Summary
Background
Cancer survival is a key measure of the effectiveness of health-care systems. Persistent regional and international differences in survival represent many avoidable deaths. Differences in survival have prompted or guided cancer control strategies. This is the first study in a programme to investigate international survival disparities, with the aim of informing health policy to raise standards and reduce inequalities in survival.
Methods
Data from population-based cancer registries in 12 jurisdictions in six countries were provided for 2·4 million adults diagnosed with primary colorectal, lung, breast (women), or ovarian cancer during 1995–2007, with follow-up to Dec 31, 2007. Data quality control and analyses were done centrally with a common protocol, overseen by external experts. We estimated 1-year and 5-year relative survival, constructing 252 complete life tables to control for background mortality by age, sex, and calendar year. We report age-specific and age-standardised relative survival at 1 and 5 years, and 5-year survival conditional on survival to the first anniversary of diagnosis. We also examined incidence and mortality trends during 1985–2005.
Findings
Relative survival improved during 1995–2007 for all four cancers in all jurisdictions. Survival was persistently higher in Australia, Canada, and Sweden, intermediate in Norway, and lower in Denmark, England, Northern Ireland, and Wales, particularly in the first year after diagnosis and for patients aged 65 years and older. International differences narrowed at all ages for breast cancer, from about 9% to 5% at 1 year and from about 14% to 8% at 5 years, but less or not at all for the other cancers. For colorectal cancer, the international range narrowed only for patients aged 65 years and older, by 2–6% at 1 year and by 2–3% at 5 years.
Interpretation
Up-to-date survival trends show increases but persistent differences between countries. Trends in cancer incidence and mortality are broadly consistent with these trends in survival. Data quality and changes in classification are not likely explanations. The patterns are consistent with later diagnosis or differences in treatment, particularly in Denmark and the UK, and in patients aged 65 years and older.
Funding
Department of Health, England; and Cancer Research UK.
Introduction
Survival is a key index of the overall effectiveness of health services in the management of patients with cancer. Substantial differences in survival have been reported for adult patients with cancer who were diagnosed in many countries in the early 1990s (CONCORD)1 and in Europe up to 2002 (EUROCARE).2 Survival has improved, but substantial differences still exist within and between countries with similar health systems and wealth, such as between Denmark and the UK and other European countries.2–5 Findings from one study suggest that for patients diagnosed up to 1999, about 11 400 more patients with cancer died per year within 5 years of diagnosis in England, Scotland, and Wales than if 5-year survival had been as high as the highest levels achieved in 13 other countries in Europe;6 cancers of the breast, colorectum, and lung accounted for about half the avoidable deaths. Avoidable deaths also arise from inequalities in survival within countries; even in Finland, with some of the highest survival levels in Europe, 4–7% of cancer deaths have been attributed to inequalities in 5-year survival between educational groups.7 However, the CONCORD and EUROCARE studies relate to patients diagnosed at least 8 years ago, and many countries have implemented cancer control plans since then.
Survival patterns help to drive national cancer strategies (references in webappendix p 2). In Denmark, the first National Cancer Plan (2000) focused on the survival deficit with neighbouring countries. The second plan (2005) also noted poorer survival than in other Nordic countries, especially just after diagnosis; it recommended reduction of diagnostic delay and establishment of multidisciplinary cancer groups. The Northern Ireland cancer plan (1996) introduced centres of excellence and multidisciplinary teams. The National Health Service Cancer Plan for England (2000) focused on improving cancer services and raising 5-year survival to the levels of the best-performing countries in Europe by 2010; the updated strategy (2007) also aims to raise cancer survival to the levels of the best. The 10-year strategy in Wales (2006) aims to achieve levels of survival within the top European quartile by 2015. In Australia, improving cancer services to raise survival has been a key platform of both cancer plans in New South Wales (2004–10), and the cancer action plan in Victoria (2008) aims “to increase survival rates by 10% by 2015, saving 2000 lives”.8 Norway's first national cancer plan (1999) aimed to improve cancer care; the updated strategy (2006) introduced official treatment guidelines. Canada created the independent non-profit Canadian Partnership Against Cancer to implement its cancer control strategy from 2007. A national cancer strategy for Sweden was proposed in 2009; two of the five goals are to improve survival and quality of life for patients with cancer, and to reduce regional differences in survival. One goal of the World Cancer Declaration9 is to achieve major improvements in survival worldwide by 2020.
Survival trends are also being used to assess cancer strategies. Denmark, Norway, and England introduced cancer plans in 1999–2000. Trends in short-term survival up to 2006 have suggested some benefit in Denmark, mainly for surgically treated cancers,10 but overall assessment of the cancer plans in Denmark and Norway suggests they have yet to show a distinct effect on survival trends.11 Survival was already improving in England by 2000, but evidence suggests some acceleration in the trend during 2004–07, after full implementation of the cancer plan.12
For any country, policy designed to achieve levels of cancer survival comparable with an external standard requires robust and timely evidence of the extent and causes of any current deficit. The International Cancer Benchmarking Partnership (ICBP) was designed for this purpose. It brings together epidemiologists, clinicians, other scientists, and policy makers from six countries. Countries were invited to participate on the basis of comparable wealth, universal access to health care, and longstanding, high-quality, population-based cancer registration. The aims of the ICBP are to produce up-to-date survival estimates for selected cancers, to establish whether international differences in survival have changed, and to investigate the causes of survival deficits. The goal is to provide policy makers with high-quality evidence on which they can take action. This first report is focused on survival trends.
Methods
Study design and data collection
Four index cancers were selected. Cancers of the breast, colorectum, and lung are common, whereas ovarian cancer is less common and has a complex diagnostic pathway. Data were provided from population-based cancer registries in 12 jurisdictions in six countries: Australia (New South Wales, Victoria), Canada (Alberta, British Columbia, Manitoba, Ontario), Denmark, Norway, Sweden (Uppsala-Örebro and Stockholm-Gotland health regions), and the UK (England, Northern Ireland, and Wales). Data for Denmark, Norway, England, Northern Ireland, and Wales covered the national population. Population coverage was for selected regions of Australia (11·4 million of 19·2 million; 59·7%), Canada (19·9 million of 31·0 million; 64·9%), and Sweden (3·8 million of 8·9 million; 42·8%), but the quality and completeness of all the registries is known to be high.13 In 2002, the six countries had a combined population of 122·9 million, of which 98·9 million (80·5%) were covered by the data contributed for this study.
Anonymised, individual cancer registration records were supplied for adults (aged 15–99 years) diagnosed with a primary, invasive, malignant neoplasm of the colorectum, lung, breast (women only), or ovary during the 13 years from 1995 to 2007, with follow-up to Dec 31, 2007. Data for ovarian cancer were not available for Sweden. Data were requested for in-situ and other non-invasive tumours to enable comparison of diagnostic intensity in the participating jurisdictions, but not all registries were able to provide complete data for these tumours. Datasets were received between April 12 and Aug 10, 2010.
Data were supplied with the anatomical location of tumours coded to the tenth revision of the International Classification of Diseases (ICD-10)14 or the third revision of the International Classification of Diseases for Oncology (ICD-O-3).15 Cancers were selected on the basis of these codes: colon (ICD-10 C18.0–C18.9), rectosigmoid junction (C19; ICD-O-3 C19.9), and rectum (C20; ICD-O-3 C20.9) excluding anus and anal canal (ICD-10 C21); lung and bronchus (C34.0–C34.9) excluding trachea (C33); breast (C50.0–C50.9); and ovary including the fallopian tube and adnexa (C56 [ICD-O-3 C56.9]; C57.0–C57.9). Tumour morphology and behaviour were coded to ICD-O-216 or ICD-O-3.
Data were provided for date of diagnosis, anatomical site, morphology and behaviour of the tumour, and date of birth, sex, and last known vital status of the patient. The New South Wales registry does not collect full dates (day, month, year) of birth or diagnosis, and Denmark only collected the full date of diagnosis from 2004. This limitation disabled some of the quality control checks and counts of deaths within 30 days of diagnosis. It also reduced the comparability of very short-term survival estimates.17 Four registries collect full dates but could not provide them for this study; they provided the duration of survival in days. Data were also provided on diagnostic investigations, stage at diagnosis and treatment, and availability of health-care resources such as specialist oncologists or imaging technology. These data will be the subject of further analyses.
National incidence and mortality rates for 1998–2002 were derived from CI5plus18 and from the WHO mortality databank.19 Trends in the 3-year moving average world-standardised yearly incidence and mortality rates per 100 000 population for the six countries over the period 1985–2005 were derived from the same databases and from several other official sources.
Data were transmitted via the secure web-based delivery system HyperSend, which meets the security standards for electronic transmission of health data under the US Health Insurance Portability and Accountability Act (PL104-191, 1996). Each file was encrypted and sent in a separate transmission, itself also encrypted. Passwords were obtained separately from pre-assigned contact persons in each registry. A few registries submitted password-protected encrypted files on unlabelled CDROMs via courier. Files were named with pre-assigned random codes; variable names were sequential, rather than informative. No primary identifying data (name, address, postcode, etc) were included in any file. For quality control purposes, each tumour record included a unique serial number assigned by the source registry to enable the record to be checked if errors were detected.
The London School of Hygiene and Tropical Medicine (LSHTM) is registered (number Z7513362) in the UK under the Data Protection Act 1998. The study protocol and the Cancer Research UK Cancer Survival Group's system-level security policy at LSHTM were approved for processing sensitive patient data without consent for this study by the Ethics and Confidentiality Committee of the statutory National Information Governance Board (NIGB) on April 21, 2010 (extension of PIAG 1-05(c)2007). The NIGB noted that “it would be necessary to have full dates of birth, diagnosis and death, as survival calculation required precise dates”. The study protocol was also approved by the South East Research Ethics Committee of the National Health Service on April 21, 2010 (10/H1102/19). Participating registries outside the UK obtained ethical approvals in their jurisdictions (details available on request).
Quality control
Each dataset was examined for adherence to protocol and the distribution of key variables. Results were discussed with each registry and any corrections agreed. In many cases, revised datasets were submitted. All tumour records were then subjected to standardised quality control procedures.1 The results were again discussed with participating registries; data preparation routines were adjusted when necessary to take account of local coding practices. We excluded the records of 102 305 patients whose tumour was benign (behaviour code 0), of uncertain or borderline malignancy (1), in-situ (2), or metastatic to the index organ from elsewhere (6). Most exclusions were for women with an in-situ neoplasm of the breast (52 705 patients, 5·8% of all 916 179 breast tumours reported) or an ovarian neoplasm of uncertain or borderline malignancy (10 409, 7·6% of all 137 199 ovarian neoplasms reported; webappendix pp 6–9).
Of the 2 504 741 adults eligible for analysis with an invasive, primary, malignant neoplasm of the colorectum, lung, breast, or ovary diagnosed during 1995–2007, we excluded a further 102 997 (4·1%). We excluded registrations made from a death certificate only (DCO) from survival analyses because the date of diagnosis and thus the duration of survival were unknown. They accounted for 73 603 (2·9%) of eligible cases: 2·4% (18 269/759 097) for colorectal cancer, 5·1% (38 561/758 792) for lung cancer, 1·5% (12 592/860 971) for breast cancer, and 3·3% (4181/125 881) for ovarian cancer. We excluded a further 3208 patients (0·13% of those eligible) because their vital status at Dec 31, 2007, was unknown, and 26 186 (1·0%) because of unknown sex, age 100 years or older at diagnosis, invalid dates or sequence of dates, diagnosis made only at autopsy, or a previous primary cancer at the same anatomical site.
Overall, data quality was high. The proportion of patients included in the analyses varied very little during 1995–2007. The lowest proportion for any period was 92·6% (104 858/113 181) for lung cancer in 1995–96 (details of exclusions by jurisdiction and calendar period available on request). For patients diagnosed during 2000–07, morphological verification of the primary cancer diagnosis was available for 91·4% (428 464/468 986) of colorectal cancers, 75·1% (339 053/451 329) of lung cancers, 94·4% (506 225/536 257) of breast cancers, and 84·5% (64 262/76 011) of ovarian cancers.
All patients with a qualifying index cancer were included in the analyses, irrespective of whether it was their first, second, or higher-order cancer (webappendix p 19). Inclusion of all primary cancers in survival estimates is important for public health because the number of people who develop a second cancer will continue to rise with ageing populations and improvements in survival. To the extent that second tumours confer shorter survival than first tumours, inclusion of all primary cancers also reduces any bias in survival comparisons between recent and long- established registries. Newer registries are less likely to identify a second tumour correctly if the first tumour occurred years before the registry was established. With the exception of Northern Ireland (1993), however, all the registries in this study have been operating for decades, starting between 1942 (Denmark) and 1972 (New South Wales). In this study, for example, a patient diagnosed with breast cancer in 1995 after a melanoma of the skin in 1990 would be included, but a patient with separate cancers of the same organ (eg, consecutive breast cancers) would be included in these analyses only for the first breast cancer, and only if that cancer was diagnosed during 1995–2007.
Statistical analysis
We estimated relative survival, which is the standard approach for population-based cancer survival.20 It is the ratio of the survival observed in patients with cancer and the survival that would have been expected if they had experienced only the all-cause death rates (background mortality) of the general population where they lived. Relative survival is interpretable as survival from the cancer after adjustment for other causes of death. It is required for international comparisons because it removes differences in the survival of patients with cancer that are unrelated to their cancer. We estimated survival for each cancer in each jurisdiction; for Australia, Canada, and the UK, we also estimated survival with the pooled data from contributing registries.
Background mortality in the general population of each jurisdiction was taken from life tables of all-cause death rates by sex, single year of age, and calendar year during 1995–2007. We constructed these tables from raw data on the numbers of deaths and the populations for selected years, by sex and single year of age or 5-year age group. The data were obtained from participating registries or vital statistics offices. Abridged (5-year) life tables were smoothed to complete (single-year-of-age) life tables and extended up to age 99 years with use of Poisson regression, with flexible link functions to model the death rates. Life tables for each year were then produced by linear interpolation between period-specific life tables. The most recent data on deaths were for 2005 or 2006, enabling construction of unique life tables for up to 11 of the 13 years during the period 1995–2007; 252 life tables were constructed to capture year-to-year changes in background mortality by age and sex in all jurisdictions.
All-cause and relative survival were estimated for adults of all ages combined at 1, 2, 3, 4, 5, 6, 9, and 12 months after diagnosis, then every 6 months to 5 years after diagnosis. We used the maximum likelihood approach to survival estimation with individual records,21 implemented in the open-source program strel22 with the statistical package Stata (version 11.1). Survival was estimated separately for the age groups 15–44, 45–54, 55–64, 65–74, and 75–99 years, but at wider intervals: 1, 2, 3, 4, 5, 6, 9, and 12 months, then every 6 months to 3 years, then every year to 5 years after diagnosis. If survival could not be estimated for a specific age group, we used the adjacent age-specific estimate. Age-standardisation of relative survival was done with the international cancer survival standard weights.23
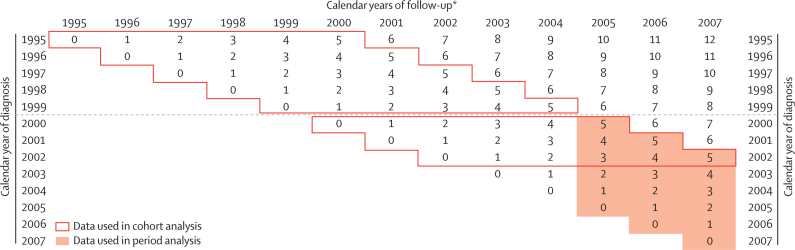
The cohort approach was used for 1995–99 and 2000–02, since all patients diagnosed during these periods had at least 5 years' potential follow-up by the end of 2007. The period approach24 was used to provide short-term predictions of 5-year survival for patients diagnosed during 2005–07. Period survival estimates combine the partial probabilities of survival up to each anniversary of diagnosis (here, up to 5 years) that were observed during the most recent period for which adequate follow-up data were available (in this report, the period 2005–07 for patients diagnosed during 2000–07; shaded area in figure 1). This approach mirrors the standard predictions of life expectancy at birth from the most recently recorded death rates at each age. Period survival estimates represent a prediction of the 5-year survival of patients diagnosed in 2005–07 under the assumption that the most recently observed partial probabilities of survival will remain constant throughout the follow-up period of interest (for these patients, up to 2012). In most cases they will rise, so the period estimates for 2005–07 are inherently conservative predictions of the (cohort) survival that will eventually be recorded when the data become available around 2014. Comparisons between jurisdictions are unbiased, since they are based on the same method for each calendar period.
Figure 1.
Structure of cohort and period approaches to survival analysis for patients diagnosed during 1995–2007 and followed up to Dec 31, 2007
*Calendar years from which the probabilities of conditional survival in each follow-up interval are combined to produce cumulative survival estimates. Numbers in the cells indicate the minimum number of years of follow-up completed by patients surviving to the end of a specific calendar year (columns) who were diagnosed in the index year (rows). Cohort approach: all patients diagnosed in a specific period were followed up for at least 5 years (full lines). Period approach: survival estimates from most recent follow-up data (shaded orange).
We report age-standardised relative survival at 1 year and 5 years after diagnosis. We also report relative survival at the fifth anniversary of diagnosis in patients who survived at least 1 year after their diagnosis. This conditional 5-year survival enables international comparisons while keeping to a minimum the effect of factors that mainly affect survival in the first year after diagnosis. Advice on study design, protocol, and methodology was provided by independent experts (Epidemiology Reference Group).
Role of the funding source
The Department of Health (London, UK) funded coordination and analysis, but had no role in preparing the protocol, or in collection, preparation, or analysis of the data. Cancer Research UK has funded the Cancer Survival Group at LSHTM (C1336/A5735 and A11700) since April, 2005, but had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Neither funding source had any role in the decision to submit the report for publication. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
2 401 744 adults diagnosed with one of the four index cancers during 1995–2007 were included in the analyses (95·9% of those eligible; table 1). The age profiles of patients with each cancer were similar in all 12 jurisdictions (webappendix pp 12–17).
Table 1.
Adults (15–99 years) diagnosed with colorectal, lung, breast (women), or ovarian cancer between 1995 and 2007 and followed up to Dec 31, 2007
| Eligible for analysis |
Excluded |
Included in analyses | ||||
|---|---|---|---|---|---|---|
| DCO* | Multiple same site† | Other‡ | ||||
| Australian registries | ||||||
| New South Wales | 145 174 | 1·0% | 0·0% | 0·2% | 143 537 (98·9%) | |
| Victoria | 110 844 | 1·8% | 0·0% | 0·3% | 108 551 (97·9%) | |
| Canadian registries | ||||||
| Alberta | 61 046 | 0·2% | 1·2% | 0·2% | 60 003 (98·3%) | |
| British Columbia | 97 762 | 1·4% | 0·0% | 0·6% | 95 829 (98·0%) | |
| Manitoba | 28 752 | 1·2% | 0·0% | 0·3% | 28 327 (98·5%) | |
| Ontario | 285 803 | 1·7% | 0·0% | 0·0% | 280 892 (98·3%) | |
| Denmark | 153 442 | 0·5% | 0·5% | 1·2% | 150 108 (97·8%) | |
| Norway | 111 314 | 0·8% | 1·3% | 0·5% | 108 452 (97·4%) | |
| Swedish regions§ | 71 321 | 0·0% | 1·0% | 0·0% | 70 554 (98·9%) | |
| UK registries | ||||||
| England | 1 312 523 | 4·4% | 0·6% | 1·0% | 1 234 029 (94·0%) | |
| Northern Ireland | 38 863 | 1·3% | 0·3% | 0·6% | 38 007 (97·8%) | |
| Wales | 87 897 | 4·7% | 0·2% | 0·1% | 83 455 (94·9%) | |
| Total | 2 504 741 | 2·9% | 0·5% | 0·7% | 2 401 744 (95·9%) | |
Data show number and percentage of patients eligible, excluded, and included in analyses, by jurisdiction.
Registration from a death certificate only (DCO).
Multiple tumours at same anatomical site.
Aged 100 years or older at diagnosis, sex or last vital status unknown, autopsy only, sex-site error, invalid dates, or duplicate registration.
Autopsy-only cases not provided.
The trends and geographical patterns in 1-year, 5-year, and conditional 5-year survival differed between the four cancers, but some features were consistent. Survival rose in all six countries, for all four cancers (table 2, figure 2). Survival was generally higher in Australia, Canada, and Sweden, intermediate in Norway, and lower in Denmark and the UK. Survival estimates for the three UK nations (England, Northern Ireland, and Wales) were mostly within 2–3% of each other. Survival in Alberta was often 3–5% lower than in the three other participating Canadian provinces. Webappendix pp 21–26 shows relative survival by age, sex, jurisdiction, and calendar period.
Table 2.
Age-standardised relative survival (%) at 1 and 5 years, and 5-year survival conditional on survival to the first anniversary of diagnosis
|
Australia |
Canada |
Denmark | Norway | Swedish regions |
UK |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Australian registries | New South Wales | Victoria | Canadian registries | Alberta | British Columbia | Manitoba | Ontario | UK registries | England | Northern Ireland | Wales | |||||
| Colorectal cancer | ||||||||||||||||
| 1 year | ||||||||||||||||
| 1995–99 | 80·0% | 80·3% | 79·6% | 79·1% | 77·6% | 80·7% | 79·8% | 78·7% | 71·7% | 78·6% | 81·8% | 70·2% | 70·2% | 74·3% | 68·3% | |
| 2000–02 | 82·5% | 83·2% | 81·6% | 81·5% | 79·8% | 82·4% | 80·4% | 81·7% | 73·9% | 78·7% | 82·8% | 73·0% | 72·9% | 76·8% | 72·3% | |
| 2005–07 | 84·9% | 84·7% | 85·1% | 83·5% | 80·4% | 84·3% | 82·1% | 83·9% | 77·7% | 82·4% | 83·8% | 74·7% | 74·7% | 76·2% | 73·6% | |
| 5 years | ||||||||||||||||
| 1995–99 | 60·0% | 61·2% | 58·4% | 58·1% | 56·3% | 59·8% | 57·6% | 58·0% | 48·2% | 56·9% | 58·5% | 47·8% | 47·8% | 51·1% | 45·9% | |
| 2000–02 | 63·4% | 65·1% | 61·1% | 60·9% | 58·0% | 61·5% | 59·6% | 61·3% | 51·7% | 58·8% | 60·6% | 51·3% | 51·2% | 54·3% | 50·3% | |
| 2005–07 | 65·9% | 66·4% | 65·5% | 63·7% | 58·3% | 64·0% | 63·3% | 64·9% | 55·8% | 62·0% | 62·6% | 53·6% | 53·7% | 55·2% | 52·3% | |
| Conditional 5-year | ||||||||||||||||
| 1995–99 | 75·0% | 76·2% | 73·4% | 73·6% | 72·5% | 74·2% | 72·2% | 73·8% | 67·3% | 72·3% | 71·6% | 68·1% | 68·2% | 68·8% | 67·2% | |
| 2000–02 | 76·7% | 78·2% | 74·9% | 74·7% | 72·5% | 74·8% | 74·0% | 75·2% | 70·1% | 74·7% | 73·3% | 70·3% | 70·3% | 71·1% | 69·7% | |
| 2005–07 | 77·7% | 78·4% | 76·8% | 76·4% | 72·3% | 75·9% | 77·1% | 77·4% | 72·1% | 75·4% | 74·8% | 71·8% | 71·8% | 73·0% | 71·1% | |
| Lung cancer | ||||||||||||||||
| 1 year | ||||||||||||||||
| 1995–99 | 38·2% | 38·6% | 37·8% | 38·7% | 36·4% | 36·6% | 41·7% | 39·6% | 26·4% | 32·3% | 35·7% | 24·3% | 24·3% | 27·4% | 23·0% | |
| 2000–02 | 40·9% | 41·5% | 39·9% | 39·7% | 36·3% | 37·5% | 44·1% | 40·5% | 31·3% | 32·2% | 36·6% | 27·5% | 27·5% | 28·3% | 25·9% | |
| 2005–07 | 42·8% | 42·9% | 42·7% | 43·1% | 41·5% | 43·0% | 42·7% | 43·4% | 34·9% | 39·2% | 43·6% | 29·7% | 29·7% | 30·6% | 28·5% | |
| 5 years | ||||||||||||||||
| 1995–99 | 13·9% | 14·2% | 13·4% | 15·7% | 13·8% | 13·9% | 16·6% | 16·6% | 8·0% | 11·0% | 12·7% | 7·0% | 6·9% | 9·7% | 7·5% | |
| 2000–02 | 15·1% | 16·2% | 13·6% | 15·9% | 13·1% | 14·0% | 19·4% | 16·7% | 9·6% | 11·0% | 11·6% | 8·1% | 8·0% | 9·7% | 7·6% | |
| 2005–07 | 17·0% | 17·6% | 16·2% | 18·4% | 15·1% | 17·7% | 20·1% | 19·1% | 10·9% | 14·4% | 16·3% | 8·8% | 8·7% | 11·0% | 9·0% | |
| Conditional 5-year | ||||||||||||||||
| 1995–99 | 35·1% | 35·9% | 34·0% | 39·9% | 37·0% | 37·2% | 39·5% | 41·3% | 28·6% | 32·1% | 35·1% | 26·9% | 26·6% | 33·8% | 29·2% | |
| 2000–02 | 36·0% | 38·1% | 33·0% | 39·4% | 35·6% | 36·5% | 44·2% | 40·5% | 29·4% | 32·8% | 31·5% | 27·6% | 27·4% | 31·8% | 27·7% | |
| 2005–07 | 38·6% | 40·1% | 36·6% | 42·1% | 35·4% | 40·3% | 47·4% | 43·5% | 30·3% | 35·0% | 36·5% | 28·2% | 27·9% | 33·8% | 30·2% | |
| Breast cancer (women) | ||||||||||||||||
| 1 year | ||||||||||||||||
| 1995–99 | 95·8% | 95·8% | 95·6% | 95·9% | 95·4% | 97·1% | 96·2% | 95·5% | 93·0% | 95·4% | 97·6% | 90·4% | 90·5% | 92·6% | 88·6% | |
| 2000–02 | 96·3% | 96·5% | 96·0% | 96·2% | 95·9% | 96·5% | 96·4% | 96·0% | 94·3% | 95·8% | 98·4% | 92·4% | 92·5% | 95·0% | 89·8% | |
| 2005–07 | 96·7% | 96·5% | 97·1% | 96·3% | 95·9% | 97·1% | 96·7% | 96·1% | 95·0% | 96·6% | 98·0% | 94·2% | 94·3% | 95·0% | 93·4% | |
| 5 years | ||||||||||||||||
| 1995–99 | 85·0% | 85·5% | 84·4% | 85·3% | 83·4% | 87·1% | 86·0% | 84·9% | 76·9% | 81·8% | 86·7% | 74·8% | 74·8% | 77·5% | 73·5% | |
| 2000–02 | 87·0% | 87·4% | 86·5% | 86·4% | 84·9% | 87·5% | 83·9% | 86·6% | 81·5% | 83·8% | 89·3% | 78·8% | 78·8% | 81·6% | 76·7% | |
| 2005–07 | 88·1% | 87·8% | 88·5% | 86·3% | 82·6% | 89·1% | 86·8% | 86·4% | 82·4% | 85·5% | 88·5% | 81·6% | 81·6% | 84·1% | 81·0% | |
| Conditional 5-year | ||||||||||||||||
| 1995–99 | 88·8% | 89·1% | 88·2% | 88·9% | 87·3% | 89·7% | 89·4% | 88·9% | 82·6% | 85·6% | 88·8% | 82·5% | 82·4% | 83·6% | 82·7% | |
| 2000–02 | 90·3% | 90·6% | 90·0% | 89·8% | 88·5% | 90·6% | 87·0% | 90·2% | 86·4% | 87·3% | 90·6% | 85·0% | 85·0% | 86·3% | 85·1% | |
| 2005–07 | 91·0% | 90·9% | 91·2% | 89·6% | 86·0% | 91·8% | 89·7% | 89·8% | 86·6% | 88·4% | 90·2% | 86·4% | 86·4% | 88·9% | 86·4% | |
| Ovarian cancer | ||||||||||||||||
| 1 year | ||||||||||||||||
| 1995–99 | 70·4% | 70·4% | 70·4% | 71·8% | 69·4% | 74·0% | 68·9% | 71·9% | 63·4% | 70·4% | .. | 59·6% | 59·8% | 62·8% | 56·6% | |
| 2000–02 | 71·1% | 72·8% | 68·9% | 73·3% | 71·0% | 75·3% | 74·1% | 73·1% | 67·9% | 74·3% | .. | 62·4% | 62·6% | 64·9% | 60·0% | |
| 2005–07 | 73·5% | 73·3% | 73·7% | 75·2% | 69·7% | 77·6% | 68·0% | 75·9% | 70·6% | 75·2% | .. | 65·0% | 65·4% | 63·9% | 60·5% | |
| 5 years | ||||||||||||||||
| 1995–99 | 36·1% | 35·9% | 36·4% | 38·2% | 41·4% | 35·1% | 32·7% | 39·0% | 31·5% | 37·2% | .. | 32·6% | 32·5% | 39·0% | 31·5% | |
| 2000–02 | 37·1% | 39·6% | 34·1% | 38·4% | 34·9% | 37·8% | 37·1% | 39·1% | 33·7% | 40·2% | .. | 34·3% | 34·3% | 37·8% | 33·8% | |
| 2005–07 | 37·5% | 39·9% | 34·2% | 41·9% | 36·9% | 44·1% | 28·8% | 43·2% | 36·1% | 39·7% | .. | 36·4% | 36·4% | 36·5% | 36·3% | |
| Conditional 5-year | ||||||||||||||||
| 1995–99 | 48·8% | 48·6% | 49·2% | 51·9% | 58·3% | 45·7% | 43·7% | 53·3% | 48·9% | 50·6% | .. | 53·1% | 52·8% | 63·0% | 53·8% | |
| 2000–02 | 50·4% | 53·0% | 47·0% | 50·9% | 46·4% | 49·1% | 48·1% | 52·2% | 47·2% | 52·2% | .. | 52·8% | 52·6% | 55·0% | 53·9% | |
| 2005–07 | 48·7% | 52·7% | 43·5% | 54·4% | 50·8% | 55·8% | 39·1% | 55·7% | 48·8% | 50·9% | .. | 53·8% | 53·5% | 55·7% | 57·4% | |
Data are for period of diagnosis, cancer, and jurisdiction. Some age-standardised estimates arise from imputed age-specific estimates. Estimates for conditional 5-year survival include only patients who survived at least 1 year after diagnosis. Analyses for 2005–07 are period estimates for patients diagnosed during 2005–07, or diagnosed earlier but alive on Jan 1, 2005. Ovarian cancer data were not supplied by Sweden.
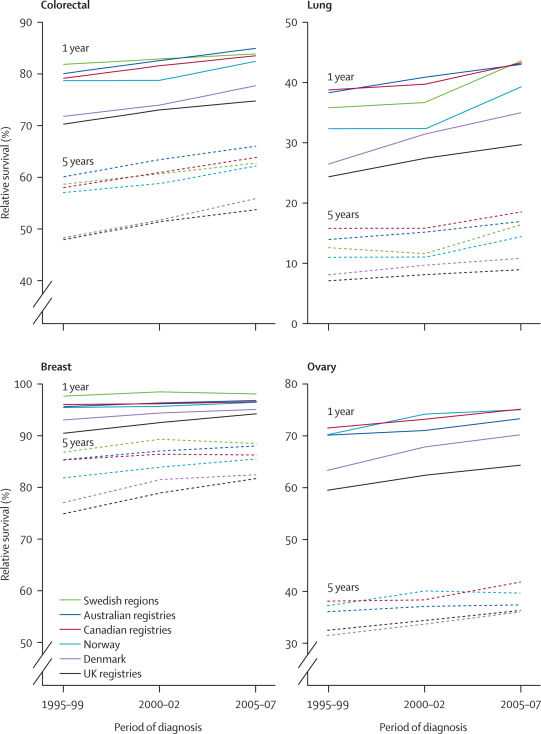
Figure 2.
Age-standardised 1-year and 5-year relative survival trends 1995–2007, by cancer and country
Data are for adults (15–99 years) diagnosed with colorectal, lung, breast, or ovarian cancer in 1995–99 and 2000–02 (cohort approach), and short-term prediction of survival for those diagnosed in 2005–07 (period approach). Ovarian cancer data were not supplied by Sweden.
Of 732 005 adults with a colorectal cancer diagnosed during 1995–2007, the mean age at diagnosis was 70·9 years (range between the 12 jurisdictions 69·2–72·0; webappendix p 18). For 58 634 patients (8·0%, range 3·5–14·8), colorectal cancer was not their first cancer (webappendix p 19). Excluding New South Wales and Denmark, death within 1 month of diagnosis was reported for 57 648 of 629 693 patients (9·2%, range 4·2–11·0%; webappendix p 20).
Colorectal cancer survival increased at a similar pace in all six countries. The differences in 1-year, 5-year, and conditional 5-year relative survival between Australia, Canada, Norway, and Sweden (higher-survival group) and Denmark and the UK (lower-survival group) persisted throughout 1995–2007 (table 2, figure 2). Most of the differences arose in the first year after diagnosis. The survival deficit in the UK and Denmark for 2005–07 (period estimate), compared with Australia, Canada, and Sweden, was 8–10% at both 1 and 5 years. For patients aged 65 years and older, these differences reached 10–15% (webappendix p 25).
Of 715 330 patients with lung cancer, the mean age at diagnosis was 70·3 years (range 68·3–71·2; webappendix p 18). For 63 480 patients (8·9%, range 4·1–17·9), lung cancer was not their first cancer (webappendix p 19). Death within 1 month of diagnosis was reported for 141 596 of 628 701 patients (22·5%, range 12·8–26·3; webappendix p 20).
Almost all the improvement in lung cancer survival is attributable to an increase in 1-year survival, for which the geographical differences in survival were widest in 1995–99 (table 2, figure 2). For 2005–07, 1-year survival was around 30% in the UK nations, 35% in Denmark, and 39–44% in Australia, Canada, Norway, and Sweden. 5-year survival was low at 9–11% in the UK and Denmark versus 15–20% in the other four countries. For 1995–99, 5-year survival was highest in Manitoba, but differences from other jurisdictions were small by 2005–07. The international range in conditional 5-year survival seems to have widened, with increases of 1·4–7·9% in Australia, Canada, and the Nordic countries, 0·0–1·3% in the three UK nations, and a small decrease (−1·6%) in Alberta. Survival in Denmark and the UK was lower than in other jurisdictions at all ages throughout 1995–2007, especially for those aged 65 years and older (webappendix p 25).
Of 833 350 women with breast cancer, the mean age at diagnosis was 62·5 years (range 60·6–63·9; webappendix p 18). For 41 798 women (5·0%, range 1·8–11·0), breast cancer was not their first cancer (webappendix p 19). Death within 1 month of diagnosis was reported for 14 749 of 730 290 women (2·0%, range 0·3–2·5; webappendix p 20).
5-year survival in the UK and Denmark improved more than in the other four countries, so the international range in breast cancer survival has narrowed since 1995–99 (table 2, figure 2). This finding might be a so-called ceiling effect, in that relative survival was more than 90% at 1 year and 82–88% at 5 years. 5-year and conditional 5-year estimates for 2005–07 in the UK and Denmark were still lower than in Australia, Canada, and Sweden, with Norway in an intermediate position. Survival in the UK was lower in all age groups during 1995–99, and up to 20% lower than in Australian and Canadian jurisdictions for women aged 65 years or older at diagnosis (webappendix p 26).
Of 121 059 women with ovarian cancer, the mean age at diagnosis was 64·0 years (range 61·1–65·2; webappendix p 18). For 9437 women (7·8%, range 6·1–13·5), ovarian cancer was not their first cancer (webappendix p 19). Death within 1 month of diagnosis was reported for 11 994 of 107 503 women (11·2%, range 6·3–12·9; webappendix p 20).
The geographical differences in survival for ovarian cancer generally resembled those for colorectal cancer, with 1-year survival during 2005–07 generally lower in the UK and Denmark than in the other countries (table 2). However, geographical differences in 1-year survival and the upward trend during 1995–2007 were much greater than for 5-year survival. Conditional 5-year survival for ovarian cancer in all three UK nations was higher than in New South Wales and Victoria, and close to the levels recorded in British Columbia and Ontario (table 2). Ovarian cancer survival at 1 year in Manitoba has fluctuated since the late 1990s and was 68% in 2005–07, 15–20% lower than in other jurisdictions (table 2). The differences were greater in women aged 55 years and older (webappendix p 26).
Discussion
This study provides population-based survival estimates for 2·4 million adults diagnosed with a cancer of the colorectum, lung, breast, or ovary up to 2007, in six countries (panel). Of the combined population of 123 million, 81% were covered by participating cancer registries. Data quality control and analyses were both done centrally with a common protocol, overseen by external experts. Data quality was high; less than 5% of eligible records (102 997/2 504 741) had to be excluded. Participating registries judged the survival estimates to be compatible with their own results, despite methodological differences.
Panel. Research in context.
Previous evidence
The CONCORD1 and EUROCARE2 studies have shown wide international differences in population-based cancer survival in patients diagnosed from the 1980s to 2002. The differences represent many avoidable premature deaths, and they have helped to drive national cancer control strategies since 2000. This international study is the first phase in a programme to identify the reasons for persistent inequalities in survival, as the evidence base for health policy to reduce them.
Interpretation
We compared survival from cancers of the colorectum, lung, breast, and ovary in six countries for patients diagnosed between 1995 and 2007. The countries selected for study have similar wealth, universal health coverage, and high-quality cancer registration. Survival has continued to improve for each cancer in all six countries, but generally remains higher in Australia, Canada, and Sweden, intermediate in Norway, and lower in Denmark and the UK. The patterns are consistent with later stage at diagnosis or differences in treatment, particularly in Denmark and the UK and in older patients, and they prompt further examination of stage and treatment by the International Cancer Benchmarking Partnership to explain the differences in survival.
Survival improved for all four cancers in all six countries during 1995–2007, although there was some fluctuation within countries. Generally, Australia, Canada, and Sweden had the highest survival, with Norway somewhat lower. Survival was generally lowest in Denmark and the three UK nations. The largest gains were recorded for colorectal cancer, and the smallest for lung and ovarian cancer. Relative survival from breast cancer at 1 year exceeded 90% in all six countries; gains were smaller, and the international range in both 1-year and 5-year survival diminished. For cancers of the colorectum, lung, and ovary, survival at 1 year was generally lower in Denmark and the three UK nations, suggesting that late diagnosis is still a problem in both countries; however, 1-year survival has increased more in Denmark since 2000–02 than in the UK.
The incidence of invasive ovarian malignancy has been falling in all six countries since 1985 (webappendix p 28). The decrease since 2000 might be partly attributable to reclassification of some tumours of borderline malignancy from invasive, malignant behaviour (code 3) in ICD-O-2 to uncertain behaviour (code 1) in ICD-O-3, but the protective effect of oral contraception and increased diagnostic intensity, with earlier diagnosis and removal of borderline or premalignant lesions, could have contributed in the long term.25 However, survival analyses were restricted to invasive cancers, so this shift would tend to reduce overall survival in the tumours still classified as invasive, by removing up to 10% of tumours with good survival. Since survival actually rose in most jurisdictions, any effect of this change seems to have been small.
We included both first and second (or higher-order) primary cancers in the analyses. The highest proportion of second and higher-order cancers was recorded in Manitoba, one of the oldest registries, and for three of the four cancers, the lowest proportion was in Northern Ireland, the newest registry (webappendix p 19). Sensitivity analyses will be done to show the effect on survival estimates of including these patients. The effect on 5-year survival is unlikely to exceed 1%.12
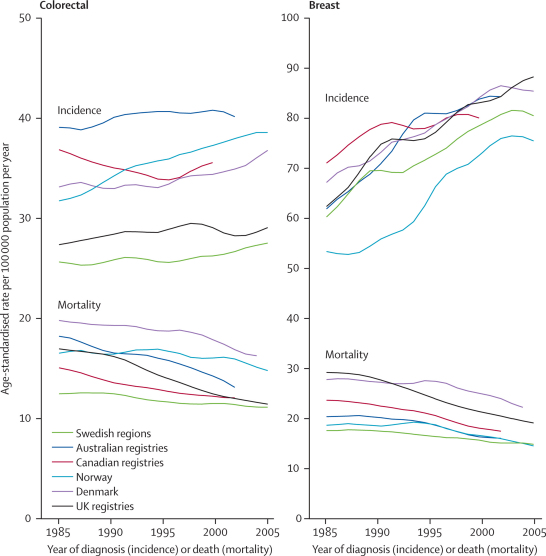
International differences in survival cannot simply be attributed to poor-quality cancer registration, as has been suggested for breast cancer in the UK in particular.26 Incidence, survival, and mortality trends are coherent for breast and other cancers (webappendix p 28), as they were when this issue was last discussed 10 years ago.27,28 For example, although breast cancer mortality in the UK has decreased more quickly than in Sweden since 1985, it is still higher, whereas incidence has risen roughly in parallel, and the difference in survival has decreased (compare figure 2 and figure 3). The Danish cancer registry is also highly regarded, with high-quality data, yet cancer survival patterns and trends in Denmark have been similar to those in the UK for several years, and the survival deficit with respect to other Nordic countries has motivated cancer strategy in Denmark, as in Britain.
Figure 3.
3-year moving-average world-standardised incidence and mortality rates per 100 000 population per year, 1985–2005, by country, for breast cancer in women and colorectal cancer in men and women combined
Incidence for Canada excludes Quebec; colorectal cancer includes cancers of the anus and anal canal; lung cancer includes cancers of the trachea. Incidence data labelled UK are for England only; mortality data labelled UK are for England and Wales only.
Mortality-incidence ratios for breast cancer in 2000 are also somewhat higher in Denmark and the UK than in other countries (webappendix p 27). A large study has shown that breast cancer incidence recorded independently in primary care in the UK during 1990–96 was very similar to incidence in the national cancer registry.29 Several studies have shown that access to radiotherapy varies widely in the UK; that manpower was low in the 1990s; and that elderly people and less affluent groups were affected by late diagnosis, treatment delays, and a survival deficit.30,31 Late-stage diagnosis accounts for much of the European variation in survival. High-resolution studies of hospital medical records (rather than cancer registry data) have shown that women with breast cancer in the UK were operated less often, have axillary dissection less often, and have fewer nodes sampled than do women in other countries.32 A study of more than 13 000 medical records has shown striking variation in breast cancer treatment in Europe in the late 1990s.33 Errors do arise in cancer registration, but the UK survival deficit is not explained by routine registration of recurrences as new diagnoses, or by failing to register long-term survivors. We will investigate this topic in a separate report.
Other studies have suggested that the wide differences in survival within Europe could be explained by large variation in diagnostic and surgical practice for colorectal cancer34 and in stage at diagnosis and treatment for breast cancer.32,35 Women older than 65 years with breast cancer in the late 1990s in England were less likely than younger women to receive triple assessment or guideline radiotherapy after conservative surgery.36 Chemotherapy and radiotherapy were later shown to increase long-term survival in early breast cancer. In Europe more generally, relative survival has improved more for women younger than 65 years at diagnosis than for older women; the widening deficit in survival in older women arises mainly in the first year after diagnosis, suggesting later diagnosis or less access to treatment.37 Survival from colorectal and breast cancers in England and Denmark is lower than in Australia, Canada, and most Nordic and western European countries.1,2,5,11 Improvements in colorectal cancer survival in England seem coherent with incidence and mortality trends.38 The lung cancer survival deficit in Denmark has been attributed to later stage at diagnosis and to the distribution of morphological types.39
The findings in this paper comprise a public health comparison of the survival of all patients with cancer in each jurisdiction, irrespective of stage at diagnosis or treatment, but after international differences in non-cancer mortality by age, sex, and calendar period are taken into account. Differences in individual, health-system, and clinical factors—such as public awareness of cancer, diagnostic delay, stage, comorbidity, and access to optimal treatment—are all potential explanations for the overall differences in relative survival. We will examine later the extent to which differences in stage at diagnosis, morphological type, and treatment for each patient can explain the survival disparities reported in this study, to provide evidence for strategy on how best to reduce the differences. Obesity, physical activity, and other lifestyle variables might also affect outcome.
Indicators of trend in incidence, survival, and mortality are all required for cancer control. Incidence rates refer to patients with cancer who are diagnosed in a specific year. Almost all are registered, but those who are not might be a biased subset. Most cancers are registered from more than one data source, with pathological evidence of the diagnosis, but a small proportion are registered only from a death certificate. Data quality control is extensive and well documented.13
Survival also refers to patients diagnosed in a specific year. Relative survival is not subject to errors in certification of the cause of death, and is corrected for background mortality, but it is subject to lead-time bias. It reflects the overall outcome for all patients with cancer, not only those in clinical trials or those diagnosed early enough for treatment of curative intent. Relative survival is a measure of the overall effectiveness of the entire health system, not only of treatment.
Mortality is a function of both incidence and survival. Mortality rates refer to the number of people who die in a specific year. Lung cancer survival has been low for many years, so mortality trends are largely parallel with incidence trends (webappendix p 28). By contrast, more than 40% of women who die of breast cancer in a specific year will have been diagnosed at least 5 years previously, so trends in mortality provide a delayed and imprecise picture of any trends in survival. It is not possible to draw conclusions about trends in survival from trends in mortality alone. Unlike relative survival, mortality rates are subject to errors in certification of the cause of death, particularly in elderly patients. Autopsies are infrequent, and quality control of death certificate data is far more limited than that of incidence data. Mortality data are nevertheless almost complete, and long time series are available. They are an invaluable public health resource.
The consistency of incidence, survival, and mortality trends in Europe has led other investigators to conclude that joint interpretation of these indicators is the best guide to policy for prevention, screening, treatment, and the organisation of health-care systems.40 We agree with this conclusion. Primary prevention to reduce incidence remains the best long-term strategy to reduce the cancer burden; incidence trends will thus remain a crucial policy guide. Until the causes of cancer are more fully understood, however, and effective strategies for prevention are fully implemented, millions of people will continue to be diagnosed with cancer every year. Therefore, for policy makers who have to deliver effective care to these patients, population-based survival will also remain a key indicator of progress. Even small improvements in survival from common cancers can prevent large numbers of premature deaths.41 Cancer registration systems should be supported to improve the quality and completeness of their data. Systematic collection of data for comorbidity and other possible determinants of survival should also be introduced. Clinicians should be encouraged to record the data that are needed for population-based comparisons more systematically. Information systems should be improved to help with recording of these data.
Additional indicators for routine monitoring of progress in cancer control are needed. For example, differences in relative survival between two countries or regions can be expressed as the number of cancer-related (excess) deaths in patients with cancer in one country that would be avoidable within, say, 5 years of diagnosis if relative survival were as high as in the other country.6 A decrease in the number of such avoidable, premature deaths can provide a useful comparative index of progress; it incorporates the relative effectiveness of both health systems, remains applicable when survival is improving in both countries, and might be more readily understood by the general public.
Survival trends can help both in the formulation of strategies for cancer control and in the assessment of their effectiveness. The International Cancer Benchmarking Partnership aims to provide robust and timely comparison of trends in cancer survival between countries or regions with good population-based data. Later phases of this partnership will explore the effect on international survival disparities of differences in public awareness and beliefs about cancer, duration of symptoms, diagnosis in primary and secondary care, comorbidity, and access to treatment. The goal is to identify remediable causes of survival deficits, to inform the development of new cancer control policy in participating countries.
Acknowledgments
Acknowledgments
This study was funded by the Department of Health, England (London, UK), and Cancer Research UK (London, UK). We thank the cancer registry staff in all jurisdictions, whose sustained efforts in data collection and quality control over many years have enabled trends in cancer patient survival to be analysed and compared in this study. We are grateful for the expert contributions from our Epidemiology Reference Group, who advised on protocol, study design, and analytical methods: Bruce Armstrong (Department of Public Health, University of Sydney, Sydney, NSW, Australia); Paul Dickman (Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden); Paul Lambert (Biostatistics Group, Department of Health Sciences, University of Leicester, Leicester, UK); Dianne O'Connell (Cancer Council New South Wales, Sydney, NSW, Australia); Milena Sant (Fondazione IRCCS, Istituto Nazionale dei Tumori, Milan, Italy); and Hannah Weir (Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, Atlanta, GA, USA). We also thank Jacques Ferlay (IARC, Lyon, France) for providing the incidence and mortality data for figure 3, and Max van Eijk (McKinsey) for sustained logistical support. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of any government agency.
Contributors
Programme Board members (OA, HB, DC, AG, GG, DH, JHan, SK, LdN, MAR, and TS) identified the questions, cancers, and countries to be studied; supervised the study; and contributed to drafting of the report. Module 1 leaders (JA, HB, MC, DF, MLG, TBJ, MLa, DM, RM, JSh, JSt, and ET) contributed to study design and drafting of the report. Cancer registry co-authors (JA, FB, HB, AC, MC, DD, GE, ME, RE, CF, MLG, CH, JHat, TBJ, DL, GLa, KL, MLa, CEM, DM, EJM, JM, KM, LM, RM, DN, CR, ASm, MS, JSh, JSt, DT, ET, JT, and CW) collected, prepared, and transmitted raw data for the study; advised on the protocol; corrected data after quality control; and checked the results of the analyses. Clinical Committee members (JBe, JBo, JBu, MB, PC, BE, HF, PF, TFi, TFo, CH, LH, NH, TH, KK, JL, GLi, MLee, MLev, PBL, IM, TM, UM, AN, JP, MP, AR, BRo, ASj, ASp, FW, GW, IW, and MW) advised about methodology, interpreted the findings, and drafted the report. The Central Analytic Team (CA, MPC, LE, CM, UN, MQ, BRa, and SW) drafted the protocol; obtained statutory and ethics approvals; had access to all the raw data; did the data preparation, quality control, and analyses; and drafted the report. All authors had access to the results of all steps of data preparation, quality control, and analyses; and contributed to writing the final report.
ICBP Module 1 Working Group
Programme Board Ole Andersen (National Board of Health, Health Planning Division, Copenhagen, Denmark); Heather Bryant (Canadian Partnership Against Cancer, Toronto ON, Canada); David Currow (Cancer Institute NSW, Sydney, NSW, Australia); Anna Gavin (Northern Ireland Cancer Registry, Belfast, UK); Gunilla Gunnarsson (Swedish Association of Local Authorities and Regions, Stockholm, Sweden); Jane Hanson (Cancer Services Co-ordinating Group, Cardiff, UK); David Hill (Cancer Council Victoria, Melbourne, VIC, Australia); Stein Kaasa (University Hospital of Trondheim, Trondheim, Norway); Lone de Neergaard (National Board of Health, Health Planning Division, Copenhagen, Denmark); Michael A Richards (National Cancer Action Team, Department of Health, London, UK; Chair); Terry Sullivan (Cancer Care Ontario, Toronto, ON, Canada). Module 1 leaders Jan Adolfsson (Karolinska University Hospital, Stockholm, Sweden); Heather Bryant (Canadian Partnership Against Cancer, Toronto, ON, Canada); Michael Coory (Cancer Council Victoria, Melbourne, VIC, Australia); David Forman (International Agency for Research on Cancer, Lyon, France; Chair); Marianne L Gjerstorff (Danish Cancer Registry, National Board of Health, Copenhagen, Denmark); Tom Borge Johannesen (Norwegian Cancer Registry, Oslo, Norway); Mats Lambe (Regional Oncological Centre, Uppsala University Hospital, Uppsala, and Karolinska Institutet, Stockholm, Sweden); David Meechan (Trent Cancer Registry, Sheffield, UK); Richard Middleton (Northern Ireland Cancer Registry, Belfast, UK); Janey Shin (Canadian Partnership Against Cancer, Toronto, ON, Canada); John Steward (Welsh Cancer Intelligence and Surveillance Unit, Cardiff, UK); Elizabeth Tracey (Cancer Institute NSW, Sydney, NSW, Australia). Cancer registries Juanita Hatcher, Carol Russell (Alberta Health Services, Edmonton, AB, Canada); Andy Coldman, Mark Elwood, David Levy, Colleen E McGahan (British Columbia Cancer Agency, Vancouver, BC, Canada); Dhali Dhaliwal, Donna Turner (CancerCare Manitoba, Winnipeg, MB, Canada); Loraine Marrett, John McLaughlin, Kamini Milnes, Diane Nishri, Michael Sherar (Cancer Care Ontario, Toronto, ON, Canada); Gerda Engholm (Department of Cancer Prevention and Documentation, Danish Cancer Society, Copenhagen, Denmark); Eva J Morris, James Thomas (Northern and Yorkshire Cancer Registration and Information Service, Leeds, UK); Karen Linklater (Thames Cancer Registry, London, UK); Rebecca Elleray, David Meechan, Andy Smith (Trent Cancer Registry, Sheffield, UK); Gill Lawrence (West Midlands Cancer Intelligence Unit, Birmingham, UK); Finian Bannon, Colin Fox (Northern Ireland Cancer Registry, Belfast, UK); Ceri White (Welsh Cancer Intelligence and Surveillance Unit, Cardiff, UK). Clinical Committee Jonas Bergh (Karolinska Institutet, Stockholm, Sweden); John Boyages (Westmead Breast Cancer Institute, Sydney, NSW, Australia); Michel Boyer (Sydney Cancer Centre, Sydney, NSW, Australia); John Butler (Department of Health, London, UK); Peter Clark (Clatterbridge Centre for Oncology, Liverpool, UK); Bill Evans (Juravinski Cancer Centre at Hamilton Health Sciences, Cancer Care Ontario, Toronto, ON, Canada); Hilary Fielder (Velindre NHS Trust, Cardiff, UK); Tony Fields (Alberta Health Services, Edmonton, AB, Canada); Paul Finan (Leeds Teaching Hospitals NHS Trust, Leeds, UK); Tommy Fornander (Södersjukhuset, Stockholm, Sweden); Neville Hacker (University of New South Wales and Royal Hospital for Women, Sydney, NSW, Australia); Louise Hanna (Velindre NHS Trust, Cardiff, UK); Thomas Hogberg (Skåne University Hospital, Lund, Sweden); Charlotte Hosbond (National Board of Health, Copenhagen, Denmark); Karl Kölbeck (Karolinska University Hospital, Stockholm, Sweden); Martin Lee (University Hospitals Coventry and Warwickshire NHS Trust, Coventry, UK); Peter Barrett Lee (Velindre NHS Trust, Cardiff, UK); Jason Lester (Velindre NHS Trust, Cardiff, UK); Mark Levine (McMaster University, Hamilton, ON, Canada); Gudrun Lindmark (Skåne University Hospital, Malmö-Lund, Sweden); Tim Maughan (Velindre NHS Trust, Cardiff, UK); Usha Menon (University College London, London, UK); Ian Moneypenny (Cardiff & Vale NHS Trust, Cardiff, Wales, UK); Andy Nordin (East Kent University Hospitals, Canterbury, UK); Julietta Patnick (NHS Cancer Screening, Sheffield, UK); Mick Peake (NHS Cancer Improvement, National Cancer Intelligence Network, Department of Respiratory Medicine, Leicester, UK); Andrew Radcliffe (Llandough Hospital, Penarth, UK); Barry Rosen (University of Toronto, Toronto, ON, Canada); Annika Sjövall (Karolinska University Hospital, Stockholm, Sweden); Allan Spigelman (University of New South Wales, Sydney, NSW, Australia); Gunnar Wagenius (Uppsala University Hospital, Uppsala, Sweden); Fredrik Wärnberg (Uppsala University Hospital, Uppsala, Sweden); Michael Williams (Addenbrooke's, Cambridge, UK); Ian Williamson (Royal Gwent Hospital, Newport, UK). Central Analytic Team (Cancer Research UK Cancer Survival Group, London School of Hygiene and Tropical Medicine, London, UK) Claudia Allemani (also Analytical Epidemiology Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy), Michel P Coleman, Libby Ellis, Camille Maringe, Ula Nur, Manuela Quaresma, Bernard Rachet, Sarah Walters.
Conflicts of interest
MAR is the National Cancer Director (England, funded by the Department of Health); and JBu is Clinical Advisor to the Medical Director of the NHS (England, funded by the Department of Health). Other authors declare that they have no conflicts of interest.
Web Extra Material
References
- 1.Coleman MP, Quaresma M, Berrino F, the CONCORD Working Group Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 2.Berrino F, De Angelis R, Sant M, the EUROCARE Working Group Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–783. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 3.Berrino F, Sant M, Verdecchia A, editors. Survival of cancer patients in Europe: the EUROCARE study (IARC Scientific Publications number 132) International Agency for Research on Cancer; Lyon: 1995. [Google Scholar]
- 4.Berrino F, Capocaccia R, Estève J, editors. Survival of cancer patients in Europe: the EUROCARE-2 study (IARC Scientific Publications number 151) International Agency for Research on Cancer; Lyon: 1999. [PubMed] [Google Scholar]
- 5.Sant M, Aareleid T, Berrino F. EUROCARE-3: survival of cancer patients diagnosed 1990–94—results and commentary. Ann Oncol. 2003;14(suppl 5):61–118. doi: 10.1093/annonc/mdg754. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Rahman MA, Stockton DL, Rachet B. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer. 2009;101(suppl 2):115–124. doi: 10.1038/sj.bjc.6605401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pokhrel A, Martikainen P, Pukkala E. Education, survival and avoidable deaths in cancer patients in Finland. Br J Cancer. 2010;103:1009–1114. doi: 10.1038/sj.bjc.6605861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Human Services . Victoria's cancer action plan 2008–2011. Victorian Government Department of Human Services; Melbourne: 2008. [Google Scholar]
- 9.Cavalli F. The World Cancer Declaration: a roadmap for change. Lancet Oncol. 2008;9:810–811. doi: 10.1016/S1470-2045(08)70213-4. [DOI] [PubMed] [Google Scholar]
- 10.Storm HH, Gislum M, Engholm G. Cancer survival before and after initiating the Danish Cancer Control plan [In Danish, English abstract] Ugeskr Laeger. 2008;170:3065–3069. [PubMed] [Google Scholar]
- 11.Storm HH, Engholm G, Hakulinen T. Survival of patients diagnosed with cancer in the Nordic countries up to 1999–2003 followed to the end of 2006. A critical overview of the results. Acta Oncol. 2010;49:532–544. doi: 10.3109/02841861003801148. [DOI] [PubMed] [Google Scholar]
- 12.Rachet B, Maringe C, Nur U. Population-based cancer survival trends in England and Wales up to 2007: an assessment of the NHS cancer plan for England. Lancet Oncol. 2009;10:351–369. doi: 10.1016/S1470-2045(09)70028-2. [DOI] [PubMed] [Google Scholar]
- 13.Curado MP, Edwards BK, Shin HR, editors. Cancer incidence in five continents (vol IX). IARC Scientific Publications number 160. International Agency for Research on Cancer; Lyon: 2007. [Google Scholar]
- 14.WHO . International statistical classification of diseases and related health problems, tenth revision. World Health Organisation; Geneva: 1994. [PubMed] [Google Scholar]
- 15.Fritz A, Percy C, Jack A, editors. International Classification of Diseases for Oncology (ICD-O) 3rd edn. World Health Organization; Geneva: 2000. [Google Scholar]
- 16.Percy C, Van Holten V, Muir CS, editors. International Classification of Diseases for Oncology (ICD-O) 2nd edn. World Health Organization; Geneva: 1990. [Google Scholar]
- 17.Dickman PW, Hakulinen T. The accuracy of index dates and calculation of survival time from cancer registry data. J Epid Biostat. 1997;2:87–94. [Google Scholar]
- 18.Ferlay J, Parkin DM, Curado MP. Cancer incidence in five continents, vol I–IX: IARC CancerBase number 9. Lyon. 2010. http://ci5.iarc.fr (accessed Dec 10, 2010).
- 19.WHO WHO Statistical Information System (WHOSIS), WHO Mortality Database. April 14, 2008. http://www.who.int/healthinfo/morttables/en/index.html (accessed Dec 10, 2010).
- 20.Ederer F, Axtell LM, Cutler SJ. The relative survival: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 21.Estève J, Benhamou E, Croasdale M. Relative survival and the estimation of net survival: elements for further discussion. Stat Med. 1990;9:529–538. doi: 10.1002/sim.4780090506. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Research UK Cancer Survival Group . strel computer program version 5.8 for cancer survival analysis. London School of Hygiene and Tropical Medicine; London: 2010. http://www.lshtm.ac.uk/ncdeu/cancersurvival/tools.htm (accessed Dec 10, 2010). [Google Scholar]
- 23.Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Brenner H, Gefeller O. An alternative approach to monitoring cancer patient survival. Cancer. 1996;78:2004–2010. [PubMed] [Google Scholar]
- 25.Skírnisdóttir I, Garmo H, Wilander E. Borderline ovarian tumors in Sweden 1960–2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer. 2008;123:1897–1901. doi: 10.1002/ijc.23724. [DOI] [PubMed] [Google Scholar]
- 26.Beral V, Peto R. UK cancer survival statistics are misleading and make survival look worse than it is. BMJ. 2010;341:c4112. doi: 10.1136/bmj.c4112. [DOI] [PubMed] [Google Scholar]
- 27.Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 28.Coleman MP, Babb P, Stockton DL, Møller H, Forman D. Trends in breast cancer incidence, survival and mortality. Lancet. 2000;356:590–591. doi: 10.1016/S0140-6736(00)02593-9. [DOI] [PubMed] [Google Scholar]
- 29.Kaye JA, Derby LE, del Mar Melero-Montes M. The incidence of breast cancer in the General Practice Research Database compared with national cancer registration data. Br J Cancer. 2000;83:1556–1558. doi: 10.1054/bjoc.2000.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams MV, Drinkwater KJ. Geographical variation in radiotherapy services across the UK in 2007 and the effect of deprivation. Clin Oncol. 2009;21:431–440. doi: 10.1016/j.clon.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Rachet B, Ellis L, Maringe C. Socioeconomic inequalities in cancer survival in England after the NHS Cancer Plan. Br J Cancer. 2010;103:446–453. doi: 10.1038/sj.bjc.6605752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sant M, Allemani C, Capocaccia R. Stage at diagnosis is a key explanation of differences in breast cancer survival across Europe. Int J Cancer. 2003;106:416–422. doi: 10.1002/ijc.11226. [DOI] [PubMed] [Google Scholar]
- 33.Allemani C, Storm H, Voogd AC. Variation in ‘standard care’ for breast cancer across Europe: a EUROCARE-3 high resolution study. Eur J Cancer. 2010;46:1528–1536. doi: 10.1016/j.ejca.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Gatta G, Zigon G, Aareleid T. Patterns of care for European colorectal cancer patients diagnosed in 1996–98: a EUROCARE high-resolution study. Acta Oncol. 2010;49:776–783. doi: 10.3109/02841861003782009. [DOI] [PubMed] [Google Scholar]
- 35.Sant M, for the EUROCARE Working Group Differences in stage and therapy for breast cancer across Europe. Int J Cancer. 2001;93:894–901. doi: 10.1002/ijc.1408. [DOI] [PubMed] [Google Scholar]
- 36.Lavelle K, Todd C, Moran A. Non-standard management of breast cancer increases with age in the UK: a population based cohort of women ≥65 years. Br J Cancer. 2007;96:1197–1203. doi: 10.1038/sj.bjc.6603709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quaglia A, Tavilla A, Shack LG. The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur J Cancer. 2009;45:1006–1016. doi: 10.1016/j.ejca.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Sanjoaquin MA, Choodari-Oskooei B, Dolbear C. Colorectal cancer incidence, mortality and survival in South-east England between 1972 and 2001. Eur J Cancer Prev. 2007;16:10–16. doi: 10.1097/01.cej.0000228398.30235.f5. [DOI] [PubMed] [Google Scholar]
- 39.Storm HH, Dickman PW, Engeland A. Do morphology and stage explain the inferior lung cancer survival in Denmark? Eur Respir J. 1999;13:430–435. doi: 10.1183/09031936.99.13243099. [DOI] [PubMed] [Google Scholar]
- 40.Karim Kos HE, de Vries E, Soerjomataram I. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Richards MA, Stockton DL, Babb P. How many deaths have been avoided through improvements in cancer survival? BMJ. 2000;320:895–898. doi: 10.1136/bmj.320.7239.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.