Abstract
Aims
Poor blood flow and hypoxia/ischemia contribute to many disease states and may also be a factor in the decline of physical and cognitive function in aging. Nitrite has been discovered to be a vasodilator that is preferentially harnessed in hypoxia. Thus, both infused and inhaled nitrite are being studied as therapeutic agents for a variety of diseases. In addition, nitrite derived from nitrate in the diet has been shown to decrease blood pressure and improve exercise performance. Thus, dietary nitrate may also be important when increased blood flow in hypoxic or ischemic areas is indicated. These conditions could include age-associated dementia and cognitive decline. The goal of this study was to determine if dietary nitrate would increase cerebral blood flow in older adults.
Methods and Results
In this investigation we administered a high vs. low nitrate diet to older adults (74.7 ± 6.9 years) and measured cerebral perfusion using arterial spin labeling magnetic resonance imaging. We found that the high nitrate diet did not alter global cerebral perfusion, but did lead to increased regional cerebral perfusion in frontal lobe white matter, especially between the dorsolateral prefrontal cortex and anterior cingulate cortex.
Conclusion
These results suggest that dietary nitrate may be useful in improving regional brain perfusion in older adults in critical brain areas known to be involved in executive functioning.
Keywords: Nitric Oxide, Nitrite, Nitrate, Cerebral Blood Flow, Aging, Magnetic Resonance Imaging
1. Introduction
Until recently, nitrite was thought to be relatively inert biologically [1]. However, nitrite infusions leading to slightly supraphysiologic levels of plasma nitrite, from 180 nM at baseline to 2,600 nM, elevated forearm blood flow and was associated with nitric oxide (NO) formation [2]. Further research showed that a smaller rise in plasma nitrite to only 350 nM also increased forearm blood flow [3]. Levels of nitrite in plasma that are shown to improve blood flow can be achieved by consuming foods high in nitrate [4]. Once ingested, nitrate is absorbed from the upper part of the intestine, and transported via plasma into salivary glands where it is concentrated and released into saliva. Nitrate is subsequently reduced to nitrite by symbiotic, oral bacteria. The nitrite is swallowed and ultimately absorbed from the intestine into the circulatory system [4, 5]. Several groups have shown that ingesting diet sources high in nitrate leads to substantial increases in plasma nitrite [6–9]. This conversion of nitrate to nitrite can be eliminated when volunteers either expectorate or use mouthwash which kills oral bacteria that are key to the conversion process [6, 8]. Physiologic effects from increasing dietary nitrate and plasma nitrite include reduction in blood pressure [6, 8], improvement in intestinal health [10], and increases in exercise performance [9] which are all attributed to the further reduction of nitrite to NO. Interestingly, nitrite is being investigated for use in a variety of conditions, including ischemic-reperfusion injury, pulmonary hypertension, stroke, sickle cell disease, and gastric diseases [5, 11]. A major feature of nitrite’s ability to increase blood flow is that it acts preferentially in hypoxic conditions, allowing nitrite to increase blood flow precisely in the areas where it is needed most [2, 12, 13]. We hypothesized that we could use this feature to increase cerebral blood flow in older adults.
As the average age in the United States continues to rise, there has been increasing interest in gaining a better understanding of the aging brain and the neurological morbidities that accompany aging. Cognitive decline in general and dementia in particular, are sources of morbidity and dependency for older adults. These conditions also place an enormous burden on the family and society. It has been shown that diminished blood flow to the brain contributes to cognitive impairment [14]. In addition, from the Rotterdam Study, it has been shown that cerebral hypoperfusion precedes and probably contributes to the onset of clinical dementia [15]. Moreover, diminished cerebral blood flow has been linked to poorer cognition, such as a reduction in information processing speed, and to dementia [16, 17]. A ubiquitous finding in neurocognitive disorders associated with aging is the so-called “white matter hyperintensity” (WMH). Chronic ischemia appears to be the fundamental process that leads to WMHs, often referred to as leukoaraiosis. Several underlying mechanisms have been shown to contribute to this chronic ischemic state, including abnormal arterioles, capillaries and venules, and increased tortuosity of arterioles as we age [18–20]. Chronic ischemia in the white matter appears to be the complex endgame leading to the WMHs and is associated with cognitive decline [21]. In particular, age-related white matter degeneration has been linked to poor executive functioning as assessed by measures of working memory [22, 23] dichotic listening [24], task switching [25] and episodic retrieval [26].
The current analyses are on 2 separate studies. The first was a preliminary study that tested the time course of plasma nitrate and nitrite levels over a 3-hour period following consumption of a high nitrate breakfast, which included beetroot juice. This provided us with a target time for measuring cerebral blood flow in the subsequent study based on the peak level achieved and kinetics of the plasma nitrate and nitrite concentrations. The subsequent and primary study examined in a within subject design the effect of two levels of dietary nitrate (high vs. low) over a 24-hour feeding period on acute changes in cerebral blood flow in older adults as measured by magnetic resonance (MR) imaging. Each person was provided both dietary conditions in a randomized order on separate days and blood flow was assessed with each diet. The hypothesis for the primary study was that a diet high in nitrate containing foods and beverages would produce changes in cerebral blood flow in key and critical areas of the brain, principally those associated with executive function, compared to a diet of foods that are low in nitrate. The fact that nitrite-based increases in blood flow targets areas of hypoxia [2, 12, 13, 27–29] supports the hypothesis that increasing plasma nitrite will increase cerebral brain perfusion in these areas where it is needed most
2. Methods
2.1 Subject Description
Subject selection for both studies followed the same criteria with an age cutoff of ≥ 70 years old. In the preliminary study, a total of 5 individuals were recruited, and the primary aim for the larger trial was addressed by recruiting a total of 16 older adults to undergo both a high nitrate and a low nitrate dietary intervention as approved by the Institutional Review Board at Wake Forest University. The investigations conformed to the principles outlined in the Declaration of Helsinki. A phone screen was administered to interested individuals for eligibility requirements. Exclusion criteria included those with systemic uncontrolled diseases, use of medications that may be contraindicated or interact with the high nitrate diet, such as nitroglycerin or nitrate preparations used for angina, or phosphodiesterase type 5 (PDE5) inhibitors, including sildenafil (Viagra®). Individuals with clinical hypotension or those with a resting blood pressure less than 100/60 were also excluded. In addition, individuals had to agree to report to the testing facilities, had no avoidance to the foods provided, had a Mini-Mental State Exam (MMSE, [30]) score of at least 24 (older adults with a score below 24 would not be able to complete the assessments reliably), and had no history of medical conditions that could impair cognition, such as head injury, stroke or dementia. They were also appropriately screened to be eligible for MR imaging.
2.2 Study Design
For the preliminary time course study, participants reported to the laboratory after an overnight fast. Blood was drawn and then they were fed a high nitrate breakfast that contained 500 ml of beetroot juice (see high nitrate breakfast in Table 1 for specific foods). At 30-minutes, and 1, 2, and 3 hours after finishing the meal, blood was drawn to determine plasma levels of nitrate and nitrite.
Table 1.
High and low nitrate diets
| Low Nitrate Diet (2,000 kcals/day) | High Nitrate Diet (2,000 kcals/day) |
|---|---|
|
|
In the cerebral perfusion trial, all participants received both treatments in a within-subject research design with diet conditions being delivered in a Latin-Square arrangement, that is, on days 1 and 2, one-half of the participants received the low nitrate diet first and the other half received the high nitrate diet first, and then for days 3 and 4 they switched treatments (see section below for description of the diets). Comparisons were made between the low and high nitrate diets with the low nitrate diet serving as the control treatment. The timeline for the study design is shown in Figure 1. All participants reported to the laboratory on 4 consecutive days; each diet treatment lasted two days. On day 1, participants arrived at the laboratory in the morning following a 10-hour overnight fast and completed an informed consent and provided a medical history and health status report. Participants were then fed breakfast comprised of foods from either the low nitrate or the high nitrate diet for their respective treatment. Foods for lunch and dinner were given to the individuals to take home to eat for the rest of day 1 following the same diet treatment given at breakfast (low or high nitrate). They reported back to the laboratory after a 10-hour overnight fast for breakfast on day 2. The same diet as for day 1 (low or high nitrate) was consumed for breakfast on day 2. Blood was drawn prior to breakfast on day 2 and at 1-hour after finishing breakfast. Brain imaging occurred immediately after the 1-hour post-breakfast blood draw. These times were based on the results from the preliminary time course study. They reported back to the laboratory on day 3 and performed the second experimental condition: either low or high nitrate diet. This followed the same pattern as day 1 with breakfast consumed in the laboratory, and food for lunch and dinner, which was provided to them, on day 3 consumed at home. On day 4, they reported back to the laboratory after a 10-hour fast, had blood drawn prior to eating, then ate breakfast of their second experimental condition. One hour after eating, participants had another blood draw, proceeded by brain imaging following the same procedure as in the first experimental condition. The time from after the imaging on day 2 to breakfast on day 3 was considered the washout period and participants resumed their normal diet during this period. This provided a 24 hour washout period between diet treatments (breakfast of day 2 until breakfast of day 3), as well as 48 hours between assessments (1-hour after breakfast on day 2 until fasting pre-breakfast assessment on day 4). No blood assessments were performed on day 1 or 3 prior to starting the respective treatments since individuals were on varied diets and nitrate and nitrite levels would have reflected their typical intake; thus not providing a control level for comparison purposes within treatment groups of the outcome measures.
Figure 1.
Timeline of study design for the larger study that included MRI scans.
Compliance to the diet treatments was determined based on direct observation by research staff of food and drink consumption at breakfast on all days, as well as obtaining a diet record for lunch, dinner, and snacks on days 1 and 3. Participants were asked to record any foods they did not consume that were part of the diet as well as record any foods they ate in addition to provided foods.
2.3 Diets (See Table 1 for complete listing of foods)
The low nitrate diet was low in fruits and vegetables and contained primarily grains, meats, and dairy products. Processed meats high in nitrates and nitrites, such as hotdogs, bacon, and sausage were avoided. The high nitrate diet contained foods high in vegetables, particularly leafy green vegetables like spinach, lettuce, and broccoli. For the high nitrate breakfast, individuals also consumed 500 ml of beet juice (Biotta, Inc., Carmel, IN). Food and beverages were liquefied and assayed for their nitrate and nitrite levels using a Sievers Nitric Oxide Analyzer. For the beet juice, nitrate and nitrite concentrations were 17 mM and 31 nM, respectively. The beet juice thus provided 8.5 mmol of nitrate (530 mg). The values measured for the high vs. low nitrate foods, respectively, were 3.9 mmol vs. 0.089 mmol for nitrate, and 20 µmol vs. 0.4 µmol for nitrite. Thus, the two diets were similarly low in nitrite content, but the high nitrate diet had a total of 12.4 mmol compared to 0.089 mmol for the low nitrate diet, nearly a 150 fold difference. For both diets, distilled water was provided to drink.
2.4 Measures
For both studies, blood was collected using similar techniques. Blood was taken from an antecubital vein and collected in two 4 mL Lithium heparin vials. The tubes were immediately centrifuged at 5,000 rpm for 2 min, plasma removed, immediately frozen on dry ice in aliquots with ~0.4 mL of plasma, and stored in a −80° C freezer. For the time course study, plasma nitrate and nitrite were determined from blood obtained before and then at 30-minutes and hourly for 3-hours after eating the high nitrate meal. In the perfusion study, age, gender, medical history, and MMSE were obtained on day 1 at the first laboratory visit. Blood was obtained on days 2 and 4 prior to breakfast and at 1-hour after eating.
Plasma and diet nitrite and nitrate levels were measured using chemiluminescence-based Nitric Oxide Analyzers (Sievers, Inc) according to instructions of the manufacturer. For all measurements, standard curves were obtained and used for quantitative measurements.
Cerebral blood flow (CBF) was determined from MR images collected 1 hour following breakfast on days 2 and 4. All scans were performed on a 1.5T GE scanner using an 8-channel head coil (GE Medical Systems, Milwaukee, WI) and included anatomic imaging (3D BRAVO) and perfusion imaging (PASL Q2TIPS, TR3000, voxel size 3.75mm × 3.75mm × 8mm). Non-invasive multi-slice quantitative CBF was measured with Quantitative Imaging of Perfusion using a Single Subtraction with Thin Slice TI1 Periodic Saturation (QUIPSS II TIPS a.k.a. Q2TIPS) [31] with a Flow-sensitive Alternating Inversion Recovery (FAIR) [32]. In our implementation of Q2TIPS, saturation pulses were Very Selective Suppression (VSS) radio frequency pulses [33], which were applied every 25 ms between 800 ms (TI1) and 1200ms (TI1s) and which saturated a 2 cm slab of tissue with a 1 cm gap between the saturation slab and the first imaging slice. Other imaging parameters were as follows: TE 28 ms, TI1 800ms, TI1s 1200 ms, TI 2000 ms, TR 3000 ms, receiver bandwidth 62.5 kHz, flip angle 90 degrees, FOV 24 cm (frequency) × 18 cm (phase), an acquisition matrix 64 × 48 (11 slices, 8 mm thickness, 0 mm slice gap), and frequency encoding direction anterior/posterior. A diffusion gradient with an equivalent b value of 5.25 sec/mm2 was added to suppress intra-arterial spins [34]. An inversion time of 2000 ms was used for both groups.
Q2TIPS-FAIR acquired data in label/control (slice selective inversion/global inversion) pairs. We acquired 60 label/control pairs to obtain perfusion weighted images with good signal-to-noise ratio in a time of 6 minutes 30 seconds. The first 30 seconds (10 volumes) was used to establish steady state and to acquiring a proton density (M0) image. The M0 image served as an internal reference to scale the perfusion weighted images appropriately to obtain quantitative CBF maps.
Quantitative CBF maps require that the T1 of the tissue be measured at each voxel. T1 maps were calculated from data acquired from a separate inversion recovery EPI imaging experiment. Twelve inversion times were acquired logarithmically from 10 milliseconds to 6 seconds with a TR of 10 seconds. A total of 13 imaging volumes were acquired in a total scan time of 2 minutes and 10 seconds. All other imaging acquisition parameters (FOV, matrix size, TE, flip angle, slice thickness, and slice location, etc) were identical to the Q2TIPS-FAIR protocol described previously.
Perfusion imaging preprocessing
Prior to statistical analyses, perfusion data were processed using the following algorithms [35]. Motion correction was applied to the perfusion weighted volumes with a six-parameter rigid body transformation using SPM5. After motion correction the difference images were averaged together, and quantitative perfusion maps were calculated from the equation:
where CBF is the cerebral blood flow, ΔM(TI2) is the mean difference in the signal intensity between the label and control images, M0,blood is the equilibrium magnetization of blood, α is the tagging efficiency, TI1 is the time duration of the tagging bolus, TI2 is the inversion time of each slice, and T1,blood is the longitudinal relaxation time of blood, qp is a correction factor that accounts for the difference between the T1 of blood and the T1 of brain tissue. The M0,blood was approximated from the M0, white matter, which was measured directly from the M0 image acquired with the perfusion weighted images. The correction factor, qp, required that the T1 of the brain tissue be measured at each voxel, which was measured with a separate inversion recovery (IR) EPI imaging experiment. All other parameters are known or assumed to be a constant (TI1=800ms, TI1S=1200ms, T1,blood=1200ms). These maps measured perfusion in standard units of milliliters per 100 grams of tissue per minute.
2.5 Statistical Analysis
Nitrite and nitrate values are reported as the mean ± one standard deviation. Repeated measures analysis of variance was used to compare the 5 time points for plasma nitrate and nitrite in the time course study. Paired t-tests were used to compare differences between the low and high diet conditions for the plasma nitrite and nitrate in the perfusion study. Cerebral perfusion analyses were performed using SPM 5 within Matlab. Imaging data were normalized to standard space within SPM to allow for perfusion comparisons in each voxel. Voxel-wise paired t-tests were performed to evaluate the effects of nitrate in an unbiased manner due to a lack of a priori knowledge of where the effects of nitrate would be observed. The voxel wise statistics can result in large numbers of false positives due to multiple comparisons. Therefore, data were corrected for multiple comparisons by first applying a relatively stringent threshold (p<0.005), and then applying an extent correction such that significant areas had to contain a minimum of 180 contiguous voxels.
3. Results
For both studies presented, the beetroot beverage and diets were generally well-tolerated by participants. The common expected side effects of red stools and urine associated with drinking beetroot juice (beeturia) were experienced and reported. One individual in the perfusion study was excluded from the analysis as they refused to drink the beetroot juice on day 2 of the high nitrate diet. No other symptoms, such as dizziness, orthostatic hypotension, or headaches were reported by participants. Compliance to the diet was high as the foods were well tolerated and no participant reported consuming additional food outside of the diet provided during testing days.
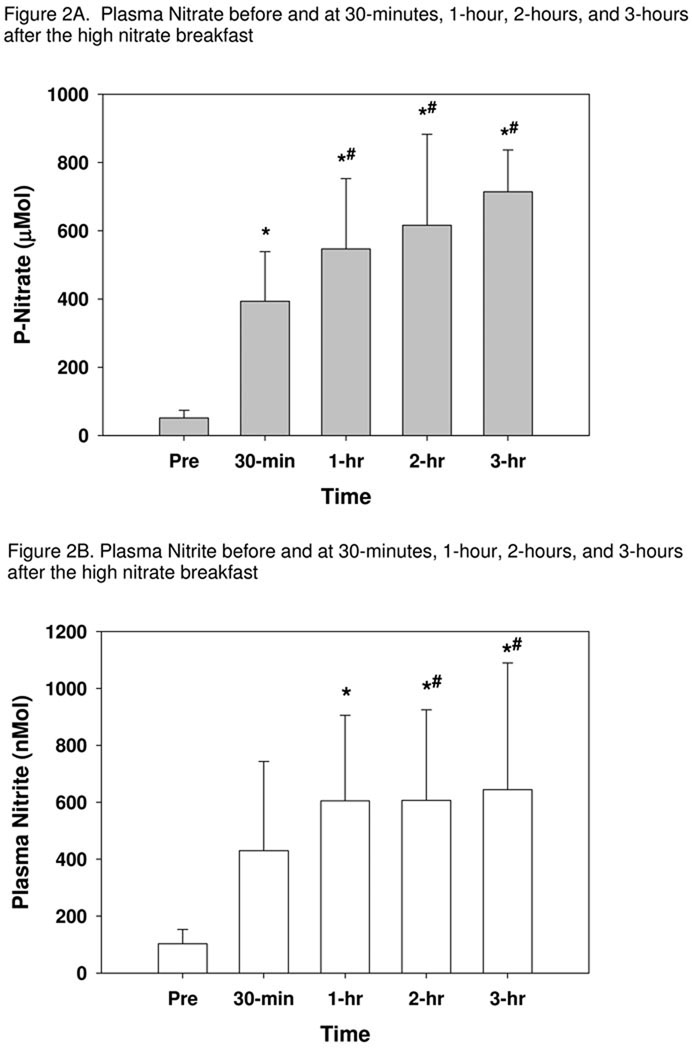
Five individuals completed the time course study. Figure 2 demonstrates the mean values for both plasma nitrate and nitrite at each data collection time. Following the overnight fast, plasma nitrate was 51.1 ± 22.3 µM and it increased at 30-minutes and remained elevated above the initial fasting draw throughout the 3-hours, F(4,16)=21.31, p<0.001. There were no differences between 1, 2, and 3 hours for nitrate, and all were higher than at 30-minutes. A similar increase in plasma nitrite was seen over time, F(4,16)=9.90, p<0.001. Post hoc comparisons showed a marginally significant trend for the 30-minute plasma to be higher than the pre-breakfast blood draw (p=0.058). By 1-hour, nitrite was elevated above both pre-breakfast and 30-minutes, and it remained higher than the fasting draw throughout the 3 hours.
Figure 2.
Plasma nitrate (A) and nitrite (B) levels prior to and at 30-minutes, 1-hour, 2-hours, and 3-hours following a high nitrate breakfast in the time course study. Plasma nitrite and nitrate were measured after consumption of a high nitrate breakfast following overnight fast. Averages and standard deviations are shown (n=5).
* indicates significantly different than fasting values
# indicates significantly different than at 30-minutes
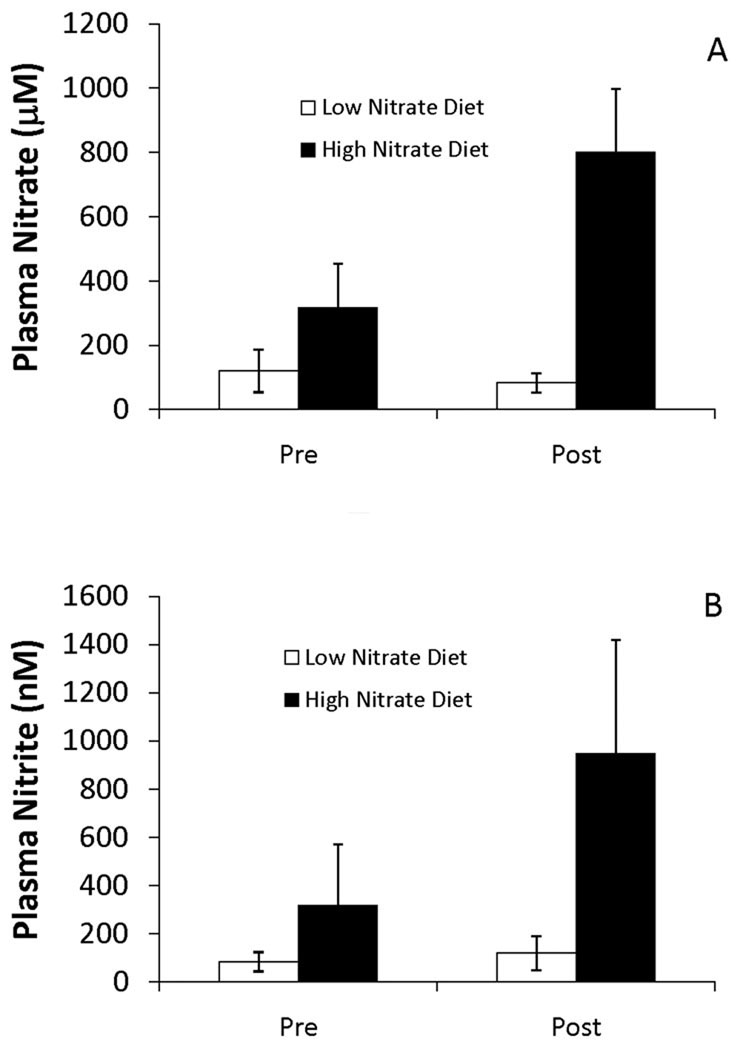
A total of 16 individuals were recruited for the perfusion study with 14 completing all components (mean age = 74.7 ± 6.9 years). One subject was excluded as they did not drink the beetroot juice on the second day for the high nitrate diet. A second subject was excluded due to a large susceptibility artifact in the MR images. Plasma nitrite and nitrate levels were measured both prior to the last breakfast (Pre) and one hour after (Post) the breakfast on days 2 and 4 of the study. As shown in Figure 3, consumption of the high nitrate diet resulted in substantially greater plasma nitrate (Figure 3A) and nitrite (Figure 3B) levels at both time points (Pre and Post) compared to low nitrate diet. The average nitrate levels one hour after consuming the high nitrate breakfast on the test day was 750 ± 230 µM compared to 80 ± 30 µM measured one hour after consumption of the low nitrate breakfast, over a 9 fold change (t(14)=-11.1, p<0.001). Average plasma nitrite level one hour after consuming the high nitrate breakfast on the test day was 950 ± 470 nM compared to 120 ± 70 nM measured one hour after consumption of the low nitrate breakfast, nearly an 8 fold change (t(14)=−7.1, p<0.001). Interestingly, plasma nitrite and nitrate levels were also higher after the overnight fast on the high nitrate diet compared to the low nitrate diet (compare Pre for high vs. low nitrate diets). The average ± standard deviation plasma nitrate values measured in these post-overnight fast, pre-breakfast time points were 300 ± 140 µM for the high nitrate breakfast compared to 120 ± 60 µM for the low nitrate breakfast (t(14)=−5.0, p<0.001). The average plasma nitrite values measured in this post-overnight fast, pre-breakfast draws were 320 ± 250 nM for the high nitrate breakfast compared to 84 ± 40 nM for the low nitrate breakfast (t(14)=−3.8, p=0.002). Interestingly, eating the low nitrate breakfast increased plasma nitrite but decreased plasma nitrate (nitrite goes from 84 ± 40 nM to 120 ± 70 nM (p = 0.005) and nitrate goes from 120 ± 46 µM to 80 ± 30 µM (p = 0.01)). These data for plasma nitrate and nitrite are pooled and are not based on treatment order, but on treatment assignment. Further analysis to examine for first order effect showed no differences in outcome variables based on treatment order. That is, participants exposed to the high nitrate diet first did not show a significant increase in plasma nitrate and nitrite during their low nitrate diet treatment for either pre or post measures as compared to those that received the low nitrate diet first. Most importantly, the data in Figure 3 demonstrate that dietary nitrate in our study of older adults led to dramatic increases in plasma nitrite just prior to imaging; levels one hour after eating the high nitrate breakfast were several times higher than one hour after eating the low nitrate breakfast (see Post data in Figure 3B).
Figure 3.
Plasma nitrate (A) and nitrite (B) levels in the perfusion study. The levels reflect measures on days and 2 and 4 of the study, either before (Pre) or one hour after (Post) consuming breakfast on the high or low nitrate diet. Subjects consumed either a high or low nitrate diet starting on days 1 and 3 that continued through the breakfast before and after which blood was drawn. Values plotted are averages ± one standard deviation.
* indicates significantly different than fasting values
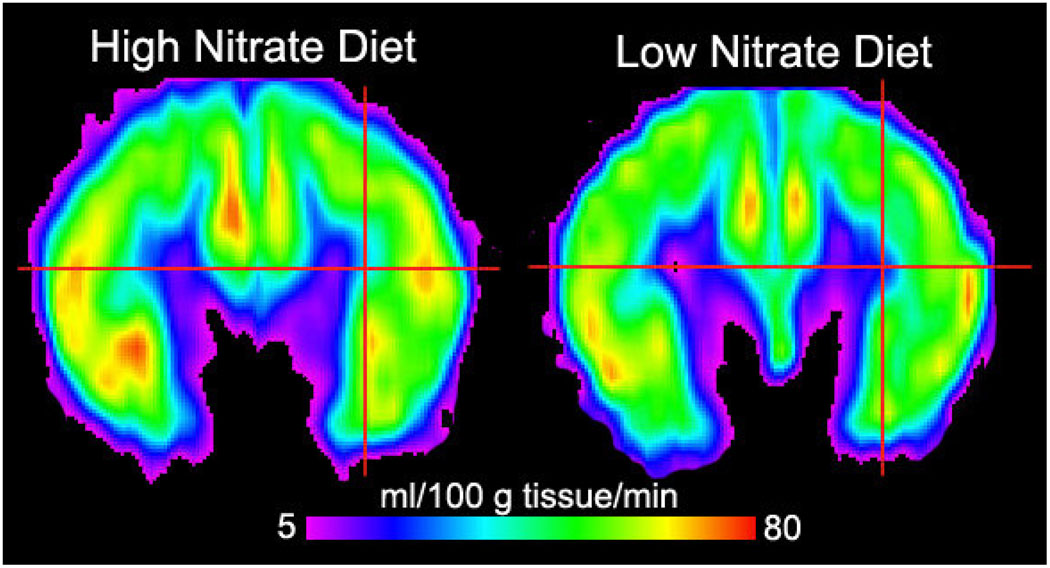
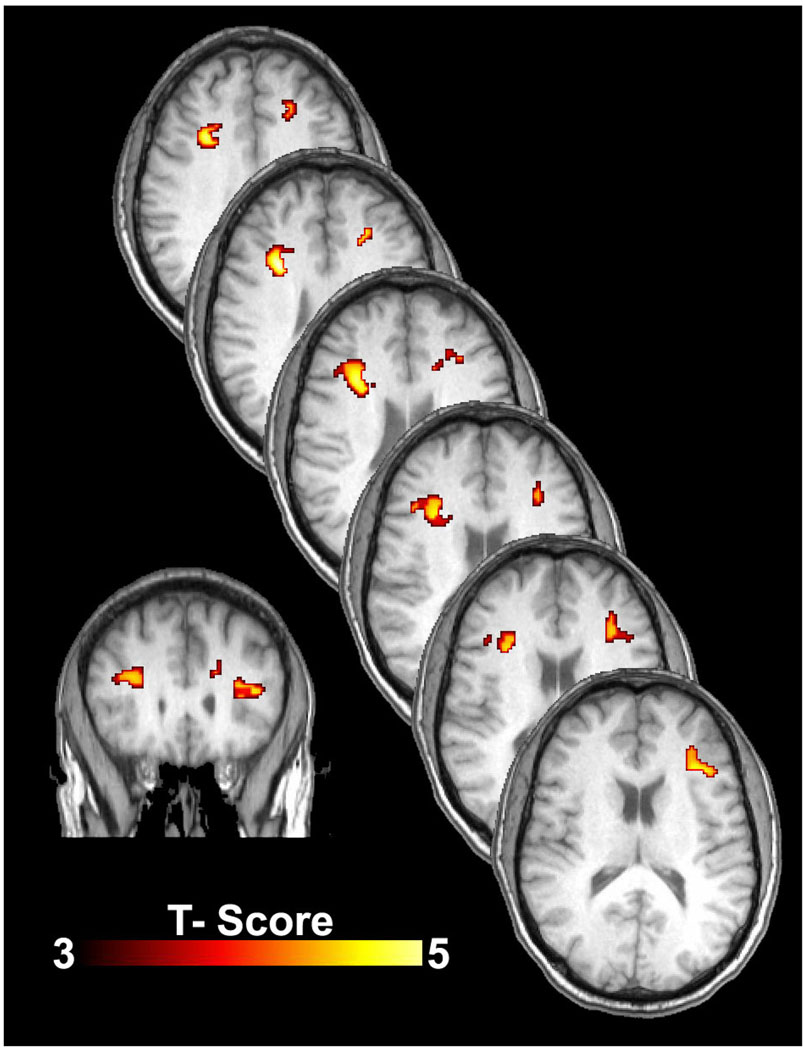
There were no global perfusion differences when the 14 subjects were on their low nitrate and high nitrate diets. Specifically, average global CBF was 43 ± 10 ml/100g/min on the low nitrate diet and 44 ± 10 on the high nitrate diet. However, when a voxel wise analysis was performed (comparison of each brain voxel across subjects), the subjects demonstrated increased CBF within the subcortical and deep white matter of the frontal lobe (Figures 4 and 5). These differences were located between the dorsolateral prefrontal cortex region and anterior cingulate gyrus. Figure 4 shows CBF on a coronal slice through the frontal lobes for the 14 subjects on the high nitrate and low nitrate diets. Note the subtle increased perfusion within the frontal lobe gray matter and increased perfusion at the gray-white junction and within the white matter itself (smaller blue and purple regions). Figure 5 shows the statistical comparison of the 14 subjects on the high versus low diets. While there were regions in the frontal lobe gray matter that showed increased flow in the high nitrate state, these areas did not reach statistical significance. However, there were four statistically significant areas of increased CBF within the bilateral white matter of the frontal lobes, areas known to be at risk for chronic ischemia in the elderly. The peak location, mean perfusion, and standard deviation for these areas are listed in Table 2. The cross-hairs on the native CBF maps in Figure 4 were placed to illustrate those regions in Figure 5 where significant increases in CBF were present on the high nitrate diet. A post-hoc analysis evaluating the effects of diet order was performed on the mean measures from the right anterior region-of-interest. This analysis showed that perfusion was greater in the high nitrate diet compared to the low nitrate diet independent of order.
Figure 4.
Group cerebral blood flow maps (in ml/100g tissue/minute) for the 14 subjects on the high nitrate diet (left) and low nitrate diet (right). Both images are from a coronal slice through the frontal lobes. There is subtle increased perfusion within the frontal lobe gray matter and at the gray-white junction and within the white matter itself (smaller blue and purple regions). The cross-hairs were placed to illustrate those regions in Figure 5 where statistically significant increases in CBF were present on the high nitrate diet.
Figure 5.
Cerebral blood flow (CBF) differences between the high nitrate diet and low nitrate diet states. Statistical maps show significant differences in regional blood flow for the n=14 subjects on the high nitrate diet versus on the low nitrate diet. Note the increased CBF (ml/100g/min) within the bilateral white matter of the frontal lobes, areas known to be at risk for chronic ischemia in the elderly. The bottom left image is a coronal slice at the level of genu of the corpus callosum. The diagonal stacked images are axial slices extending from the uppermost portions of the lateral ventricles superiorly to the basal ganglia/mid-body of the lateral ventricles inferiorly. Although there are some asymmetries to the findings, the effects on CBF from the high nitrate diet clearly manifest bilaterally within the white matter. Statistical analyses were performed at p<0.005, extent corrected at 180 voxels. Color scale represents the t-score from a voxel-wise paired-samples t-test.
Table 2.
The mean and standard deviation of the perfusion (ml/100g tissue/min) was calculated for a 10mm diameter region-of-interest centered at each the peak of significance. Values are presented for low and high nitrate diets in each of the four regions.
| peak location |
mean perfusion | stdev | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | high diet |
low diet | high diet |
low diet | ||||
| right | anterior | 30 | 14 | 28 | 31.32731 | 22.35293 | 11.93273 | 9.847101 | ||
| posterior-superior | 12 | −6 | 58 | 45.34016 | 37.55213 | 9.450536 | 10.78369 | |||
| left | anterior | −32 | 26 | 14 | 40.2891 | 32.50811 | 8.964576 | 13.94549 | ||
| posterior-superior | −16 | −8 | 56 | 37.5261 | 29.78261 | 9.404393 | 10.36062 | |||
4. Discussion
In a study led by Gladwin in 2003, it was shown that slightly supraphysiological amounts of infused nitrite (going from 180 nM at baseline to 2,600 nM after infusion) led to increased forearm blood flow [2]. More recently, the Gladwin lab showed that infusion of nitrite to achieve a level of only 350 nM results in increased forearm blood flow [3]. Others have since confirmed that nitrite acts as a vasodilator in various tissues and that its activity is heightened in hypoxia [36–40]. Rifkind and coworkers showed that nitrite infusions led to increases in cerebral blood flow in rats as measured by laser Doppler flowmetry [38]. Thus, there is growing evidence that nitrite, rather than being relatively biologically inert, can act as a potent vasodilator harnessed primarily under hypoxic conditions. In this paper, we demonstrated the ability of oral nitrate to increase plasma nitrite and to increase cerebral blood flow within white matter in older adult humans using perfusion MRI.
Others have previously shown that substantial elevations in plasma nitrite occur through increasing dietary nitrate intake [4]. Nitrate from the diet, once absorbed from the intestine, is taken up from the plasma by salivary glands and concentrated in saliva; nitrate is subsequently reduced to nitrite by symbiotic, oral bacteria and ultimately absorbed into the circulatory system [4, 5]. Documented physiological effects from increasing dietary nitrate include reduction in blood pressure [6, 8], improvement in intestinal health [10], and increases in exercise performance [9]. These effects are eliminated when volunteers either spit or use mouthwash, thereby implicating the importance of nitrate reduction to nitrite by oral bacteria. The level of nitrate utilized in the current study (12.4 mmol) from the dietary manipulations falls within the range shown by others to reduce blood pressure and improve exercise performance (22.5 mmol and 5.5 mmol, respectively).
To our knowledge, we are the first to demonstrate that these pathways also function in older adults. One might think that due to xerostomia, altered oral bacterial colonization, and increased gastric pH, the effect of dietary nitrate on plasma nitrite levels of older adults would not be as great as for younger adults. However, the increases in plasma nitrite that we observed in these older adults (an average of 630 nM increase one hour after the high nitrate breakfast compared to before the breakfast, and an average of 830 nM increase in post high nitrate breakfast compared to post low nitrate breakfast, Figure 3) are comparable to what has been observed in younger adults [6, 8]. Moreover, the plasma levels of nitrite achieved in our study using a dietary nitrate intervention (950 ± 470 nM, with a range of 280 to 1890 nM measured 1 hour after the high nitrate breakfast) are similar in magnitude to those obtained in nitrite infusions which led to increased forearm blood flow [2, 3]. Thus, as dietary nitrate is a natural variant, our data support the idea that nitrite is a physiological (rather than simply therapeutic) modulator of blood flow.
Results from the initial kinetics study presented here demonstrate the time course of changes in nitrate and nitrite after consuming the high nitrate breakfast containing 500 ml of beetroot juice. There was an increase in plasma nitrite levels by over 5 fold above fasting values in one hour, and this level was maintained for at least an additional two hours. These findings suggest that the plasma nitrite concentrations are maintained at a high level for the time participants in the perfusion study underwent imaging for perfusion measures.
In fact, the effects of dietary nitrate on plasma nitrite and nitrate levels were observed to be sustained after the overnight fast (compare levels of different diets pre-breakfast in Figure 2), suggesting that the effects of dietary nitrate are prolonged. Consumption of the low nitrate breakfast led to increases in plasma nitrite, but nitrate levels decreased. This could possibly be due to increased swallowing and gastric activity leading to more nitrate conversion to nitrite without consumption of substantial amounts of additional nitrate.
Although the effects of the nitrate in the diet on plasma nitrite and nitrate levels was highly significant, there was a substantial amount of variability, particularly in the levels of nitrite. The increase in individual subjects in plasma nitrite after the high nitrate breakfast compared to the low nitrate breakfast ranged from none in one case and a few fold in a couple of cases to up to 20 fold. This variance is not grossly dissimilar to that previously reported in a study using beet juice in younger adults [8]. The cause for this large variance could be related to variability in the nitrate reductase activity of oral bacteria in different individuals, rates of nitrate uptake, or other causes. Additionally, gastric pH may lead to alterations in nitrate and nitrite metabolism. The presence of achlorhydria and use of pharmacological agents that can affect gastric pH, such as proton pump inhibitors were not controlled in the present study and this may have contributed to the variability observed. Clearly, this individual difference in response deserves further study.
There have been several mechanisms proposed for nitrite’s vasodilatory action, all involving conversion of nitrite to nitric oxide, although sometimes through the intermediacy of other nitrogen oxides [13]. Most (but not all [41]) of these involve the action of a particular protein and those proposed include hemoglobin [2, 42], myoglobin [43, 44], xanthine oxidoreductase [45, 46], nitric oxide synthase [47], cytochrome c oxidase [48, 49], aldehyde oxidase [50, 51] and cytochrome c [52, 53]. It is likely that several of these act to reduce nitrite in different tissues and different conditions. Importantly, all proposed mechanisms of nitrite reduction to nitric oxide include a potentiation under hypoxic conditions, consistent with observed physiological nitrite action.
Earlier work has shown that aging is associated with progressive impairment of endothelial function [54]. This may lead to a reduction in NO produced from arginine via the nitric oxide synthase (NOS) reaction in the endothelium and contribute to the reduced perfusion in tissues with aging. Supplementing L-arginine in older adults has produced equivocal results with some groups indicating improvements in flow-mediated dilation and other showing no effect with L-arginine supplementation [55, 56]. The current work indicates improvement in tissue perfusion via an endothelium-independent treatment. It would be informative to determine if L-arginine supplementation would increase cerebral blood flow independent of the effect from dietary nitrate, or if there is a potentiating effect from these two agents.
Historically, very high concentrations of nitrate in drinking water were thought to cause methemoglobinemia in infants, but more recent research has questioned whether nitrate or nitrite alone is the cause or if bacterial infection is also required [57, 58]. Furthermore, in the 1970s, concern over the possibility of nitrite causing cancer arose from the theoretical association between the ability of nitrite to form nitrosamines in the gut and studies showing that nitrosoamines are carcinogenic [58–61]. However, the evidence that high levels of nitrate in diet causes any type of cancer is weak and most studies have found no link between dietary nitrate and cancer at all [58, 59]. The highest sources of nitrate in our diet are found in certain vegetables like spinach, celery, and beetroot, and epidemiologic evidence does not support these foods as causing diseases from nitrate. It may be that in addition to nitrate, vegetables also have antioxidants which may protect against nitrosamine formation. This being said, it is possible that some human subpopulations may be especially sensitive to cancer causing effects of nitrate and continued monitoring of this potential untoward health effect is advisable.
Chronic ischemia in the white matter is associated with aging. Chronic ischemia appears to be the fundamental process that leads to so-called white matter hyperintensities (WMHs). Several underlying mechanisms have been shown to contribute to this chronic ischemic state, including abnormal arterioles, capillaries and venules, and increased tortuosity of arterioles as we age [18–20]. However, whatever the cause and regardless of the clinical diagnosis, chronic ischemia in the white matter appears to be the complex endgame leading to cognitive decline [21]. As poor cerebral perfusion and ischemia have been associated with cognitive decline and dementia [14–21], our results support the proposal that oral nitrate therapy may be beneficial in treating cognitive decline that is often observed with aging. Towards that end, we show a direct effect of dietary nitrate on cerebral blood flow within the subcortical and deep white matter of the frontal lobes. This finding is intriguing as there is evidence for an anterior-posterior gradient in age-related degeneration of white matter [62, 63] suggesting frontal regions are particularly compromised by aging. Moreover, there is a strong relationship between losses in white matter integrity and declines in aspects of executive function, including working memory, task-switching and episodic memory retrieval [25, 26, 62], which are important for older adults’ performance of instrumental activities of daily living, such as writing checks, using appliances, and shopping [64].
As already discussed, nitrite has been shown to not only increase blood flow to certain areas of the body, but also acts preferentially in hypoxic conditions, allowing nitrite to increase blood flow precisely in the areas where it is needed [2, 12, 13]. Based on this notion, our data suggest that a diet high in nitrate might allow increased perfusion to those areas of the brain known to be at risk in the elderly and important for cognitive function —the deep white matter in the frontal lobe.
The diets used in our studies were designed to be isocaloric with similar protein levels, but differing levels of nitrate. The measured levels of nitrate were more than 1,000 fold different but the nitrite levels were similar between the low and high nitrate treatments. Based on the different food groups that compose each diet, it is expected that the level of antioxidants and other phytochemicals in the diets differ. We recognize that other dietary components besides nitrate may be partially responsible for the differential response in cerebral perfusion we observed. However, previous studies using a treatment of beetroot juice only or nitrate by itself have found that minimizing the conversion of nitrate to nitrite, by using mouthwash to kill bacteria or spitting to lower the absorption of nitrite, eliminated the hypotensive effect of the nitrate or juice [6, 8]. Thus, we suggest that the differences in nitrate in the diet and nitrite in the plasma are primarily responsible for the physiological effect we observed. Nevertheless, using a bactericidal mouthwash or spitting would need to be performed to substantiate the effect observed with the current diet is from the nitrate content in the diet.
5. Conclusions
We have shown, as previously demonstrated in younger adults, that consumption of a high nitrate diet results in substantial increases in plasma nitrite in older adults. The increase in plasma nitrite and nitrate is sustained, even after overnight fast. Generally, we hypothesize that dietary nitrate may be beneficial in compensating for age-related endothelial dysfunction and associated pathology. In this study we focused on cerebral blood flow and found that perfusion increased in frontal lobe white matter after consumption of the high nitrate diet compared to the low nitrate diet. Further studies are required to confirm that the effect we observed is due to nitrate and to refine the nitrate diet. Such work has potential to lead to interventions that could improve cognitive and physical functional health in older adults.
Acknowledgments
We thank Erin Reddan, Alesia Goodman, Rebecca Patten, Rudayna Chanouha, Kati Gigler, Andrea Worsham, Sara Turner, Aasha Anderson, and Michael Font for technical assistance.
Funding
This work was supported by the Translational Science Center on the Reynolda Campus at Wake Forest University, The Science Research fund of Wake Forest University, and National Institutes of Health [grant numbers HL058091; HL62198].
Abbreviations
- NO
Nitric Oxide
- CBF
Cerebral Blood Flow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Dr. Kim-Shapiro is listed as a co-author on a patent application entitled "USE OF NITRITE SALTS FOR THE TREATMENT OF CARDIOVASCULAR CONDITIONS".
References
- 1.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu XL, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 3.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, III, Schechter AN, Gladwin MT. Nitrite Infusion in Humans and Nonhuman Primates. Endocrine Effects, Pharmacokinetics, and Tolerance Formation, Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nature Chemical Biology. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide-Biol Ch. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. New Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 8.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O-2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 10.Bjorne H, Petersson J, Phillipson M, Weltzberg E, Holm L, Lundberg JO. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J. Clin. Invest. 2004;113:106–114. doi: 10.1172/JCI200419019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon I. Richard O, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster J. Jack R, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nature Chemical Biology. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces Nitric oxide under allosteric control. J. Clin. Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Faassen EE, Babrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li HT, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as Regulator of Hypoxic Signaling in Mammalian Physiology. Medicinal Research Reviews. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer JS, Rogers RL, Judd BW, Mortel KF, Sims P. Cognition and cerebral blood flow fluctuate together in multi-infarct dementia. Stroke. 1988;19:163–169. doi: 10.1161/01.str.19.2.163. [DOI] [PubMed] [Google Scholar]
- 15.Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 16.Rabbitt P, Scott M, Thacker N, Lowe C, Jackson A, Horan M, Pendleton N. Losses in gross brain volume and cerebral blood flow account for age-related differences in speed but not in fluid intelligence. Neuropsychology. 2006;20:549–557. doi: 10.1037/0894-4105.20.5.549. [DOI] [PubMed] [Google Scholar]
- 17.Spilt A, Weverling-Rijnsburger AW, Middelkoop HA, van Der Flier WM, Gussekloo J, de Craen AJ, Bollen EL, Blauw GJ, van Buchem MA, Westendorp RG. Late-onset dementia: structural brain damage and total cerebral blood flow. Radiology. 2005;236:990–995. doi: 10.1148/radiol.2363041454. [DOI] [PubMed] [Google Scholar]
- 18.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology. 1995;194:469–476. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 19.Moody DM, Brown WR, Challa VR, Ghazi-Birry HS, Reboussin DM. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer's disease. Ann N Y Acad Sci. 1997;826:103–116. doi: 10.1111/j.1749-6632.1997.tb48464.x. [DOI] [PubMed] [Google Scholar]
- 20.Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 21.Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlton RA, Schiavone F, Barrick TR, Morris RG, Markus HS. Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. Journal of Neurology Neurosurgery and Psychiatry. 2010;81:13–19. doi: 10.1136/jnnp.2008.167288. [DOI] [PubMed] [Google Scholar]
- 23.Oosterman JM, van Harten B, Weinstein HC, Scheltens P, Sergeant JA, Scherder EJA. White matter hyperintensities and working memory: An explorative study. Aging Neuropsychology and Cognition. 2008;15:384–399. doi: 10.1080/13825580701879998. [DOI] [PubMed] [Google Scholar]
- 24.Gootjes L, Scheltens P, Van Strien JW, Bouma A. Subcortical white matter pathology as a mediating factor for age-related decreased performance in dichotic listening. Neuropsychologia. 2007;45:2322–2332. doi: 10.1016/j.neuropsychologia.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical "disconnection" as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- 26.Nordahl CW, Ranganath C, Yonelinas AP, DeCarlil C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. Journal of Cognitive Neuroscience. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gladwin MT. Haldane, hot dogs, halitosis, and hypoxic vasodilation: the emerging biology of the nitrite anion. J. Clin. Invest. 2004;113:19–21. doi: 10.1172/JCI200420664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro D, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat. Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 29.Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N. The reaction between nitrite and hemoglobin: The role of nitrite in hemoglobin-mediated hypoxic vasodilation. J Inorg Biochem. 2005;99:237–246. doi: 10.1016/j.jinorgbio.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. MINI-MENTAL STATE - PRACTICAL METHOD FOR GRADING COGNITIVE STATE OF PATIENTS FOR CLINICIAN. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41:1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 32.Kim SG, Tsekos NV. Perfusion imaging by a flow-sensitive alternating inversion recovery (FAIR) technique: application to functional brain imaging. Magn Reson Med. 1997;37:425–435. doi: 10.1002/mrm.1910370321. [DOI] [PubMed] [Google Scholar]
- 33.Tran TK, Vigneron DB, Sailasuta N, Tropp J, Le Roux P, Kurhanewicz J, Nelson S, Hurd R. Very selective suppression pulses for clinical MRSI studies of brain and prostate cancer. Magn Reson Med. 2000;43:23–33. doi: 10.1002/(sici)1522-2594(200001)43:1<23::aid-mrm4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Frank JA, Hou L, Ye FQ, McLaughlin AC, Duyn JH. Multislice imaging of quantitative cerebral perfusion with pulsed arterial spin labeling. Magn Reson Med. 1998;39:825–832. doi: 10.1002/mrm.1910390520. [DOI] [PubMed] [Google Scholar]
- 35.Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- 36.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GVR, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 37.Rogers SC, Khalatbari A, Datta BN, Ellery S, Paul V, Frenneaux MP, James PE. NO metabolite flux across the human coronary circulation. Cardiovasc. Res. 2007;75:434–441. doi: 10.1016/j.cardiores.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Rifkind JM, Nagababu E, Barbiro-Michaely E, Ramasamy S, Pluta RM, Mayevsky A. Nitrite infusion increases cerebral blood flow and decreases mean arterial blood pressure in rats: A role for red cell NO. Nitric Oxide-Biol Ch. 2007;16:448–456. doi: 10.1016/j.niox.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Langston W, Teng XJ, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozlov AV, Costantino G, Sobhian B, Szalay L, Umar F, Nohl H, Bahrami S, Redl H. Mechanisms of vasodilatation induced by nitrite instillation in intestinal lumen: Possible role of hemoglobin. Antioxid Redox Sign. 2005;7:515–521. doi: 10.1089/ars.2005.7.515. [DOI] [PubMed] [Google Scholar]
- 41.Zweier JL, Wang PH, Samouilov A, Kuppusamy P. Enzyme-Independent Formation of Nitric-Oxide in Biological Tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 42.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 43.Shiva S, Huang Z, Grubina R, Sun JH, Ringwood LA, MacArthur PH, Xu XL, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 44.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin - Oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 2007;100:1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 45.Li HT, Samouilov A, Liu XP, Zweier JL. Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J. Biol. Chem. 2004;279:16939–16946. doi: 10.1074/jbc.M314336200. [DOI] [PubMed] [Google Scholar]
- 46.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 47.Gautier C, van Faassen E, Mikula I, Martasek P, Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem. Biophys. Res. Commun. 2006;341:816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 48.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Castello PR, Woo DK, Ball K, Wojcik J, Liu L, Poyton RO. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proceedings of the National Academy of Sciences. 2008;105:8203–8208. doi: 10.1073/pnas.0709461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li HT, Kundu TK, Zweier JL. Characterization of the Magnitude and Mechanism of Aldehyde Oxidase-mediated Nitric Oxide Production from Nitrite. J. Biol. Chem. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric Oxide Production from Nitrite Occurs Primarily in Tissues Not in the Blood: CRITICAL ROLE OF XANTHINE OXIDASE AND ALDEHYDE OXIDASE. J. Biol. Chem. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YR, Chen LC, Liu X, Li HT, Zweier JL, Mason MP. Involvement of protein radical, protein aggregation, and effects on NO metabolism in the Hypochlorite-mediated oxidation of mitochondrial cytochrome c. Free Radical Biol. Med. 2004;37:1591–1603. doi: 10.1016/j.freeradbiomed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 53.Basu S, Azarova NA, Font MD, King SB, Hogg N, Gladwin MT, Shiva S, Kim-Shapiro DB. Nitrite Reductase Activity of Cytochrome c. J. Biol. Chem. 2008;283:32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyons D, Roy S, Patel M, Benjamin N, Swift CG. Impaired nitric oxide-mediated vasodilatation and total body nitric oxide production in healthy old age. Clinical Science. 1997;93:519–525. doi: 10.1042/cs0930519. [DOI] [PubMed] [Google Scholar]
- 55.Bode-Boger SM, Muke J, Surdacki A, Brabant G, Boger RH, Frolich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vascular Medicine. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 56.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol. 2007;102:63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- 57.Avery AA. Infantile methemoglobinemia: Reexamining the role of drinking water nitrates. Environ Health Persp. 1999;107:583–586. doi: 10.1289/ehp.99107583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilchrist M, Winyard PG, Benjamin N. Dietary nitrate – Good or bad? Nitric Oxide. 2009 doi: 10.1016/j.niox.2009.10.005. In Press. [DOI] [PubMed] [Google Scholar]
- 59.Milkowski A, Garg HK, Coughlin JR, Bryan NS. Nutritional Epidemiology in the Context of Nitric Oxide Biology: A Risk-Benefit Evaluation for Dietary Nitrite and Nitrate. Nitric Oxide. 2009 doi: 10.1016/j.niox.2009.08.004. In press. [DOI] [PubMed] [Google Scholar]
- 60.Tannenbaum SR, Weisman M, Fett D. EFFECT OF NITRATE INTAKE ON NITRITE FORMATION IN HUMAN SALIVA. Food and Cosmetics Toxicology. 1976;14:549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- 61.Spiegelhalder B, Eisenbrand G, Preussmann R. INFLUENCE OF DIETARY NITRATE ON NITRITE CONTENT OF HUMAN SALIVA - POSSIBLE RELEVANCE TO INVIVO FORMATION OF N-NITROSO COMPOUNDS. Food and Cosmetics Toxicology. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 62.Madden DJ, Bennett IJ, Song AW. Cerebral White Matter Integrity and Cognitive Aging: Contributions from Diffusion Tensor Imaging. Neuropsychology Review. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weiner MW, Chui HC, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cahn-Weiner D, Boyle P, Malloy P. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Applied Neuropsychology. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]