. [DOI] [PubMed] [Google Scholar]
- 18.Tikunova SB, Davis JP, Rall JA. Engineering cardiac troponin C (cTnC) Mutants with dramatically altered Ca2+ dissociation rates as molecular tools to study cardiac muscle relaxation. Biophys J. 2004;86(1):394a. [Google Scholar]
- 19.Kreutziger KL, Piroddi N, Belus A, Poggesi C, Regnier M. Ca2+-binding kinetics of troponin C influence force generation kinetics in cardiac muscle. Biophys J. 2007;92:477a. [Google Scholar]
- 20.Kreutziger KL, Piroddi N, Belus A, Scellini B, Poggesi C, Regnier M. Effect of TnC with altered Ca2+ binding kinetics on force generation in striated muscle. J Muscle Res Cell Motil. 2006;27:501–2. [Google Scholar]
- 21.Dong W, Rosenfeld SS, Wang CK, Gordon AM, Cheung HC. Kinetic studies of calcium binding to the regulatory site of troponin C from cardiac muscle. J Biol Chem. 1996 Jan 12;271(2):688–94. doi: 10.1074/jbc.271.2.688. [DOI] [PubMed] [Google Scholar]
- 22.Tikunova SB, Rall JA, Davis JP. Effect of hydrophobic residue substitutions with glutamine on Ca(2+) binding and exchange with the N-domain of troponin C. Biochemistry. 2002 May 28;41(21):6697–705. doi: 10.1021/bi011763h. [DOI] [PubMed] [Google Scholar]
- 23.Gomes AV, Venkatraman G, Davis JP, Tikunova SB, Engel P, Solaro RJ, et al. Cardiac troponin T isoforms affect the Ca(2+) sensitivity of force development in the presence of slow skeletal troponin I: insights into the role of troponin T isoforms in the fetal heart. J Biol Chem. 2004 Nov 26;279(48):49579–87. doi: 10.1074/jbc.M407340200. [DOI] [PubMed] [Google Scholar]
- 24.Kohler J, Chen Y, Brenner B, Gordon AM, Kraft T, Martyn DA, et al. Familial hypertrophic cardiomyopathy mutations in troponin I (K183Delta, G203S, K206Q) enhance filament sliding. Physiol Genomics. 2003 Jul 7;14(2):117–28. doi: 10.1152/physiolgenomics.00101.2002. [DOI] [PubMed] [Google Scholar]
- 25.Piroddi N, Belus A, Eiras S, Tesi C, van der Velden J, Poggesi C, et al. No direct effect of creatine phosphate on the cross-bridge cycle in cardiac myofibrils. Pflugers Arch. 2006 Apr;452(1):3–6. doi: 10.1007/s00424-005-0008-7. [DOI] [PubMed] [Google Scholar]
- 26.Brandt PW, Colomo F, Piroddi N, Poggesi C, Tesi C. Force regulation by Ca2+ in skinned single cardiac myocytes of frog. Biophys J. 1998 Apr;74(4):1994–2004. doi: 10.1016/S0006-3495(98)77906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martyn DA, Chase PB, Hannon JD, Huntsman LL, Kushmerick MJ, Gordon AM. Unloaded shortening of skinned muscle fibers from rabbit activated with and without Ca2+ Biophys J. 1994 Nov;67(5):1984–93. doi: 10.1016/S0006-3495(94)80681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesi C, Colomo F, Nencini S, Piroddi N, Poggesi C. The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys J. 2000 Jun;78(6):3081–92. doi: 10.1016/S0006-3495(00)76845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tesi C, Piroddi N, Colomo F, Poggesi C. Relaxation kinetics following sudden Ca(2+) reduction in single myofibrils from skeletal muscle. Biophys J. 2002 Oct;83(4):2142–51. doi: 10.1016/S0006-3495(02)73974-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis JP, Norman C, Kobayashi T, Solaro RJ, Swartz DR, Tikunova SB. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys J. 2007 May 1;92(9):3195–206. doi: 10.1529/biophysj.106.095406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tikunova SB, Liu B, Swindle N, Little SC, Gomes AV, Swartz DR, et al. Effect of calcium-sensitizing mutations on calcium binding and exchange with troponin C in increasingly complex biochemical systems. Biochemistry. 2010 Mar 9;49(9):1975–84. doi: 10.1021/bi901867s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martyn DA, Gordon AM. Influence of length on force and activation-dependent changes in troponin c structure in skinned cardiac and fast skeletal muscle. Biophys J. 2001 Jun;80(6):2798–808. doi: 10.1016/S0006-3495(01)76247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martyn DA, Regnier M, Xu D, Gordon AM. Ca2+ - and cross-bridge-dependent changes in N- and C-terminal structure of troponin C in rat cardiac muscle. Biophys J. 2001 Jan;80(1):360–70. doi: 10.1016/S0006-3495(01)76020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang YP, Fuchs F. Length, force, and Ca(2+)-troponin C affinity in cardiac and slow skeletal muscle. Am J Physiol. 1994 Apr;266(4 Pt 1):C1077–82. doi: 10.1152/ajpcell.1994.266.4.C1077. [DOI] [PubMed] [Google Scholar]
- 35.Davis JP, Rall JA, Alionte C, Tikunova SB. Mutations of hydrophobic residues in the N-terminal domain of troponin C affect calcium binding and exchange with the troponin C-troponin I96–148 complex and muscle force production. J Biol Chem. 2004 Apr 23;279(17):17348–60. doi: 10.1074/jbc.M314095200. [DOI] [PubMed] [Google Scholar]
- 36.Lu QW, Hinken AC, Patrick SE, Solaro RJ, Kobayashi T. Phosphorylation of cardiac troponin I at protein kinase C site threonine 144 depresses cooperative activation of thin filaments. J Biol Chem. 2010 Apr 16;285(16):11810–7. doi: 10.1074/jbc.M109.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner B, Chalovich JM. Kinetics of thin filament activation probed by fluorescence of N-((2-(Iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1, 3-diazole-labeled troponin I incorporated into skinned fibers of rabbit psoas muscle: implications for regulation of muscle contraction. Biophys J. 1999 Nov;77(5):2692–708. doi: 10.1016/S0006-3495(99)77103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzsimons DP, Patel JR, Campbell KS, Moss RL. Cooperative mechanisms in the activation dependence of the rate of force development in rabbit skinned skeletal muscle fibers. J Gen Physiol. 2001 Feb;117(2):133–48. doi: 10.1085/jgp.117.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razumova MV, Bukatina AE, Campbell KB. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys J. 2000 Jun;78(6):3120–37. doi: 10.1016/S0006-3495(00)76849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988 May;85(9):3265–9. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kress M, Huxley HE, Faruqi AR, Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–42. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- 42.Huxley AF, Simmons RM. Rapid ‘give’ and the tension ‘shoulder’ in the relaxation of frog muscle fibres. J Physiol. 1970 Sep;210(1):32P–3P. [PubMed] [Google Scholar]
- 43.Luo Y, Davis JP, Tikunova SB, Smillie LB, Rall JA. Myofibrillar determinants of rate of relaxation in skinned skeletal muscle fibers. Adv Exp Med Biol. 2003;538:573–81. doi: 10.1007/978-1-4419-9029-7_51. discussion 81–2. [DOI] [PubMed] [Google Scholar]
- 44.Lim CC, Yang H, Yang M, Wang CK, Shi J, Berg EA, et al. A novel mutant cardiac troponin C disrupts molecular motions critical for calcium binding affinity and cardiomyocyte contractility. Biophys J. 2008 May 1;94(9):3577–89. doi: 10.1529/biophysj.107.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang B, Chung F, Qu Y, Pavlov D, Gillis TE, Tikunova SB, et al. The familial hypertrophic cardiomyopathy related cardiac troponin C mutation L29Q affects Ca2+ binding and myofilament contractility. Physiol Genomics. 2008 Feb 19;33(2):257–66. doi: 10.1152/physiolgenomics.00154.2007. [DOI] [PubMed] [Google Scholar]
- 46.Landstrom AP, Parvatiyar MS, Pinto JR, Marquardt ML, Bos JM, Tester DJ, et al. Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. J Mol Cell Cardiol. 2008 Aug;45(2):281–8. doi: 10.1016/j.yjmcc.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neulen A, Stehle R, Pfitzer G. The cardiac troponin C mutation Leu29Gln found in a patient with hypertrophic cardiomyopathy does not alter contractile parameters in skinned murine myocardium. Basic Res Cardiol. 2009 Jun 9; doi: 10.1007/s00395-009-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biesiadecki BJ, Kobayashi T, Walker JS, John Solaro R, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007 May 25;100(10):1486–93. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.