Abstract
Lipid overload resulting in lipotoxicity is prominent in a number of chronic diseases and has been associated with cellular dysfunction and cell death. This study characterizes palmitic acid-induced lipotoxicity (PA-LTx) in Schwann cell cultures grown in normal and high glucose concentrations. The study shows for the first time that Schwann cell (SC) cultures exposed to elevated levels of PA exhibit a dose and time –dependent loss in cell viability. Hoescht and Annexin V/7AAD staining confirmed cell death through apoptosis and the lipotoxic effect was more dramatic in SC cultures grown under high glucose conditions. The first indication of cellular dysfunction in treated SC cultures was a decrease in Ca++ levels in the endoplasmic reticulum (ER, [Ca++]ER) observed five minutes following the initial challenge with PA. This decrease in [Ca++]ER was followed by a significant increase in the expression of ER stress signature genes CHOP, Xbp1 and GRP78. The early ER stress response induced by PA-LTx was followed by a strong mitochondrial membrane depolarization. Flow cytometry using 2’, 7’-dichlorodihydrofluorescein diacetate (H2DCF-DA) showed an increase in oxidative stress within three to six hours after PA treatment. Treatment of cultures undergoing PA-LTx with the calcium chelator BAPTA-AM and the antioxidant MC1-186 significantly reversed the lipotoxic effect by decreasing the generation of ROS and significantly increasing cell viability. We conclude that lipotoxicity in Schwann cells results in cellular dysfunction and cell death that involves a robust ER stress response, mitochondrial dysfunction and an augmented stated of cellular oxidative stress (ASCOS).
Keywords: Lipotoxicity, Schwann Cells, Peripheral neuropathy, ER stress, Type 2 diabetes
1. Introduction
Normal cellular functions depend on the availability of adequate levels of free fatty acids (FFA) that are an important for critical normal metabolic activities (Bezuglov et al., 1998). However, a significant metabolic disarray and cellular dysfunction can occur when tissues and cells are chronically exposed to elevated levels of FFAs as observed in connection with type 2 diabetes and obesity (Ghosh and Rodrigues, 2006; Ioannidis, 2008 ; Kusminski et al., 2009 ; Unger, 2008 ; Wilding, 2007). While adipocytes have the proper cellular machinery to store and safely utilize high amount of these FFAs, non-adipose cells are vulnerable to lipid overload which can induce lipotoxicity and apoptotic cell death (Listenberger et al., 2003; van Herpen and Schrauwen-Hinderling, 2008) Lipotoxicity in non-nerve cells is well documented in the literature (El-Assaad et al., 2003 ; Li et al., 2008 ; Rho et al., 2007 ; Wei et al., 2006). For instance, pancreatic beta cells exposed to high levels of palmitic acid (PA) exhibit a dramatic dysfunction and apoptotic cell death (Kato et al., 2008 ; Koshkin et al., 2008 ; Lupi et al., 2002 ; Shimabukuro et al., 1998). It has been shown that lipotoxicity-induced apoptosis and beta cell death in the pancreas precedes hyperglycemia suggesting that can be an important factor in the onset of type 2 diabetes (Schaffer, 2003 ; Unger, 1997 ; Unger and Zhou, 2001 ; van Herpen and Schrauwen-Hinderling, 2008)
Nerve cells are exposed to multiple pathologies that can lead to lipid overload and lipotoxicity that can result in lipid peroxidation and lipotoxicity (Almaguel et al., 2009 ; Ilieva et al., 2007 ; Simonian and Coyle, 1996 ; Taghibiglou et al., 2009) During ischemia, there is a significant increase in lipid peroxidation in membranes of the endoplasmic reticulum (ER), mitochondria and lysosomes which may contribute to the marked dysfunction and cell death observed in this condition (Hayashi et al., 2005). Ceramide, a lipid species that forms from fatty acyl CoA and sphingosine, is elevated in the white matter of post-mortem brains of patients with Alzheimer’s disease (Grimm et al., 2005 ; He et al., 2010). Traumatic injuries in the brain and spinal cord result in a significant increase of FFAs that is believed to play a role in the extensive amount of cell death and tissue damage occurring during the secondary phase of the condition (Conti et al., 1998 ; Newcomb et al., 1999) (Malecki et al., 2000).
Our laboratory is interested in examining the role and impact of lipotoxicity in the nervous system. Previous reports have shown that stearic acid and PA trigger a strong apoptotic cell death response in nerve growth factor differentiated pheochromocytoma cells (PC12 cells) and cortical cells cultures (Ulloth et al., 2003, Almaguel et al., 2009, 2010). This lipotoxic effect was shown to be specific for saturated fatty acids such as palmitic (PA) and stearic acid, because treatment with oleic nor arachidonic acids at the same concentrations did not trigger a lipotoxic response (Ulloth et al., 2003). The lipotoxic cell death process is caspase-independent and involves a significant differential expression in gene expression of fas ligand/receptor and members of the Bcl2 family (Ulloth et al., 2003). Caspase-independent cell death can occur through significant dysfunction of vital organelles such as the endoplasmic reticulum, lysosomes and mitochondria suggesting that calcium and oxidative cellular discruption may play a key cellular role in this process (Ulloth et al., 2003, Almaguel et al., 2009, 2010). The aim of this study was to assess whether ER stress and ROS production are principal contributors to the PA-LTx process in Schwann cells grown under hyperglycemic and euglycemic conditions. This study shows for the first time that Schwann cells (SC) chronically exposed to PA overload exhibit dramatic lipotoxicity and apoptotic cell death by a mechanism that require ER stress followed by an augmented state of cellular oxidative stress (ASCOS) and mitochondrial dysfunction. Further, PA-LTx occurred in a dose and time dependent manner, and is significantly stimulated by high gluc.ose. These data is important considering that pathological conditions that lead to lipid overload can result in SC dysfunction, demyelination and axon atrophy, three prominent abnormalities associated with peripheral neuropathies and axon degeneration (Dyck and Giannini, 1996 ; Sango et al., 2006 ; Song et al., 2003 ; Welcher et al., 1991)
2. Results
2.1 Cell viability and lipotoxicity in immortalized Schwann cells (iSC) cultures in different glucose concentrations
Chronic elevated plasma levels of saturated FFA have been reported to cause dysfunction and injury in affected tissues and cells (Cnop, 2008 ; McGarry, 2002 ; Paolisso et al., 1995 ; Reaven et al., 1988 ; Unger, 2008). Previous reports from our laboratory have shown that PA (PA:BSA 2:1, see Experimental Procedure) induces lipotoxicity and cell death in nerve growth factor differentiated PC12 cells and rat cortical cells (Almaguel et al., 2009 ; Almaguel et al., 2010 ; Ulloth et al., 2003). This experimental paradigm exposed cells to a 10 nM range of unbound FFA concentration which parallel to levels found in conditions such as diabetes and ischemic injury (Almaguel et al., 2009 ; Bazan, 1970 ; Cistola et al., 1990 ; Thomas et al., 2002).
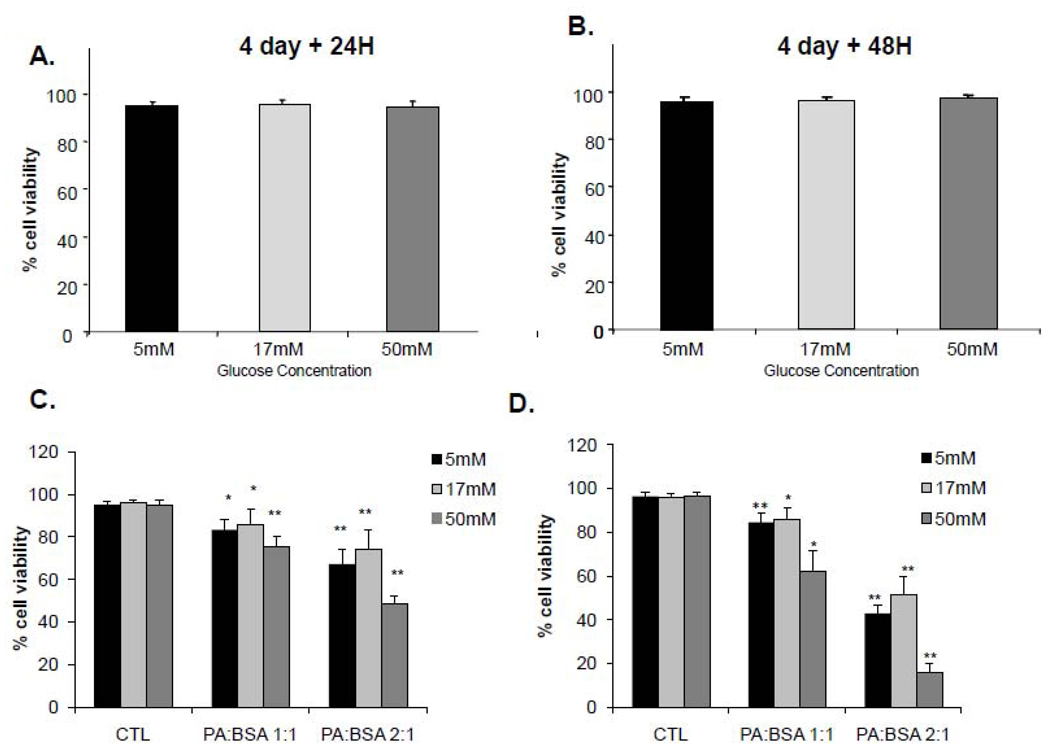
Our initial experiments determined the changes in cell viability under three different conditions: 1) hypoglycemic (5mM glucose), 2) euglycemic (17mM glucose) and 3) hyperglycemic (50 mM glucose) (Vincent et al., 2005). In all of our studies, cells were cultured in the indicated glucose concentrations for 4 days before the exposure to PA. Our results show that glucose level alone did not affect viability of iSC cells at any of the concentrations tested (Figure 1A, B). To explore any potential synergistic detrimental effects of FFA and glucose, we performed a series of experiments using trypan blue assays to assess viability of iSC after exposure to PA at a dose of 1:1 or 2:1 together with these three non-damaging glucose concentrations. Figure 1C and 1D shows that under each of these glucose concentrations, PA caused a dose- and time-dependent decrease in cell viability. Cell loss observed in cell cultures exposed to PA under hypoglycemic (5 mM glucose) and euglycemic (17 mM glucose) were similar, however the dose and time-dependent increase in cell death was significantly greater in hyperglycemic cultures (Figure 1C, D). Cell cultures exposed to PA for 24 hr under hyperglycemic condition showed a 24.3 ± 4.9 % and 60.6 ±19.0 % induction of cell death at PA:BSA 1:1 and 2:1 treatments respectively. At 48 hr, hyperglycemic conditions showed dramatically increased cell death in PA:BSA 1:1 and 2:1 treatments, up to 32.7 ± 12.2 % and 83.9± 4.0 % respectively (Figure 1D).
Figure 1. Cell viability under varied glucose and PA concentrations.
iSC cells were cultured in medium containing 5, 17 or 50 mM glucose for 4 days before the addition of PA. After 4 days of pre-conditioning under varied glucose concentrations, BSA control (A) or PA:BSA (1:1 or 2:1) (B) was added to the cells. Cell viability was assessed by trypan blue assay after 24 and 48 hr. Statistical analysis was performed using student’s t-test.. *p< 0.05, **p<0.005, N=4
2.2 iSC cells undergo apoptosis during PA-LTx
As shown by Hoescht staining in Figure 2, numerous cells in the PA: BSA 1:1 and PA: BSA 2:1 panels exhibit nucleus condensation and apoptotic bodies while those in untreated cultures show normally dispersed chromatin and intact nuclear membranes. The next series of experiments further quantified this apoptotic process by taking advantage of established flow cytometric methods using Annexin V FITC and 7 AAD to measure early (Annexin V positive cell populations) and late apoptosis (7AAD positive cell populations). Figure 3A shows that there is a significant increase in the number of apoptotic iSC cells at 6 hr following exposure to PA in euglycemic cultures (8.69% early apoptotic, 5.38% late apoptotic cells) and the proportion of apoptotic cells continued to rise up until 48 hr of PA treatment (26.60% early apoptotic, 21.95 % late apoptotic cells). In contrast to the euglycemic conditions, cultures grown in hyperglycemic conditions exhibited an earlier and more pronounced increase in cell death. Cell death in the hyperglycemic conditions occurred as early as 3 hr (3.40% early apoptotic, 4.40% late apoptotic cells) and continued to increase reaching a peak at 48 hr (41.98% early apoptotic, 24.80% late apoptotic cells) Figure 3B.
Figure 2. PA-LTx induced apoptotic features in iSC cells.
To determine nuclear morphology, Hoescht staining was performed after 48 hr PA treatment. Nuclear condensation is indicated with white arrows. Representative micrographs of five independent experiments are shown.
Figure 3. Time-dependent induction of apoptosis by PA-LTx.
Time course experiments of Annexin V and 7AAD flow cytometric assay of apoptosis were performed on PA treated iSC cells cultured in (A) euglycemic and (B) hyperglycemic media. Early apoptotic translocation of phosphotidyl cistein to the outer cell membrane renders the cell Annexin V+, while late apoptosis is detected by entry and retention of 7AAD in the nucleus. The percentages of cells that are Annexin V−/7AAD− (non apoptotic). Annexin V+/7AAD− (early stage apoptosis) and Annexin V+/7AAD+ (late stage apoptosis) are graphed. Statistical analysis was performed using student’s t-test. *p< 0.05, **p<0.005, N=4
2.3 Early mediators of PA-LTx in iSC cells: Potential role of intracellular calcium
In the nervous system, neurons and associated supportive cells like Schwann cells are vulnerable to conditions that generate calcium overload and ROS as is evident in ischemic and traumatic injuries (Barber and Shaw, 2010 ; Dugan et al., 2009 ; Lu et al., 2009). For example, FFA overload has been associated with significant ER and mitochondrial dysfunction which may result in Ca++ release and potential toxicity and cell death (Almaguel et al., 2009 ; Borradaile et al., 2006 ; Karaskov et al., 2006 ; Wei et al., 2006). The first series of experiments examined the potential role of calcium overload by examining releasable [Ca++] in the ER, the cellular organelle that is most responsible for controlling free Ca++ concentrations in the cytosol. Cell cultures were exposed to 2:1 PA: BSA and Ca++ levels in the ER ([Ca++]ER) were analyzed at 5 min, 15 min, 30 min and 3, 6, 12 hr. Figure 4A shows that PA-LTx reduced [Ca++]ER in a time dependent manner in both 17 and 50 mM glucose conditions, suggesting that PA was able to impair the capacity of the ER to sequester Ca++. In euglycemic cultures, a significant decrease of [Ca++]ER was present at 30 min after PA treatment, while in hyperglycemic treatment, a significant reduction in [Ca++]ER was observed as early as 15 min after PA treatment and the [Ca++]ER was reduced to approximately 25% by 12 hr (Figure 4A). The limit in the capacity of the ER to store Ca++ imposed by PA-LTx results in an increase of free Ca++ in the cytoplasm which could lead to cellular toxicity. To test this hypothesis we used a well established Ca++ chelator, BAPTA-AM, to assess whether we could reduce the lipotoxic injury. Figure 4B and 4C shows that BAPTA-AM, as expected, reversed the loss of cell viability triggered by PA-LTx in both glucose concentrations and was able to reverse the LTx effect at both 24 and 48 hr (Figure 4B C). These data suggest that PA-LTx in iSC cells induces an early ER dysfunction that compromises the ability of this organelle to sequester Ca++; resulting in levels of cytosolic Ca++ that can cause cell injury. It is important to note that elevated levels of glucose alone did not significantly affect [Ca++]ER in iSC cells (data not shown).
Figure 4. Ca++ concentration in the ER is reduced with PA treatment.
(A) The iSC cells were cultured in 17 mM or 50 mM glucose and treated with PA:BSA 2:1. [Ca++]ER, reference to control level, was determined at 5, 15, 30 min and 3, 6, 12 hr after treatment. (B)(C) Cells were treated with PA:BSA 2:1 in the presence or absence of BAPTA-AM (5µM) or D-AP5 (50µM) for 24 and 48 hr. Assessment of cell viability was performed by crystal violet assays. Statistical analysis was performed using student’s t-test..*p< 0.05, **p< 0.005, N=3
To assess the potential sources of the elevated levels of intracellular Ca++ observed under PA-LTx conditions it was important to also examine the possible contribution of influx of Ca++ from the extracellular space. A co-treatment of an NMDAR inhibitor, D-AP5 (50µM) with PA (2:1) was performed in order to test for the contribution of NMDA receptors. Our data demonstrated that D-AP5 was able to significantly increase cell viability in euglycemic and hyperglycemic iSC at 24hr only. However, no increase in cell viability was observed at 48hr (Figure 4B, C).
As shown above, the effect of PA-LTx on ER dysfunction and Ca++ released into the cytosol is the earliest event of PA-LTx that was observed in iSC cells. To further examine the effects of PA-LTx on the ER, the mRNA expression of well-established ER stress response proteins such as CHOP, GRP78 and Xbp1 were examined (Eizirik et al., 2008 ; Malhotra and Kaufman, 2007 ; Xu et al., 2005). CHOP, also known as Ddit3, is a transcription factor that mediates the ER stress-induced apoptotic pathways. The mechanism by which CHOP induces apoptosis remains unclear although it has been implicated in the inhibition of the transcription of anti-apoptotic Bcl-2 proteins (Eizirik et al., 2008 ; Malhotra and Kaufman, 2007). ER stress genes were examined using quantitative real time PCR at 0, 3, 6, 12, 24, and 48 hr following exposure to PA:BSA 2:1. We found that PA-LTx triggers a robust up-regulation of CHOP mRNA at 6, 12, and 24 hr in euglycemic and hyperglycemic conditions (Figure 5A and 5B). Spliced Xbp1 is a transcriptional activator that induces genes that encode for ER-associated degradation, chaperones, and lipid synthesis (Malhotra and Kaufman, 2007 ; Yasuda et al., 2003). PA-LTx induced a modest increase in mRNA levels of Xbp1 at 6 hr and reached a peak at 24 hr in euglycemic cells. However, Xbp1 was only significantly up-regulated at 24 and 48hr in hyperglycemic cells (Figure 5A and 5B). Grp78 is a 78 kDa glucose regulated protein that resides in the ER and tends to be up-regulated when the cell is exposed to environmental stressors (Dey et al., 2006 ; Hiramatsu et al., 2006 ; Rao and Bredesen, 2004). Interestingly, mRNA levels of GRP78 were significantly down-regulated at 24 and 48 hr in euglycemic and had a maximum 5-fold up-regulation in hyperglycemic conditions at 48 hr. (Figure 5A and 5B).
Figure 5. ER stress genes are regulated with PA treatment.
Quantitative RT-PCR was performed to measure mRNA levels of ER stress genes CHOP, Xbp1, and Grp78 in iSC cells at 0 (CTL), 3, 6, 12, 24 and 48 hr after adding PA:BSA 2:1 in euglycemic (A) and hyperglycemic conditions (B). In addition, ER stress genes where also evaluated after a co-treatment of PA and BAPTA-AM in both euglycemic (C) and hyperglycemic (D) conditions. Statistical analysis was performed using student’s t-test. *p<0.05, **p<0.005, N=3
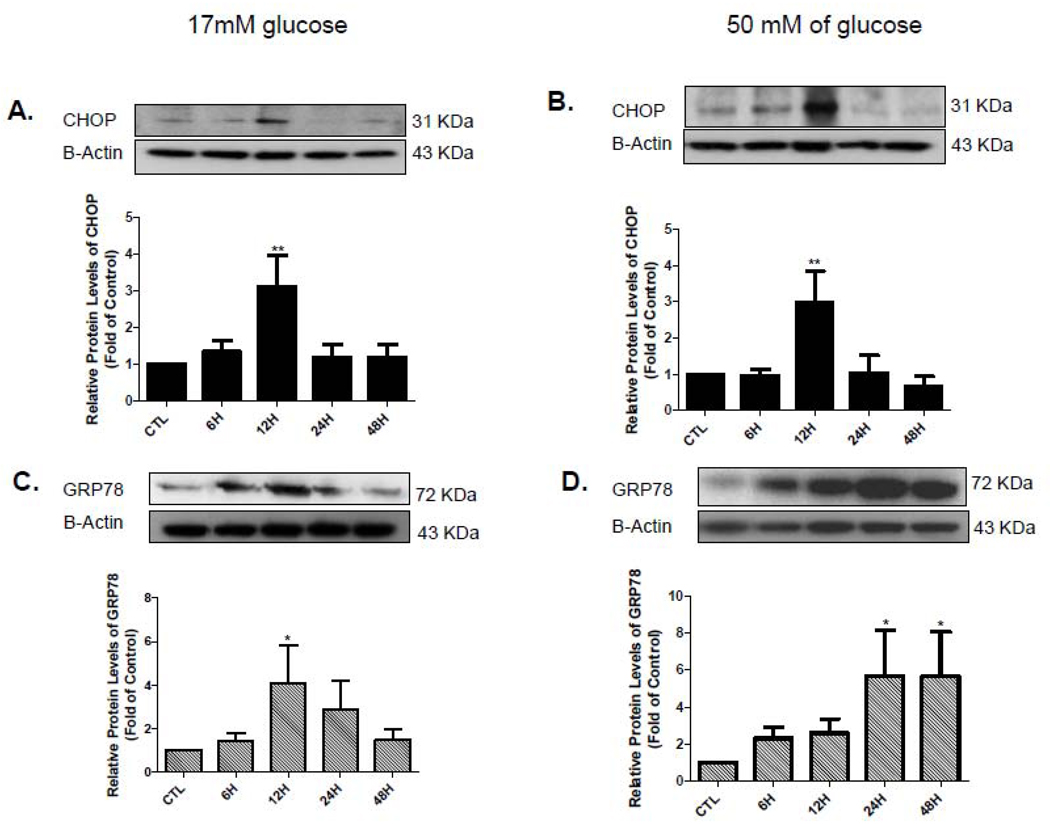
Furthermore, we also examined the effect of PA on the protein levels of these ER stress genes at 0, 6, 12, 24, and 48 hr by using Western blots. We found that PA-LTx triggers a robust up-regulation of CHOP protein levels at 12 hrs which returned to normal levels at 24 and 48 hr in euglycemic and hyperglycemic culture conditions (Figure 6A and 6B). In addition, the proteins levels of GRP78 were significantly up-regulated at 12 hr and returned to control expression at 24 hrs and 48 hr in the euglycemic condition (Figure 6C). However, in hyperglycemic condition we observed a significant increased in the protein levels of GRP78 at 24 hrs that remained elevated at 48 hrs (latest time point measured, Figure 6D). We were not able to performed Western experiments for the XBP1 protein because unable to obtain a good antibody.
Figure 6. ER stress proteins are altered after PA treatment.
Cells lysates of iSC from euglycemic and hyperglycemic condition at 0 (CTL),6, 12, 24 and 48 hr after adding PA:BSA 2:1. were prepared and subjected to Western blot analysis using specific antibodies against CHOP (A,B top) and GRP78(C,D top). The proteins were quantified by densitometric scanning (A–D, bottom). Statistical analysis was performed using student’s t-test. *p<0.05, **p<0.005, N=4
ER stress genes were also examined in the presence of BAPTA-AM. In both euglycemic and hyperglycemic conditions, BAPTA-AM stabilized GRP78 mRNA and maintained its level similar to control through 48 hr after PA treatment (Figure 5C and 5D). For XBP1 mRNA levels, a 50% up-regulation of was observed in euglycemic cells at 12, 24, 48 hr and a 20% increase showed at 12 hr in hyperglycemic cells during co-treatment situation (Figure 5C and 5D). PA in the presence of BAPTA-AM still produced a significant up-regulation of CHOP mRNA in iSC cells cultured in euglycemic environment, however it was not comparable to the levels found with PA treated alone (Figure 5A and 5C). In hyperglycemic conditions, a significant up-regulation of CHOP was observed at 24 hr followed by a significant reduction at 48hr (Figure 5D).
2.4 Early mediators of PA-LTx in iSC cultures: Potential role of reactive oxygen species
We assessed the generation of ROS using 2’, 7’ dichlorodihydrofluorecein diacetate (H2DCFDA) and flow cytometry. Flow cytometry analysis was performed at 0, 3, 6, 12, 24, and 48 hr following exposure to PA. In agreement with our earlier experiments (see figure 1), hyperglycemic conditions in our cell model did not elicit an increase in ROS levels (Figure7A). However, the addition of PA:BSA 2:1 induced a significant increase of ROS as early as 3hr, followed by further increases at 6, 12, 24, and 48 hr under 17 mM glucose condition (Figure 7B). With a lower dose of PA (PA:BSA 1:1), the ROS levels were shown to significantly increase at 12, 24 and 48 hr only (Figure 7B). In hyperglycemic system, this increase in ROS was observed as early as 3 hr after PA exposure and continued to elevate up to 48 hr (Figure 7C). The magnitude of the ROS increase was also concentration-and time-dependent (Figure 7 C). Next, we evaluated the physiological relevance of this ROS elevation by treating the culture with MCI-186, a well characterized free radical scavenger (Takahashi et al., 2004). Our data show that control euglycemic cell cultures treated with 0.1 mM of MCI-186 exhibited a 38% reduction in the ROS levels with approximately 93% of the cells viable at 24 hr (Figure 8A and 8C). Subsequently, co-treatment of PA and MCI-186 in euglycemic cell cultures resulted in a 46% reduction in ROS as compared to PA treatment alone (Figure 8A). Similarly, the addition of 1 mM MCI-186 to cultures grown under hyperglycemic conditions reduced ROS levels by 16% in control and by 47% in PA-treated cells at 24 hr (Figure 8B). MCI-186 was found to be toxic in both euglycemic and hyperglycemic cultures at 48 hr (data not shown). To examine the effects of this reduction in ROS on viability of PA-treated iSC cells, crystal violet viability assays were performed. Figure 8C shows that iSC cell viability was increased by 20% in PA-LTx cells co-treated with 0.1mM of MCI-186 under euglycemic conditions (Figure 8c) and completely restored to normal levels when 1 mM of MC-186 was added to cells grown in hyperglycemic conditions (Figure 8D).
Figure 7. ROS analysis using H2DCFDA flow cytometry.
iSC cells were cultured in hypoglycemic (5 mM glucose), euglycemic (17 mM glucose), and hyperglycemic (50 mM glucose) media for at least 4 days before analyzing ROS levels by H2DCFDA assay. The increase in fluorescence is indicative of an increase in ROS within the cell. Cells treated with 300 µM of H2O2 for 15 min were used as a positive control. (A) Representative flow cytometry graphs of iSC cells cultured in different concentrations of glucose. No increase in ROS was observed. ROS analysis was performed on iSC cells treated with PA:BSA 1:1 and 2:1 in (B) 17 mM glucose and (C) 50 mM glucose conditions. Flow cytometry data was quantified using Cell Quest Pro® and Flow-Jo® software. Statistical analysis was performed using student’s t-test..*p< 0.05, **p< 0.005, N=3
Figure 8. MCI-186 and BAPTA-AM reduce ROS levels and increases cell viability in PA-LTx iSC cells.
The iSC cells were cultured in 17 mM glucose (A, C, E) or 50 mM glucose (B,D, F) concentrations and treated with PA:BSA 2:1 (PA) in the presence or absence of 100 µM or 1 mM MCI-186 or 5µM BAPTA-AM. ROS analysis using H2DCFDA flow cytometry (A, B) and Crystal violet assay for cell viability (C, D) were performed at 24 hr. Cells cultured in euglycemic (E) and hyperglycemic (F) conditions were co-treated with BAPTA-AM for 24 and 48H and analyzed for ROS levels. Statistical analysis was performed using student’s t-test.*p< 0.05, **p< 0.005, N=3
Given that BAPTA-AM increased cell viability at 24 and 48hr after PA treatment, it was of interest to also evaluate levels of ROS. Our data demonstrates that co-treatment of 5µM BAPTA-AM with 300µM PA was able to significantly decrease ROS levels at 24 and 48hr (Figure 8E and 8F).
The significant generation of ROS observed after exposure to PA suggests a potential increase in mitochondrial permeability and dysfunction. We performed a series of flow cytometry experiments using JC-1 to evaluate mitochondrial function. Cell cultures were treated with PA:BSA 2:1 and analyzed by JC-1 flow cytometry at 30 min, 1, 3, 6 and 12 hr after the initial exposure to PA. Figure 9A and 9C show that cells grown in euglycemic conditions exhibit significant mitochondria membrane depolarization at 6 hr and reached a peak at 12 hr after the initial exposure to PA (Figures 9A and 9C). Interestingly, cells grown under hyperglycemic conditions show this dysfunction as early as one hour after exposure to PA:BSA 2:1 treatment (Figure 9B and 9D). Hyperglycemia alone did not result in significant mitochondrial depolarization in iSC cells (data not shown).
Figure 9. PA-LTx induces mitochondrial depolarization in iSC cells.
Mitochondrial membrane depolarization was examined by flow cytometric analysis using JC-1 fluorescent dye. Representative flow cytometric plots of control (CTL) and 12 hr PA:BSA 2:1 treatment (12H PA) under (A) 17 mM glucose condition and (B) 50 mM glucose condition are shown. R1 quadrant indicates cells with intact mitochondria while R2 quadrant represents cells with depolarized mitochondria, i.e. apoptotic cells. Quantitative analysis of JC-1 data using Flow JO® software is shown in (C) 17 mM glucose condition and (D) 50 mM glucose condition. Statistical analysis was performed using student’s t-test.. *p<0.05, **p<0.005 N=4
3. Discussion
The present study shows for the first time that exposure to high levels of PA results in a strong lipotoxic process that result in apoptotic cell death and is mediated though ER stress, ROS generation and mitochondria depolarization. This lipotoxic insult increases when cells are cultured under high glucose conditions. Interestingly, the calcium chelator BAPTA-AM and free radical scavenger MC1-186 are powerful inhibitors of PA-LTx and cell death. Our findings also show that ER stress precedes mitochondrial dysfunction and ROS generation after exposure to PA.
Previous studies have examined the role of high glucose in inducing Schwann cell (SC) dysfunction and elucidated its potential role in the development of peripheral neuropathy (PN), a common type 2 diabetes morbidity (Eckersley, 2002) (Vincent et al., 2005) ; (Yu et al., 2008). However, because of controversies as to whether hyperglycemia is the primary upstream factor responsible of SC dysfunction in PN, another contributor, dyslipidemia, is attracting further attention (Cameron and Cotter, 2008 ; Obrosova et al., 2007 ; Vincent et al., 2009 ; Wiggin et al., 2009). Type 2 diabetes is characterized by a serious dysregulation in lipid metabolism resulting in chronic elevated levels of FFA in the plasma (Ioannidis, 2008 ; McGarry, 2002 ; Paolisso et al., 1995 ; Reaven et al., 1988 ; Unger, 2008). Thus, it is important to assess the pathological implications of hyperglycemia in combination with high levels of FFA in SC viability. This report shows that chronic hyperglycemia alone does not induce iSC cell death but its presence strongly magnified the effects of lipotoxic injury. This is consistent with observations that cultures treated with high glucose alone retard neurite outgrowth, slows down proliferation and delays migration without inducing cell death in isolated neonatal SC cultures (Gumy et al., 2008). PA-LTx has also been shown to be enhanced by high glucose in pancreatic β-cell cell death (Okuyama et al., 2003). The failure of hyperglycemia to induce death of iSC may be due to the activation of cellular pro-survival pathways known to be present when cells are exposed to low stress insults. This induced protective status includes the generation of anti-apoptotic pro-survival proteins that counteract pro-apoptotic pathways stimulated by the low threshold stimuli. The exposure to an additional insult such as lipid overload may result in an override of the cell defensive response and result in cell death and apoptosis (Cheng and Zochodne, 2003 ; Kamiya et al., 2005 ; Sango et al., 2002).
Long chain saturated free fatty acids (LCSFFA) like stearic acid and PA are strong inducers of lipotoxicity and cell death in neurons and other cells (Almaguel et al., 2009 ; Almaguel et al., 2010 ; Ulloth et al., 2003). Cell membranes containing phospholipids enriched with LCSFFA exhibit lower fluidity (Borradaile et al., 2006). These LCSFFA may accumulate in membrane lipid rafts, alter the lipid environment and affect important functions of key receptors and other membrane proteins (Hon et al., 2009 ; Horrobin, 1998 ; Innis and Clandinin, 1981 ; Stubbs and Smith, 1990). These LCSFFA also undergo esterification with less efficiency as a result of possible low efficiency of the enzymes involved (Cnop, 2008 ; Gelb et al., 1964 ; Listenberger et al., 2003 ; Maedler et al., 2003). Furthermore, these LCFFA accumulate in the cell and form diaglycerides and ceramide, which may contribute to the cellular dysfunction observed (Hardy et al., 2003 ; Prentki and Madiraju, 2008 ; Ricchi et al., 2009). Ceramide is a byproduct produced by saturated fatty acids and is a key mediator of cytotoxicity and apoptosis (Brugg et al., 1996 ; German et al., 2006 ; Wiesner and Dawson, 1996). This may be one of the apoptotic pathways that are involved in the PA-induced cell death demonstrated in numerous studies using non-nerve cells and in the present study (Belosludtsev et al., 2006 ; Chakrabandhu et al., 2007 ; Okuyama et al., 2003).
Our data show that PA-LTx in the presence of high glucose results in an early and more robust release of Ca++ into the cytosol from the ER. The data points to the ER as the earliest organelles to be affected by the lipid overload. Studies have shown that PA-LTx may affect cellular organelles like the ER and the mitochondria (Almaguel et al., 2009 ; Borradaile and Schaffer, 2005 ; Li et al., 2008). In the mitochondria, Ca++ is important for the generation of ATP and Ca++ sensing chaperones and binding proteins are equally important in regulating free cytosolic Ca++ concentrations (Csordas et al., 2006 ; Paschen, 2003 ; Pinton et al., 2008). The data is consistent with a model in which PA initially injures the ER membranes, resulting in the release of Ca++ into the cytosol and overloading the mitochondrial matrix. Although not measured in detail in the present study, these series of events would lead to the translocation of AIF and EndoG to the nucleus, thus inducing chromatin condensation and DNA fragmentation (van Gurp et al., 2003). Moreover, injury to the mitochondria induces the release of cytrochrome c into the cytosol, which may contribute to the formation of the apoptosome (cyto-c +Apaf-1 + caspase 9) (Kroemer et al., 1997 ; Kruman and Mattson, 1999 ; McConkey and Orrenius, 1997).
Previous work from our laboratory demonstrated that inhibition of caspase activation did not block the lipotoxic process but the present study found that reducing excessive cytosolic Ca++ levels using BAPTA-AM inhibited the cell death process. Furthermore, our data demonstrates that BAPTA-AM is able to reduce ROS level in both euglycemic and hyperglycemic iSC at 24 and 48hr. Previous studies in the literature have shown that buffering calcium with agents such as BAPTA-AM prevented the induction of apoptosis in different cellular models by preventing mitochondrial membrane depolarization (Deniaud et al., 2008 ; Kruman and Mattson, 1999 ; Kruman et al., 1998 ; Paschen et al., 2003). Deniaud et al (2008) et al examined the inner mitochondrial membrane permeabilization of HeLa cells and found that after 24 hr treatment with ER-stress inducers, such as thapsigargin and tunicamycin, inner mitochondrial membrane permeabilization was reduced by BAPTA-AM. (Deniaud et al., 2008). This effect of BAPTA-AM was highly significant even though this drug has a modest effect on ROS levels in control cells in control group (Ahluwalia et al., 2001 ; Takadera et al., 2010).
Besides BAPTA-AM, another factor that can increase cell viability is the inhibition of NMDA receptors (Novelli et al., 1988 ; Peng et al., 2009 ; Yu et al., 2002). A study by Yu et al., (2002) found that activation of NMDA receptors are needed in order to induce the translocation of AIF from the mitochondria to the nucleus, thus leading to apoptosis. In our hands, inhibiting NMDA receptors with D-AP5 increased cell viability at 24hr in both euglycemic and hyperglycemic conditions. We speculate that by inhibiting the NMDA receptors, translocation of AIF to the nucleus may have been prevented and future experiments will address this question. The inability of D-AP5 to increase cell viability at 48hr may be explained by the overall severity of cellular injury that drives the cell towards cellular death regardless of the inhibition of these receptors.
We found that the depletion of [Ca++]ER by PA not only affects normal mitochondrial function but also alters the expression of ER stress genes and proteins such as CHOP, Xbp1, and GRP78. In euglycemic conditions, PA-LTx triggers the up-regulation of Xbp1 and CHOP mRNA levels, simultaneously, before significant cell death was observed. It was also observed that protein levels of CHOP were increased at 12hr. The pattern of gene regulation observed in cells undergoing PA-LTx suggests that SC increases the expression of genes that encode for the ER stress response, such as chaperones, and transcription factors. These in turn function to decrease the load on the ER, therefore promoting cell survival (Malhotra and Kaufman, 2007 ; Paschen et al., 2001 ; Sundar Rajan et al., 2007 ; Xu et al., 2005). In conjunction, these cells also promote cell death through up-regulation of CHOP, which down-regulates Bcl-2 expression, depletes cellular levels of glutathione, translocates BAX from the cytosol to the mitochondria, and induces TRB3, an AKT inhibitor that plays a role in ER stress-induced cell death (Eizirik et al., 2008 ; Malhotra and Kaufman, 2007 ; Schapansky et al., 2007). In addition, the unfolding protein response can activate IRE-1, a transmembrane ER stress sensor, that will engage TNF receptor-associated factor 2 (TRAF2) which can mobilize apoptosis signal-regulating kinase (ASK1) resulting in the activation of c-Jun amino terminal kinase (JNK), a powerful pro-apoptotic kinase that interacts with Bcl-2 family proteins (Lee et al., 2003 ; Szegezdi et al., 2009 ; Urano et al., 2000 ; Yoneda et al., 2008). Hence, there is a period of time where the cell promotes cell survival and cell death simultaneously (Chauhan and Anderson, 2003 ; Lee et al., 2007). Ultimately, as time progresses, one pathway will dominate and govern the fate of the cell. In this manner, PA treated cells will begin to express significantly higher mRNA levels of CHOP, in which Xbp-1 can no longer exert its influence, resulting in cellular death.
An interesting finding was the up-regulation of GRP78 mRNA and protein expression in the hyperglycemic state at 24 and 48 hr after PA treatment since up-regulation of GRP78 is frequently correlated with cell survival (Jamora et al., 1996 ; Zhang et al., 2006). Elevation of GRP78 expression has also been observed at times when cells are undergoing apoptosis (Li et al., 2008 ; Ohse et al., 2006 ; Yoneda et al., 2008). In the spontaneous hypertensive rat model, cardiomyocytes that suffered from prolonged hypertension (32 weeks) demonstrated an increase in apoptotic cells and expression of significant levels of GRP78, caspase 3 and caspase 12 (Sun et al., 2008).
Treatment of iSC cells with BAPTA-AM resulted in the stabilization of mRNA levels of pro-survival genes such as GRP78, upregulation of XBP1 in a euglycemic and hyperglycemic conditions. The decrease in pro-aoptotic ER stress genes such as CHOP was observed in both euglycemic and hyperglycemic conditions. Thus, reducing the stress on the ER by BAPTA-AM, may assist the cell in its attempt to survive by promoting anti-apoptotic pathways.
The mechanisms by which hyperglycemia potentiates PA injury has not been studied in SC. Cell culture studies using beta cells have found that PA increases nitric oxide (NO) while high glucose increases levels of superoxide (O2−). However, when the cells are exposed to both insults the production of both radicals can lead to the production of peroxynitrite (OOON−), a powerful free radical known to cause DNA damage and apoptosis (Okuyama et al., 2003). Hyperglycemia can also activate PARP which can deplete NAD levels and lead to dysfunction of glycolitic and mitochondrial respiration pathways in diabetic neuropathy (Obrosova et al., 2004 ; Pacher et al., 2005 ; Sharma et al., 2008 ; Virag et al., 2002).
Our findings indicate that the ER response is the earliest event that follows PA-LTx. The ER response resuls in Ca++ to be released into the cytosol, affecting the mitochondria and leading to mitochondrial membrane depolarization. Consequently, mitochondrial dysfunction will result in an increase in levels of ROS and cellular metabolic derangements. Moreover, pro-apoptotic and anti-apoptotic pathways will be activated simultaneously. As the injury continues, a full ASCOS condition will be in place affecting proteins, lipids, and DNA. Further, when high glucose levels are incorporated, other factors such as a decrease in anti-oxidant molecules, disruption of metabolic activities, including glycolysis and respiratory pathways, and possibly the activation of inflammatory mediators can dramatically augment the injury caused by PA. Increasing number of studies are linking ER stress to a decrease in myelin production in the nervous system (Lin et al., 2006 ; Shin et al., 2010). The data is consistent with these studies and agrees with these published results and further show that the combination of hyperglycemia and palmitic acid overload can cause SC dysfunction and SC death. We propose that similar mechanism my be happening in vivo that may result in SC dysfunction, cell death, focal demyelination resulting in abnormal nerve conduction and synaptic transmission.
4. Experimental Procedure
4.1 Cell Culture
The Schwann cells used in this study were a generous gift from Dr. Laurel Bolin (Bolin et al., 1992). This “spontaneous immortalized SC clone” (iSC) was shown to express markers specific to primary SC such as S100β, p75 NGFR and Vimentin (Bolin et al., 1992 ; Cochard and Paulin, 1984 ; De Leon et al., 1991 ; Heumann et al., 1987). Cells were maintained in culture medium prepared from Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM/F12) 50/50 mix without glutamine (Mediatech, Herndon, VA). This medium contains 17 mM glucose (i.e. the euglycemic condition for this study). Ten percent horse serum (Invitrogen, Carlsbad, CA), 2 mM L-glutamine (Mediatech), 100 units/mL penicillin and 100 µg/mL streptomycin (Mediatech) were supplemented to the media. iSC cells were incubated at 37° C with 5% CO2. Culture media was changed every three days. Cells were used at a maximum of 7 passages. To achieve desired hyperglycemic and hypoglycemic state of the culture media, we also prepared DMEM beginning with powder DMEM (Mediatech) without glucose, L-glutamine, phenol red, pyruvate or sodium bicarbonate. These were added at the appropriate concentrations after DMEM was dissolved in sterile deionized water. To make hyperglycemic media, 45 mM dextrose (equals to 90 mM glucose) was supplemented and no additional glucose was added to make hypoglycemic media. Next, DMEM was filtered and mixed with equal volume of Ham’s F12 (contains 10 mM glucose) to make final DMEM/F12 (50/50) media with 50 mM glucose (hyperglycemic) or 5 mM glucose (hypoglycemic) respectively. Further, 10% horse serum, 2 mM L-glutamine, 100 units/mL penicillin and 100 µg/mL streptomycin were also added.
4.2 Fatty Acid Treatment
Treatments were done in serum free media. Serum free media consisted of DMEM/F12 (50/50), 2 mM L-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin, and 1X N-2 supplement (Gibco, Invitrogen Corp.). The iSC cells were plated in full serum media, placed in the incubator and allowed to attach for 5 hr. The media was removed, washed with Dulbecco’s Phosphate-Buffered Saline (DPBS) and transferred to serum free media. After 12 hr in serum free media, cells were treated with the selected concentrations of PA complexed with BSA as described previously (Almaguel et al., 2009 ; Ulloth et al., 2003). The PA (Sigma-Aldrich, St. Louis, MO) stock was prepared in 100% ethanol at a concentration of 300 mM. Fatty acid-free BSA (EMD Biosciences, San Diego, CA) was used as a buffer to ensure that concentrations of unbound free PA in the media were in the 10 nM range during the course of incubation (Ulloth et al., 2003). In brief, serum free medium containing 150 µM fatty acid-free BSA was prepared and warmed up to 37°C to aid in the complete disassociation of PA. Once media was warm, PA stock was added while vortexing to avoid clumping of PA. Two treatment concentrations were used: 150 µM PA (PA:BSA, 1:1) and 300 µM PA (PA:BSA, 2:1). Treatment media were place in the water bath at 37°C for 30 min before they were added to the cells.
4.3 Cell Viability Assays
Trypan Blue Exclusion Assay
iSC cells were plated in T25 flask at a density of 2.5 × 105 cells/flask. After PA/BSA treatment, cells were trypsinized and centrifuged at 700 × g for 5 min and resuspended in 500 µl of DPBS with 10% glycerol. Trypan Blue 0.4% solution (500 µl, Sigma-Aldrich) was added immediately before counting cells using an inverted microscope. At least 1000 cells were counted for each flask. Percent of blue cells (damaged cells) and clear cells (viable cells) in a given field were recorded.
Crystal Violet Assay
Briefly, iSC cells were plated in 96 well- tissue culture plates at the density of 1.0 × 104 cells/well. Subsequent to 24 or 48 hr treatment of PA, 100 µl/well of 4% formaldehyde was added. Following a 5 min incubation period at room temperature, the fixative was removed and cells were washed twice with distilled water. Afterward, cells were post-fixed with 4% formaldehyde for another 30 min. iSC cells were then washed twice with distilled water and allowed to dry completely. Once dried, 100µl/well of crystal violet dye (Accustain®, Sigma-Aldrich) was added and incubated for 30 min at room temperature. Cells were washed with distilled water to remove any unbound stain and dried. The bound crystal violet was then dissolved with 100µl/well of 10% acetic acid solution and place in rocker for 10 min at room temperature. The plate was analyzed by μQuant (Bio-Tek Instruments, Winooski, VT) using an optical desity (O.D.) of 570 nm. Data was examined using KCjunior™ software (Bio-Tek Instruments).
WST-1 Assay
Similar to crystal violet assay, iSC cells were plated in 96 well plates. After treatment, WST-1 (10 µl, Roche Applied Science, Indianapolis, IN) in 100 µl of serum free media was added to each well. The plate was incubated at 37°C with 5% CO2 for 2 hr and O.D. of 450 nm was determined using the μQuant plate reader. Data was examined using KCjunior™ software.
4.4 Nuclear Morphology
Hoechst 33258 dye was used to assess chromatin condensation in iSC cells. The dye (10 µg/ml) was added to the cells and incubated for 10 min at 37°C with 5% CO2. Nuclear morphology of the cells was then photographed using an Olympus fluorescent microscope (excitation/emission wavelength of 365/420 nm). Cells categorized as apoptotic demonstrated apoptotic bodies and increased chromatin condensation.
4.5 Assessment of Apoptosis by Flow Cytometry
Annexin V (BD Biosciences, San Diego, CA) and 7AAD (eBioscience, San Diego, CA) were used to detect apoptotic changes occurring in PA-LTx in iSC cells. iSC cells (1.25 × 104) were plated in a 6 well plate. After treatment, cells were trypsinized and collected. They were then resuspended in 40 µl of binding buffer with 2 µl Annexin V FITC. Cells were incubated for 15 min in the dark at room temperature. After incubation, 160 µl of binding buffer and 2 µl of 7AAD were added. The cells were incubated for 5 min and additional 200 µl of binding buffer was added. Before analyzing, cells were filtered through a cell strainer cap that was fitted to a polystyrene round bottom flow cytometric tube. Cells were analyzed using the Becton-Dikinson FACSCalibur® flow cytometer (Becton-Dikinson, San Fransisco, CA). A total of 100,000 events were measured per sample. Annexin V was detected in the FL-1 channel (530/30nm) while 7AAD was detected in FL-3 channel (650nm). Data was collected in log scale and analyzed using Cell Quest Pro® software and Flow-Jo® software.
4.6 Analysis of Mitochondrial Membrane Permeabilization
Disruption of the mitochondrial membrane potential was assessed using the lipophilic cationic probe 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylben-zimidazol-carbocyanine iodide (JC-1, MitoScreen kit, BD Biosciences) as described previously (Almaguel et al., 2009). Briefly, unfixed cells were washed with PBS and resuspended in 1X JC-1 assay buffer supplemented with 10 µg/mL of JC-1. Cells were then incubated for 15 min at 37°C, washed, and resuspended in 1X JC-1 assay buffer for immediate FACSCalibur® flow cytometry analysis. JC-1 in healthy cells was aggregated in mitochondria and detected in the FL-2 channel while JC-1 in apoptotic cells with depolarized mitochondria membrane potential was indicated by a reduced fluorescence in the FL-2 channel. The percentage of cells with disrupted mitochondrial membrane potential was calculated using the Flow-Jo® software.
4.7 Measurements of [Ca++]ER
To measure the [Ca++] in ER after addition of PA in iSC cells, the Fluo-4 NW Calcium Assay kit (Invitrogen) was used. Briefly, 1.0 ×104 cells were plated in 96 well poly-D-lysine coated plates. Cells were treated accordingly. Afterward, 100 µl of the dye, which included Probenecid (2.5 mM) was added to each well. The plates were incubated at 37°C for 30 min, then at room temperature for another 30 min. In the last 2 min of room temperature incubation, Thapsigargin (1µM) or DMSO (vehicle) was added to the cells in order to deplete Ca++ from ER. Cells were analyzed using the Envison HTS Microplate reader (Perkin-Elmer ® Life and Analytical sciences, Shelton, CT). Excitation/emission parameters were 480 nm and 510 nm respectively.
4.8 Ca++ chelating by BAPTA-AM
BAPTA-AM (1,2-bis-(o-Aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid tetraacetoxymethyl ester, Invitrogen) was used as a Ca++ chelator to reduce intracellular Ca++ levels in iSC cells while undergoing PA treatment. BAPTA-AM was dissolved in DMSO and co-treatment of 5 µM BAPTA-AM and 300 µM PA was used. Cell viability was assessed by crystal violet after 24 and 48 hr treatment.
4.9 Inhibition of NMDAR by AP5
D-AP5, D-(−)-2-amino-5-phosphonopentanoic acid, (Tocris, Bristol, UK) was used to inhibit NMDA receptors in iSC cells. D-AP5 was dissolved in ddH20 and a co-treatment of 50µM AP5 and 300µM PA was utilize. Cell viability was analyzed using crystal violet assays after 24 and 48hr.
4.10 Quantitative RT-PCR
Total RNA was extracted using TRI-Reagent (Molecular Research Center, Cincinnati, OH). RNA quantification was done by measuring O.D. at 260 nm and stored at −80°C. Two-step RT-PCR was performed. First, RNA (0.5~ 1µg) was used to make cDNA using the iScrip cDNA synthesis kit (Bio-Rad, Hercules, CA). Next, real-time PCR with Syber Green was conducted using CFX96 Real-Time System (Bio-Rad). For that, CHOP reverse primer 5’-TCC-TCA-TAC-CAG-GCT-TCC-AG-3’ and forward primer 5’-CAG-CGA-CAG-AGC-CAA-AAT-AA-3’, GRP78 reverse primer 5’-ATA-GGG-CTC-TGC-TGG-AGT-CA-3’ and forward primer 5’-CTA-CCC-ACC-TTT-TGC-CAC-TC-3’, Xbp1 reverse primer 5’-TTT-CTA-TCT-CGC-GCA-GTC-TGT-3’ and forward primer 5’-CCC-CCA-AAG-TGC-TAC-TCC-TA-3’ were used. β-actin was selected as a housekeeping gene (reverse primer 5’-GCG GCA GTG GCC ATC TC-3’ and forward primer 5’-GGG AAA TCG TGC GTG ACA TT-3’). The relative amount of mRNA was calculated using the 2 -ΔΔCT formula.
4.11 Reactive Oxygen Species (ROS) Detection
Detection of oxidative stress was done by staining the iSC cells with 20 µM of 2’, 7’ dichlorodihydrofluorecein deacetate (H2DCFDA, Invitrogen) for 20 min at 37° C. Cells were then detached by trypsinization and washed twice with DPBS. After filtered through cell strainer cap, cells were analyzed using a FACSCalibur® flow cytometer. A total of 10,000 events were measured per sample. Excitation/Emission wavelengths were 488 nm and 530/30 nm respectively. Data was collected in log scale and analyzed using Cell Quest Pro® software and Flow-Jo® software. Antioxidant MCI-186 (Biomol Research Laboratories, Plymouth Meeting, PA) was used to reduce ROS during PA-LTx. MCI-186 was first dissolved in DMSO and warmed up to 37° C to ensure the complete disassociation. MCI-186 stock solution (or DMSO as a control) was then diluted into the PA/BSA treatment media to attain final concentration of 100 µM or 1 mM.
4.12 Western Blots
Western procedures have been described elsewhere (Ulloth et al., 2003; Liu et a., 2008). Protein extract from iSC were separate on a SDS-PAGE gel and electrophoretically transferred to a nitrocellulose membrane. After transfer, the membranes were blocking with 5% milk in Tris-buffered saline (TTBS) with 0.05% Tween 20, PH 7.4 at room temperature for 1 hr. The membranes were then incubated with specific antibodies against different protein, GRP78 and CHOP/Ddit3 (Abcam) in TTBPS containing 0.05% Tween 20 at 4°C overnight. Subsequently the membranes were washed three times with TTBS and incubated with HRP-anti mouse or HRP-anti rabbit (GE Healthcare Bioscience) for 1 hr at room temperature, followed by three washes with TTBS. The signal was then detected by ECL-plus (GE Healthcare Bioscience). Quantitative analysis of the protein was performed by densitometric scanning of the autoradiographs by employing the using ChemiImager™ 4000 (Alpha Innotech Corporation, San Leandro, CA, USA).
4.13 Statistical Analysis
All the experiments were repeated independently at least three times. Values represent means ± SE. Statistical comparisons were made using Student t test. Significance was accepted at p < 0.05.
Research Highlights.
Lipotoxicity (LTx) triggers a strong apoptotic cell death in Schwann cells
The LTx process includes an early upstream endoplasmic reticulum (ER) stress response
Downstream events include ROS generation and mitochondrial dysfunction
Hyperglycemia exacerbates the cellular effects of LTx and increases apoptotosis
Ca++ chelator BAPTA-AM and antioxidant MCI-186 inhibit LTx in Schwann cells
Acknowledgments
This work has been supported by NIH awards 5P20MD001632 and 5R25GM060507. We would like to thank Drs. Jo-Wen Liu for her valuable input in preparing the final version of the manuscript and Liming Bu for his technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahluwalia JP, Topp JD, Weirather K, Zimmerman M, Stamnes M. A role for calcium in stabilizing transport vesicle coats. J Biol Chem. 2001;276:34148–34155. doi: 10.1074/jbc.M105398200. [DOI] [PubMed] [Google Scholar]
- Almaguel FG, Liu JW, Pacheco FJ, Casiano CA, De Leon M. Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. J Neurosci Res. 2009;87:1207–1218. doi: 10.1002/jnr.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaguel FG, Liu JW, Pacheco FJ, De Leon D, Casiano CA, De Leon M. Lipotoxicity-mediated cell dysfunction and death involve lysosomal membrane permeabilization and cathepsin L activity. Brain Res. 2010;1318:133–143. doi: 10.1016/j.brainres.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SC, Shaw PJ. Oxidative stress in ALS: Key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Bazan NG., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970;218:1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- Belosludtsev K, Saris NE, Andersson LC, Belosludtseva N, Agafonov A, Sharma A, Moshkov DA, Mironova GD. On the mechanism of palmitic acid-induced apoptosis: the role of a pore induced by palmitic acid and Ca2+ in mitochondria. J Bioenerg Biomembr. 2006;38:113–120. doi: 10.1007/s10863-006-9010-9. [DOI] [PubMed] [Google Scholar]
- Bezuglov VV, Bobrov M, Archakov AV. Bioactive amides of fatty acids. Biochemistry (Mosc) 1998;63:22–30. [PubMed] [Google Scholar]
- Bolin LM, Iismaa TP, Shooter EM. Isolation of activated adult Schwann cells and a spontaneously immortal Schwann cell clone. J Neurosci Res. 1992;33:231–238. doi: 10.1002/jnr.490330206. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Schaffer JE. Lipotoxicity in the heart. Curr Hypertens Rep. 2005;7:412–417. doi: 10.1007/s11906-005-0035-y. [DOI] [PubMed] [Google Scholar]
- Brugg B, Michel PP, Agid Y, Ruberg M. Ceramide induces apoptosis in cultured mesencephalic neurons. J Neurochem. 1996;66:733–739. doi: 10.1046/j.1471-4159.1996.66020733.x. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets. 2008;9:60–67. doi: 10.2174/138945008783431718. [DOI] [PubMed] [Google Scholar]
- Chakrabandhu K, Herincs Z, Huault S, Dost B, Peng L, Conchonaud F, Marguet D, He HT, Hueber AO. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 2007;26:209–220. doi: 10.1038/sj.emboj.7601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Anderson KC. Mechanisms of cell death and survival in multiple myeloma (MM): Therapeutic implications. Apoptosis. 2003;8:337–343. doi: 10.1023/a:1024164700094. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes. 2003;52:2363–2371. doi: 10.2337/diabetes.52.9.2363. [DOI] [PubMed] [Google Scholar]
- Cistola DP, Sacchettini JC, Gordon JI. 13C NMR studies of fatty acid-protein interactions: comparison of homologous fatty acid-binding proteins produced in the intestinal epithelium. Mol Cell Biochem. 1990;98:101–110. doi: 10.1007/BF00231373. [DOI] [PubMed] [Google Scholar]
- Cnop M. Fatty acids and glucolipotoxicity in the pathogenesis of Type 2 diabetes. Biochem Soc Trans. 2008;36:348–352. doi: 10.1042/BST0360348. [DOI] [PubMed] [Google Scholar]
- Cochard P, Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J Neurosci. 1984;4:2080–2094. doi: 10.1523/JNEUROSCI.04-08-02080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Raghupathi R, Trojanowski JQ, McIntosh TK. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed posttraumatic period. J Neurosci. 1998;18:5663–5672. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon M, Van Eldik LJ, Shooter EM. Differential regulation of S100 beta and mRNAs coding for S100-like proteins (42A and 42C) during development and after lesion of rat sciatic nerve. J Neurosci Res. 1991;29:155–162. doi: 10.1002/jnr.490290204. [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Dey A, Kessova IG, Cederbaum AI. Decreased protein and mRNA expression of ER stress proteins GRP78 and GRP94 in HepG2 cells over-expressing CYP2E1. Arch Biochem Biophys. 2006;447:155–166. doi: 10.1016/j.abb.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, Behrens MM. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol. 1996;55:1181–1193. doi: 10.1097/00005072-199612000-00001. [DOI] [PubMed] [Google Scholar]
- Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int Rev Neurobiol. 2002;50:293–321. doi: 10.1016/s0074-7742(02)50081-7. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, Joly E, Dbaibo G, Rosenberg L, Prentki M. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- Gelb AM, Davidson MI, Kessler JI. Effect of Fasting on Esterification of Fatty Acids by the Small Intestine in Vitro. Am J Physiol. 1964;207:1207–1210. doi: 10.1152/ajplegacy.1964.207.6.1207. [DOI] [PubMed] [Google Scholar]
- German OL, Miranda GE, Abrahan CE, Rotstein NP. Ceramide is a mediator of apoptosis in retina photoreceptors. Invest Ophthalmol Vis Sci. 2006;47:1658–1668. doi: 10.1167/iovs.05-1310. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Rodrigues B. Cardiac cell death in early diabetes and its modulation by dietary fatty acids. Biochim Biophys Acta. 2006;1761:1148–1162. doi: 10.1016/j.bbalip.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Gumy LF, Bampton ET, Tolkovsky AM. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci. 2008;37:298–311. doi: 10.1016/j.mcn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Hardy S, El-Assaad W, Przybytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278:31861–31870. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab. 2005;25:41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol Aging. 2010;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu N, Kasai A, Hayakawa K, Yao J, Kitamura M. Real-time detection and continuous monitoring of ER stress in vitro and in vivo by ES-TRAP: evidence for systemic, transient ER stress during endotoxemia. Nucleic Acids Res. 2006;34:e93. doi: 10.1093/nar/gkl515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon GM, Hassan MS, van Rensburg SJ, Abel S, van Jaarsveld P, Erasmus RT, Matsha T. Red blood cell membrane fluidity in the etiology of multiple sclerosis. J Membr Biol. 2009;232:25–34. doi: 10.1007/s00232-009-9213-1. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30:193–208. doi: 10.1016/s0920-9964(97)00151-5. [DOI] [PubMed] [Google Scholar]
- Ilieva EV, Ayala V, Jove M, Dalfo E, Cacabelos D, Povedano M, Bellmunt MJ, Ferrer I, Pamplona R, Portero-Otin M. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain. 2007;130:3111–3123. doi: 10.1093/brain/awm190. [DOI] [PubMed] [Google Scholar]
- Innis SM, Clandinin MT. Dynamic modulation of mitochondrial membrane physical properties and ATPase activity by diet lipid. Biochem J. 1981;198:167–175. doi: 10.1042/bj1980167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis I. The road from obesity to type 2 diabetes. Angiology. 2008;59:39S–43S. doi: 10.1177/0003319708318583. [DOI] [PubMed] [Google Scholar]
- Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Zhangm W, Sima AA. Apoptotic stress is counterbalanced by survival elements preventing programmed cell death of dorsal root ganglions in subacute type 1 diabetic BB/Wor rats. Diabetes. 2005;54:3288–3295. doi: 10.2337/diabetes.54.11.3288. [DOI] [PubMed] [Google Scholar]
- Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- Kato A, Minoshima Y, Yamamoto J, Adachi I, Watson AA, Nash RJ. Protective effects of dietary chamomile tea on diabetic complications. J Agric Food Chem. 2008;56:8206–8211. doi: 10.1021/jf8014365. [DOI] [PubMed] [Google Scholar]
- Koshkin V, Dai FF, Robson-Doucette CA, Chan CB, Wheeler MB. Limited mitochondrial permeabilization is an early manifestation of palmitate-induced lipotoxicity in pancreatic beta-cells. J Biol Chem. 2008;283:7936–7948. doi: 10.1074/jbc.M705652200. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- Kruman II, Mattson MP. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Cherla RP, Tesh VL. Simultaneous induction of apoptotic and survival signaling pathways in macrophage-like THP-1 cells by Shiga toxin 1. Infect Immun. 2007;75:1291–1302. doi: 10.1128/IAI.01700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Srivastava S, Findlan R, Chan C. Using dynamic gene module map analysis to identify targets that modulate free fatty acid induced cytotoxicity. Biotechnol Prog. 2008;24:29–37. doi: 10.1021/bp070120b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-W, Almaguel FG, Bu L, De Leon DD, De Leon M. Expression of E-FABP in PC12 cells increases neurite extension during differentiation: involvement of n-3 and n-6 fatty acids. Journal of Neurochem. 2008;1006:2015–2029. doi: 10.1111/j.1471-4159.2008.05507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Xu XL, He Z, Huang XJ, Guo LJ, Wang HX. Guattegaumerine protects primary cultured cortical neurons against oxidative stress injury induced by hydrogen peroxide concomitant with serum deprivation. Cell Mol Neurobiol. 2009;29:355–364. doi: 10.1007/s10571-008-9327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- Malecki A, Garrido R, Mattson MP, Hennig B, Toborek M. 4-Hydroxynonenal induces oxidative stress and death of cultured spinal cord neurons. J Neurochem. 2000;74:2278–2287. doi: 10.1046/j.1471-4159.2000.0742278.x. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun. 1997;239:357–366. doi: 10.1006/bbrc.1997.7409. [DOI] [PubMed] [Google Scholar]
- McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Newcomb JK, Zhao X, Pike BR, Hayes RL. Temporal profile of apoptotic-like changes in neurons and astrocytes following controlled cortical impact injury in the rat. Exp Neurol. 1999;158:76–88. doi: 10.1006/exnr.1999.7071. [DOI] [PubMed] [Google Scholar]
- Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988;451:205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of "healthy" diet and aldose reductase inhibition. Diabetes. 2007;56:2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- Ohse T, Inagi R, Tanaka T, Ota T, Miyata T, Kojima I, Ingelfinger JR, Ogawa S, Fujita T, Nangaku M. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int. 2006;70:1447–1455. doi: 10.1038/sj.ki.5001704. [DOI] [PubMed] [Google Scholar]
- Okuyama R, Fujiwara T, Ohsumi J. High glucose potentiates palmitate-induced NO-mediated cytotoxicity through generation of superoxide in clonal beta-cell HIT-T15. FEBS Lett. 2003;545:219–223. doi: 10.1016/s0014-5793(03)00534-9. [DOI] [PubMed] [Google Scholar]
- Pacher P, Obrosova IG, Mabley JG, Szabo C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- Paschen W. Shutdown of translation: lethal or protective? Unfolded protein response versus apoptosis. J Cereb Blood Flow Metab. 2003;23:773–779. doi: 10.1097/01.WCB.0000075009.47474.F9. [DOI] [PubMed] [Google Scholar]
- Paschen W, Hotop S, Aufenberg C. Loading neurons with BAPTA-AM activates xbp1 processing indicative of induction of endoplasmic reticulum stress. Cell Calcium. 2003;33:83–89. doi: 10.1016/s0143-4160(02)00195-1. [DOI] [PubMed] [Google Scholar]
- Paschen W, Mengesdorf T, Althausen S, Hotop S. Peroxidative stress selectively down-regulates the neuronal stress response activated under conditions of endoplasmic reticulum dysfunction. J Neurochem. 2001;76:1916–1924. doi: 10.1046/j.1471-4159.2001.00206.x. [DOI] [PubMed] [Google Scholar]
- Peng LL, Shen HM, Jiang ZL, Li X, Wang GH, Zhang YF, Ke KF. Inhibition of NMDA receptors underlies the neuroprotective effect of ginsenoside Rb3. Am J Chin Med. 2009;37:759–770. doi: 10.1142/S0192415X09007223. [DOI] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Madiraju SR. Glycerolipid metabolism and signaling in health and disease. Endocr Rev. 2008;29:647–676. doi: 10.1210/er.2008-0007. [DOI] [PubMed] [Google Scholar]
- Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16:653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM, Chang H, Ho H, Jeng CY, Hoffman BB. Lowering of plasma glucose in diabetic rats by antilipolytic agents. Am J Physiol. 1988;254:E23–E30. doi: 10.1152/ajpendo.1988.254.1.E23. [DOI] [PubMed] [Google Scholar]
- Rho MC, Ah Lee K, Mi Kim S, Sik Lee C, Jeong Jang M, Kook Kim Y, Sun Lee H, Hyun Choi Y, Yong Rhim B, Kim K. Sensitization of vascular smooth muscle cell to TNF-alpha-mediated death in the presence of palmitate. Toxicol Appl Pharmacol. 2007;220:311–319. doi: 10.1016/j.taap.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, Lonardo A, Carulli N, Loria P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- Sango K, Horie H, Saito H, Ajiki K, Tokashiki A, Takeshita K, Ishigatsubo Y, Kawano H, Ishikawa Y. Diabetes is not a potent inducer of neuronal cell death in mouse sensory ganglia, but it enhances neurite regeneration in vitro. Life Sci. 2002;71:2351–2368. doi: 10.1016/s0024-3205(02)02040-4. [DOI] [PubMed] [Google Scholar]
- Sango K, Suzuki T, Yanagisawa H, Takaku S, Hirooka H, Tamura M, Watabe K. High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. J Neurochem. 2006;98:446–458. doi: 10.1111/j.1471-4159.2006.03885.x. [DOI] [PubMed] [Google Scholar]
- Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- Schapansky J, Olson K, Van Der Ploeg R, Glazner G. NF-kappaB activated by ER calcium release inhibits Abeta-mediated expression of CHOP protein: enhancement by AD-linked mutant presenilin 1. Exp Neurol. 2007;208:169–176. doi: 10.1016/j.expneurol.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Sharma SS, Kumar A, Kaundal RK. Protective effects of 4-amino1,8-napthalimide, a poly (ADP-ribose) polymerase inhibitor in experimental diabetic neuropathy. Life Sci. 2008;82:570–576. doi: 10.1016/j.lfs.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Jang SY, Lee HK, Jung J, Suh DJ, Seo SY, Park HT. Pathological adaptive responses of Schwann cells to endoplasmic reticulum stress in bortezomib-induced peripheral neuropathy. Glia. 2010;58:1961–1976. doi: 10.1002/glia.21065. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Song Z, Fu DT, Chan YS, Leung S, Chung SS, Chung SK. Transgenic mice overexpressing aldose reductase in Schwann cells show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress. Mol Cell Neurosci. 2003;23:638–647. doi: 10.1016/s1044-7431(03)00096-4. [DOI] [PubMed] [Google Scholar]
- Stubbs CD, Smith AD. Essential fatty acids in membrane: physical properties and function. Biochem Soc Trans. 1990;18:779–781. doi: 10.1042/bst0180779. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu G, Song T, Liu F, Kang W, Zhang Y, Ge Z. Upregulation of GRP78 and caspase-12 in diastolic failing heart. Acta Biochim Pol. 2008;55:511–516. [PubMed] [Google Scholar]
- Sundar Rajan S, Srinivasan V, Balasubramanyam M, Tatu U. Endoplasmic reticulum (ER) stress & diabetes. Indian J Med Res. 2007;125:411–424. [PubMed] [Google Scholar]
- Szegezdi E, Macdonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–C953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- Taghibiglou C, Bradley CA, Gaertner T, Li Y, Wang Y, Wang YT. Mechanisms involved in cholesterol-induced neuronal insulin resistance. Neuropharmacology. 2009;57:268–276. doi: 10.1016/j.neuropharm.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Takadera T, Ohtsuka M, Aoki H. Chelation of extracellular calcium-induced cell death was prevented by glycogen synthase kinase-3 inhibitors in PC12 cells. Cell Mol Neurobiol. 2010;30:193–198. doi: 10.1007/s10571-009-9442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi G, Sakurai M, Abe K, Itoyama Y, Tabayashi K. MCI-186 reduces oxidative cellular damage and increases DNA repair function in the rabbit spinal cord after transient ischemia. Ann Thorac Surg. 2004;78:602–607. doi: 10.1016/j.athoracsur.2004.02.133. [DOI] [PubMed] [Google Scholar]
- Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol. 2002;283:F640–F647. doi: 10.1152/ajprenal.00001.2002. [DOI] [PubMed] [Google Scholar]
- Ulloth JE, Casiano CA, De Leon M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J Neurochem. 2003;84:655–668. doi: 10.1046/j.1471-4159.2003.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH. How obesity causes diabetes in Zucker diabetic fatty rats. Trends Endocrinol Metab. 1997;8:276–282. doi: 10.1016/s1043-2760(97)00094-5. [DOI] [PubMed] [Google Scholar]
- Unger RH. Reinventing type 2 diabetes: pathogenesis, treatment, and prevention. JAMA. 2008;299:1185–1187. doi: 10.1001/jama.299.10.1185. [DOI] [PubMed] [Google Scholar]
- Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50 Suppl 1:S118–S121. doi: 10.2337/diabetes.50.2007.s118. [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487–497. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]
- van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–241. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri v, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. Faseb J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- Virag L, Szabo E, Bakondi E, Bai P, Gergely P, Hunyadi J, Szabo C. Nitric oxide-peroxynitrite-poly(ADP-ribose) polymerase pathway in the skin. Exp Dermatol. 2002;11:189–202. doi: 10.1034/j.1600-0625.2002.110301.x. [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- Welcher AA, Suter U, De Leon M, Bitler CM, Shooter EM. Molecular approaches to nerve regeneration. Philos Trans R Soc Lond B Biol Sci. 1991;331:295–301. doi: 10.1098/rstb.1991.0020. [DOI] [PubMed] [Google Scholar]
- Wiesner DA, Dawson G. Staurosporine induces programmed cell death in embryonic neurons and activation of the ceramide pathway. J Neurochem. 1996;66:1418–1425. doi: 10.1046/j.1471-4159.1996.66041418.x. [DOI] [PubMed] [Google Scholar]
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58:1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med. 2007;24:934–945. doi: 10.1111/j.1464-5491.2007.02186.x. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69:229–285. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Yoneda Y, Steiniger SC, Capkova K, Mee JM, Liu Y, Kaufmann GF, Janda KD. A cell-penetrating peptidic GRP78 ligand for tumor cell-specific prodrug therapy. Bioorg Med Chem Lett. 2008;18:1632–1636. doi: 10.1016/j.bmcl.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Rouen S, Dobrowsky RT. Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination. Glia. 2008;56:877–887. doi: 10.1002/glia.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jiang Y, Jia Z, Li Q, Gong W, Wang L, Wei D, Yao J, Fang S, Xie K. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 2006;23:401–410. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]