Abstract
AIM: To assess the effectiveness and safety of imaging-guided percutaneous cecostomy in the management of pediatric patients with organic fecal incontinence.
METHODS: Twenty three cecostomies were performed on 21 children with organic fecal incontinence (13 males, 8 females), aged from 5 to 16 years (mean 9.5 years). Thirteen patients had neurogenic fecal incontinence and 8 patients had anorectal anomalies. Procedures were performed under general anesthesia and fluoroscopic guidance. Effectiveness and complication data were obtained for at least 1 year after the procedure.
RESULTS: Cecostomy was successful in 20 patients (primary technical success rate 95%). Cecostomy failed in one patient due to tube breakage (secondary technical success rate 100%). The tubes were in situ for an average of 18 mo (range 12-23 mo). Eighteen patients (87%) expressed satisfaction with the procedures. Resolution of soiling was achieved in all patients with neurogenic fecal incontinence (100%) and in 5 of 8 patients with anorectal anomalies (62.5%). Eleven patients (52%) experienced minor problems. No major complications were noted.
CONCLUSION: Percutaneous cecostomy improves the quality of life in children with organic fecal incontinence. A satisfactory outcome is more prevalent in patients with neurogenic fecal incontinence than anorectal anomalies.
Keywords: Cecostomy, Fecal incontinence, Interventional radiology, Pediatric radiology
INTRODUCTION
Fecal incontinence refers to the inability to hold feces in the rectum. This is due to failure of voluntary control over the anal sphincters, permitting untimely passage of feces and gas. Though most children become toilet trained between 2 and 4 years of age, there are some situations in which children do not develop complete control of their stool elimination. This leads to stool leaking from the rectum at unexpected times. Fecal incontinence is a devastating disability that is not uncommon in the pediatric population. Fecal incontinence can be one of the most psychologically and socially debilitating conditions in an otherwise healthy individual. It can lead to social isolation, loss of self-esteem and self-confidence, and depression[1].
Fecal incontinence can be associated with peristalsis, sensation, sphincter control, anatomy, or psychosocial disorders. Most cases of fecal soiling in children are functional and are usually associated with severe constipation. Organic fecal incontinence may be permanent, as in patients with myelodysplasia, self-limiting, as in patients who have fecal soiling after a pull-through operation for Hirschsprung’s disease, or partial, as with many patients who have undergone repair of an anorectal malformation[2]. Recently, Di Lorenzo et al[3] classified pediatric fecal incontinence into four main categories according to pathophysiology. The first category is functional fecal retention due to withholding of feces because of fear of painful defecation, which results in constipation and overflow soiling. The second category is functional nonretentive fecal soiling due to antidiarrheal agents, which can increase the consistency of stools and facilitate continence. The third category is organic fecal insentience due to congenital anorectal anomalies. The last category is organic neurogenic fecal insentience, which occurs in children with spina bifida or spinal injuries.
Traditional treatments of fecal incontinence include dietary modification, laxatives, suppositories, enemas, manual disimpaction, biofeedback and electrostimulation. Despite these efforts, many patients do not achieve fecal continence. Fecal incontinence can be treated by emptying the bowels with a rectal enema. This treatment is effective, but it can also be messy, time-consuming, inconvenient, and difficult (or impossible) for some patients to do by themselves.
Surgically created cecostomies have been attempted for many years. In 1986, Casola et al[4] described a percutaneous approach for the placement of a cecostomy catheter for colonic decompression in adults. In1988, Ganc et al[5] described a technique for transcolonoscopic extraperitoneal cecostomies as an effective new therapeutic technique for the management of fecal incontinence. In 1990, Malone et al[6] described antegrade colonic enema (MACE) as a surgical procedure where the appendix is used to form a cutaneous cecostomy for fluid irrigation of the colon. However, these surgically created cecostomies carry risks of stomal stenosis, stomal leakage, appendiceal necrosis, general anesthesia and bowel perforation.
In 1996, Shandling et al[7] described a pilot study for a percutaneous image-guided approach to gain access to the cecum for the introduction of an antegrade enema. This study was later followed by other studies that showed this percutaneous procedure represents a less invasive alternative to a surgical procedure[8-12]. Percutaneous endoscopic cecostomy is a viable alternative to surgically or fluoroscopically placed cecostomy in a select group of patients with recurrent colonic pseudo-obstruction or chronic intractable constipation[13]. In general, and according to the pathophysiology of pediatric fecal incontinence, functional fecal retention is treated by dietary changes, use of drugs, anorectal biofeedback and cognitive and behavioral interventions, such as toilet training. On the other hand, patients with organic fecal incontinence benefit from techniques that teach them how to defecate, such as with cecostomy, which completely cleanses the colon, increases the child’s autonomy, and decreases the chance of soiling. However, there is great variation in the postsurgical functional outcomes of these procedures[3,8-12].
The purpose of our study was to assess the effectiveness and safety of imaging-guided percutaneous cecostomy in the management of pediatric patients with organic fecal incontinence.
MATERIALS AND METHODS
From January 2005 to December 2009, 23 imaging-guided percutaneous cecostomy tube placements were performed on 21 children with organic fecal incontinence. The patients included 13 males and 8 females aged from 5 to
16 years (mean 9.5 years). Thirteen patients suffered from neurogenic fecal incontinence secondary to spinal etiology (6 patients had myelomeningocele, 2 had sacral agenesis and 5 had dorso-lumbar spinal injuries with paraplegia). The other 8 patients had fecal incontinence secondary to anorectal anomalies after failure of medical and surgical management. This study was approved by the credential and privileges committee in our hospital. Informed consents were obtained from the parents or guardians. All patients had lax anal sphincter by digital rectal examination and had marked cecal dilatation 13 ± 3.5 cm as seen in plain abdominal radiographs. Ten patients underwent a barium enema study before admission to detect the position of the cecum.
Technique of percutaneous cecostomy
Pre-procedure preparation: The patients consumed a fluid diet for 2 d before the percutaneous cecostomy procedure. Usually the patients were admitted 1-d before the procedure. Forty five milliliters sodium phosphate solution (Phospho-Soda oral laxative; Fleet, Lynchburg, Va) was administered orally the night of admission. Radiography of the abdomen was performed just prior to the procedure to assess bowel cleansing, and a repeat dose of the phosphate solution was given, if necessary. Complete blood analysis, clotting and coagulation times were checked before the procedure. Abdominal and pelvic ultrasonography (Voluson 730 Expert, GE, Austria) was performed before the procedure to identify fluid collections and the positions of major organs, including the gallbladder, liver, and urinary bladder. Glucagon hydrochloride (Eli Lilly, Scarborough, Ontario, Canada) was administered intravenously at a dose of 0.5-1.0 mg before the procedure to inhibit colonic peristalsis. A prophylactic antibiotic regimen was given to all patients before and after the procedure. The antibiotic regimen included three intravenously administered antibiotics: 2.5 mg/kg gentamicin (Gentamicin Injection USP; Sabex, Boucherville, Quebec, Canada) every 8 h, 10 mg/kg metronidazole (Metronidazole Injection; Abbott, St Laurent, Quebec, Canada) every 8 h, and 20 mg/kg ampicillin (Ampicillin Sodium for Injection USP; Novofarm, Toronto, Ontario, Canada) every 6 h.
Procedure: The procedures were performed under general anesthesia in all patients. Imaging was performed on a C-arm fluoroscopic table with tilting capabilities (Siemens Neurostar, Siemens Medical Systems, Munich, Germany).We used the technique that was described in detail by Chait et al[14] in 2003.
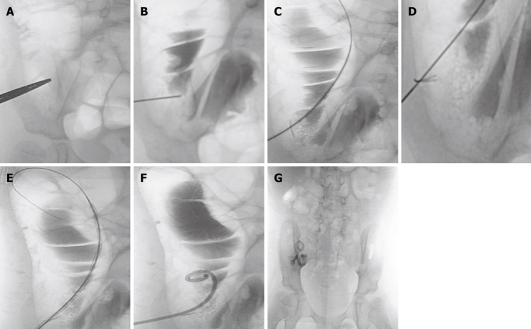
Briefly, the cecum was distended with air by using a 22-F silicone (latex-free) Foley catheter (Rusch-Pilling, Kamunting, Malaysia) that was inserted through the rectum. The skin entrance site of the air-filled cecum was localized under fluoroscopic guidance (Figure 1A). A puncture was made using a Majestic 18-gauge, 7-cm, one-wall needle with a Seldinger shield (Merit Medical, South Jordan, Utah). To confirm the intraluminal position of the needle, 5 mL of water soluble contrast material (Omnipaque; Nycomed Imaging, Oslo, Norway) was injected under fluoroscopic guidance (Figure 1B). A 0.035-inch guide wire (Cook, Bloomington, MA) was inserted and the needle was advanced into the cecum (Figure 1C). A T-fastener (Meditech/Boston Scientific, Watertown, MA) was then deployed to fix the anterior wall of the cecum to the anterior abdominal wall (Figure 1D). The retention suture threads were clamped with mosquito forceps. The tract was dilated by the use of an 8-F Coons dilator (Cook, Bloomington, MA; Figure 1E). After dilatation, a 8.5-F, 15-cm latex-free Dawson Mueller Mac-Loc locking pigtail catheter (Cook, Bloomington, MA) was inserted (Figure 1F). The catheter was connected to a drainage bag into which liquid drained to empty the colon and decrease the risk of leakage around the cecostomy tube site.
Figure 1.
Procedure of percutaneous cecostomy. A: Skin entrance site is localized over the air-filled distended cecum; B: After needle insertion into the cecum, contrast is injected to confirm its location within the cecal lumen; C: Guide wire insertion into the distended right side of the colon; D: T-fastener deployed to fix the anterior wall of the cecum to the abdominal wall; E: The insertion tract is dilated before placement of the cecostomy tube; F: Locking pigtail catheter is inserted into the cecum; G; After 6 wk, the Dawson Mueller catheter is exchanged for the permanent Chait Trapdoor cecostomy catheter.
Post procedure care
The antibiotic regimen was continued for 48 h and followed by orally administered metronidazole (10 mg/kg every 8 h) for 5 d. Analgesia after the procedure comprised intravenously administered morphine (0.05 mg/kg) every 4 h, as needed, for 12 h, followed by orally administered acetaminophen (15 mg/kg) every 4 h as needed. The patients could eat after 12 h, if there was no paralitic ileus and early ambulation was advised as tolerated. Patients who were free of complications were discharged within 2 d or 3 d. Patients that suffered from postprocedure paralytic ileus were usually discharged on the 5th or 6th day after the procedure. When patients were discharged from the hospital, the drainage bags were removed at the time of discharge, and the patients were asked to flush the catheter by 5 mL of saline twice daily. A week after the insertion of the cecostomy tube, antegrade enema irrigations began according to individual needs. Patients and their relatives were trained on how to use the two irrigation bags for routine antegrade colonic evacuation and cleansing. The first bag was a sodium phosphate enema (Merck-Frost, Mississauga, Ontario, Canada), which was introduced at a dose of 2 mL/kg up to a total dose of 130 mL. After 15 min, the second bag (200-400 mL saline solution) was introduced, which could be easily prepared (10 mL salt for one liter of water). The retention sutures were cut 10 d after the procedure.
Long-term care
After 6 wk, the Dawson Mueller Mac-Loc catheter was exchanged by the permanent Chait Trapdoor cecostomy catheter (Cook, Bloomington, MA). The exchange was performed over a guidewire under fluoroscopic guidance. No sedation or antibiotic coverage was necessary, and the procedure was performed on an outpatient basis (Figure 1G). The Chait Trapdoor catheter is to be replaced every year or when a problem arises. Catheter replacement was performed by cutting the old one below the “trapdoor” and advancing the new catheter over a guidewire. This displaced the distal part of the old catheter into the ascending colon, and then it passed easily through the rectum. Long-term care of the cecostomy patients was performed by a team comprising an interventional radiologist, a general surgeon and a pediatrician. Data regarding effectiveness, complications and satisfaction of treatment were obtained by interviewing the children and their parents during follow-up consultation.
RESULTS
Twenty three cecostomy tube placements were performed on 21 children with organic fecal incontinence. In all cases, an 8.5-F Dawson-Mueller catheter were placed in the cecum and exchanged after 6 wk with a cecostomy button (Trapdoor catheter). Tube placement was successful in 20 patients (primary success rate is 95%). Cecostomy tube insertion failed in one patient due to tube breakage during its insertion and replacement by another tube was successfully done in the same setting. Another patient required repeated tube placement after accidental dislodgement of the previously inserted tube after 5 d (secondary success rate is 100%). Functional results (effectiveness, complications and satisfaction) were obtained at a follow-up period of at least 1 year after the procedure. The cecostomy catheters were in situ for an average of 18 mo (range 12-23 mo). Eighteen of the 21 patients (87%) expressed satisfaction in the effectiveness of the procedure. Resolution of continuous soiling was achieved in all patients with neurogenic fecal incontinence (100%) and in 5 out of 8 patients with anorectal anomalies (62.5%). Antegrade enemas were administered according to the needs of the patient (range, every 12-48 h; mean, every 18 h).
Eleven patients (52%) experienced minor cecostomy tube related problems at some point during the follow-up period; a cecostomy tube infection occurred in 2 patients, which required oral antibiotic treatment, clogging of the cecostomy tube in 2 patients, partial dislodgment of the tube in 2 patients, the development of granulation tissue accompanied with redness at the cecostomy site appeared in 4 patients and vomiting related to the phosphate enema occurred in one patient. These problems were easily corrected at home and did not require surgical intervention. Among the patients that underwent cecostomies, no procedure related death or major complication requiring surgical intervention or hospital admission was noted.
DISCUSSION
Fecal incontinence is a major health problem in that it affects the quality of life and causes significant embarrassment. In childhood and adolescence, fecal soiling represents a psychologically devastating problem and there is both physical and emotional distress associated with daily rectal enemas. Pediatric fecal incontinence is classified into organic or true fecal incontinence, which is either neurogenic or secondary to anorectal anomalies, and functional fecal incontinence, which is either retention-type due to fecal withholding with overflow or nonretentive-type of fecal soiling with antidiarrheal agents[3]. The majority of cases of fecal incontinence in children are functional, which is fortunately a self-limiting problem and usually disappears at puberty. Organic fecal incontinence may be permanent, as with myelodysplasia; self-limiting, as after a pull-through operation for Hirschsprung’s disease; or partial, as with an anorectal malformation[2]. Traditional treatment options for fecal incontinence include dietary modification, drugs, retrograde enemas, manual disimpaction, biofeedback, electrostimulation and cognitive and behavioral interventions that facilitate continence. These options work well in controlling functional fecal incontinence[3,15,16]. However, these options are ineffective in controlling organic fecal incontinence, which may benefit from techniques that completely cleanse the colon and decrease the chance of soiling such as with percutaneous cecostomy. Administration of antegrade enemas through a cecostomy is a therapeutic option for children with severe fecal incontinence. However, there is great variation in postsurgical functional outcomes of these procedures.
In this study, the primary and secondary technical success rates of insertion of cecostomy tubes were 95% and 100%, respectively, which are similar to other studies[8-11,14,15,17]. Long term follow up of patients showed resolution of continuous soiling in all patients with neurogenic fecal incontinence. These results confirm the effectiveness of cecostomy in the management of patients with neurogenic fecal incontinence secondary to spina bifida or traumatic spinal injury, which was reported in other studies[3,18,19]. On the other hand, resolution of continuous soiling was achieved in 5 of 8 patients with anorectal anomalies (62.5%). This result is lower than that of Sierre et al[20] in which 90% of their 20 patients (18 of them had anorectal anomalies) reported satisfaction with the procedure. However, our results are similar to other studies that noted there is great variation in postsurgical functional outcomes of cecostomy in the management of patients with anorectal malformations[3,21,22]. The reason for this relatively low success rate can be explained by the complexity of anorectal anomalies, such as the presence of distal rectovesical or rectovaginal fistulae that may cause soiling.
Regarding post procedure complications in this study, there were no major complications and 11 patients (52%) experienced minor cecostomy tube related problems at some point during the follow-up period. These problems were easily corrected at home and did not require medical or surgical intervention. These complications are within the range of complications noticed in other studies[17,22].
In conclusion, imaging-guided percutaneous cecostomy and antegrade enemas improved symptoms and the quality of life in children with organic fecal incontinence with minor early and late complications. The success rate was more evident in patients with neurogenic fecal incontinence rather than those with anorectal anomalies. However, further evaluation by double-blinded, randomized controlled trials is recommended to define the role of cecostomy in the management of different kinds of organic fecal incontinence.
COMMENTS
Background
In childhood, fecal incontinence represents a psychologically devastating problem. Pediatric fecal incontinence is classified into organic or true fecal incontinence, which is either neurogenic or secondary to anorectal anomalies, and functional fecal incontinence, which is either retention-type due to fecal withholding with overflow or a nonretentive-type of fecal soiling with antidiarrheal agents. The majority cases of fecal incontinence in children are functional, which a self-limiting problem, while organic fecal incontinence is permanent and needs surgical intervention.
Research frontiers
Traditional treatment options of fecal incontinence work well in controlling functional fecal incontinence. These options are ineffective in controlling organic fecal incontinence, which may benefit from techniques that completely cleanse the colon, such as surgical or imaging-guided percutaneous cecostomy.
Innovations and breakthroughs
Imaging-guided percutaneous cecostomy and antegrade enemas improved symptoms and the quality of life in children with organic fecal incontinence with minor early and late complications.
Applications
Administration of antegrade enemas through a percutaneous cecostomy is a therapeutic option for children with organic fecal incontinence.
Terminology
Percutaneous cecostomy is imaging-guided construction of an opening into the cecum with a tube through the abdominal wall. It is an alternative to the traditional surgical technique for cecal decompression.
Peer review
The manuscript is accepted without modification.
Footnotes
Peer reviewer: Alain Chapel, PhD, Institut de Radioprotection et de S reté Nucléaire, DPHD, IRSN. B.P. no 17, F-92262 Fontenay-Aux-Roses, France
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
References
- 1.Bishop PR, Nowicki MJ. Defecation disorders in the neurologically impaired child. Pediatr Ann. 1999;28:322–329. doi: 10.3928/0090-4481-19990501-13. [DOI] [PubMed] [Google Scholar]
- 2.Rintala RJ. Fecal incontinence in anorectal malformations, neuropathy, and miscellaneous conditions. Semin Pediatr Surg. 2002;11:75–82. doi: 10.1053/spsu.2002.31805. [DOI] [PubMed] [Google Scholar]
- 3.Di Lorenzo C, Benninga MA. Pathophysiology of pediatric fecal incontinence. Gastroenterology. 2004;126:S33–S40. doi: 10.1053/j.gastro.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Casola G, Withers C, vanSonnenberg E, Herba MJ, Saba RM, Brown RA. Percutaneous cecostomy for decompression of the massively distended cecum. Radiology. 1986;158:793–794. doi: 10.1148/radiology.158.3.3945754. [DOI] [PubMed] [Google Scholar]
- 5.Ganc AJ, Netto AJ, Morrell AC, Plapler H, Ardengh JC. Transcolonoscopic extraperitoneal cecostomy. A new therapeutic and technical proposal. Endoscopy. 1988;20:309–312. doi: 10.1055/s-2007-1018203. [DOI] [PubMed] [Google Scholar]
- 6.Malone PS, Ransley PG, Kiely EM. Preliminary report: the antegrade continence enema. Lancet. 1990;336:1217–1218. doi: 10.1016/0140-6736(90)92834-5. [DOI] [PubMed] [Google Scholar]
- 7.Shandling B, Chait PG, Richards HF. Percutaneous cecostomy: a new technique in the management of fecal incontinence. J Pediatr Surg. 1996;31:534–537. doi: 10.1016/s0022-3468(96)90490-x. [DOI] [PubMed] [Google Scholar]
- 8.Chait PG, Shandling B, Richards HM, Connolly BL. Fecal incontinence in children: treatment with percutaneous cecostomy tube placement--a prospective study. Radiology. 1997;203:621–624. doi: 10.1148/radiology.203.3.9169678. [DOI] [PubMed] [Google Scholar]
- 9.Chait PG, Shandling B, Richards HF. The cecostomy button. J Pediatr Surg. 1997;32:849–851. doi: 10.1016/s0022-3468(97)90634-5. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox DT, Kiely EM. The Malone (antegrade colonic enema) procedure: early experience. J Pediatr Surg. 1998;33:204–206. doi: 10.1016/s0022-3468(98)90432-8. [DOI] [PubMed] [Google Scholar]
- 11.Malone PS, Curry JI, Osborne A. The antegrade continence enema procedure why, when and how? World J Urol. 1998;16:274–278. doi: 10.1007/s003450050066. [DOI] [PubMed] [Google Scholar]
- 12.Soulsby R, Radley S. Simple equipment for decompression of the colon during laparotomy for large bowel obstruction. Colorectal Dis. 2002;4:262–263. doi: 10.1046/j.1463-1318.2002.00347.x. [DOI] [PubMed] [Google Scholar]
- 13.Lynch CR, Jones RG, Hilden K, Wills JC, Fang JC. Percutaneous endoscopic cecostomy in adults: a case series. Gastrointest Endosc. 2006;64:279–282. doi: 10.1016/j.gie.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 14.Chait PG, Shlomovitz E, Connolly BL, Temple MJ, Restrepo R, Amaral JG, Muraca S, Richards HF, Ein SH. Percutaneous cecostomy: updates in technique and patient care. Radiology. 2003;227:246–250. doi: 10.1148/radiol.2271020574. [DOI] [PubMed] [Google Scholar]
- 15.Mousa HM, van den Berg MM, Caniano DA, Hogan M, Di Lorenzo C, Hayes J. Cecostomy in children with defecation disorders. Dig Dis Sci. 2006;51:154–160. doi: 10.1007/s10620-006-3101-7. [DOI] [PubMed] [Google Scholar]
- 16.Byrne CM, Solomon MJ, Young JM, Rex J, Merlino CL. Biofeedback for fecal incontinence: short-term outcomes of 513 consecutive patients and predictors of successful treatment. Dis Colon Rectum. 2007;50:417–427. doi: 10.1007/s10350-006-0846-1. [DOI] [PubMed] [Google Scholar]
- 17.Wong AL, Kravarusic D, Wong SL. Impact of cecostomy and antegrade colonic enemas on management of fecal incontinence and constipation: ten years of experience in pediatric population. J Pediatr Surg. 2008;43:1445–1451. doi: 10.1016/j.jpedsurg.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Vande Velde S, Van Biervliet S, Van Renterghem K, Van Laecke E, Hoebeke P, Van Winckel M. Achieving fecal continence in patients with spina bifida: a descriptive cohort study. J Urol. 2007;178:2640–2644; discussion 2644. doi: 10.1016/j.juro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Goepel M, Sperling H, Stöhrer M, Otto T, Rübben H. Management of neurogenic fecal incontinence in myelodysplastic children by a modified continent appendiceal stoma and antegrade colonic enema. Urology. 1997;49:758–761. doi: 10.1016/S0090-4295(96)00623-1. [DOI] [PubMed] [Google Scholar]
- 20.Sierre S, Lipsich J, Questa H, Bailez M, Solana J. Percutaneous cecostomy for management of fecal incontinence in pediatric patients. J Vasc Interv Radiol. 2007;18:982–985. doi: 10.1016/j.jvir.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Altomare DF, Rinaldi M, Rubini D, Rubini G, Portincasa P, Vacca M, Artor NA, Romano G, Memeo V. Long-term functional assessment of antegrade colonic enema for combined incontinence and constipation using a modified Marsh and Kiff technique. Dis Colon Rectum. 2007;50:1023–1031. doi: 10.1007/s10350-006-0863-0. [DOI] [PubMed] [Google Scholar]
- 22.Nanigian DK, Nguyen T, Tanaka ST, Cambio A, DiGrande A, Kurzrock EA. Development and validation of the fecal incontinence and constipation quality of life measure in children with spina bifida. J Urol. 2008;180:1770–1773; discussion 1773. doi: 10.1016/j.juro.2008.03.103. [DOI] [PubMed] [Google Scholar]