Abstract
Interferons (IFNs) are a first line of defense against viral infection. Herein we describe the use of an adenovirus vectored mouse IFN alpha gene (mDEF201) as a prophylactic and treatment countermeasure in a SARS-CoV-infected BALB/c mouse model. Complete survival protection was observed in mice given a single dose of mDEF201 administered intranasally 1, 3, 5, 7, or 14 days prior to lethal SARS-CoV challenge (p < 0.001), and body weights of these treated mice were unaffected by the challenge. In addition, low doses of mDEF201 protected lungs in a dose dependent manner as measured by a reduction in gross pathology. Intranasal treatment with mDEF201 ranging from 106 to 108 PFU significantly protected mice against a lethal SARS-CoV infection in a dose dependent manner up to 12 h post infection (p < 0.001). The data suggest that mDEF201 is a new class of antiviral agent further development as treatment for SARS-CoV infections.
Keywords: Interferon, mDEF201, SARS, Treatment, Prophylaxis
1. Introduction
Severe acute respiratory syndrome (SARS) is an emerging infectious disease caused by a novel human coronavirus (SARS-CoV), which results in severe pulmonary pathological failure (Drosten et al., 2003, Ksiazek et al., 2003, Peiris et al., 2003, Katze et al., 2003). Due to its high morbidity and mortality, SARS has been considered as an important global respiratory disease, with risk of epidemics even in populations with no new infections. Therefore, development of new anti-SARS-CoV agents and further experimental and clinical research are warranted to control future outbreaks.
Since SARS poses a significant threat for public health, and represents a challenge for antiviral drug development and administration (Groneberg et al., 2003, Groneberg et al., 2004), numerous types of agents have been tested against SARS-CoV both in vitro and in vivo (Barnard and Kumaki, 2009). Notably, antibodies to the SARS-CoV spike protein have been shown to block entry (Sui et al., 2004) and small peptides derived from the heptad repeat (HR) regions of SARS-CoV S protein have been shown to inhibit SARS-CoV infection by the interference of SARS-CoV fusion with target cells (Bosch et al., 2004, Ho et al., 2006). In addition, the main protease of SARS-CoV, which is essential for the replication cycle of SARS-CoV, has been a key target for the development anti-SARS drugs (Anand et al., 2003, Yang et al., 2003). Antisense RNA and RNA interference (siRNA) technologies have shown potential in treating some severe diseases including SARS-CoV infection (Ahlquist, 2002, Gibson, 1994, Johnson-Saliba and Jans, 2001, Leonard and Schaffer, 2006). Using siRNAs to inhibit SARS-CoV infection in Rhesus macaques, it has been demonstrated that siRNAs are effective both prophylactically and therapeutically in vivo (Chang et al., 2007). This effect was likely due to virus specific inhibition and not due to induction of interferon because the siSC2-5 and siCONa-b used in the study contained neither the 5′-UGUGU-3′ nor the 5′-GUCCUUCAA-3′ motifs suspected to be immunostimulatory elements when used with transfection reagents (Judge et al., 2005). In addition, there are also significant concerns with regard to toxicity issues and the use of siRNAs (Frantz, 2006).
We have previously described the inhibitory role of compounds approved for therapeutic use in humans, and the evaluation of promising in vitro inhibitors of SARS-CoV in mouse models and have shown that activation of the IFN pathways results in significant protection (Barnard et al., 2006). Both IFN alpha (IFN-α) B/D, and an IFN inducer, Ampligen (poly I:poly C124), were shown to potently inhibit virus titers in the lungs of infected mice (Barnard et al., 2006). Furthermore, IFN-alfacon 1 inhibits SARS-CoV infection in human bronchial epithelial Calu-3 cells (Kumaki et al., 2008). More recently, we have also shown that intranasal treatment with an interferon inducer, polyriboinosinic–polyribocytidylic acid stabilized with poly-l-lysine and carboxymethyl cellulose (poly ICLC, Hiltonol) was effective in protecting mice against a lethal infection with mouse-adapted SARS-CoV and reduced viral lung titers (Kumaki et al., 2010).
Recombinant interferons have been extensively researched for their therapeutic properties. IFN-α is an effective and safe recombinant human protein with broad clinical appeal (Brassard et al., 2002). However, while recombinant IFN-α has great therapeutic value, its usefulness is hindered by its short half-life. The terminal half-life of interferon alfacon 1 following subcutaneous dosing was 1.3 h in golden Syrian hamsters and 3.4 h in rhesus monkeys (Blatt et al., 1996). Due to rapid decay, multiple injections are required. To address the rapid degradation, PEGylated rIFN-α have been introduced with half-lives that are on the order of days instead of hours, thus reducing the number of injections to once per week (Bell et al., 2008). To circumvent the fast in vivo decay of rIFN-α, we developed a Adenovirus5 (Ad5) gene delivery platform to deliver the mouse IFN-α gene (subtype 5) that constitutively drives IFN-α production in situ. As IFN is species specific, the mouse IFN gene was required, and is denoted as Ad5-mIFN-α (or mDEF201). mDEF201 has normally been delivered intranasally, as studies have suggested that intranasal administration will prevent the host immune system from recognizing the Ad5 vector, thereby bypassing any pre-existing immunity present in the clinical scenario (Croyle et al., 2008). In addition, intranasal administration allows for needle-free dosing, which reduces the need for medical personnel, thereby facilitating mass distribution in the event of an epidemic (Weaver and Barry, 2008). The Ad5-mIFN-α has been shown by Defense Research and Development Canada (DRDC, Suffield) to provide both pre- and post-exposure protection against Western Equine Encephalitis virus (WEEV) (Wu et al., 2007). Thus, their findings illustrated the functionality of the mDEF201 system in a murine model, and suggested that adenovirus-mediated expression of IFN-α could be an alternative approach for the prevention and treatment of other viral infections.
In this report, we evaluate the use of mDEF201 as a prophylactic treatment and a therapeutic countermeasure for treating lethal SARS infections in BALB/c mice caused by a mouse-adapted SARS-CoV.
2. Materials and methods
2.1. Cells
Vero 76 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA), and were routinely grown in minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Thermo Fisher Scientific Co., Logan, UT). For in vitro antiviral assays, the serum was reduced to 2% FBS and gentamicin was added to the medium up to a final concentration of 50 μg/ml.
2.2. SARS-CoV Urbani strain and mouse-adapted SARS-CoV
SARS-CoV, strain Urbani (200300592), was obtained from Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA). This strain was propagated and titrated in Vero 76 cells.
The mouse-adapted SARS-CoV strain has previously been described (Day et al., 2009). The virus used for this experiment was passaged 25 times through BALB/c mice and has been verified as SARS-CoV by ELISA and PCR. The virus has 9 mutations, one of which affected two reading frames, resulting in changes to 10 amino acid residues compared with the wild type Urbani. Four mutations occur in the spike protein (S) region (Day et al., 2009). A putative G-A mutation has been observed in base pair 22,722 with some replicates, but it was not found in RNA from infected mouse lungs. The mutations were compared to the MA15 lethal strain (Roberts et al., 2007), revealing interesting similarities and differences in the mutation spectra associated with mouse adaptation in young BALB/c mice. In the structural genes, the Y436H mutation in the v2163 spike region was conserved in strain MA15, and the M-mutation was in a similar location on both lethal strains (S6L for v2163, E11K in MA15). In the replicase genes, the nsp9 mutation (E4185D) was located near an nsp9 mutation (T4184A) found in strain MA15, and both lethal strains had an nsp13 mutation. In the mouse model, infected animals die between days 4 and 8, with 90–100% mortality achieved by day 8 (Day et al., 2009).
In an infected mouse, gross lung pathology is characterized by a severely inflamed lung surface with severe discoloration; most of the lung is dark plum-colored. Weight loss is excessive relative to virus exposure. The occasional surviving animal may lose 25% or more of its weight, but surviving mice regain all of the initial weight by day 9 or 10 and live at least 21 days or more. Virus titers in the lungs exceed 107/ml, the titers peaking at days 3–4. In mice that survive, virus lung titers persist at least until day 7 (Day et al., 2009). An LD95 dose (2.5 × 103 PFU) was used to infect mice.
All experiments involving infectious viruses were conducted in an approved select agent-approved, biosafety level 3+ laboratory using appropriate personal protective equipment.
2.3. Test articles
Construction of mDEF201 has been described previously (Wu et al., 2007), briefly the mouse interferon alpha 5 gene was cloned into a replication deficient Ad5 vector (deletions of E1 and E3 genes), amplified in 293 cells and purified by cesium chloride gradient centrifugation. Stock solutions of mDEF201 (Ad5-mIFN alpha, Defyrus Inc., Toronto, Ontario, Canada) were provided at 3.11 × 109 PFU/ml and stored at −80 °C. The IFN inducer, polyriboinosinic–polyribocytidylic acid stabilized with poly-l-lysine and carboxymethyl cellulose (poly ICLC) was obtained from Andres M. Salazar (Oncovir, Inc., WA, DC, 20008). Both mDEF201 and poly ICLC were diluted in physiologically sterile saline (PSS) for in vivo experiments just before use.
2.4. Animals
Specific pathogen-free female 18–20 g BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA) for this study. They were maintained on Wayne Lab Blox and fed with standard mouse chow and tap water ad libitum. The BALB/c mice were quarantined for 24 h prior to use. The animal studies were carried out in a certified bio-safety level 3+ animal facility. Personnel entering the facility wore powered air-purifying respirators (3 M HEPA Air-Mate; 3 M, Saint Paul, MN) and Tyvek body protection suits. Animal studies were approved by Utah State University Animal Care Committee.
2.5. Experimental design
The general experimental design is described below. The BALB/c mice were anesthetized with a 0.1 ml intraperitoneal injection of 20 mg/kg of Ketamine® and challenged with SARS-CoV intranasally (i.n.) in a volume of 0.05 ml. Groups of 10 mice were administered mDEF201 (doses and times of administration varied as described in Section 3) intranasally and challenged with 2.5 × 103 PFU of mouse-adapted SARS-CoV. Poly ICLC was administered i.n. 24 h before virus exposure and 8 h after exposure to virus and served as a positive control for controlling viral infection. Fifteen mice were treated i.n. with vehicle placebo (PSS or PBS) or empty vector not expressing mDEF201 at various times prior to or after virus exposure, depending on the experiment. Mice in these groups represented the placebo controls. SARS-CoV-infected and mock-infected mice were weighed every day and clinical signs of disease were also observed and recorded daily. Animal deaths were recorded for up to 21 days post virus exposure. Animals that lost greater than 30% of their initial body weight were humanely euthanized by CO2 asphyxiation, and the day of euthanization was designated as the day of death due to infection.
2.6. Compound toxicity determination
For mDEF201, a dose range finding experiment was carried out to determine the effect of i.n. administration on the animals’ body weight and overt measurable toxicology. Three mice were used per treatment group, and toxicity was evaluated in terms of weight change and adverse events. Mice were weighed every day from 24 h prior to virus infection to day 21 post exposure. Adverse events for which observations were made included ruffling of fur, lethargy, paralysis, incontinence, repetitive circular motion, and aggression. No overt toxicity or adverse events were detected.
2.7. Lung score determinations
Five mice from each low dose group (105, 104, 103, 102) were sacrificed on day 3 and lung samples from each lobe were weighed and placed in a Petri dish. Lungs were scored based on surface appearance and were assigned a score ranging from 0 to 4, with 0 indicating normal appearance and 4 denoting that the entire surface area of the lungs exhibited discoloration. The lungs were scored with 1–4. This score represented the observed gross pathology of the lung surface. Thus, 25–100% of the surface of the lungs exhibited plum-colored discoloration and/or hemorrhaging (Sidwell et al., 1995). Significant differences were analyzed by the Kruskal–Wallis test followed by Dunn's posttest for evaluating the significant pairwise comparisons.
2.8. Survival and weight change analyses
Mice were weighed in groups prior to treatment and then every day thereafter to determine the average weight change for all animals in each treatment group. Weights were expressed as group averages for each day and evaluated for statistical significance by the two-way analysis of variance. Since statistical significance was achieved, significant differences between two treatment groups were analyzed by Bonferroni's pairwise comparison tests.
Survival analyses were first conducted using the Kaplan–Meier method and a Logrank test. When significant differences among the treatment groups were observed, pairwise comparisons of survivor curves (PSS vs. any treatment) were analyzed by the Gehan-Breslow-Wilcoxon test, and the relative significance was adjusted to a Bonferroni-corrected significance threshold for the number of treatment comparisons done. Mean day of death (deaths caused by disease) was calculated and analyzed by the Kruskal-Wallis test followed by Dunn's posttest for evaluating the significant pairwise comparisons. Live numbers per total mice in a group differences were evaluated by contingency table analysis.
3. Results
3.1. Effects of mDEF201 on weight change of uninfected female BALB/c mice
In a preliminary study to determine the toxicity of mDEF201 by recording weight changes and observing adverse advents, uninfected mice randomly assigned to anmDEF201 treatment group. Mice were administered intranasally meDEF201 106 or 105 PFU at 24 h prior to challenge with mouse-adapted virus. All mDEF201-treated mice gained weight at rates indistinguishable to mice receiving PSS (data not shown). Mice treated with poly ICLC were the only group to lose noticeable amounts of weight, but regained the lost body weight after day 2 and by the end of the experiment their gain was equal to the other groups. No adverse events were observed for any of the toxicity control mice used in the experiment (data not shown).
3.2. mDEF201 protects BALB/c mice against a lethal dose of SARS-CoV-infection
mDEF201 at 106 or 105 PFU or placebo (PSS or PBS) intranasally administered (25 μl/nare) 24 h prior to challenge with 2.5 × 103 PFU of mouse-adapted SARS-CoV. An IFN inducer, poly ICLC (1 mg/kg), was given intranasally to mice 24 h before and 8 h after virus exposure. The survival rates of both groups were monitored daily and determined at day 21 after infection. Treatments with either mDEF201 or poly ICLC by the intranasal route were effective in protecting mice against a lethal infection with mouse-adapted SARS-CoV (p < 0.001, Fig. 1 ). The mice receiving the mDEF201 treatments were also significantly protected against infection related weight loss (data not shown, p < 0.05–p < 0.001, days 2–7, mDEF201 either dose vs. mDEF201 empty vector or PSS). Several mice in the placebo groups also survived the challenge, probably due to natural susceptibility variation in this species of mouse.
Fig. 1.
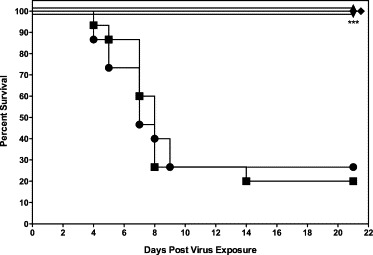
Effects of mDEF201 on survival of female BALB/c mice infected with a lethal dose of mouse-adapted SARS-CoV. A total of 106 or 105 PFU of mDEF201 or vehicle placebo were administered intranasally 24 h prior to challenge with mouse-adapted virus. Experimental groups, n = 10; placebo group, n = 15. Data were statistically analyzed by Logrank test followed by Gehan–Breslow–Wilcoxon pairwise comparison tests. Relative significance was adjusted to a Bonferroni-corrected significance threshold for the number of treatment comparisons done. (●) PSS (−24 h), (■) PSS (−24 h, +8 h), (▴) mDEF201 (106 PFU, −24 h), (▾) mDEF201 mDEF201 (105 PFU, −24 h), (♦) Poly ICLC (1 mg/kg, −24 h, +8 h). *** p < 0.001 each compound vs. PSS.
3.3. Extended prophylaxis with mDEF201 protects SARS-CoV-infected BALB/c mice from mortality
A dose of 106 PFU of mDEF201 or vehicle placebo was administered intranasally at 14, 7, 5 or 3 days prior to challenge with 2.5 × 103 PFU of mouse-adapted SARS-CoV. Complete survival was recorded for mice treated with a single dose of mDEF201 at all time points prior to challenge. (p < 0.001, Fig. 2A). The mice receiving the prophylactic mDEF201 were also significantly protected against weight loss due to the infection (p < 0.001, Fig. 2B). One mouse survived in the placebo-treated group and regained weight as has previously been described (Day et al., 2009).
Fig. 2.
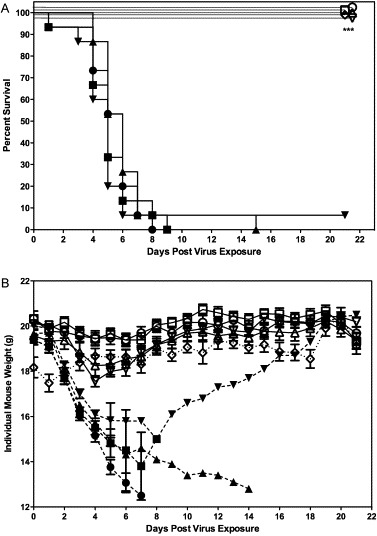
(A) Effects of mDEF201 on survival of female BALB/c mice infected with a lethal dose of mouse-adapted SARS-CoV. A total of 106 PFU of mDEF201 or vehicle placebo were administered intranasally 14, 7, 5 and 3 day prior to challenge with mouse-adapted virus. Experimental groups, n = 10; placebo group, n = 15. Data were statistically by Logrank test followed by Gehan–Breslow–Wilcoxon pairwise comparison tests. Relative significance was adjusted to a Bonferroni-corrected significance threshold for the number of treatment comparisons done. *** p < 0.001 each compound vs. respective PSS. (●) PSS (−14 days), (■) PSS (−7 day), (▴) PSS (−5 days), (▾) PSS (−3 days), (○) mDEF201 (−14 days), (□) mDEF201 (−7 days), (▵) mDEF201 (−5 days), (▿) mDEF201 (−3 days), (♢) Poly ICLC (1 mg/kg, −24 h, +8 h). * p < 0.001 each compound vs. respective PSS. (B) Effects of mDEF201 on individual mouse weight of female BALB/c mice infected with a lethal dose of mouse-adapted SARS-CoV. Data were statistically analyzed by two-way analysis of variance. Post-tests were done using Bonferroni's pairwise comparison tests. (●) PSS (−14 days), (■) PSS (−7 days), (▴) PSS (−5 days), (▾) PSS (−3 days), (○) mDEF201 (−14 days), (□) mDEF201 (−7 days), (▵) mDEF201 (−5 days), (▿) mDEF201 (−3 days), (♢) Poly ICLC (1 mg/kg, −24 h, +8 h). *** p < 0.001 each compound vs. respective PSS.
3.4. Effective dose range of mDEF201 as prophylactic against SARS-CoV
SARS-CoV-infected female BALB/c mice were administered with mDEF201 (105, 104, 103 and 102 PFU) or vehicle placebo intranasally 24 h prior to challenge with mouse-adapted virus. The mice were weighed every day until day 21, and both weight loss and clinical signs were observed. Only mice receiving a prophylactic dose of mDEF201 at 105 PFU were significantly protected from mortality (p < 0.001, Fig. 3A).
Fig. 3.
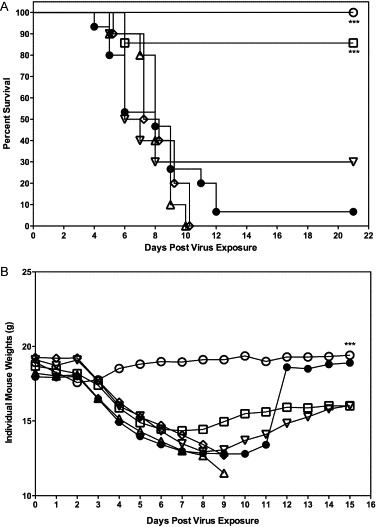
(A) Dose–response of mDEF201 on survival of female BALB/c mice infected with a lethal dose of mouse-adapted SARS-CoV. SARS-CoV-infected BALB/c mice were administered with mDEF201 (105, 104, 103 and 102 PFU) or vehicle placebo intranasally 24 h prior to challenge with mouse-adapted virus. Experimental groups, n = 10; placebo group, n = 15. Data were statistically by Logrank test followed by Gehan–Breslow–Wilcoxon pairwise comparison tests. Relative significance was adjusted to a Bonferroni-corrected significance threshold for the number of treatment comparisons done. (●) PSS, (□) mDEF201 (105 PFU), (▵) mDEF201 (104 PFU), (▿) mDEF201 (103 PFU), (♢) mDEF201 (102 PFU), ○ Poly ICLC (1 mg/kg). *** p < 0.001 each compound vs. PSS. (B) Effects of mDEF201 on individual mouse weights of female BALB/c mice infected with a lethal dose of mouse-adapted SARS-CoV. Data were statistically analyzed by two-way analysis of variance. Post-tests were done using Bonferroni's pairwise comparison tests. (●) PSS, (□) mDEF201 (105 PFU), (▵) mDEF201 (104 PFU), ▿ mDEF201 (103 PFU), ♢ mDEF201 (102 PFU), (○) Poly ICLC (1 mg/kg). *** p < 0.001 Poly ICLC vs. PSS.
Infected mice receiving mDEF201 at 105 PFU were also protected against the serious weight loss suffered by mice receiving the placebo, PSS (Fig. 3B). Surviving mice receiving mDEF201 (105 PFU) or poly ICLC went on to recover some weight by the end of the experiment (Fig. 3B). Mice treated with 105 PFU mDEF201 trended towards less weight loss relative to other mice in other treatment groups and the placebo-treated mice. In contrast, the lower doses of mDEF201 did not significantly protect against death and did not ameliorate weight loss in infected, mice through the critical days of infection from day 3 to 9 (Fig. 3B).
3.5. Effects of mDEF201 on lung scores in a murine SARS-CoV model
The efficacy described above was also supported by the gross pathology scores of the lungs from low dose mDEF201-treated infected mice, which were recorded for each dose at day 3 after virus exposure (Fig. 4 ). At day 3, mDEF201 (105 PFU) afforded significant protection against the surface hemorrhaging observed for infected, vehicle placebo-treated mice (p < 0.01, Fig. 4). Compared to placebo-treated mice, mice receiving the mDEF201 (104 or 103 PFU) also displayed significantly less surface pathology (p < 0.01) as well mice receiving poly ICLC (p < 0.001). There were no effects on virus lung titers, a phenomenon that has been reported by others when using interferon to treat SARS infections in animals (Smits et al., 2010).
Fig. 4.
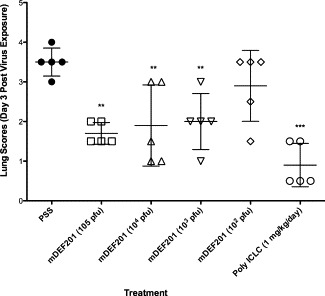
Effects of mDEF201 on lung scores of female BALB/c mice infected with a lethal dose of mouse-adapted SARS-CoV at day 3. Each mouse lung lobe was removed, weighed, placed in a plastic dish, and then assigned a score ranging from 0 (normal appearing lung) to 4 (maximal plum coloration in 100% of lung). Scatter plots are reported as individual values and mean ± SD are indicated by horizontal bars. The significance of the lung score differences between treatment groups was determined using a Kruskal–Wallis test, followed by Dunn's post-tests for evaluating significant pairwise comparisons. Significant lung weight differences compared to the placebo-treated mice were evaluated by analysis of variance, after which individual treatment values were compared to the PSS control using Newman–Keuls pair-wise comparison tests. (●) PSS, (■) mDEF201 (105 PFU, (▴) mDEF201 (104 PFU), (▾) mDEF201 (103 PFU), (□) mDEF201 (−106 PFU, +12 h), (▵) mDEF201 (−106 PFU, +24 h), (▿) mDEF201 (−105 PFU, +6 h), (○) mDEF201 (−105 PFU, +12 h), (*) mDEF201 (−105 PFU, +24 h), (♢) Poly ICLC (1 mg/kg, −24 h, +8). ** p < 0.01, *** p < 0.001 each compound vs. PSS.
3.6. Protection of SARS-CoV-infected mice against death with higher doses of mDEF201 administered post virus exposure
The efficacy of mDEF201 as a treatment for SARS-CoV infection was evaluated with 105 or 106 PFU of intranasal mDEF201 at 6, 12 or 24 h post-challenge. The 106 PFU dose of mDEF201 resulted in 90% survival when administered at 6 h post-challenge, at a time when clinical signs (weight loss) of SARS were being manifested in the untreated BALB/c mice (p < 0.001, Fig. 5 ). At this dose or the 105 PFU dose, mDEF201 was not protective when given 12 or 24 h after SARS-CoV infection (Fig. 5). In a subsequent therapeutic experiment, even higher doses of 107 or 108 PFU of mDEF201 were administered intranasally at 6, 12 or 24 h post-challenge with an empty Ad5 vector as a negative control. All animals treated with mDEF201 at 108 PFU at 6 h post infection survived, and significantly protected against mice against weight loss from days 3–13 post virus exposure (p < 0.05–p < 0.001, data not shown). In addition, animals treated with 108 PFU of mDEF201 showed a marked increase in survival when treated as late as 24 h post infection (Fig. 6 ). Although we observed a trend towards protection against weight loss, a statistically significant protection was not observed at later time points.
Fig. 5.
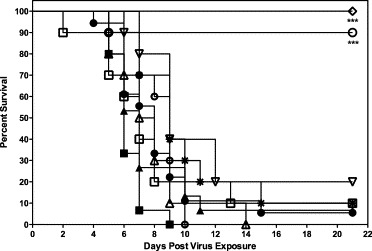
Therapeutic effects of mDEF201 on survival of female BALB/c mice infected with a lethal dose of mouse-adapted SARS-CoV. A total of 106 or 105 PFU of mDEF201 or vehicle placebo were administered intranasally 6, 12 or 24 h after challenge with mouse-adapted virus. Experimental groups, n = 10; placebo group, n = 15. Data were statistically by Logrank test followed by Gehan–Breslow–Wilcoxon pairwise comparison tests. Relative significance was adjusted to a Bonferroni-corrected significance threshold for the number of treatment comparisons done. (●) PSS (+6 h), (■) PSS (+12 h), (▴) PSS (+24 h), (○) mDEF201 (106 PFU, +6 h), (♢) Poly ICLC (1 mg/kg, −24 h, +8 h). *** p < 0.001 each compound vs. respective PSS.
Fig. 6.
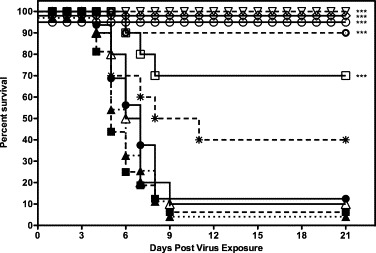
Therapeutic effects of high dose mDEF201 on survival of female BALB/c mice infected with a lethal dose of mouse-adapted SARS-CoV. Data were statistically by Logrank test followed by Gehan–Breslow–Wilcoxon pairwise comparison tests. Relative significance was adjusted to a Bonferroni-corrected significance threshold for the number of treatment comparisons done. (●) PSS (+6 h), (■) PSS (+12 h), (▴) PSS (+24 h), (○) mDEF201 (−107 PFU, +6 h), (□) mDEF201 (107 PFU, +12 h), (▵) mDEF201 (107 PFU, +24 h), (▿) mDEF201 (−108 PFU, +6 h), (○) mDEF201 (108 PFU, +12 h), (*) mDEF201 (108 PFU, +24 h), (♢) Poly ICLC (1 mg/kg, −24 h, +8 h). *** p < 0.0001, each compound vs. mDEF201 empty vector.
4. Discussion
In an effort to recapitulate the human disease, we have employed the SARS-CoV infected BALB/c mouse model as a surrogate for SARS-CoV infection of humans, with the respiratory tract as the site of virus inoculation (Day et al., 2009). Our previous data revealed a peak of viral load (106/ml) approximately 3–4 days post SARS-CoV infection (Kumaki et al., 2010). Extrapolating the rodent data, in the macaque model (Macaca fascicularis), IFN-α dosing protected SARS-CoV-infected animals when given prophylactically (Haagmans et al., 2004). In a preliminary human clinical study, Loutfy et al. (2003) tested IFN-alfacon 1 combined with corticosteroids to assess potential clinical benefit and safety for SARS patients. This data demonstrated significant benefits to patients receiving IFN alfacon 1 and suggests that repeated doses of IFN alone or with corticosteroids may function as an antiviral therapeutic for the treatment of SARS patients (Loutfy et al., 2003).
In the current study, mice administered mDEF201 as a prophylactic up to two weeks prior to viral challenge survived the virus infection. In addition, mDEF201 therapeutically protected 70–100% of animals when administered up to 12 h after challenge in a dose dependent manner. We believe that the constitutive expression of IFN-α provided by mDEF201 stimulates the innate immune response to effectively suppress the SARS-CoV challenge and results in these significant survival benefits, especially when administered prophylactically.
The normal host innate immune response against virus insult includes the production of IFN type I (IFN-α/β), which is initiated to limit viral replication. Virus-infected cells usually cause the activation of several transcription factors, such as IFN regulatory factor 3 (IRF-3), which play a central role in downstream gene activation (Lin et al., 1998). Once IFN is synthesized and secreted from the cells, it binds to cell surface receptors and induces transcription, which results in an anti-viral state in the target cells. However, the production of IFN type I by SARS-CoV-infected cells is limited (Chen and Subbarao, 2007, Spiegel et al., 2005) and neither endogenous IFN transcripts nor IFN promoter activity are detected (Spiegel et al., 2005). This lack of IFN activity has been attributed to proteins, perhaps virion and non-structural proteins of the SARS-CoV, which antagonize IFN and block transcription factors necessary for the expression of IFN (Kopecky-Bromberg et al., 2007).
Some proteins of SARS-CoV have been reported to antagonize the production and the immunomodulatory effect of endogenous IFN. The nucleocapsid protein (N) of SARS-CoV has been shown to inhibit IFN-beta (IFN-β) induced by poly IC (Lu et al., 2009). In that study, the authors found that the N protein inhibition of IFN-β production was not due to overexpression of downstream signaling molecules of the toll-like receptor 3 (TLR3)- or the RIG-I-like receptors (RLR)-dependent pathways. It was likely that SARS-CoV N protein targeted the initial step, probably the cellular PRRs (pattern recognition receptors)-RNAs-recognition step in the innate immune pathways, to suppress IFN expression responses (Lu et al., 2009). In addition, a nonstructural protein of SARS-CoV, nsp1, also has been found to modulate interferon induction. The nsp1 protein has been shown to suppress type I interferon production, by promoting host mRNA degradation and inhibiting host translation in infected cells (Kamitani et al., 2009). Yet despite these inhibitory molecules, the virus remains susceptible to exogenous IFNs (Chen and Subbarao, 2007). It is interesting that neither of these wild type virus gene products that modulate IFN pathways or IFN expression were altered in the mouse adapted Urbani strain of SARS-CoV used in the current study (Day et al., 2009).
IFN-α/β have previously been shown to be effective against SARS-CoV infection (Cinatl et al., 2003). In vitro studies report a potent inhibition of SARS-CoV infection and replication by exogenous IFN-β, and, less efficiently by IFN-α (Chen et al., 2004, Cinatl et al., 2003, Dahl et al., 2004, Tan et al., 2004, Zheng et al., 2004). In terms of IFN inducers, we recently reported that intranasal treatment with poly ICLC protected mice against a lethal mouse-adapted SARS-CoV infection with reduced viral load in the lungs (Kumaki et al., 2010). Poly ICLC was effective when therapy was initiated 24 h or more before infection or within 8 h after virus inoculation. In the current study, similar efficacy was achieved in protecting mice against death not only with the interferon inducer but also with mDEF201 vector that constitutively expresses interferon, but also without substantially reducing viral lung titers. Similar results were obtaineod by others when using interferon to treat SARS infections (Ströher et al., 2004, Smits et al., 2010). In this regard, its worth noting that mice infected with non-mouse adapted strains of SARS-CoV always support vigorous replication of virus in the lungs with virus lung titers peaking at 24 h post virus exposure and continuing to remain at comparable levels through day 3 or 4 of the infection. At day 6 of the infection, virus replication ceases or dramatically slows down and virus is cleared within 1 or two days thereafter (Subbarao et al., 2004, Rockx et al., 2009). All the while, no pathogenesis is detectable. It could be that in the current study later induction of other interferon pathways upregulated by meDEF1 are sufficient to control damage to host and yet allow a certain amount of virus replication. For example, despite virus sensitivity to IFN-α/β (Cinatl et al., 2003), SARS-CoV still replicates rapidly in lungs in mice as previously mentioned. It has previously been shown that, in non-lymphatic cells infected with SARS-CoV, neither dimerization nor stable nuclear accumulation of IFN-regulatory factor 3 (IRF-3) occurs (Spiegel et al., 2005). IRF-3 is a protein with a key role in the transactivation of the IFN-β promoter. In addition, the initial production of interferon normally triggers expression of a related factor, IRF-7, but not in a SARS-CoV infection (Honda et al., 2005, Kuri et al., 2009. In the latter study, Kuri et al. (2009) recently showed results that suggest that interferon produced by plasmacytoid dendritic cells could enable cells to launch a host response to SARS-CoV, that IRF-3 and IRF-7 may be active at subdetectable levels and still function to promote interferon responses, and that SARS-CoV infection does not activate IRF-7. Thus, active IRF-7 and IRF-3 might be necessary to reduce virus lung titers early in the infection, but later induction of other interferon pathways might be sufficient to control the damaging effects of virus replication in the lung triggered by a virus-induced a cytokine storm and ARD (Rockx et al., 2009, Smits et al., 2010).
What advantage does mDEF-1 have over other methods of interferon delivery? Paragas et al. (2005) reported that IFN-alfacon 1 had anti-SARS-CoV activity only prior to infection in a cell-based model in vitro. In this case, IFN-alfacon 1 likely induced an antiviral state in the target cells, which produced a cellular environment that was not suitable for viral replication. However, IFN-alfacon 1 did not protect the cells when added post infection, whereas mDEF201 afforded compete survival even when added 12 h after virus infection and gave partial protection at very high doses out to 24 h post virus exposure. This illustrates the benefit of constitutive expression of interferon using the AD5 vector. Further studies need to be conducted in which direct comparisons of IFN protein and mDEF201 to elucidate this further. In addition, it is likely that lower doses given over a period of weeks, as is the case for the DEF201 concept, could counter the rapid degradation and toxicity associated with bolus dosing of interferon. Thus, the benefits of the mDEF201 approach over conventional therapy with recombinant human IFN or interferon inducers include: (a) single, intranasal dose vs. repeated injected dosing and (b) an extended therapeutic window. These benefits, if extrapolated to a clinical setting, could have utility during a large-scale SARS epidemic as a post-exposure prophylactic or treatment as it facilitates quick and easy dissemination to an at-risk population, such as medical chain workers. In short, this approach retains the proven clinical benefits of IFN-α in managing SARS-CoV infections, but overcomes the short half-life limitations of exogenous IFN or IFN inducers. More broadly, given the efficacy profile of mDEF201 shown here and that of Wu et al. (2007), mDEF201 has significant potential as a broad spectrum, host-directed antiviral.
Although IFNs could theoretically be ideal antiviral agents, their therapeutic value is limited since they are effective only during relatively short periods and at high doses they have serious toxic effects on the host. Therefore, attempts to use exogenous IFN for the treatment of human viral diseases had also been met with limited success. To address this issue Defyrus has developed a technology that utilizes the Adenovirus5 (Ad5) gene delivery platform to deliver the human IFN-alpha gene that drives IFN-alpha production in situ. The intranasal (i.n.) administration of the Ad5-IFN-a prevents its recognition by the host immune system. In addition, the shelf-stable, powdered formulation administered i.n. allows for easy storage and administration in the event of the need for mass distribution.
Acknowledgments
We thank Michael A. Morrey for kindly providing technical assistance, Heather L Greenstone for scientific discussion. We are grateful to other colleagues for their support. This work was supported by a contract N01-AI-15435 from the Virology Branch, National Institute of Allergic and Infectious Diseases, National Institutes of Health. Joseph K.-K. Li was also supported in parts by AES Project 538.
Contributor Information
Jane Ennis, Email: jane.ennis@defyrus.com.
Dale L. Barnard, Email: dale.barnard@usu.edu.
References
- Ahlquist P. RNA-dependent RNA polymerases, viruses, and RNA silencing. Science. 2002;296:1270–1273. doi: 10.1126/science.1069132. [DOI] [PubMed] [Google Scholar]
- Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Barnard D.L., Kumaki Y. Development in the search for the small-molecule inhibitors for treatment of severe acute respiratory syndrome coronavirus. In: LaFemina R.L., editor. Antiviral Research: Strategies in Antiviral Drug Discovery. ASM Press; Washington, DC: 2009. pp. 209–222. [Google Scholar]
- Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L., Chan P.K., Sidwell R.W. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir. Chem. Chemother. 2006;17:275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- Bell S.J., Fam C.M., Chlipala E.A., Carlson S.J., Lee J.I., Rosendahl M.S., Doherty D.H., Cox G.N. Enhanced circulating half-life and antitumor activity of a site-specific pegylated interferon-alpha protein therapeutic. Bioconjug. Chem. 2008;19:299–305. doi: 10.1021/bc070131q. [DOI] [PubMed] [Google Scholar]
- Blatt L.M., Davis J.M., Klein S.B., Taylor M.W. The biologic activity and molecular characterization of a novel synthetic interferon-alpha species, consensus interferon. J. Interferon Cytokine Res. 1996;16:489–499. doi: 10.1089/jir.1996.16.489. [DOI] [PubMed] [Google Scholar]
- Bosch B.J., Martina B.E., Van Der Zee R., Lepault J., Haijema B.J., Versluis C., Heck A.J., De Groot R., Osterhaus A.D., Rottier P.J. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassard D.L., Grace M.J., Bordens R.W. Interferon-alpha as an immunotherapeutic protein. J. Leukoc. Biol. 2002;71:565–581. [PubMed] [Google Scholar]
- Chang Z., Babiuk L.A., Hu J. Therapeutic and prophylactic potential of small interfering RNAs against severe acute respiratory syndrome: progress to date. BioDrugs. 2007;21(1):9–15. doi: 10.2165/00063030-200721010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Subbarao K. The immunobiology of SARS*. Annu. Rev. Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- Chen F., Chan K.H., Jiang Y., Kao R.Y., Lu H.T., Fan K.W., Cheng V.C., Tsui W.H., Hung I.F., Lee T.S., Guan Y., Peiris J.S., Yuen K.Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle M.A., Patel A., Tran K.N., Gray M., Zhang Y., Strong J.E., Feldmann H., Kobinger G.P. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008;3:e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H., Linde A., Strannegard O. In vitro inhibition of SARS virus replication by human interferons. Scand. J. Infect. Dis. 2004;36(11–12):829–831. doi: 10.1080/00365540410021144. [DOI] [PubMed] [Google Scholar]
- Day C.W., Baric R., Cai S.X., Frieman M., Kumaki Y., Morrey J.D., Smee D.F., Barnard D.L. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. 2009;395:210–222. doi: 10.1016/j.virol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Frantz S. Safety concerns raised over RNA interference. Nat. Rev. Drug. Discov. 2006;5:528–529. doi: 10.1038/nrd2104. [DOI] [PubMed] [Google Scholar]
- Gibson I. Antisense DNA and RNA strategies: new approaches to therapy. J. R. Coll. Physicians Lond. 1994;28:507–511. [PMC free article] [PubMed] [Google Scholar]
- Groneberg D.A., Witt C., Wagner U., Chung K.F., Fischer A. Fundamentals of pulmonary drug delivery. Respir. Med. 2003;97:382–387. doi: 10.1053/rmed.2002.1457. [DOI] [PubMed] [Google Scholar]
- Groneberg D.A., Fischer A., Chung K.F., Daniel H. Molecular mechanisms of pulmonary peptidomimetic drug and peptide transport. Am. J. Respir. Cell Mol. Biol. 2004;30:251–260. doi: 10.1165/rcmb.2003-0315TR. [DOI] [PubMed] [Google Scholar]
- Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G., van Riel D., de Jong T., Itamura S., Chan K.H., Tashiro M., Osterhaus A.D. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Wei Y.C., Cheng S.E., Chang Y.H., Liu H.J., Hsiang C.Y. Design and biological activities of novel inhibitory peptides for SARS-CoV spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2006;69:70–76. doi: 10.1016/j.antiviral.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N., Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:7034. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Johnson-Saliba M., Jans D.A. Gene therapy: optimising DNA delivery to the nucleus. Curr. Drug Targets. 2001;2:371–399. doi: 10.2174/1389450013348245. [DOI] [PubMed] [Google Scholar]
- Judge A.D., Sood V., Shaw J.R., Fang D., McClintock K., MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Kamitani W., Huang C., Narayanan K., Lokugamage K.G., Makino S. A two-pronged strategy to suppress host protein synthesis by SARS coronavirus Nsp1 protein. Nat. Struct. Mol. Biol. 2009;16:1134–1140. doi: 10.1038/nsmb.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M.G., Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Kopecky-Bromberg S.A., Martinez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kumaki Y., Day C.W., Wandersee M.K., Schow B.P., Madsen J.S., Grant D., Roth J.P., Smee D.F., Blatt L.M., Barnard D.L. Interferon alfacon 1 inhibits SARS-CoV infection in human bronchial epithelial Calu-3 cells. Biochem. Biophys. Res. Commun. 2008;371:110–113. doi: 10.1016/j.bbrc.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaki Y., Day C.W., Bailey K.W., Wandersee M.K., Wong M.H., Madsen J.R., Madsen J.S., Nelson N.M., Hoopes J.D., Woolcott J.D., McLean T.Z., Blatt L.M., Salazar A.M., Smee D.F., Barnard D.L. Induction of interferon-gamma-inducible protein 10 by SARS-CoV infection, interferon alfacon 1 and interferon inducer in human bronchial epithelial Calu-3 cells and BALB/c mice. Antivir. Chem. Chemother. 2010;20:169–177. doi: 10.3851/IMP1477. [DOI] [PubMed] [Google Scholar]
- Kuri T., Zhang X., Habjan M., Martinez-Sobrido L., Garcia-Sastre A., Yuan Z., Weber F. Interferon priming enables cells to partially overturn the SARS coronavirus-induced block in innate immune activation. J. Gen. Virol. 2009:2686–2694. doi: 10.1099/vir.0.013599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J.N., Schaffer D.V. Antiviral RNAi therapy: emerging approaches for hitting a moving target. Gene Ther. 2006;13:532–540. doi: 10.1038/sj.gt.3302645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Heylbroeck C., Pitha P.M., Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., Pham D.H., Deif H., LaMere E.A., Chang M., Kain K.C., Farcas G.A., Ferguson P., Latchford M., Levy G., Dennis J.W., Lai E.K., Fish E.N. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Lu X., Pan J., Tao J., Guo D. SARS-CoV nucleocapsid protein antagonizes IFN-beta response by targeting initial step of IFN-beta induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2009 doi: 10.1007/s11262-010-0544-x. (Epub date: 2010/10/27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paragas J., Blatt L.M., Hartmann C., Huggins J.W., Endy T.P. Interferon alfacon1 is an inhibitor of SARS-corona virus in cell-based models. Antiviral Res. 2005;66:99–102. doi: 10.1016/j.antiviral.2005.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Deming D., Paddock C.D., Cheng A., Yount B., Vogel L., Herman B.D., Sheahan T., Heise M., Genrich G.L., Zaki S.R., Baric R., Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3(1):e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T., Frieman M., Dyer M.D., Teal T.H., Proll S., van den Brand J., Baric R. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R.W., Bailey K.W., Wong M.H., Huffman J.H. In vitro and in vivo sensitivity of a non-mouse-adapted influenza A (Beijing) virus infection to amantadine and ribavirin. Chemotherapy. 1995;41:455–461. doi: 10.1159/000239382. [DOI] [PubMed] [Google Scholar]
- Smits S.L., de Lang A., van den Brand J.M.A., Leijten L.M., van Ijcken W.F., Eijkemans M.J.C., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D.M.E., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6:e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M., Pichlmair A., Martinez-Sobrido L., Cros J., Garcia-Sastre A., Haller O., Weber F. Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 2005;79:2079–2086. doi: 10.1128/JVI.79.4.2079-2086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströher U., DiCaro A., Li Y., Strong J.E., Aoki F., Plummer F., Jones S.M., Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-α. J. Infect. Dis. 2004;189:1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K., Packard M., Shieh W.J., Zaki S., Murphy B. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S., Olurinde M., Choe H., Anderson L.J., Bellini W.J., Farzan M., Marasco W.A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E.L., Ooi E.E., Lin C.Y., Tan H.C., Ling A.E., Lim B., Stanton L.W. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver E.A., Barry M.A. Effects of shielding adenoviral vectors with polyethylene glycol on vector-specific and vaccine-mediated immune responses. Hum. Gene Ther. 2008;19:1369–1382. doi: 10.1089/hum.2008.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.Q., Barabe N.D., Huang Y.M., Rayner G.A., Christopher M.E., Schmaltz F.L. Pre- and post-exposure protection against Western equine encephalitis virus after single inoculation with adenovirus vector expressing interferon alpha. Virology. 2007;369:206–213. doi: 10.1016/j.virol.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Yang H., Yang M., Ding Y., Liu Y., Lou Z., Zhou Z., Sun L., Mo L., Ye S., Pang H., Gao G.F., Anand K., Bartlam M., Hilgenfeld R., Rao Z. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., He M.L., Wong K.L., Lum C.T., Poon L.L., Peng Y., Guan Y., Lin M.C., Kung H.F. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma) J. Interferon Cytokine Res. 2004;24:388–390. doi: 10.1089/1079990041535610. [DOI] [PubMed] [Google Scholar]


