Abstract
Cellular α-glucosidases I and II are enzymes that sequentially trim the three terminal glucoses in the N-linked oligosaccharides of viral envelope glycoproteins. This process is essential for the proper folding of viral glycoproteins and subsequent assembly of many enveloped viruses, including dengue virus (DENV). Imino sugars are substrate mimics of α-glucosidases I and II. In this report, we show that two oxygenated alkyl imino sugar derivatives, CM-9-78 and CM-10-18, are potent inhibitors of both α-glucosidases I and II in vitro and in treated animals, and efficiently inhibit DENV infection of cultured human cells. Pharmacokinetic studies reveal that both compounds are well tolerated at doses up to 100mg/kg in rats and have favorable pharmacokinetic properties and bioavailability in mice. Moreover, we showed that oral administration of either CM-9-78 or CM-10-18 reduces the peak viremia of DENV in mice. Interestingly, while treatment of DENV infected mice with ribavirin alone did not reduce the viremia, combination therapy of ribavirin with sub-effective dose of CM-10-18 demonstrated a significantly enhanced antiviral activity, as indicated by a profound reduction of the viremia. Our findings thus suggest that combination therapy of two broad-spectrum antiviral agents may provide a practically useful approach for the treatment of DENV infection.
Keywords: α-glucosidase, imino sugar, dengue virus, ribavirin, combination
1. Introduction
Dengue virus (DENV) is a mosquito-borne flavivirus that causes lethal hemorrhagic fever in people and is currently classified as a re-emerging infectious disease of bioterror concern (Mackenzie, Gubler, and Petersen, 2004). Effective antiviral therapies and vaccines are not yet available to treat or prevent DENV infection. Because the life-threatening Dengue hemorrhagic diseases are considered to be due to a higher titer of viremia, antivirals that reduce viral load by 10-fold or greater are anticipated to decrease mortality of DENV infection (Noble et al., 2010; Vaughn et al., 2000).
Concerning the antiviral strategies to inhibit DENV infection, one of the most obvious approaches is to directly target virus encoded enzymes and other functions. Alternatively, inhibition of host cellular functions required for DENV replication or activation of host cellular intrinsic antiviral programs should also impair the viral replication. In fact, compared to targeting viral functions, one of the major advantages of targeting host functions is the lower likelihood of the emergence of drug-resistant mutants (Noble et al., 2010).
α-glucosidases I and II are endoplasmic reticulum (ER) resident enzymes responsible for the sequential hydrolysis of glucose residues from the asparagine-linked (N-linked) oligosaccharides on glycoprotein precursors (Helenius and Aebi, 2004). Removal of the glucose residues is essential for the glycoproteins to interact with ER chaperones, which ensures the proper folding of the glycoproteins (Dwek et al., 2002). Imino sugars, represented by deoxynojirimycin (DNJ), are glucose mimics that act as competitive inhibitors of α-glucosidases I and II. It has been demonstrated, by others and us that imino sugar derivatives selectively inhibited the virion assembly and secretion of many enveloped viruses, including DENV (Block et al., 1998; Block et al., 1994; Chang et al., 2009; Dwek et al., 2002; Gu et al., 2007; Jordan et al., 2002). However, the essential role of cellular α-glucosidase II in DENV infection was only recently demonstrated in a genome-wide siRNA knockdown effort to identify host factors required for or restricting the virus infection (Sessions et al., 2009).
In our effort to search for imino sugar derivatives with superior antiviral activity against DENV, we found previously that two pharmacophores of oxygenated alkyl imino sugar derivatives, CM-9-78 and CM-10-18, potently inhibit bovine viral diarrhea virus (BVDV) infection in cell cultures (Chang et al., 2009). In this study, we further demonstrate that both compounds are glucosidase inhibitors in vitro and in treated mice. Interestingly, although monotherapy of DENV-infected mice with either CM-9-78 or CM-10-18 modestly reduced the viremia, combination therapy with a clinically approved broad-spectrum antiviral compound, ribavirin, greatly improved the antiviral activity of the imino sugar. Our results imply that combination therapy of DENV infection with two mechanistically distinct antiviral compounds may provide an option for the treatment of Dengue fever and Dengue hemorrhagic diseases.
2. Materials and methods
2.1. In vitro α-glucosidases I and II enzymatic assay
Free oligosaccharide (FOS), Glc3Man5GlcNAc1, a substrate for glucosidase I, and Glc1Man5GlcNAc1, a substrate of glucosidase II, were purified as previously described (Alonzi et al., 2008). Following 2-AA (2-aminobenzoic acid)-fluorescent labeling of the FOS, purified α-glucosidase I or glucosidase II (from rat liver) and various concentration of CM-9-78 or CM-10-18 were added. Separation of the hydrolysis products was performed using Normal phase-HPLC (NP-HPLC, Waters). Measurement of areas under the peak area was used as quantification of the hydrolysis product by α-glucosidases I or II. Dose-response curves were generated to calculate the concentration required to inhibit enzyme activity by 50% (IC50).
2.2. In vitro antiviral assay against DENV infection
A cell-based flavivirus immunodetection (CFI) assay was used to determine the in vitro anti-dengue activity, as previously described (Wang et al., 2009). Briefly, 2×104 of A549 cells were seeded in 96-well plate and cultured overnight, before they were infected with DENV (serotype II, TSV01 strain, virus was produced as previously described (Schul et al., 2007)) at multiplicity of infection (MOI) of 0.3 for 1 hour, followed by incubation in absence or presence of indicated doses of test compounds for 2 days. The cells were then fixed and incubated with primary antibody specific for flavivirus envelope (E) proteins (mouse monoclonal antibody 4G2, ATCC), followed by incubation with secondary horseradish peroxidase-conjugated anti-mouse immunoglobulin G. After addition of substrate, the plate was read at 450nm to quantify the DENV E protein. Dose-response curves were generated to calculate 50% effective concentration (EC50) values.
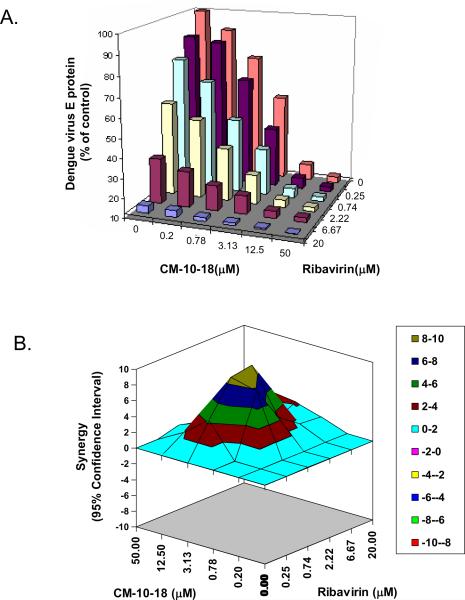
CFI assay was also used as in vitro antiviral assay to evaluate synergistic effect between imino sugar and ribavirin. CM-10-18 or ribavirin, were used either alone or in combination at indicated doses, in a checkerboard dilution matrix format (Buckwold et al., 2003). This experiment was performed in 8 replicates for each dose condition to generate statistically sufficient data to facilitate subsequent application of MacSynergy II, a program allowing for the plotting of synergistic effect (Prichard et al. MacSynergy II. User's manual. University of Michigan, Ann Arbor, 1993). Briefly, if the drug-drug interaction is additive, this program will generate a horizontal surface, however, if the interaction is greater-, or less-than-expected, it will generate peak above or below the surface to indicate a synergy or antagonism interaction.
Cell viability assay was performed using propodium iodide staining to monitor the cytotoxicity of test compounds. Dose-response curves were generated to calculate 50% cytotoxic concentration (CC50).
2.3. Pharmacokinetic (PK) Analysis
Female CD-1 mice were used to generate PK parameters shown in Tab. 2. CM-10-18 was formulated in Phosphate Buffered Saline (PBS) pH 7.4, and dosed at 32.4mg/Kg (oral), or 6.5mg/Kg intravenously (i.v.). Blood samples were collected at 0.02 (i.v. only), 0.08 (oral only), 0.25, 0.5, 1, 2, 4, 8, and 24h (oral only) post administration. PK parameters were calculated as previously described (Yin et al., 2009). Three mice were included in each dosing group.
Table 2.
Pharmacokinetic properties of CM-10–18 in mice dosed orally at 32.4 mg/kg or intravenously (i.v.) at 6.5mg/kg
| Parameters | Unit | Value |
|---|---|---|
| Vss (i.v.) | L/kg | 1.02 |
| CL (i.v.) | ml/h/kg | 1466.27 |
| T1/2 (i.v.) | h | 2.63 |
| Tmax (oral) | h | 0.25 |
| Cmas (oral) | μg/ml | 4.20 |
| AUC (oral) | mg*h/ml | 12.15 |
| F | % | 56 |
Vss, Volume of distribution at steady state;
CL, plasma clearance;
T1/2, elimination half time;
Tmax, time to maximum concentration in plasma;
Cmax, maximum concentration in plasma;
AUC, Area Under the Curve (t=0 to infinite);
F, percent of bioavailability
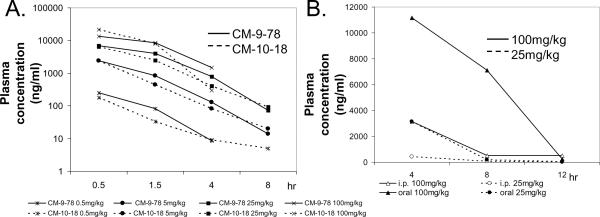
Dose escalation studies of CM-9-78 and CM-10-18 were performed to measure PK and preliminary toxicity, with Spague-Dawley rats (BASi) (Fig. 2A). Compounds in PBS were given via intra-peritoneal (i.p) route, through an implanted catheter. Three rats per compound were given vehicle plus four escalating doses (0.5, 5, 25, and 100mg/kg, all dosed at 5ml/kg) of test compounds every 48 to 72 hours. To monitor systemic compound concentration, blood samples were collected following each dosing, at time points indicated in Fig. 2A. Tissue concentrations (liver, kidney, brain and abdominal fat) from samples collected four hours post-dose following i.p. administration of 100mg/kg CM-9-78 or CM-10-18 were also measured. Analysis of compounds was performed using a gradient HILIC LC-MS/MS technique. Animals were monitored with each dose to assess possible toxic response post-administration.
Fig. 2. Pharmacokinetic properties of CM-9-78 and CM-10-18.
(A) Dose escalation studies of CM-9-78 and CM-10-18. Escalating doses, as indicated, were given to rats every 48 to 72 hours, via i.p. route. Blood samples were harvested at 0.5, 1.5, 4 and 8 (except at dose 100mg/kg) hours after injection to measure the plasma concentration (ng/ml) of the test compounds. Each data points represent average values obtained from 3 rats. (B) Single dose administration via oral gavage or i.p. injection of CM-10-18 in mice. CM-10-18 was given at 25mg/kg or 100mg/kg. Blood samples were drawn at 4, 8, and 12 hours post administration to measure the plasma concentration of compound (ng/ml). Each data points represent average values obtained from 2 mice.
For experiment shown in Fig. 2B, 6 week-old S129 mice (AG129 mice used in animal efficacy studies are derived from S129 mice) were used. 25 or 100mg/kg of CM-9-78 were given by oral gavage or i.p. injection. Each group contained 6 mice. Blood samples were collected at 4, 8 and 12 hours post dosing. Analysis of compounds was performed by BASi, using similar method developed for rat experiments.
2.4. Antiviral efficacy in DENV infected mice
The in vivo efficacy experiments were largely performed as previously described, using a dengue viremia model in AG129 mouse (defective of both type I and type II interferon receptors) (Schul et al., 2007). Each experiment contained 6 mice (7 to 8 week-old) per dosing group. The mice were challenged with DENV (serotype 2, TSV01 strain), at 5×106 pfu/mouse via i.p. injection. Imino sugars were dosed twice daily at 12 hr intervals, and ribavirin was dosed once daily, for 3 consecutive days post-infection. Blood samples were drawn 3 days post-infection for determination of plasma virus titer by plaque assay, as previously published (Schul et al., 2007).
2.5. Detection of free oligosaccharides (FOS) in plasma from treated mice
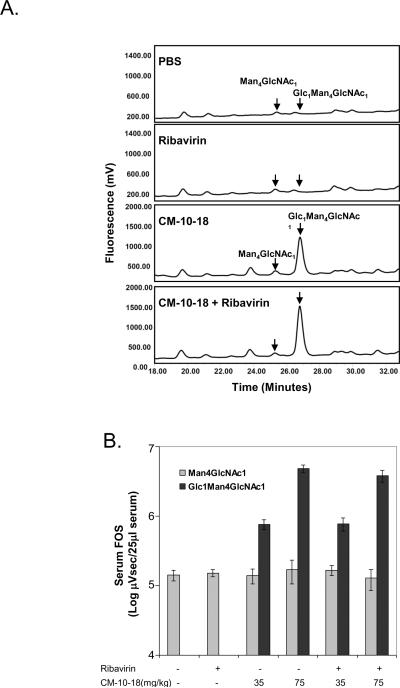
FOS was isolated from 25μl of plasma samples from mice treated with indicated compounds, using a procedure described previously (Alonzi et al., 2008). After labeling of the FOS with 2-AA, a NP-HPLC analysis was performed to separate individual FOS as shown in Fig. 6A. The peak areas of two 2-AA-labelled FOS (Glc1Man4GlcNAc1 and Man4GlcNAc1) were measured using Waters Empower software to generate quantitative values of each FOS, and expressed as μVsec (Fig. 6B). While Man4GlcNAc1 FOS serves as internal control, Glc1Man4GlcNAc1, a representative FOS of terminal mono- glucose retention as a result of glucosidase II inhibition, is the indicator of the effect of imino sugar on glucosidases activities in vivo (Alonzi et al., 2008).
Fig. 6. Imino sugar treatment inhibited α-glucosidase II in mice.
Blood samples obtained from in vivo efficacy study, described in Fig. 5B, were also analyzed for the presence of specific FOS (Glc1Man4GlcNAc1) as representative of glucosidase inhibition. A corresponding FOS (Man4GlcNAc1) was used as internal control, (A) Representative fluorescent Peak Time plots of FOS from samples obtained from mice treated with PBS, ribavirin (40mg/kg), CM-10-18 (75mg/kg) as well as combination of the two treatments. (B) Quantitation of the peak areas of the two FOS (Glc1Man4GlcNAc1 and Man4GlcNAc1) (Log μVsec/25ml of plasma) in samples from mice treated with ribavirin, indicated doses of CM-10-18 or combination. Values represent average and standard deviation from results obtained from 6 mice per treatment group.
3. Results
3.1. Imino sugar derivatives CM-9-78 and CM-10-18 inhibit α-glucosidase I and II enzymatic activities
We have previously reported a novel series of imino sugar derivatives with nitrogen linked 5 or 6 carbon alkyl side chain and variable terminal ring structures (Chang et al., 2009; Gu et al., 2007). CM-9-78 and CM-10-18 are two representative compounds from this class (Table 1). Although we have demonstrated previously that both CM-9-78 and CM-10-18 inhibited the virion assembly and secretion of BVDV, which is in agreement with the inhibition of cellular α-glucosidases (Chang et al., 2009), we have now obtained direct evidence with an in vitro enzymatic assay that both CM-9-78 and CM-10-18 are α-glucosidase I inhibitors with IC50 values of 0.42μM and 0.46μM, respectively and inhibit α-glucosidase II activity with IC50 values of 2.63 μM and 1.55 μM, respectively (Tab. 1). These results demonstrated that CM-9-78 and CM-10-18 are inhibitors of both α-glucosidases I and II.
Table 1.
Inhibition of α-glucosidases I and II enzymatic activity
| Compound | CM-9–78 | CM-10–18 |
|---|---|---|
| Structure |
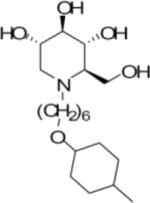
|
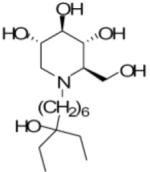
|
| Enzyme: α-Glucosidase I Substrate: Glc3Man5GlcNAc1 IC50 (μM) | 0.42 ± 0.15 | 0.46 ± 0.18 |
| Enzyme: α-Glucosidase II Substrate: Glc1Man5GlcNAc1 IC50 (μM) | 2.63 ± 0.25 | 1.55 ± 0.21 |
3.2. In vitro antiviral activity of CM-9-78 and CM-10-18
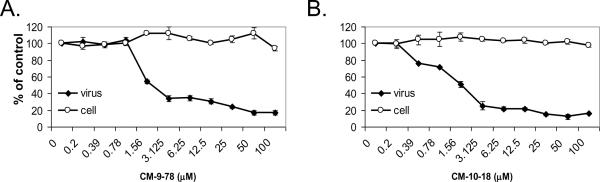
We reported previously that CM-9-78 inhibited DENV and WNV infection in African green monkey kidney (Vero) cells (Chang et al., 2009). Since imino sugars target the host α-glucosidases, ideally, their antiviral activity would be tested in cultured human cells. To this end, the antiviral efficacy of CM-9-78 and CM-10-18 was further evaluated in A549 cells, a human lung cancer-derived cell line. As shown in Fig. 1, both CM-9-78 and CM-10-18 significantly inhibited DENV infection of A549 cells, with EC50 values of 1.5μM and 1.1μM, respectively. In agreement with previous results obtained from Vero cells, the two compounds are well tolerated by human cells with CC50 values of >100μM.
Fig. 1. Antiviral activity of CM-9-78 and CM-10-18 against DENV infection in vitro.
A549 cells were infected with DENV at MOI of 0.3, and treated with indicated concentration of CM-9-78 or CM-10-18 for two days. Dengue E protein was assayed by CFI assay as a measure of DENV replication (labeled as virus). Cytotoxicity was assay by propodium iodide staining (labeled as cell). Both parameters were presented as percentage of values from the treated cells compared with that from control (no treatment) cells. Values represent average from 2 independent experiments.
3.3. PK property and preliminary in vivo toxicity of CM-9-78 and CM-10-18
In order to evaluate the antiviral efficacy of the two novel imino sugars in a mouse model, we first performed PK studies to determine the proper administration route, dose range, and frequency of administration.
The PK parameters of CM-10-18 were determined in mice, dosed either orally at 32.4mg/kg or i.v. at 6.5mg/kg (Tab. 2). After i.v. injection, CM-10-18 demonstrated a high volume of distribution and a low plasma clearance rate, leading to a moderate elimination half life (T1/2) of 2.63 h. By comparison with NBDNJ, (a well studied imino sugar derivative approved for treatment of type I Gaucher disease, but with less antiviral potency (Fischl et al., 1994)) which is less hydrophobic than CM-10-18, a faster clearance rate in mouse was observed, with a T1/2 of 1.34 h (Alonzi et al., 2008). The compound CM-10-18 was rapidly absorbed following oral administration, reaching peak plasma concentration (Tmax) at 0.25 h, at a plasma concentration (Cmax) of 4.20μg/ml (10.7μM) and bioavailability of 56%.
Furthermore, a dose escalation study of CM-9-78 and CM-10-18 was performed in rats, to measure pharmacokinetics and toxicity. Following i.p. injection of the compounds with four escalating doses (0.5, 5, 25 and 100mg/kg) every 48 to 72 hours, no significant signs of toxicity were observed. For both compounds, systemic exposure increased roughly proportional to dose over the range from 0.5mg/kg to 100mg/kg. Moreover, both compounds had very similar absorption and elimination rates (Fig. 2A). Tissue concentration from samples collected 4 h post administration of 100mg/kg showed that for both compounds, the liver and kidney had the greatest concentration (data not shown).
The PK parameters calculated from the rat dose escalation study indicated that absorption from the peritoneal cavity was rapid, with the maximum plasma concentrations occurring with the first collected plasma sample at 0.5 h post-dose. Moreover, at 5mg/kg dose via i.p. injection, the AUC value was 2.4mg*h/ml for CM-10-18, which was significantly lower than that observed in mice dose orally with similar dose (12.15 mg*h/ml), indicating i.p. administration may have disadvantage over oral administration for maintaining sustained drug concentration in blood.
We therefore directly compared the systematic compound concentration following i.p. or oral administration, with particular focus on the later time points to determine the level and duration of the compound to be maintained in the plasma. In addition, in order to test the drug metabolism in the most relevant animals, this experiment was performed in S129 mice, since the AG129 mice to be used in the in vivo animal efficacy study are derived from S129 mice.
As shown in Fig. 2B, comparison of single dose oral gavage and i.p. injection of S129 mice revealed that CM-10-18 was very quickly cleared from circulation following i.p. injection. However, oral administration of 100 mg/kg resulted in a slower clearance from the plasma. At 11 hours post administration, the plasma concentration of CM-10-18 was 2000ng/ml (5μM), which is still significantly higher than its EC50 value (1.1μM) to inhibit DENV infection in A549 cells.
In summary, the results obtained from PK studies indicate that CM-9-78 and CM-10-18 have good in vivo pharmacokinetic properties and are biologically available through oral administration. Based on their PK parameters, we reasoned that oral administration of the two imino sugars at a dose equal to or less than 100mg/kg, twice daily (every 12 h), should be a proper starting dosing strategy for in vivo efficacy studies.
3.4. CM-9-78 and CM-10-18 reduce DENV viremia in mice
Infection of AG129 mice with DENV leads to viremia, peaking at day 3 post infection (Schul et al., 2007). Since the viremia is an indicator of in vivo viral replication and directly correlates with the severity of DENV-induced diseases, this mouse model is a useful system to test antiviral effect of small molecule using plasma virus titer as a readout (Williams et al., 2009; Yin et al., 2009).
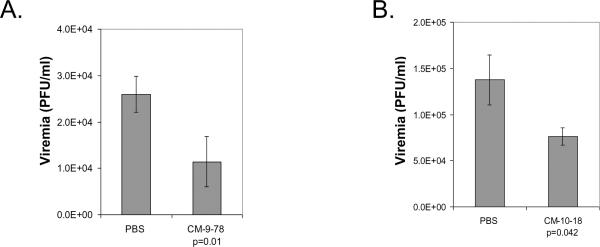
CM-9-78 or CM-10-18 was given orally immediately after infection at 75mg/kg, twice daily at 12 h intervals, for three consecutive days. At 3 days after infection, blood samples were collected to determine virus titers by plaque assay. As shown in Fig. 3A and B, treatment with either CM-9-78 or CM-10-18 reduced the viremia by 2.3-fold (P=0.01) or 1.8-fold (p=0.042), respectively. Both reductions are modest, but statistically significant. Lower doses of CM-9-78 (10mg/kg and 25mg/kg) were also tested using the same dosing schedule, no statistically significant reduction on viremia was achieved (data not shown).
Fig. 3. CM-9-78 or CM-10-18 monotherapy reduced viremia in DENV infected mice.
CM-9-78 (A) or CM-10-18 (B) was given orally at 75mg/kg twice daily at 12 hour interval, starting immediately after infection, to AG129 mice. PBS was given to control mice. 3 days after infection, the blood samples were drawn to determine virus titer by plaque assay. Values represent average and standard deviation from results obtained from 6 mice per treatment group. p values were calculated using student t test.
3.5. Combination of CM-10-18 with ribavirin synergistically inhibited DENV infection in vitro
As shown in the above in vivo efficacy studies, the glucosidase inhibitors reported thus far only demonstrated a modest antiviral effect against DENV in vivo. Although our effort to search for imino sugars with superior glucosidase inhibitory activity is still under way, we are now exploring an alternative approach to find compounds that act in combination with glucosidase inhibitors to synergistically inhibit DENV replication. In principle, combination of two mechanistically distinct antiviral compounds has the potential to result in a synergistic antiviral effect. However, due to the limited options and considering the potential clinical application, we first tested if ribavirin, a broad spectrum antiviral nucleoside analogue that is currently used in combination with IFN-α to treat chronic hepatitis C (Fried et al., 2002), in combination with CM-10-18 could synergistically inhibit DENV infection in cultured cells.
Effects of ribavirin and CM-10-18, either alone or in combination were tested with serial concentrations of the compounds in A549 cells. While ribavirin by itself was active against DENV, with an EC50 of 3μM, combination of CM-10-18 with ribavirin demonstrated a clear enhancement in the reduction of virus replication (Fig. 4A). To distinguish synergistic and additive effect, we used the MacSynergy II program which uses algorithms to subtract additive interactions from the observed surface to reveal regions of statistically significant greater-than-expected (synergy) interactions shown as peaks above the horizontal plane in the plots (MacSynergy II. Version 1.0 User's manual. University of Michigan, Ann Arbor (Prichard, Aseltine, and Shipman, 1993)). As shown in Fig. 4B, in the 95% confidence interval difference plot, combination of CM-10-18 (from 0.78μM to 12.5μM) and ribavirin (from 0.25μM to 6.67μM) demonstrated a clear peak above the calculated additive surface, with the maximum interaction occurring between 3.13μM of CM-10-18 and 0.74μM or 2.22μM of ribavirin. The synergy score calculated by the MacSynergy II program was 111. Notably, over the ranges of doses tested, there was no evidence of potential antagonist effect observed (below the surface or negative value in synergy plot). Based on the PK parameters of CM-10-18 reported herein and published PK information of ribavirin in human (which achieved a systematic concentration of ~15μM) (Lindahl et al., 2005), a synergistic antiviral effect of CM-10-18 and ribavirin may occur at pharmacologically achievable concentrations of both compounds in vivo.
Fig. 4. Combination of imino sugar and ribavirin synergistically inhibited DENV infection in vitro.
Antiviral activities of indicated doses of CM-10-18 and ribavirin, either alone or in combination in a checkboard dilution format, were performed with CFI assay in A549 cells. (A) Effect on DENV E protein, shown as percentage of no treatment control; (B) Synergy analysis was performed with MacSyngergy II. The peak above the surface represents the degree of synergy with 95% confidence interval, calculated from 8 independent experiments.
3.6. Ribavirin significantly improved the antiviral efficacy of CM-10-18 in mice
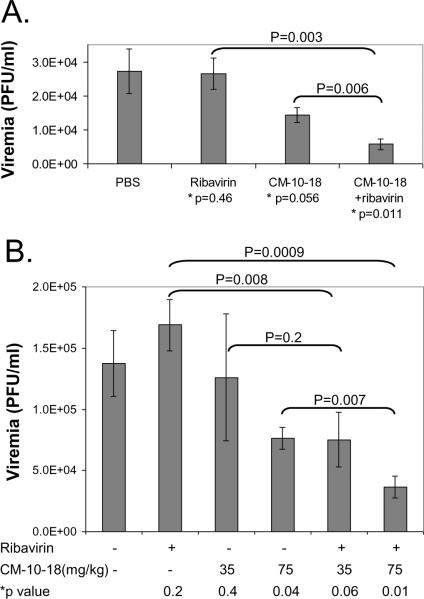
To test the antiviral efficacy of ribavirin and CM-10-18 combination therapy in DENV infected AG129 mice, treatment immediately started after the virus infection for three consecutive days. CM-10-18 was given orally at 75mg/kg twice daily at 12 h interval. Ribavirin was given orally at 40mg/kg once daily. At 3 days after infection, plasma was collected to determine the virus titers. As shown in Fig. 5A, while ribavirin at 40mg/kg by itself did not reduce viremia, and 75 mg/kg of CM-10-18 treatment only modestly reduced the viremia by 1.9-fold, combination of the two compounds reduced the viremia by 4.7-fold.
Fig. 5. Ribavirin significantly improved imino sugar's antiviral activity in DENV infected mice.
AG129 mice were treated with (A) either 40mg/kg of ribavirin, 75mg/kg of CM-10-18 or a combination of the two compounds; (B) 40mg/kg of ribavirin, doses of CM-10-18, or combination of two compounds, as indicated. PBS was given to control mice. 3 days after infection, the blood samples were drawn to determine virus titer by plaque assay. Values represent average and standard deviation from results obtained from 6 mice per treatment group. P values were calculated using student t test. * indicate P values compared to PBS control.
The in vivo combination effect of the two compounds was further tested with additional doses. While increase of ribavirin dose from 40mg/kg to 80mg/kg did not improve its antiviral effect (data not shown), as shown in Fig. 5B, increase of CM-10-18 dose from 35mg/kg to 75kg/mg markedly enhanced the antiviral effect. Interestingly, combination of 40mg/kg ribavirin with increasing dose of CM-10-18 (35mg/kg or 75mg/kg) significantly reduced the viremia.
Taken together, the results presented above demonstrate that although ribavirin inhibited DENV virus infection in cultured cells it was ineffective in reducing viremia in monotherapy, however, the drug improved the antiviral efficacy of both a sub-effective dose (35mg/kg) and an effective dose (75 mg/kg) of CM-10-18 in dengue infected mice.
3.7. Evidence that CM-10-18 inhibits α-glucosidase activity in mice
To confirm that the imino sugar derivatives indeed inhibited glucosidases in vivo and determine if the observed synergistic antiviral effect in glucosidase inhibitor and ribavirin combination therapy is due to an enhanced inhibition of glucosidase activity, we measured the amount of FOS containing terminal glucose residues in the plasma, as indicator of ER-α-glucosidases inhibition.
It is well-known that the first step N-linked glycosylation of protein is the attachment of a preformed oligosaccharides structure with 3 terminal glucose residues (Glc3). During the maturation of glycoproteins, the three terminal glucose residues are sequentially removed by α-glucosidases I and II. Inhibition of α-glucosidases, such as imino sugar treatment, results in the retention of glucose residues on N-linked oligosaccharides of glycoproteins, which leads to the misfolding and subsequent degradation of glycoproteins. As a result of the misfolded glycoprotein degradation, free oligosaccharides (FOS) containing terminal mono- , bi-and tri- glucose residue(s) are produced. A method to quantitatively measure these FOS inside the cells and in the tissue or plasma of animals treated with imino sugars has been described (Alonzi et al., 2008; Mellor et al., 2004). Here we monitored a mono-glucosylated FOS (Glc1Man4GlcNAc1) as marker to analyze the effect of glucosidase inhibition in imino sugar treated mice. A corresponding non-glucosylated free FOS (Man4GlcNAc1), which is a result of normal protein metabolism and is abundant and readily detectable, was used as control (Alonzi et al., 2008). Plasma samples were obtained from mice treated in the in vivo efficacy study, shown in Fig. 5B. As shown in a representative result obtained from chromatography analysis (Fig. 6A), a peak representing a mono-glucosylated FOS (Glc1Man4GlcNAc1) was readily detectable in the plasma of mice treated with CM-10-18. On the contrary, this peak could not be detected in the plasma from PBS and ribavirin treated mice. Quantitative analyses of Glc1Man4GlcNAc1 from 6 mice of each treatment group were summarized and presented in Fig. 6B. These results clearly indicate that treatment of mice with CM-10-18, but not ribavirin, resulted in an increased level of plasma Glc1Man4GlcNAc1 in a dose-dependent manner. Moreover, despite of a dramatic improvement in antiviral efficacy, combination therapy with CM-10-18 and ribavirin did not demonstrate an enhanced inhibition of glucosidase activity, suggesting ribavirin's effect is independent of glucosidase inhibition. The absence of tri-glucosylated FOS following inhibitor treatment suggests that at the doses used, there might be insufficient cellular concentrations reached to inhibit α-glucosidase I. Compound CM-10-18 has a respectable inhibition constant for glucosidase I using in vitro assays (Tab. 1) and is thus predicted to have a similar mechanism for action as NBDNJ, where glucosidase inhibition in cells or in vivo is biased towards concentration-dependent selectivity for α-glucosidase II (Alonzi et al., 2008).
4. Discussion
In the absence of effective vaccines and specific treatment, the presence of DENV infection in more than 100 countries demands a critical need for antiviral drugs. While drugs that specifically target the virus could be more potent and potentially less toxic, the rapid development of resistant mutants due to the error-prone nature of viral RNA polymerase may ultimately limit their antiviral efficacy (Noble et al., 2010). targeting the host factors required for viral propagation poses an attractive alternative. This is especially the case for a short duration of treatment during DENV infection, since the toxicity associated with inhibition of a host function can be significantly limited.
Cellular α-glucosidases are considered as antiviral targets for many enveloped viruses (Dwek et al., 2002). In fact, α-glucosidase inhibitors, such as imino sugar derivatives, have already been demonstrated to be broad-spectrum antiviral agents against multiple families of enveloped viruses, including hepatitis B virus, human immunodeficiency virus, herpes simplex virus, influenza virus, and several members of Flaviviridae family (Block et al., 1998; Block et al., 1994; Bridges et al., 1995; Chang et al., 2009; Datema et al., 1984; Dwek et al., 2002; Gu et al., 2007; Jordan et al., 2002). Moreover, one of the enzymes, glucosidase II, has been identified recently by a genome-wide siRNA screening approach as essential host factors required for DENV infection (Sessions et al., 2009).
Although sound in mechanism, the preclinical and clinical development of glucosidase inhibitors as antiviral drugs is hampered by their poor efficacy in vivo. For example, a phase II clinical trial evaluating NBDNJ as an anti-HIV agent showed that although well tolerated, the compound did not demonstrate any antiviral efficacy. The reason for the failure is that the compound could not achieve a sufficient antiviral concentration in the circulation of treated individuals (Fischl et al., 1994). In preclinical studies, only modest antiviral effects of glucosidase inhibitors against dengue virus (Schul et al., 2007; Whitby et al., 2005) and Japanese encephalitis virus (Wu et al., 2002) in mice as well as woodchuck hepatitis virus in woodchuck (Block et al., 1998) were reported.
Our current study was intended to test the antiviral efficacy of two novel imino sugar derivatives against DENV infection in mice. Although it is evident that under the treatment conditions, the host cellular α-glucosidase activity was inhibited, the two compounds only modestly reduced the viremia. Based on our results obtained from the PK studies, it is possible that the modest antiviral efficacy of the compounds is due to the relatively higher EC50 value and/or lower level of plasma drug concentration or low concentration of the drug in the virus-replicating cells, possibly due to low uptake or plasma protein binding. Hence, increase of dosing frequency or targeted delivery of the compounds may enhance its antiviral efficacy in vivo. In addition, extensive medicinal chemistry effort has been under way in our laboratory to search for imino sugars with better antiviral activity against DENV(Block and Jordan, 2001; Gu et al., 2007; Mehta et al., 2002). As a result of this study, we recently discovered a new class of imino sugar derivatives with extraordinary antiviral activity against DENV in cultured cells (Chang et al., 2009).
As an alternative approach, we also explored the possibility to enhance the antiviral efficacy of imino sugars by combination with one or more antivirals with distinct antiviral mechanisms. As a proof of concept study, we show in this report that while ribavirin monotherapy did not significantly reduce viremia (which is consistent with a previous report (Schul et al., 2007)), combination therapy with CM-10-18 and ribavirin resulted in an enhanced and statistically significant reduction of the viremia.
The major goal of therapy for DENV infection is to prevent patients from developing severe hemorrhagic clinical manifestation. To achieve this by virological suppression, it has generally been assumed that it is necessary to suppress viremia by 10-fold or greater (Noble et al., 2010; Vaughn et al., 2000); however, as little as 2 to10-fold suppression in viremia has been reported to be associated with protection of animal death in a lethal DENV infection mouse model (Yin et al., 2009). Therefore, although only a limited amount of viral suppression was achieved with imino sugars and combination of ribavirin, it is possible that this level of suppression can achieve a threshold of efficacy in disease progression. In the future, in order to further evaluate its therapeutic value, the combination therapy regime will be tested in lethal DENV infection mouse model, with treatment starting not only immediate after infection, but also with several days delay.
In addition to ribavirin, there are many other candidates to be tested for their ability to compliment imino sugar against DENV (Markland et al., 2000; Ouzounov et al., 2002; Qing et al., 2009; Rothwell et al., 2009; Stevens et al., 2009; Zhang et al., 2009). Moreover, encouraged by these results, we are currently also identifying rational partners of imino sugars through a high throughput screening effort.
Although imino sugar targets host enzymes, it has shown very limited side effects. NBDNJ has been used in human to treat Gaucher's disease for a decade without serious adverse effects, as reported in the European Union Intensive Safety Surveillance Scheme. There are cases of transient, mild osmotic diarrhea, due to inhibition of gut disaccharidases. However, this effect can be simply monitored and is manageable with anti-diarrhea agent such as Loperamide (Butters, 2007). It is our hope that by improvement of imino sugars and/or searching for more potent partners of imino sugars, glucosidase inhibitors will finally be developed as antiviral drugs, either for monotherapy or as a vital component of combination therapy.
ACKNOWLEDGEMENT
This work was supported by NIH grants (AI061441 and AI084267-0109) and by the Hepatitis B Foundation through an appropriation from the Commonwealth of Pennsylvania. TDB, DA, and GR thank the Glycobiology Institute for support.
Abbreviations
- DENV
dengue virus
- ER
endoplasmic reticulum
- DNJ
deoxynojirimycin
- BVDV
bovine viral diarrhea virus
- FOS
Free oligosaccharide
- IC50
50% inhibitory concentration
- EC50
50% effective concentration
- CC50
50% cytotoxicity concentration
- CFI
cell-based flavivirus immunodetection
- PK
Pharmacokinetic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonzi DS, Neville DC, Lachmann RH, Dwek RA, Butters TD. Glucosylated free oligosaccharides are biomarkers of endoplasmic- reticulum alpha-glucosidase inhibition. Biochem J. 2008;409(2):571–80. doi: 10.1042/BJ20070748. [DOI] [PubMed] [Google Scholar]
- Block TM, Jordan R. Iminosugars as possible broad spectrum anti hepatitis virus agents: the glucovirs and alkovirs. Antivir Chem Chemother. 2001;12(6):317–25. doi: 10.1177/095632020101200601. [DOI] [PubMed] [Google Scholar]
- Block TM, Lu X, Mehta AS, Blumberg BS, Tennant B, Ebling M, Korba B, Lansky DM, Jacob GS, Dwek RA. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nat Med. 1998;4(5):610–4. doi: 10.1038/nm0598-610. [DOI] [PubMed] [Google Scholar]
- Block TM, Lu X, Platt FM, Foster GR, Gerlich WH, Blumberg BS, Dwek RA. Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin. Proc Natl Acad Sci U S A. 1994;91(6):2235–9. doi: 10.1073/pnas.91.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CG, Ahmed SP, Kang MS, Nash RJ, Porter EA, Tyms AS. The effect of oral treatment with 6-O-butanoyl castanospermine (MDL 28,574) in the murine zosteriform model of HSV-1 infection. Glycobiology. 1995;5(2):249–53. doi: 10.1093/glycob/5.2.249. [DOI] [PubMed] [Google Scholar]
- Buckwold VE, Wei J, Wenzel-Mathers M, Russell J. Synergistic in vitro interactions between alpha interferon and ribavirin against bovine viral diarrhea virus and yellow fever virus as surrogate models of hepatitis C virus replication. Antimicrob Agents Chemother. 2003;47(7):2293–8. doi: 10.1128/AAC.47.7.2293-2298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters TD. Pharmacotherapeutic strategies using small molecules for the treatment of glycolipid lysosomal storage disorders. Expert Opin Pharmacother. 2007;8(4):427–35. doi: 10.1517/14656566.8.4.427. [DOI] [PubMed] [Google Scholar]
- Chang J, Wang L, Ma D, Qu X, Guo H, Xu X, Mason PM, Bourne N, Moriarty R, Gu B, Guo JT, Block TM. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob Agents Chemother. 2009;53(4):1501–8. doi: 10.1128/AAC.01457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R, Romero PA, Rott R, Schwarz RT. On the role of oligosaccharide trimming in the maturation of Sindbis and influenza virus. Arch Virol. 1984;81(1–2):25–39. doi: 10.1007/BF01309294. [DOI] [PubMed] [Google Scholar]
- Dwek RA, Butters TD, Platt FM, Zitzmann N. Targeting glycosylation as a therapeutic approach. Nat Rev Drug Discov. 2002;1(1):65–75. doi: 10.1038/nrd708. [DOI] [PubMed] [Google Scholar]
- Fischl MA, Resnick L, Coombs R, Kremer AB, Pottage JC, Jr., Fass RJ, Fife KH, Powderly WG, Collier AC, Aspinall RL, et al. The safety and efficacy of combination N-butyl-deoxynojirimycin (SC-48334) and zidovudine in patients with HIV-1 infection and 200–500 CD4 cells/mm3. J Acquir Immune Defic Syndr. 1994;7(2):139–47. [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr., Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Gu B, Mason P, Wang L, Norton P, Bourne N, Moriarty R, Mehta A, Despande M, Shah R, Block T. Antiviral profiles of novel iminocyclitol compounds against bovine viral diarrhea virus, West Nile virus, dengue virus and hepatitis B virus. Antivir Chem Chemother. 2007;18(1):49–59. doi: 10.1177/095632020701800105. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–49. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Jordan R, Nikolaeva OV, Wang L, Conyers B, Mehta A, Dwek RA, Block TM. Inhibition of host ER glucosidase activity prevents Golgi processing of virion-associated bovine viral diarrhea virus E2 glycoproteins and reduces infectivity of secreted virions. Virology. 2002;295(1):10–9. doi: 10.1006/viro.2002.1370. [DOI] [PubMed] [Google Scholar]
- Lindahl K, Stahle L, Bruchfeld A, Schvarcz R. High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology. 2005;41(2):275–9. doi: 10.1002/hep.20563. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(12 Suppl):S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Markland W, McQuaid TJ, Jain J, Kwong AD. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother. 2000;44(4):859–66. doi: 10.1128/aac.44.4.859-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Ouzounov S, Jordan R, Simsek E, Lu X, Moriarty RM, Jacob G, Dwek RA, Block TM. Imino sugars that are less toxic but more potent as antivirals, in vitro, compared with N-n-nonyl DNJ. Antivir Chem Chemother. 2002;13(5):299–304. doi: 10.1177/095632020201300505. [DOI] [PubMed] [Google Scholar]
- Mellor HR, Neville DC, Harvey DJ, Platt FM, Dwek RA, Butters TD. Cellular effects of deoxynojirimycin analogues: inhibition of N-linked oligosaccharide processing and generation of free glucosylated oligosaccharides. Biochem J. 2004;381(Pt 3):867–75. doi: 10.1042/BJ20031824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble CG, Chen YL, Dong H, Gu F, Lim SP, Schul W, Wang QY, Shi PY. Strategies for development of dengue virus inhibitors. Antiviral Res. 2010 doi: 10.1016/j.antiviral.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Ouzounov S, Mehta A, Dwek RA, Block TM, Jordan R. The combination of interferon alpha-2b and n-butyl deoxynojirimycin has a greater than additive antiviral effect upon production of infectious bovine viral diarrhea virus (BVDV) in vitro: implications for hepatitis C virus (HCV) therapy. Antiviral Res. 2002;55(3):425–35. doi: 10.1016/s0166-3542(02)00075-x. [DOI] [PubMed] [Google Scholar]
- Prichard MN, Aseltine KR, Shipman C., Jr. User's manual. Version 1.0 University of Michigan; Ann Arbor: 1993. MacSynergy II. [Google Scholar]
- Qing M, Yang F, Zhang B, Zou G, Robida JM, Yuan Z, Tang H, Shi PY. Cyclosporine inhibits flavivirus replication through blocking the interaction between host cyclophilins and viral NS5 protein. Antimicrob Agents Chemother. 2009;53(8):3226–35. doi: 10.1128/AAC.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell C, Lebreton A, Young Ng C, Lim JY, Liu W, Vasudevan S, Labow M, Gu F, Gaither LA. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389(1–2):8–19. doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Schul W, Liu W, Xu HY, Flamand M, Vasudevan SG. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J Infect Dis. 2007;195(5):665–74. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, Garcia-Blanco MA. Discovery of insect and human dengue virus host factors. Nature. 2009;458(7241):1047–50. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AJ, Gahan ME, Mahalingam S, Keller PA. The medicinal chemistry of dengue fever. J Med Chem. 2009;52(24):7911–26. doi: 10.1021/jm900652e. [DOI] [PubMed] [Google Scholar]
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181(1):2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- Wang QY, Patel SJ, Vangrevelinghe E, Xu HY, Rao R, Jaber D, Schul W, Gu F, Heudi O, Ma NL, Poh MK, Phong WY, Keller TH, Jacoby E, Vasudevan SG. A small-molecule dengue virus entry inhibitor. Antimicrob Agents Chemother. 2009;53(5):1823–31. doi: 10.1128/AAC.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby K, Pierson TC, Geiss B, Lane K, Engle M, Zhou Y, Doms RW, Diamond MS. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J Virol. 2005;79(14):8698–706. doi: 10.1128/JVI.79.14.8698-8706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Zompi S, Beatty PR, Harris E. A mouse model for studying dengue virus pathogenesis and immune response. Ann N Y Acad Sci. 2009;1171(Suppl 1):E12–23. doi: 10.1111/j.1749-6632.2009.05057.x. [DOI] [PubMed] [Google Scholar]
- Wu SF, Lee CJ, Liao CL, Dwek RA, Zitzmann N, Lin YL. Antiviral effects of an iminosugar derivative on flavivirus infections. J Virol. 2002;76(8):3596–604. doi: 10.1128/JVI.76.8.3596-3604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, Kondreddi RR, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JY, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi PY. An adenosine nucleoside inhibitor of dengue virus. Proc Natl Acad Sci U S A. 2009;106(48):20435–9. doi: 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XG, Mason PW, Dubovi EJ, Xu X, Bourne N, Renshaw RW, Block TM, Birk AV. Antiviral activity of geneticin against dengue virus. Antiviral Res. 2009;83(1):21–7. doi: 10.1016/j.antiviral.2009.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]