Abstract
Folate deficiency has been shown to influence carcinogenesis by creating an imbalance in the base excision repair (BER) pathway impacting BER homeostasis. The inability to mount a BER response to oxidative stress in a folate deficient environment results in the accumulation of DNA repair intermediates, i.e., DNA strand breaks. Our data indicate that upregulation in β-pol expression in response to oxidative stress is inhibited by folate deficiency at the level of gene expression. Alteration in expression of β-pol in a folate deficient environment is not due to epigenetic changes in the core promoter of the β-pol gene, i.e., the CpG islands within the β-pol promoter remain unmethylated in the presence and/or absence of folate. However, the promoter analysis studies show a differential binding of regulatory factor(s) to the −36 to −7 region (the folic acid response region, FARR) within the core promoter of β-pol. Moreover, we observe a tight correlation between the level of binding of regulatory factor(s) with the FARR and inhibition of β-pol expression. Based on these findings, we propose that folate deficiency results in an upregulation/stability of negative regulatory factor(s) interacting with FARR, repressing the upregulation of the β-pol gene in response to oxidative stress.
Keywords: Oxidative stress, DNA polymerase β, Base excision repair, cAMP response element, DNA methylation, transcriptional regulation, folate deficiency
INTRODUCTION
Bioactive food components found in normal diets have been determined to confer protection against many diseases by impacting the onset, progression and severity of the disease [1]. Studies on nutritional genomics, nutritional transcriptomics, nutritional epigenomics and proteomics provide evidence of the effect of these bioactive food components on individual biomolecules and their overall effect on health and disease. Through their effect on biomolecules, bioactive food components can regulate many different pathways such as cell growth, differentiation, DNA repair, apoptosis and carcinogenesis [2]. These essential and/or non-essential factors of the diet have been shown to be capable of regulating gene expression [1]. Folic acid, an essential water soluble vitamin and a cofactor in one-carbon metabolism, has been associated with the etiology of many chronic diseases such as cardiovascular disease, neurological degeneration and cancer. Folate deficiency has been shown to enhance the potency of carcinogenic agents [3] and increase the accumulation of preneoplastic changes in the colon and liver of animal models [3, 4]. Although the underlying mechanism of folate deficiency induced tumorigenesis is largely unknown, many different factors have been suggested as being responsible for its carcinogenic effect. Among those are epigenetic alterations, e.g., DNA methylation possibly via the reduction of S-adenosylmethionine [5, 6], uracil misincorporation into the DNA [6,7] and deoxynucleotide imbalance [7,8]. However, data from human and animal studies reject the possibility of global DNA methylation as a potential mechanism behind folate deficiency induced tumorigenesis [9].
Data from our laboratory and others suggest that apoptotic and DNA repair pathways might be potential targets of folate deficiency [8, 10]. In other words, increased accumulation of DNA damages such as DNA strand breaks, increased somatic mutations and chromosomal aberrations observed during folate deficiency can be attributed to defective DNA repair. Moreover, folate deficiency has been shown to act synergistically with DNA damaging agents to increase mutation frequency and DNA strand breaks, suggesting potential compromises in DNA repair capacity as has been discussed previously[6, 8, 11].
Base Excision Repair [BER] is a sequential pathway involved in the repair of endogenous DNA damage and damages that arise from alkylation and oxidative stress, along with misincorporated uracil [12]. Data from our laboratory and others have provided strong evidence to indicate that BER is a stress response pathway [13,14]. In addition, we have previously demonstrated deregulation of BER in response to folate deficiency [11]. We have shown that folate deficiency impacts initiation of the BER pathway, i.e., uracil DNA glycosylase activity is upregulated, while no upregulation in the DNA polymerase β ( β-pol) protein level and/or activity is observed. The inability to mount a BER response to stress in a folate deficient environment resulted in accumulation of DNA repair intermediates, i.e., DNA strand breaks which could potentially result in double strand breaks and chromosomal aberrations. The objective of this study was to elucidate the molecular mechanism by which folate status impacts the regulation of DNA polymerase β expression.
EXPERIMENTAL PROCEDURES
Animals
The experiments were performed in young (3–4 month), wild-type male C57BL/6 specific pathogen-free mice in accordance with NIH guidelines for the use and care of laboratory animals. The Wayne State University Animal Investigation Committee approved the animal protocol. The mice were maintained on a 12-hr light/dark cycle and fed standard mouse chow and water ad libitum. At 3–4 months of age, the mice were randomly assigned to two dietary groups and were fed AIN93G-purified isoenergetic diets (Dyets, Inc., Lehigh Valley, PA). Diets were stored at –20°C. 1% succinyl sulfathiazole was added to all diets. The control group received a folate adequate diet containing 2 mg/kg folic acid. The experimental group received a folate-deficient diet containing 0 mg/kg folic acid. The animals' food intake and body weights were monitored twice weekly to monitor for signs of toxicity (e.g., weight loss), and the experimental diets were continued for 8 weeks. Mice were anesthetized in a CO2 chamber and sacrificed by cervical dislocation. Harvested liver was flash frozen and stored in liquid nitrogen.
DNA damage induction
Experimental mice were intraperitonealy injected with 100 mg/kg body weight 2-nitropropane [2-NP; Aldrich Chemical Company, Chem. Abstr. Serv. Reg. No. (79-46-9)] dissolved in olive oil. Control mice were injected with olive oil vehicle intraperitonealy. Mice were sacrificed after 12hrs, 24hrs and 48hrs. The dose and exposure time were based on previous studies characterizing the effect of 2-NP on DNA damage and repair induction [15].
Analysis of 8-OHdG levels
Genomic DNA isolation was done using the NaI DNA extractor kit (Wako chemicals, Inc.,Richmond, VA) as described previously [16]. DNA oxidation that occurs during DNA isolation was minimized by the usage of NaI instead of phenol. Nuclease P1 and calf alkaline phosphatase were used to hydrolyze 30ug of DNA. The levels of both 8-OHdG and 2dG in the DNA hydrolysates were quantified using a HPLC-EC detection system with a polar mobile phase. The level of DNA oxidation is expressed as the ratio of 8-OHdG to 2dG.
Gene expression profiling
The mRNA expression level of β-pol was quantified using real-time PCR as described previously [17]. Briefly, total RNA was extracted from liver tissue of folate adequate and folate deficient control and 2-NP treated mice using the RNeasy Kit (Qiagen, Valencia, CA). First strand cDNA was synthesized from 1 µg RNA using random primers (Promega, Madison, Wisconsin) and purified using the QIAquick PCR purification kit (Qiagen, Valencia, California). Expression of β-pol was quantified using real time PCR with specific primers for the gene. The gene transcript was normalized to both Gapdh and β-actin [17]. External standards for all the genes were prepared by subcloning the amplicons, synthesized using the specific primers into PGEM-T easy vector.
Nuclear protein isolation
Nuclear extracts were isolated using a transfactor extraction kit (Clontech, Mountain View, CA) as described previously [17]. The kit uses a hypotonic buffer to lyse the cell allowing the removal of cytosolic fractions and is followed by the extraction of nuclear proteins by a high salt buffer. All samples and tubes were handled and chilled on ice, and all solutions were made fresh according to the manufacturer’s protocol. Low molecular weight contaminants were removed from extracts by dialysis in 1L of dialysis buffer (20mM Tris-HCl, pH 8.0; 100mM KCl; 10mM NaS2O5; 0.1mM DTT; 0.1mM PMSF; 1mg/ml PepstatinA) for 3 hours at 4°C using Slide-A-Lyzer® mini-dialysis units (Pierce Biotechnology, Rockford, IL) with a molecular weight cut off of 3.5kDa. Dialyzed extracts were aliquoted and flash frozen in liquid nitrogen and stored at −70°C for subsequent analyses. Protein concentrations were determined according to Bradford using Protein Assay Kit I (Bio-Rad, Hercules, CA).
Protein expression analysis
Western blot analysis was performed using 200 µg of nuclear protein as described previously [17]. Upon completion of SDS-PAGE, the region containing the protein[s] of interest was excised and prepared for western blot analysis while the remaining portion of the gel was stained with GelCode®Blue Stain Reagent (Pierce Biotechnology, Rockford, IL) to ensure equal protein loading. Anti-sera developed against β-pol were used to detect the proteins of interest followed by incubation with HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). As an internal control to ensure equal protein transfer, membranes were reprobed with PCNA antibody. The bands were visualized and quantified using a ChemiImager® System (Bio-Rad, Hercules, CA) after incubation in SuperSignal® West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL). Data are expressed as the integrated density value (I.D.V.) of the band per mg of protein loaded.
DNA Base Excision Repair Assay
The G:U mismatch repair assay is developed to measure monofunctional glycosylase-initiated base excision repair (BER) activity. Radio-end labeled 30-bp oligonucleotides (upper strand: 5'-ATACCGCGGUCGGCCGATCAAGCTTATT dd-3'; lower strand: 3'-ddTATATGGCGCCG GCCGGCTAGTTCGAATAA-5') containing a G:U mismatch and a Hpa II restriction site (CCGG) were incubated in a BER reaction mixture containing 50 µg of the nuclear protein as previously described [18]. Repair of the G:U mismatch to a correct G:C base pair was determined via treatment of the duplex oligonucleotide with 20U of HpaII (Promega, Madison, WI) for 1hour at 37°C and analysis by electrophoresis on a 20% denaturing 19:1 acrylamide/bis-acrylamide gel (SequaGel® Sequencing System, National Diagnostics, Atlanta, GA). Repair activity (presence of a 16-mer band) was visualized and quantified using a Molecular Imager® System (Bio-Rad, Hercules, CA) by calculating the ratio of the 16-mer product with the 30-mer substrate (product/substrate). Data are expressed as machine counts per microgram of protein.
Methylation Assay
Methylation status of β-pol promoter region: −643 to +44 was determined using the bisulfite genomic sequencing method to generate a methylation map with single base resolution. In brief, genomic DNA was isolated (Qiagen, Valencia, CA) from liver tissue obtained from folate adequate/deficient animals treated with/without 2-NP. DNA samples were treated with a bisulfite conversion reagent converting unmethylated cytosine residues to uracil according to the manufacturer’s protocol (Zymo Research, Orange, CA). Then, bisulfite treated DNA was PCR amplified using primers designed around CpG islands: −105 to +44 and −322 to −123 (Methprimers, San Francisco, CA) present in the β -pol promoter. The resulting amplicons were cloned into pGEM-T Easy Vector (Promega, Madison, Wisconsin). Plasmids from 7 colonies of each subclone were sequenced and the presence of preserved methylated cytosine residues representing methylation status was determined.
Cell Culture and promoter Analysis
Mouse hepatoma (hepa 1–6) cells of epithelial origin were obtained from American Type Culture Collection (ATCC). The cells were cultured in monolayer condition in a standard DMEM medium (GIBCO BRL, Grand Island, NY) containing 4.5g/L glucose, 4mg/L folic acid, or in a customized DMEM medium (GIBCO BRL, Grand Island, NY) free of folic acid and supplemented with 10% dialyzed fetal bovine serum (Thermo Scientific Hyclone, West Palm Beach, FL). Each of the growth mediums were supplemented with 1% penicillin streptomycin and maintained at 37°C in 10% CO2 and 95% relative humidity.
For the promoter analysis a number of promoter-reporter constructs were created using fragments of the β-pol promoter. The fragments −924 to +38, −602 to +38, −360 to + 38, −173 to +38, and −104 to +38 of the 5'-flanking region of the mouse β-pol gene encompassing the basal promoter and 5’ untranslated region were inserted into a pGL3 vector creating an array of promoter-reporter constructs: p924Luc, p602Luc, p360Luc, p173Luc and p104Luc, respectively. The hepa 1–6 cells maintained in a folate adequate medium were transfected with these promoter-reporter constructs using the effectene transfection reagent according to the manufacturer’s protocol [Qiagen, Valencia, CA]. After 24 hours of transfection, the cells were treated with 100µM of H2O2 for two hours. 24 hours later the cell lysates were quantified for the luciferase activity using the Luciferase assay kit (Promega, Madison, WI) utilizing a 20/20 luminometer (Turner Biosystems). β-galactosidase was used as an internal control for transfection. For determining the β-pol promoter activity during folate deficiency, hepa 1–6 cells maintained in folate adequate and folate deficient medium for 48hrs were transfected with p924Luc using the effectene transfection reagent following the manufacturer’s protocol (Qiagen, Valencia, CA). After 24 hours of transfection, the cells were treated with 100µM of H2O2 for two hours. 24 hours later, the cell lysates from each of the conditions were quantified for the luciferase activity using the Luciferase assay kit (Promega, Madison, WI) utilizing a 20/20 luminometer (Turner Biosystems, Promega, Madison, Wisconsin). β-galactosidase was used as an internal control for transfection.
DNA Foot printing
For DNA foot printing analysis fragments contained by the −924 to +311 region of the β-pol gene was amplified that included a 6-FAM labeled oligonucleotide for the forward primer. The 6-FAM labeled fragment consisted of a region of the β-pol promoter, exon 1 and a portion of intron 1. We used this fragment for DNA footprinting. Each reaction required 8µg of nuclear extract, and 50µg of template DNA. Nuclear extract, buffer and DNA template were added in this order to each tube while being kept on ice. Reactions were then transferred to a room temperature water bath and incubated for 20 minutes. Following the 20 min incubation at room temperature, 10 µl of a 1:50 dilution of DNase 1 (10 U/µl) was added to each reaction and reaction mixtures were incubated at room temperature for 1 minute. After 1 minute incubation with DNase 1, 50 µl of cold EDTA was added to each reaction, and the reactions were placed on ice. A control without nuclear extracts was also treated with DNase 1 along with the other samples. The completed reactions were then purified using the Qiaquick nucleotide removal kit (Qiagen, Valencia, CA). Fragments were separated on an ABI Prism 310 DNA genetic analyzer (Life Technologies, Carlsbad, CA) and processed by software using the Gene Scan application
Electrophoretic mobility shift assay (EMSA)
A non-radioisotopic EMSA was used to determine the protein-DNA binding activity of nuclear extracts isolated from liver tissue of folate adequate and folate deficient mice treated with and without 2-NP, according to the manufacturer's protocol (LightShift® Chemiluminescent EMSA kit, Pierce Biotechnology, Rockford, IL). Briefly, 40 fmol of biotin-end-labeled DNA −36 to −7 upstream β-pol promoter was incubated with 10 µg of nuclear extract in a 20 µl reaction mixture containing 1X binding buffer (100 mM Tris, 500 mM KCl, 10 mM DTT; pH 7.5), 2.5% glycerol, 5 mM MgCl2, 50 ng/µl poly (dI-dC), and 0.05% NP-40. Negative controls (all components except nuclear extract) were included in all experiments. In competitive assays, 100X molar excess of unlabeled −36 to −7 oligonucleotide, mutant −36 to −7 oligonucleotide or consensus CRE/CREB sequence were added to the reaction mixture. Samples were incubated for 20 min at room temperature and then resolved on a 6% non-denaturing polyacrylamide gel in 0.5X TBE buffer. After electrophoresis, samples were transferred from the gel to a positively charged nylon membrane and cross-linked. Biotin-labeled protein/DNA complexes were detected by chemiluminescence and quantified using a ChemiImager™ System (AlphaInnotech, San Leandro, CA). Data are expressed as the integrated density value (I.D.V.) of the band/mg of protein loaded.
Statistical analysis
Statistical significance between means was determined using ANOVA as described previously [17]. P-values less than 0.05 were considered statistically significant.
RESULTS
Analysis of formation of 8-OHdG, expression of DNA polymerase β and G:U mismatch BER during folate deficiency and 2-NP treatment in the liver of mice
Folate deficiency has been shown to influence carcinogenesis by creating an imbalance in the base excision repair pathway [11]. In this study we have characterized the effect of folate deficiency on BER in response to oxidative stress in vivo. We used 2-Nitropropane (2-NP, a hepatocarcinogen) to induce oxidative stress in the liver of C57BL/6 mice. 2-NP has been shown to increase mutation frequency in rodents and has been suggested to induce cancer through the formation of DNA damages such as 8-OHdG and 8-aminoguanine in liver [15, 17]. As reported previously by our laboratory [15,16], 2-NP treatment (i.p. injection of 100 mg/kg body weight) resulted in a significant increase in DNA oxidation, 8-OHdG, and an upregulation in expression of β-pol in a time dependent manner. We observed a six-fold increase (p < 0.001) in 8-OHdG levels 12 hr post treatment (Fig 1A), while the β-pol mRNA level reached its maximum at 12 hr and remained high at 24 hr post 2-NP treatment (Fig. 1B). Moreover, we show a steady increase in the level of β-pol protein in response to 2-NP (Fig. 1C). Previously, it has been shown that β-pol is a critical enzyme in BER [16,19]. As such, we observed a concurrent increase in BER activity (40%, p < 0.01) in response to 2-NP in liver tissues of the mice (Fig. 1D).
Fig. 1.
Effect of 2-NP on β-pol expression and level of 8-OHdG in liver of C57BL/6 mice. [A] The 8-OHdG levels in liver tissues of control and 2-NP treated mice were measured by HPLC and EC detector. The data are expressed as values relative to the amount of 2dG detected by UV absorbance at 290nm. [B] DNA polymerase β mRNA levels in the liver tissues of control and 2-NP treated mice were quantified using real-time PCR and normalized against Gapdh. [C] The levels of β-pol protein in 100 µg of liver nuclear extract obtained from control and 2-NP treated mice were determined by western blot analysis. The level of β-pol protein was normalized based on the amount of protein loaded on each gel. PCNA served as the nuclear protein loading control. [I.D.V.]: integrated density value corresponding to the level of β-pol protein as quantified by a Bio-Rad ChemiImager® System. Effect of folate deficiency and 2-NP on Base Excision Repair activity. [D] The in vitro G:U mismatch BER assay was conducted using nuclear extracts obtained from liver of control and 2-NP treated mice fed a folate adequate and/or folate deficient diet. The reaction products were resolved on a sequencing gel. Repair activity was visualized by the appearance of a 16b fragment. The relative level of BER was quantified using a Bio-Rad Molecular Imager® System. The data were normalized based on the amount of protein used in each reaction and expressed as machine counts per µg of protein. Values represent an average [± S.E.M.] for data obtained from 5 mice in each group and are representative of separate identical experiments. Values with different letter superscripts indicate significant differences at P < 0.05. FA: Folate adequate; FD: Folate deficient.
To further study the effect of folate deficiency on BER in response to oxidative stress, we analyzed BER activity in 2-NP treated animals fed a folate deficient diet. We performed an eight-week feeding study where the C57BL/6 mice were fed a folate deprived diet. As reported previously [10], this regimen resulted in a 90% decrease in plasma folate level and a 40% decrease in tissue folate levels with no significant change in the food intake and weight of these animals (data not shown). As such, we expected to see an increase in BER activity in these animals. However, BER activity failed to be upregulated in response to folate deficiency. Moreover, we expected to see a further increase in BER activity in folate deficient mice in response to 2-NP. Surprisingly, we observed no increase in BER activity in response to 2-NP in mice fed a folate deficient diet (Fig. 1D). In agreement, we show that the lack of inducibility of BER in response to 2-NP in a folate deficient environment is preceded by a lack of induction in β-pol expression, i.e., β-pol mRNA and protein levels (Figs. 2A and 2B). Interestingly, 2-NP treatment of folate deficient mice resulted in a significant up-regulation in the expression of two key enzymes in the BER pathway, Ung and Ape1/Ref-1 (data not shown), while, upregulation of the β-pol gene was inhibited by folate deficiency. These findings signify that the effect of folate on gene expression is tightly regulated, i.e., the impact of folate deficiency is gene dependent. Thus, the inability to induce BER in response to oxidative stress in a folate deficient environment appears to be due to attenuation in the ability of the β-pol gene to mount a response to oxidative stress. Based on these findings, it is inviting to suggest that the mechanism by which folate deficiency induces carcinogenesis in response to DNA damaging agents is due in part to the inability to induce a BER response at the level of β-pol expression, an important observation in the realm of nutrient gene interaction.
Fig. 2. Effect of folate deficiency and 2-NP on the expression of β-pol.
[A] DNA polymerase β mRNA expression level in the liver tissues of control and 2-NP treated mice fed a folate adequate and/or folate deficient diet were quantified using real-time PCR and normalized against Gapdh. [B] The levels of β-pol protein in 100 µg of liver nuclear extract obtained from control and 2-NP treated mice fed a folate adequate and/or folate deficient diet were determined by western blot analysis. The levels of β-pol protein were normalized based on the amount of protein loaded on each gel. [I.D.V.]: integrated density value corresponding to the level of β-pol protein as quantified by a Bio-Rad ChemiImager® System. Values represent an average [± S.E.M.] for data obtained from 5 mice in each group and are representative of separate identical experiments. Values with different letter superscripts indicate significant differences at P < 0.05. FA: Folate adequate; FD: Folate deficient.
Analysis of the promoter of DNA polymerase β for the methylation of CpG islands in the liver of mice
Folate deficiency has been shown to alter S-adenosylmethionine/S-adenosyl homocysteine ratio, thus impacting the levels of the universal methyl donor. As such, folate deficiency has been suggested to impact gene expression through epigenetic modulation by modifying the methylation pattern of the genome. DNA methylation generally occurs in the cytosine of CpG dinucleotides, converting the cytosine to 5-methyl cytosine. In order to dissect out the epigenetic effect of folate deficiency on β-pol expression, we analyzed the methylation status of the CpG islands found in the promoter of the β-pol gene, using the bisulfite genomic sequencing method. Using MethPrimer software [20], we identified two CpG islands within the first 687bp region of the β-pol promoter, and part of exon 1(Island 1: −105 to +44 and Island 2: −322 to −123). Subsequently, primers were designed to amplify both islands, and the methylation status of the CpG sites within these regions was determined. Herein, we demonstrate that folate deficiency does not alter the methylation pattern of the CpG islands in the promoter region of β-pol. Interestingly, no CpG sites were methylated in DNA samples obtained from liver of mice fed a folate deficient and/or adequate diet and treated with 2-NP (Fig.3). We were concerned whether the lack of methylation at the CpG sites within these two islands is due to an artifact of the assay conditions. Thus, we examined the methylation pattern of exon 8 of the p53 gene as control. Exons 5–8 of p53 has been shown to be a ‘hot spot’ involved in neoplastic transformations, and this region has been shown to undergo alteration in its methylation patterns during folate deficiency [5, 21]. Interestingly, all the CpG sites within exon 8 of the p53 gene were methylated. Our findings with respect to the methylation patterns of the β-pol promoter are consistent with the notion that promoter regions of housekeeping genes remain unmethylated. Therefore, our data illustrate that deregulation of β-pol expression by folate deficiency is not through epigenetic alteration of the core region of its promoter. It is inviting to suggest therefore that folate deficiency might be impacting β-pol expression transcriptionally by way of regulatory factors.
Fig. 3. Effect of folate deficiency and 2-NP on methylation of β-pol promoter.
Methylation of promoter/Exon1 of the β-pol gene was determined by bisulfite sequencing assay with single base resolution. The bisulfite treated DNA was PCR amplified using primers designed around CpG islands. The resulting amplicons were cloned into pGEM-T Easy Vector. Plasmids from 7 colonies of each subclone were sequenced and the presence of preserved methylated cytosine residues representing methylation status was determined. [A] Sequence of the two CpG islands [Island 1: −105 to +44 and Island 2: −322 to −123] present in the β-pol promoter/Exon1 has been illustrated. [B] Methylation patterns of the CpG sites present within the CpG islands of the β-pol promoter/Exon1 has been depicted. Open circle: unmethylated CpG site; Solid circle: methylated CpG site. FA: Folate adequate; FD: Folate deficient.
Analysis of the DNA polymerase β promoter using DNA footprinting and gel retardation assays
To further investigate the mechanism of altered transcription of DNA polymerase β gene in response to oxidative stress in folate deficient mice, the cis acting region that is involved in this regulation was analyzed. We initially determined the promoter region required for the upregulation of β-pol gene in response to oxidative stress in cultured liver cell lines, hepa 1–6. First, the effect of H2O2 on β-pol mRNA in the mouse hepa 1–6 was examined. In hepatoma cells, β-pol mRNA increased 2-fold by 100µM H2O2 treatment for 2 hr, where no sign of cellular toxicity was observed (Fig.4A). Consequently, we used a similar treatment to determine the promoter activity of the β-pol gene in cultured cells to identify the region of the promoter responsible for upregulation in response to oxidative stress. Using a promoter analysis assay, we examined the regulation of β-pol promoter activity in response to H2O2 and folate deprivation. The fragments −924 to +38, −602 to +38, −360 to + 38, −173 to +38, and −104 to +38 of the 5’-flanking region of the mouse β-pol gene were inserted into pGL3 vector creating an array of promoter-reporter constructs: p924Luc, p602Luc, p360Luc, p173Luc and p104Luc, respectively. After transfection, the luciferase activity for each construct was quantified and normalized against β-galactosidase activity. The luciferase activity in the cells transfected with p924Luc, p602Luc, p360Luc, p173Luc and p104Luc were stimulated 2 to 3.5-fold following a 2 hr treatment with 100µM H2O2. This suggests that the core promoter encumbers the necessary elements required for response to oxidative stress (Fig. 4B). Interestingly, while folate deprivation resulted in a slight increase in β-pol promoter activity in cell culture, the robust upregulation in luciferase activity in response to oxidative stress was lost when the cells were cultured in the medium without folic acid (Fig. 4C). These results suggest that the sequence −924 to +38 contains the cis-element which responds to oxidative stress as well as the elements harboring the regulatory factor(s) responding to folate deprivation. This is the first report demonstrating that the folate responsive region of the β-pol gene promoter is located within the basal promoter encumbering the CRE region.
Fig. 4. Effect folate deficiency and hydrogen peroxide on β-pol expression and promoter activity in hepa 1–6 cell line.
[A] DNA polymerase β mRNA levels in hepa 1–6 cells treated with H2O2 [0, 100, 200, 300, 500µM] were determined. Hepa 1–6 cells were treated for 2 and 4 hrs with H2O2 and the levels of β-pol mRNA were quantified using real-time PCR 24hrs post treatment. The data were normalized against Gapdh. [B] Relative promoter activity of sequential deletions of −924 to +38 upstream region of β-pol promoter transfected into hepa 1–6 cell line was determined. The transfected hepa 1–6 cells were treated with H2O2[100µM] for 2hrs. Luciferase activity was determined 24hrs post treatment and normalized by β-galactosidase activity. [C] Effect of folate deficiency and H2O2 treatment on the relative promoter activity of −924 to +38 upstream region of β-pol promoter transfected into hepa 1–6 cell line were determined. The transfected cells were treated with H2O2 [100µM] for 2 hrs as described in Methodology. Luciferase activity was determined 24hrs post treatment and normalized by β-galactosidase activity. Values represent an average [± S.E.M.]. FA: Folate adequate; FD: Folate deficient.
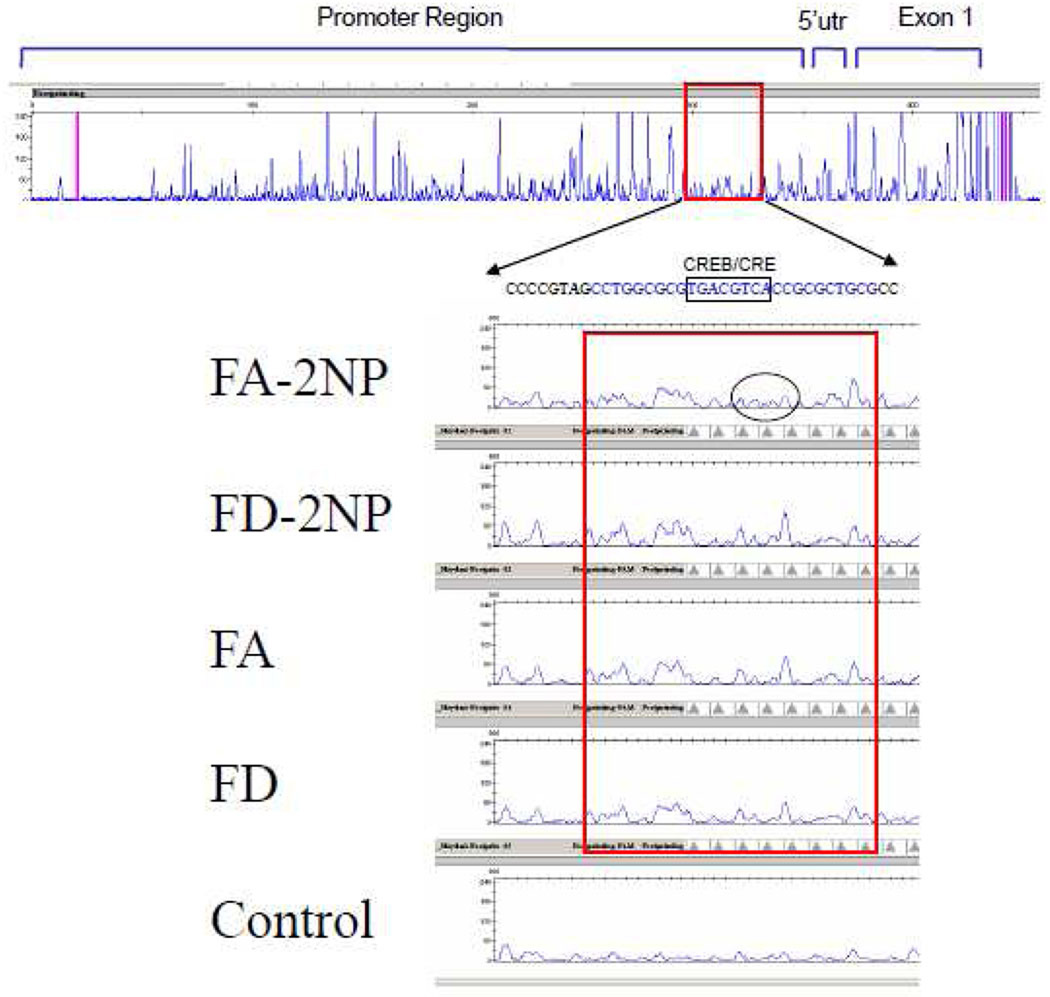
To determine the differential protein-DNA binding pattern within the β-pol promoter, we performed a footprinting assay using DNA fragments contained by the −924 to +311 region of the β-pol promoter. To elucidate the protein/DNA interactions in a physiologically relevant model, liver nuclear extracts from mice were utilized for this assay. As depicted in Fig. 5, a significant difference in protection pattern in a region previously shown to hold the core promoter activity of human β-POL [22,23] was detected. In other words, the DNA footprinting profiles generated using this fragment showed differential protein-DNA binding patterns in the −36 to −7 upstream β-pol promoter region. Interestingly extracts from 2-NP treated animals fed a folate adequate diet displayed maximum DNase I sensitivity suggesting the absence /reduced protein binding in this region (Fig. 5).
Fig. 5. β-pol promoter DNase I footprinting analysis.
DNase I footprinting assay was performed using the –360 to +97 fragment of β-pol promoter and nuclear extracts obtained from liver tissues of mice fed a folate deficient and/or folate adequate diet. DNA fragments were analyzed using ABI Prism 310 Genetic Analyzer [Life Technologies, Carlsbad, CA]. Numbers at the top of the panel represent fragment length in nucleotides. The specific protected fragment encompassing the CRE/CREB binding site is shown in an expanded view. The DNA sequence of the region is displayed above with the CRE/CREB consensus site highlighted. FA: Folate adequate; FD: Folate deficient.
The above findings provide evidence that region −36 to −7 upstream of the mouse β-pol promoter shows a differential DNA protein binding pattern in response to folate deprivation and oxidative stress. This region which we refer to as the Folic Acid Response Region (FARR) contains a binding site for CRE. CREB is a stress induced transcription factor shown to be responsible for activating gene expression when bound to the CRE region. The CRE site of the human β-POL promoter has been demonstrated to be involved in its expression both under basal conditions and when exposed to DNA alkylating agent [24–26]. Herein we attempted to identify transcription factor(s) that mediate the up-regulation of the β-pol in response to oxidative stress and potential factors that appear to have negative regulatory impact on the expression of β-pol in response to folate deficiency. We performed electrophoresis mobility shift assays (EMSA) to measure the ability of nuclear extracts isolated from the liver of mice to bind to the FARR (−36 to −7). For these experiments, nuclear extracts from the liver of mice fed a folate adequate and a folate deficient diet, were subjected to gel shift assays using the FARR probe. As shown in Fig. 6A, we observed a shifted band, complex 1. The intensity of this band was consistently stronger in extracts obtained from liver tissues of folate deficient mice as compared to mice fed a folate adequate diet and/or mice exposed to oxidative stress, 2-NP. In other words, the protein composing this shifted band appeared to be the factor that responds to oxidative stress and folate deficiency by altering its binding capability to the FARR. For example, the 2-NP treated folate adequate animals showed protein binding ∼50% lower than the controls (Fig. 6A, lane 3), whereas the folate deficient animals exposed to oxidative stress showed ∼50% increase in binding activity (Fig. 6A, lane 3). Interestingly, these data show an inverse correlation between the level of DNA binding activity to the β-pol promoter, FARR, and the β-pol mRNA expression (Fig. 1 D), i.e., the least binding activity in folate adequate 2-NP corresponds to maximal β-pol expression, and higher binding activity in folate deficient 2-NP corresponds to the lowest β-pol expression.
Fig. 6. Effect of folate deficiency and 2-NP on DNA binding activity in nuclear extracts obtained from liver tissues of C57BL/6 mice.
[A] Binding of liver nuclear extracts to the −36 to −7 region [FARR, 5’–AGCCTGGCGCGTGACGTCACCGCGCTGCGC-3’] of the β-pol promoter. Nuclear extracts were obtained from liver tissues of mice fed a folate deficient and a folate adequate diet and were analyzed [10 µg of nuclear extracts per assay] by the mobility shift assay, using biotin-end-labeled FARR oligonucleotide as described in Methodology. [B] The shifted bands observed in the Panel A [Complex 1] were quantified using a Bio-Rad ChemiImager® System. Values represent an average [± S.E.M.] for data obtained from 5 mice in each group and are representative of separate independent experiments. [C] The specificity of protein-DNA binding activity was determined by competition assays. Mobility shift assays were performed in the presence of 100x molar excess of cold FARR [Lane b, 5’– AGCCTGGCGCGTGACGTCACCGCGCTGCGC-3’], mutated FARR [Lane c, 5’-AGCCTGGCGCGTGTGGTCACCGCGCTGCGC-3’], and CRE/CREB Consensus [lanes d–f, 5’- AGAGATTGCCTGACGTCAGAGAGCTAG-3’] oligonucleotides. Lane a is a positive control and does not contain any competitor. I.D.V., integrated density value. Values with different letter superscripts indicate significant differences at P < 0.05. FA: Folate adequate; FD: Folate deficiency.
To address the specificity of the protein/DNA binding activity observed above, we performed competition assays, using cold, mutated FARR and CRE/CREB consensus oligonucleotides. Fig. 6C shows the results of a competition assay using 100X molar excess of the unlabelled probe. This competitor eradicated the shifted band indicating the binding activity to be specific. Moreover, the shifted band was also eliminated with 100X molar excess of an unlabelled mutated −36 to −7 probe (FARR with mutation in the CRE site: 5’-TGACGTCA-3’ mutated to 5’-TGTGGTCA-3’) suggesting that the binding activities seen in these conditions are not specific to the consensus CRE site (Fig. 6C lane 3). Furthermore, Fig. 6C also shows the result of a competition assay using a consensus CRE/CREB (5’-TGACGTCA-3’) probe as competitor. This competitor did not affect the formation of the shifted band, indicating that the factor involved is less likely to belong to the CREB/ATF family. We cannot, however, exclude the possibility that CREB/ATF is one of the components of this complex, given that the affinity of CREB/ATF to the CRE site of the mouse β-pol promoter is not known. Additionally, a gel shift assay performed with the labeled CRE mutated FARR showed significant DNA binding activity, confirming that the binding is not specific to CRE, while the shifted band did not show any super shifts when antibodies to ATF and AP-1 complex were added to the nuclear extracts (Data not shown). These findings indicate that the factors which show differential binding activity to the FARR segment of the β-pol promoter do not belong to the ATF or AP-1 families. Therefore, it is inviting to suggest that the proteins binding to the elements within the FARR region in nuclear extracts obtained from 2-NP treated mice fed a folate deficient diet are negative regulatory factors independent of CREB/ATF, and inhibit induction of β-pol expression in response to oxidative stress.
DISCUSSION
Folate deficiency has been associated with many types of cancer, but the underlying molecular mechanism of its tumorigenic effect has not yet been elucidated. Inconsistencies observed in studies conducted on folate deficiency and supplementation suggest that association between cancer and folate status is dependent on the timing of dietary treatment and the exposure to stress inducing agents and carcinogens [27–31]. Low plasma folate levels have been associated with increased risk of colorectal cancer development in individuals with single nucleotide polymorphism in the methylene tetrahydrofolate reductase gene [1, 32]. Folate deficiency has also been shown to incapacitate the DNA repair pathways, a potential mechanism behind the accumulation of DNA damage during this deficiency [6,8,11,33]. Moreover, agents such as methotrexate, an inhibitor of dihydrofolate reductase, have been shown to reduce the efficiency of excision repair in CHO cells [34]. Furthermore, BER, a DNA damage inducible pathway [13–15,26,35,36] has been shown to lose its ability to respond to damage during folate deficiency, leading to the accumulation of toxic intermediates [11].
Although folate deficiency has been implicated in inducing cellular stress and impacting DNA repair potential in cells and animal models, the exact mechanism is still not understood. Using microarray analysis and a proteomics approach, recent studies demonstrate the differential effect of folate deficiency on pathways associated with cancer development, DNA repair and apoptosis. A study done on colon cancer cells shows that folate deficiency alters the expression of vital genes involved in many pathways, such as DNA repair, apoptosis, angiogenesis and cell cycle control [37]. Data from our lab shows that folate deficiency in BER compromised mice suppresses DNA repair and promotes apoptosis by altering the expression of key genes involved in these pathways [10]. Furthermore, proteomic analyses, done on folate deficient rat livers, show differential expression of certain proteins involved in oxidative stress and cancer pathways [38]. Thus folate deficiency seems to affect these pathways by targeting specific genes involved in them. In line with these studies, earlier data from our lab demonstrate that folate deficiency negatively regulates BER by affecting the expression of its key enzyme DNA polymerase β, and that folate deficiency and β-pol haploinsufficiency interact to increase the accumulation of DNA damage [11]. In this study, we provide further data that sheds light on the mechanism by which folate deficiency impacts β-pol expression at the level of transcription and prevents its upregulation even under the influence of oxidative stress.
DNA polymerase β plays a very important role in embryogenesis, gap-filling repair synthesis and recombination repair [23–25]. β-pol is considered to be a housekeeping gene and is constitutively expressed in all cell cycle stages with differential expression based on the tissue type [23–25]. β-pol exhibits dual function in short patch BER, namely polymerase and dRP lyase activity, and has also been demonstrated to play a critical role in Long-Patch BER [19,39,40]. Homozygous deletion of β-pol is embryonic lethal, whereas the heterozygous knockouts survive and are fertile [41]. β-pol haploinsufficient mice display ∼ 50% reduced β-pol protein levels and significant reduction in their β-pol dependant G:U mismatch BER efficiency [15,16]. This reduction in β-pol and BER efficiency is thought to be responsible for the increase in mutation frequency seen in these animals when exposed to alkylating agents [16]. In addition, studies conducted on β-pol null cells show the accumulation of DNA damage in response to stress inducing agents [42, 43].
DNA polymerase β has been demonstrated to be a DNA damage inducible gene i.e., β-pol expression has been shown to be upregulated in response to alkylating and oxidizing agents [15,17,26]. In this study, folate deficiency is shown to inhibit the upregulation of β-pol mRNA and protein levels in response to 2-NP, an oxidizing agent, whereas, upregulation of other key enzymes in the BER pathway, namely Ung and Ape1/Ref-1, are unaffected. Thus, the impact of folate deficiency on gene expression seems to be gene specific. Folate deficiency is well known for its effect on DNA methylation via the reduction of the universal methyl donor, SAM. Alterations in DNA methylation in general have been strongly associated with cancer formation, and these alterations are believed to occur early in cancer development [44]. Due to the strong association between genomic hypomethylation and tumor formation especially in colon, stomach, thyroid, breast, uterine and prostate cancers [28,44–47], folate deficiency is suggested to cause cancer via this epigenetic effect. Here in this study, we observed no alterations in the methylation pattern of the CpG rich regions within β-pol promoter during folate adequacy and deficiency. No methylated CpG sites were found in the CpG islands identified within this region of β-pol promoter. This data suggests that epigenetic alterations, at least in the core promoter region of β-pol, are not the mechanism behind the loss of induction of β-pol during folate deficiency. This observation is in line with the notion that the promoter regions of the housekeeping genes are usually not methylated to maintain basal expression [48].
Expression of a gene can be tightly regulated at different points during its conversion from DNA to protein. Gene expression in part is controlled by the interaction of cis-elements within the promoter of the gene with their associated protein factors. Our study shows that the elements within the −104 to +38 region of a β-pol promoter are sufficient for the induction of the β-pol gene in hepa 1–6 cells exposed to oxidative stress. This finding is in agreement with previous work from other labs which showed that the first 100 nucleotides 5’ of exon 1 of human β-POL had the core promoter activity [22, 23]. Wilson et al further identified SP1 and CRE sites within this region [23]. Sp1 sites in the human core β-POL promoter have been shown to be involved in the transcriptional activation of the TATA less promoter [49]. The CRE site, the general binding site for ATF-1, CREB-1, CREM-1 and AP-1, has been shown to be involved in the expression of human β-POL during basal conditions and during stress [24–26,50]. Mouse β-pol has also been shown to have this consensus palindromic sequence for CREB, and was shown to be required for transcriptional activation of β-pol by adenovirus type 12 E1A proteins [51]. DNA alkylating agents like MNNG require the CRE region for inducing β-pol [24]. MNNG treatment increases CREB-1 levels by 10-fold in CHO cells which in turn is responsible for β-pol induction [24]. In addition, Lamph et al. have demonstrated that CREB, the CRE binding protein, can act as an activator when phosphorylated, and can also act as a repressor in the dephosphorylated state [52]. Further, the CRE consensus sequence has similarities to the AP-1 binding site (TGACTCA) [52,53] and the AP-1 complex can act both as an activator and repressor [54–56]. It has also been shown that AP-1 can bind to the CRE site and act as a repressor in the expression of MyoD1 involved in muscle cell differentiation [57]. Additionally, Wilson et al, have identified a 60Kda novel ATF protein called ATF2d in human testis with incomplete N-terminus [25]. This ATF-2d protein has been demonstrated to have the capacity to bind to a β-pol promoter and negatively regulate it [25]. Our data from hepa1–6 cells show that the promoter activity of β-pol is attenuated during folate deficiency even under oxidative stress. This correlates closely with the attenuation of β-pol expression observed in mouse liver during folate deficiency. Our footprinting analysis and gel shift assays done on mouse liver extracts show the involvement of the FARR site of β-pol promoter encompassing the CRE in the regulation of β-pol expression during folate deficiency and oxidative stress. The footprinting and gel shift assays provide evidence that the elements within the FARR site behaves as a negative regulatory site, and the factors binding to these elements act as potential repressors of β-pol expression, keeping the β-pol expression at the basal level. Furthermore, our data show that the factors binding to the FARR are not specific to the consensus CRE sequence. This observation indicates that the negative regulatory factor binding to the FARR site during folate deficiency might be independent of the CREB/ATF family.
In conclusion, folate deficiency impacts the BER capacity by regulating β-pol expression at the level of transcription. This regulation possibly goes beyond folate deficiency’s general impact on DNA methylation and controls the β-pol expression through the interplay of cis and trans acting regulatory factors. Our data provides evidence that folate deficiency potentially inhibits β-pol induction through negative regulatory factors and keeps its expression at the basal level. However, further characterization is required to identify the inhibitory factors as well as other potential regions of repression
Acknowledgments
This work was supported by grants from the National Institutes of Health [R01 CA121298 to A.R.H.] and the American Institute for Cancer Research [03A061 to A.R.H.].
Antibodies to β-pol and PCNA were generous gifts from Dr Grigory L. Dianov from Gray Institute for Radiation Oncology and Biology, University of Oxford, Oxford, UK.
We would like to acknowledge Dr Richard Caldwell from the Journal of the American College of Nutrition for critical review of the manuscript.
The abbreviations used are
- β-pol
DNA polymerase β
- BER
base excision repair
- CRE
cAMP response element
- CREB
cAMP response element binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cindy CD, Milner J. Frontiers in Nutrigenomics, proteomics, metabolomics and cancer prevention. Mut.Res. 2004;551:51–64. doi: 10.1016/j.mrfmmm.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Trujillo E, Davis C, Milner J. Nutrigenomics, Proteomics, Metabolomics, and the practice of dietetics. J. Am. Diet Assoc. 2006;106:403–413. doi: 10.1016/j.jada.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Cravo ML, Mason JB, Dayal Y, Hutchinson M, Smith D, Selhub J, Rosenberg IH. Folate deficiency enhances the development of colonic neoplasia in dimethylhydrazine-treated rats. Cancer Res. 1992;52:5002–5006. [PubMed] [Google Scholar]
- 4.James SJ, Miller BJ, Basnaknian AG, Pogribny IP, Pogribna M, Muskhelishvili I. Apoptosis and proliferation under conditions of deoxynucleotide pool imbalance in liver of folate/methyl deficient rats. Carcinogenesis. 1997;18:287–293. doi: 10.1093/carcin/18.2.287. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y-I, Pogribny IP, Basnakian AG, Miller JW, Selhub J, James SJ, Mason JB. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. 1997;65:46–52. doi: 10.1093/ajcn/65.1.46. [DOI] [PubMed] [Google Scholar]
- 6.Duthie SJ, Hawdon A. DNA instability [strand breakage, uracil misincorporation, and defective repair] is increased by folic acid depletion in human lymphocytes in vitro. Faseb J. 1998;12:1491–1497. [PubMed] [Google Scholar]
- 7.Melnyk S, Pogribna M, Miller BJ, Basnakian AG, Pogribny IP, James SJ. Uracil misincorporation, DNA strand breaks, and gene amplification are associated with tumorigenic cell transformation in folate deficient/repleted Chinese hamster ovary cells. Cancer Letters. 1999;146:35–44. doi: 10.1016/s0304-3835(99)00213-x. [DOI] [PubMed] [Google Scholar]
- 8.James SJ, Basnakian AG, Miller BJ. In vitro folate deficiency induces deoxynucleotide pool imbalance, apoptosis, and mutagenesis in Chinese hamster ovary cells. Cancer Res. 1994;54:5075–5080. [PubMed] [Google Scholar]
- 9.Kim Y-I. Folate and DNA methylation: A mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol. Biomarkers Prev. 2004;13:511–519. [PubMed] [Google Scholar]
- 10.Ventrella-Lucente LF, Unnikrishnan A, Pilling AB, Patel HV, Kushwaha D, Dombkowski AA, Schmelz EM, Cabelof DC, Heydari AR. Folate deficiency provides protection against colon carcinogenesis in DNA polymerase beta haploinsufficiency mice. J.Biol.Chem. 2010;285:19246–51928. doi: 10.1074/jbc.M109.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabelof DC, Raffoul JJ, Nakamura J, Kapoor D, Abdalla H, Heydari AR. Imbalanced base excision repair in response to folate deficiency is accelerated by polymerase β haploinsufficiency. J. Biol.Chem. 2004;279:36504–36513. doi: 10.1074/jbc.M405185200. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. 2nd edition. Washington D.C.: ASM press; 2006. Base excision repair; pp. 169–226. [Google Scholar]
- 13.Wilson DM, III, Sofinowski TM, McNeill DR. Repair mechanisms for oxidative DNA damage. Front. Biosci. 2003;8:d963–d981. doi: 10.2741/1109. [DOI] [PubMed] [Google Scholar]
- 14.Izumi T, Wiederhold LR, Roy G, Roy R, Jaiswal A, Bhakat KK, Mitra S, Hazra Tk. Mammalian DNA base excision repair repair proteins: their interactions and role in repair of oxidative DNA damage. Toxicology. 2003;193:43–65. doi: 10.1016/s0300-483x(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 15.Cabelof DC, Raffoul JJ, Yanamadala S, Guo Z, Heydari AR. Induction of DNA polymerase β-dependent base excision repair in response to oxidative stress in vivo. Carcinogenesis. 2002;23:1419–1425. doi: 10.1093/carcin/23.9.1419. [DOI] [PubMed] [Google Scholar]
- 16.Cabelof DC, Guo Z, Raffoul JJ, Sobol RW, Wilson SH, Richardson A, Heydari AR. Base excision repair deficiency caused by polymerase β haploinsufficiency: Accelerated DNA damage and increased mutational response to carcinogens. Cancer Res. 2003;63:5799–5807. [PubMed] [Google Scholar]
- 17.Unnikrishnan A, Raffoul JJ, Patel HV, Prychitko TM, Anyangwe N, Meira LB, Friedberg EC, Cabelof DC, Heydari AR. Oxidative stress alters base excision repair pathway and increases apoptotic response in apurinic/apyrimidinic endonuclaese 1/redoxfactor-1 haploinsufficient mice. Free Rad. Biol. Med. 2009;49:1488–1499. doi: 10.1016/j.freeradbiomed.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raffoul JJ, Cabelof DC, Nakamura J, Meira LB, Friedberg EC, Heydari AR. Apurinic/apyrimidinic endonuclease [APE/REF-1] haploinsufficient mice display tissue specific differences in DNA polymerase beta-dependent base excision repair. J. Biol. Chem.s. 2004;279:18425–18433. doi: 10.1074/jbc.M313983200. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava DK, VandeBerg BJ, Prasad R, Molina JT, Beard WA, Tomkinson AE, Wilson SH. Mammalian abasic site base excision repair: identification of the reaction sequence and rate-determining steps. J. Biol. Chem. 1998;273:21203–21209. doi: 10.1074/jbc.273.33.21203. [DOI] [PubMed] [Google Scholar]
- 20.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;11:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 21.Sohn K-J, Stempak JM, Reid S, Shirwadkar S, Mason JB, Kim Y-I. The eefect of dietary folate on genomic and p53-specific DNA methylation in rat colon. Carcinogensis. 2003;24:81–90. doi: 10.1093/carcin/24.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Briggs MR, Kadonaga JT, Bell SP, Tjian R. Purification and biochemical characterization of the promoter specific transcription factor, Sp1. Science. 1986;234:47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- 23.Englander EW, Wilson SH. Protein binding elements in the human β-polymerase promoter. Nucleic acids Res. 1990;18:919–928. doi: 10.1093/nar/18.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He F, Yang X-P, Srivastava DK, Wilson SH. DNA polymerase β gene expression: The promoter activator CREB-1 is upregulated in Chinese hamster ovary cells by DNA alkylating agent-induced stress. Biol. Chem. 2003;384:19–23. doi: 10.1515/BC.2003.003. [DOI] [PubMed] [Google Scholar]
- 25.Chyan Y-J, Rawson TY, Wilson SH. Cloning and characterization of a novel member of the human ATF/CREB family: ATF2 deletion, a potential regulator of the human DNA polymerase β promoter. Gene. 2003;312:117–124. doi: 10.1016/s0378-1119(03)00607-3. [DOI] [PubMed] [Google Scholar]
- 26.Fornance AJ, Jr, Zmudzka B, Hollander MC, Wilson SH. Induction of b-polymerase mRNA by DNA-damaging agents in Chinese hamster ovary cells. Mol. Cell Biol. 1989;9:851–853. doi: 10.1128/mcb.9.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y-I. Folate, colorectal carcinogenesis, and DNA methylation: Lessons from Animal studies. Environ. Mol. Mutagen. 2004;44:10–25. doi: 10.1002/em.20025. [DOI] [PubMed] [Google Scholar]
- 28.Linhart HG, Troen A, Bell GW, Cantu E, Chao W-H, Moran E, Steine E, He T, Jaenisch R. Folate deficiency induces genomic uracil misincorporation and hypomethylation but does not increase DNA point mutations. Gasteroenterology. 2009;136:227–235. doi: 10.1053/j.gastro.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, Eyssen GM, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Perason LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg R. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 31.Van Guelpen B, Hultdin J, Johansson I, Hallmans G, Stenling R, Riboli E, Winkvist A, Palmqvist R. Low folate levels may protect against colorectal cancer. Gut. 2006;55:1461–1466. doi: 10.1136/gut.2005.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eussen SJPM, Vollset SE, Igland J, Meyer K, Fredriksen A, Ueland PM, Jenab M, Slimani N, Boffetta P, Overvad K, Tjonneland A, Olsen A, Clavel-Chapelon F, Boutron-Ruault M-C, Morois S, Weikert C, Pischon T, Linseisen J, Kaaks R, Trichopoulou A, Zilis D, Katssoulis M, Palli D, Berrino F, Vineis P, Tumino R, Panico S, Peeters PHM, Bueno-de-Mesquita HB, Duijnhoven FJBV, Gram IT, Skeie G, Lund E, Gonzalez CA, Martinez C, Dorronsoro M, Ardanaz E, Navarro C, Rodriguez L, Guelpen BV, Palmqvist R, Manjer J, Ericson U, Bingham S, Khaw K-T, Norat T, Riboli E. Plasma folate, related genetic variants and colorectal cancer risk in EPIC. Cancer Epidemiol Biomarkers Prev. 2010;19:1328–1340. doi: 10.1158/1055-9965.EPI-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi S-W, Kim Y-I, Weitzel JN, Mason JB. Folate depletion impairs DNA excision repair in the colon of the rat. Gut. 1998;43:93–99. doi: 10.1136/gut.43.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borchers AH, Kennedy KA, Straw JA. Inhibition of DNA excision repair by methotrexate in Chinese hamster ovary cells following exposure to ultraviolet irradiation or ethylmethanesulfonate. Cancer Res. 1990;50:1786–1789. [PubMed] [Google Scholar]
- 35.Chen KH, Yakes FM, Srivastava DK, Singhal RK, Sobol RW, Horton JK, vanHouten B, Wilson SH. Upregulation of base excision repair correlates with enhanced protection against a DNA damaging agent in mouse cell lines. Nucleic Acids Res. 1998;26:2001–2007. doi: 10.1093/nar/26.8.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin LH, Cao S, Yu L, Cui J, Hamilton WJ, Liu PK. Upregulation of base excision repair activity for 8-hydroxy-2’deoxyguanosine in the mouse brain after forebrain ischemia reperfusion. J. Neurochem. 2000;74:1098–1105. doi: 10.1046/j.1471-4159.2000.741098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novakovic P, Stempak JM, Sohn K-J, Kim Y-I. Effects of folate deficiency on gene expression in the apoptosis and cancer pathways in colon cancer cells. Carcinogenesis. 2006;27:916–924. doi: 10.1093/carcin/bgi312. [DOI] [PubMed] [Google Scholar]
- 38.Chanson A, Sayd T, Rock E, Chambon C, Sante-Lhoutellier V, Potier de Courcy G, Brachet P. Proteomic analysis reveals changes in the liver protein pattern of rats exposed to dietary folate deficiency. J.Nutr. 2005;135:2524–2529. doi: 10.1093/jn/135.11.2524. [DOI] [PubMed] [Google Scholar]
- 39.Asagoshi K, Liu Y, Masaoka A, Lan L, Prasad R, Horton JK, Brown AR, Wang X-H, Bdour HM, Sobol RW, Taylor J-S, Yasui A, Wilson SH. DNA polymerase β-dependent long patch base excision repair in living cells. DNA Repair. 2010;9:109–119. doi: 10.1016/j.dnarep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton JK, Prasad R, Hou E, Wilson SH. protection against methylation-induced cytotoxicity by DNA polymerase β-dependent long patch base excision repair. J.Biol.Chem. 2000;275:2211–2218. doi: 10.1074/jbc.275.3.2211. [DOI] [PubMed] [Google Scholar]
- 41.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 42.Sobol RW, Wilson SH. Mammalian DNA polymerase β in base excision repair of alkylation damage. Prog. Nucleic Acid Res. Mol. Biol. 2001;68:57–74. doi: 10.1016/s0079-6603(01)68090-5. [DOI] [PubMed] [Google Scholar]
- 43.Horton JK, Joyce-Gray DF, Pachkowski BF, Swenberg JA, Wilson SH. Hypersensitivity of DNA polymerase b null mouse fibroblasts refelects accumulation of cytotoxic repair intermediates from site-specific alkyl DNA lesions. DNA Repair. 2003;2:27–48. doi: 10.1016/s1568-7864(02)00184-2. [DOI] [PubMed] [Google Scholar]
- 44.Choi S-W, Mason JB. Folate and Carcinogenesis: An integrated Scheme. J.Nutr. 2000;130:129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 45.Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 46.Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003;300:455. doi: 10.1126/science.1083557. [DOI] [PubMed] [Google Scholar]
- 47.Goelz SE, Vogelstein B, Hamilton SR, Feinberg AP. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985;228:187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- 48.Clark SJ, Melki J. DNA methylation and gene silencing in cancer: which is the guilty party? Oncogene. 2002;21:5380–5387. doi: 10.1038/sj.onc.1205598. [DOI] [PubMed] [Google Scholar]
- 49.Narayan S, Wilson SH. Kinetic analysis of Sp1-mediated transcriptional activation of the human DNA polymerase β promoter. Oncogene. 2000;19:4729–4735. doi: 10.1038/sj.onc.1203823. [DOI] [PubMed] [Google Scholar]
- 50.Yang X-P, He F, Rawson TY, Wilson SH. Human DNA polymerase-β promter: Phorbol ester activation is mediated through the cAMP response element and cAMP-response-element-binding protein. J. Biomed. Sci. 1997;4:279–288. doi: 10.1007/BF02258351. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi M, Hayashi Y, Hirose F, Shiroki K, Matsukage A. Activation of the mouse DNA polymerase β gene promoter by adenovirus type 12 E1A proteins. Nucleic Acids Res. 1992;20:2321–2325. doi: 10.1093/nar/20.9.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamph WW, Dwarki VJ, Ofir R, Montminy M, Verma IM. Negative and positive regulation by transcription factor cAMP response element-binding protein is modulated by phosphorylation. Proc. Natl. Acad. Sci. 1990;87:4320–4324. doi: 10.1073/pnas.87.11.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc. Natl. Acad. Sci. 1986;83:6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schule R, Umesono K, Mangelsdorf DJ, Bolado J, Pike JW, Evans RM. Jun-Fos and receptors for Viatmins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990;61:497–504. doi: 10.1016/0092-8674(90)90531-i. [DOI] [PubMed] [Google Scholar]
- 55.Schule R, Rangarajan P, Kliewer S, Ransone LJ, Bolado J, Verma IM, Evans RM. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62:1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- 56.Diamond MI, Miner JN, Yoshinaga SK, Yamamoto KR. Transcription factor interactions: Selectors of positive or negative regulation from a single DNA element. Science. 1990;249:1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- 57.Pedraza-Alva G, Zingg J-M, Jost J-P. Ap-1 binds to a putative cAMP response element of the MyoD1 promoter and negatively modulates MyoD1 expression in dividing myoblasts. J. Biol. Chem. 1994;269:6978–6985. [PubMed] [Google Scholar]