Abstract
Dopamine (DA) transporter (DAT) functions at the surface of dopaminergic neurons to clear extracellular DA. DAT surface levels are regulated by endocytosis. However, the endosomelysosome system is not well characterized in dopaminergic neurons and the endocytic trafficking of endogenous DAT is poorly studied. Hence we analyzed the distribution of endocytic compartments and DAT localization in cultured rat embryonic and postnatal neurons using fluorescence microscopy. Early Rab5 and EEA.1 containing endosomes were mostly found in somatodendritic regions of neurons, whereas endosomes containing recycling markers were primarily found in axons. In axons, DAT was located mainly in recycling endosomes and plasma membrane whereas in cell bodies and dendrites DAT was detected in early, late and recycling endosomal compartments. Subcellular fractionation of adult rat striatal synaptosomes demonstrated that DAT is enriched in fractions containing plasma membrane and recycling endosomes. This pattern of DAT distribution was not altered upon activation of protein kinase C in postnatal DA neurons. Altogether, our data suggest that axonal DAT mainly shuttles between the plasma membrane and recycling endosomes, whereas in the somatodendritic region of neurons DAT traffics through all conventional endosomal pathways.
INTRODUCTION
The dopamine transporter (DAT) is primarily responsible for termination of dopamine (DA) neurotransmission through reuptake of extracellular DA (Giros et al., 1996). DAT is a 12-transmembrane domain protein and a member of the SLC6 gene family of Na+/Cl−-dependent neurotransmitter transporters that also includes serotonin, GABA, norepinephrine and glycine transporters. By regulating neurotransmission through removal of signaling molecules from the extracellular space, transporters such as DAT play a critical role in numerous physiological and cognitive functions. For example, DA is involved in cognition, locomotion, motivation and reward-related behaviors. Abnormal DA signaling results in several neurological disorders such as Parkinson's disease, Schizophrenia, bipolar disorder and Attention Deficit Hyperactivity Disorder (ADHD). Additionally, psychostimulant drugs like cocaine and amphetamine are known to target DAT and modulate DA neurotransmission (Giros and Caron, 1993; Uhl et al., 1996). The importance of DAT is exemplified by the experiments in mice with genetic deletion of the DAT gene (Giros et al., 1996). These mice display severe abnormalities in cognition and locomotion. Other phenotypes of the DAT−/− mice such as hyperactivity and reduction in psychostimulant drug-induced responsiveness closely resemble those of the patients with ADHD (Gainetdinov et al., 1999). These and other studies indicate the importance of analyzing DAT modulation to further understand the role DA signaling and DAT function has in human disorders.
DAT is exclusively expressed in dopaminergic neurons (DA neurons) (Gainetdinov et al., 1999). The cell bodies of DA neurons are located in the ventral mesencephalic region of the brain consisting of the substantia nigra (SN) and ventral tegmental area (VTA). Axonal projections of DA neurons primarily innervate regions involved in controlling locomotion, reward processing and cognition, such as the dorsal striatum, nucleus accumbens and the prefrontal cortex. DAT protein is synthesized and processed in the endoplasmic reticulum (ER) and Golgi apparatus, both of which are located in the soma of DA neurons. Mature, fully N-glycosylated DAT is delivered from Golgi to the plasma membrane of the soma, dendrites and axons.
DAT can participate in the DA clearance only when it is present on the plasma membrane. DAT plasma membrane expression is regulated by the process of endocytosis (Pristupa et al., 1998; reviewed in Melikian, 2004). DAT is thought to undergo constitutive and regulated endocytosis (Zahniser and Sorkin, 2009). In the past decade, the mechanisms of phorbol ester induced endocytic trafficking of DAT were extensively studied in non-neuronal expression systems. Studies indicate that protein kinase C (PKC) activation leads to endocytosis of DAT through clathrin coated pits (Daniels and Amara, 1999; Sorkina et al., 2005) and accumulation of the transporter in early, recycling and late endosomes (Melikian and Buckley, 1999; Sorkina et al., 2005; Miranda et al., 2007).
Constitutive endocytosis of DAT was also first observed in non-neuronal cells (Chi and Reith, 2003; Loder and Melikian, 2003). Several molecular determinants in the carboxy- and amino-termini of DAT are thought to control its constitutive endocytosis (Holton et al., 2005; Sorkina et al., 2005; Sorkina et al., 2009). Constitutive internalization appears to be clathrin-dependent and leads to accumulation of internalized DAT in early, recycling and late endosomes (Loder and Melikian, 2003; Sorkina et al., 2005; Eriksen et al., 2010).
Much less is known about constitutive and regulated endocytosis of endogenous DAT in DA neurons. Electron microscopy studies in rat DA neurons demonstrated that DAT is located in the plasma membrane of juxtasynaptic areas of axons and distal dendritic processes while it is mostly present on internal membrane compartments like ER, Golgi and multivesicular bodies in the soma and proximal dendrites (Ciliax et al., 1995; Nirenberg et al., 1996; Nirenberg et al., 1997a; Nirenberg et al., 1997b). Constitutive endocytosis of exogenously expressed epitope-tagged DAT was observed in cell bodies and axonal processes of cultured embryonic DA neurons (Sorkina et al., 2006). Recent studies using fluorescent cocaine analogues revealed presence of internalized DAT in Rab5- and transferrin receptor (TfR) positive vesicles in the cell bodies of cultured postnatal DA neurons (Eriksen et al., 2009).
It should be emphasized that the endosomal system is not well characterized in DA neurons. In particular, the types of endosomes present in the long and highly complex axons of DA neurons are unknown, and the subcellular distribution of endogenous endosomal markers in these neurons has not been studied. Likewise, localization of internalized axonal DAT has not been defined. Hence, in this study we investigated the distribution of endocytic compartments and the localization of intracellular pool of DAT by analyzing the distribution of natively expressed endosomal proteins in DA neurons using fluorescent microscopy and subcellular fractionation. Our analysis showed that the axonal compartment of DA neurons is enriched in recycling endosomes but is deficient in other types of endosomes, whereas the somatodendritic compartment contains all types of endosomes. Analysis of DAT distribution revealed similar patterns with its preferential localization in recycling endosomes and plasma membrane in axons, and various types of endosomes in the soma and dendrites. Activation of PKC in postnatal DA neurons did not change this distribution pattern of DAT.
RESULTS
Endocytic compartments in different regions of rat DA neurons
We analyzed the distribution of various types of endosomes in different regions of embryonic rat DA neurons using three-dimensional (3-D) fluorescence microscopy imaging and deconvolution. DA neurons in embryonic ventral mesencephalic cultures were identified by staining for tyrosine hydroxylase (TH) (Pickel et al., 1975). TH-positive neurons were stained with endocytic markers and the staining patterns in the soma, dendrites and axons of these neurons were investigated. The somatic regions were identified by DAPI staining of cell nuclei. Tau-1 is typically utilized as a marker for axonal processes (Kempf et al., 1996). However, in postnatal DA neurons, Tau-1 antibody stained all dendritic as well as axonal processes (Supplemental Figure S1), likely because these neurons were not terminally differentiated at early developmental stages. On the other hand, MAP2 antibody specifically stained the somatodendritic compartments and not the axons. The morphological features of dendrites and axons correlated very well with MAP-2 and Tau-1 (after subtraction of the MAP-2-positive staining of dendritic processes) staining, respectively. Axonal processes were defined as very long, very thin, typically homogeneous in morphology and having characteristic small “swellings” or “varicosities” (Pickel et al., 1981; Bouyer et al., 1984; Descarries et al., 1996; Sesack et al., 1998). Growth cones were identified as axonal widenings with filopodia-like extensions normally present at the end of the axonal processes. Dendrites were typically wider than the axons, much shorter and more smooth (did not contain varicosities or spines), and displayed rather heterogeneous morphology. Because simultaneous staining of cultures with the DA neuronal marker, endosome markers and a third antibody species (to detect MAP2 and/or Tau-1) was practically impossible, in subsequent experiments of double-staining of cultures we used the above morphological features to distinguish between dendrites and axons.
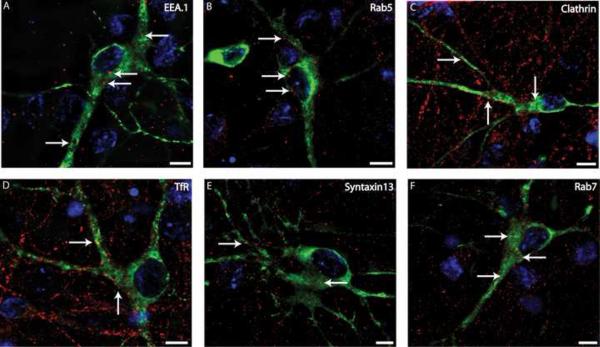
To investigate the distribution of early endosomes, immunofluorescence staining was performed with antibodies to resident early endosomal proteins, Rab5 and early endosomal antigen 1 (EEA.1) (Gorvel et al., 1991; Mu et al., 1995). Rab5 is a small GTPase protein while EEA.1 is a downstream Rab5 effector involved in endosome fusion. As shown in Figure 1A and B, Rab5- and EEA.1-positive early endosomes were found in abundance in the cell bodies of TH-positive neurons. These structures were also found in the proximal as well as distal dendritic regions. Also, numerous puncta of clathrin, which is the major structural component of coated pits and vesicles, were found in these regions of DA neurons (Figure 1 C).
Figure 1. Immunofluorescence detection of endosomal compartments in somatodendritic regions of embryonic dopaminergic rat neurons.
Embryonic ventral mesencephalic cultures were co-stained with mouse monoclonal TH antibody (green) and rabbit polyclonal antibodies (red) to EEA.1 (A), Rab5 (B), clathrin (C), TfR (D), Syntaxin13 (E) or Rab7 (F). The cell nuclei are stained with DAPI (blue). Z-stacks of images were acquired through Cy3, FITC and DAPI filter channels and the merged images are presented. Individual optical sections of deconvoluted 3-D images are shown. The images depict the cell body and dendritic regions of TH positive neurons. The arrows show examples of structures positive for the respective endocytic markers and located in TH neurons. Scale bars, 5 μm.
To characterize the distribution of recycling endosomes, neurons were stained with antibodies to transferrin receptor (TfR) and Syntaxin13. TfR is constitutively internalized and recycled, and at steady state accumulates in “late” recycling endosomes (Maxfield and McGraw, 2004). Syntaxin13 is an endosomal SNARE located on early and recycling endosomes where it facilitates recycling internalized cargo back to the plasma membrane (Advani et al., 1998; Prekeris et al., 1998). As shown in Figures 1D and E, both TfR and Syntaxin13 stained vesicular and tubular-shaped structures in the cell bodies and dendrites of TH-positive neurons.
To identify the late endocytic compartments, DA neurons were stained with antibodies to Rab7, a resident late endosomal protein (Zerial and McBride, 2001). Rab7-positive endosomes were also present in the somatodendritic regions of the DA neurons (Figure 1F). The specificity of the Rab7 antibody, which has not been well characterized, was confirmed by demonstrating the efficient staining of Rab7-GFP transiently expressed in COS1 cells (Supplemental Figure S2).
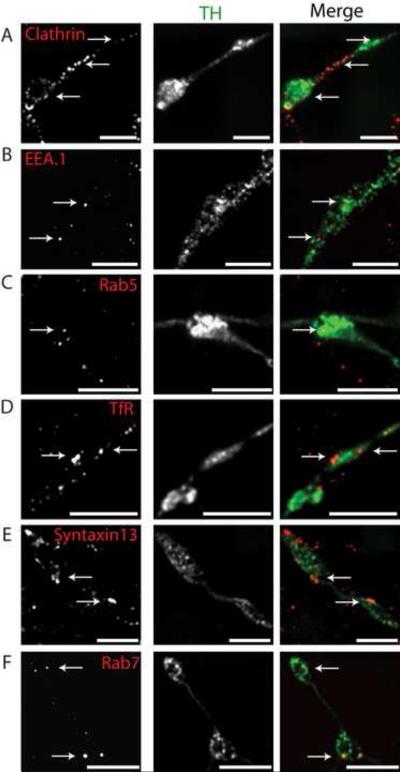
We next examined the endosomal populations in axonal processes of DA neurons. The axonal processes including the varicosities were enriched in clathrin (Figure 2A), which is consistent with the presence of high concentration of clathrin coated pits and vesicles near the active zone of the synapse. The early endosomes containing Rab5 or EEA.1 were occasionally found in the axonal varicosities and extensions (Figure 2B and C). Interestingly, TfR and Syntaxin13 positive endosomes were observed much more frequently than Rab5/EEA.1-labeled early endosomes in varicosities (Figure 2D and 2E). A pool of Rab7 positive late endosomes was also found in axons of the DA neurons (Figure 2F). The growth cones of DA neurons exhibited similar distribution patterns of different endocytic markers with the exception of Rab7 positive vesicles which were found at much lower abundance (Supplemental figure S3).
Figure 2. Endosomal compartments in the axonal processes of embryonic dopaminergic neurons.
Embryonic ventral mesencephalic cultures were co-stained with mouse monoclonal TH antibody (green) and rabbit polyclonal antibodies (red) to clathrin (A), EEA.1 (B), Rab5 (C), TfR (D), Syntaxin13 (E) or Rab7 (F). Z-stacks of images were acquired through Cy3 and FITC filter channels and the individual optical sections of deconvoluted 3-D images are shown. The images depict the axonal processes and varicosities of TH positive neurons. The arrows show examples of endocytic structures in axonal varicosities and extensions. Scale bars, 5 μm.
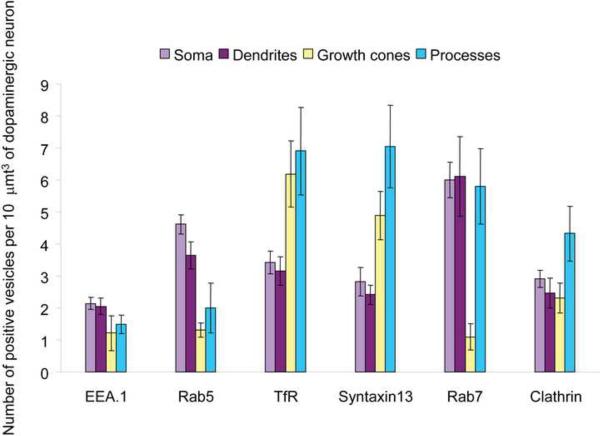
To directly compare the relative abundance of individual endosomal populations in different regions of the DA neuron, a quantitative analysis of the multiple imaging experiments represented in Figures 1, 2 and S3 was performed. To this end, the apparent concentration of endocytic vesicles positive for a marker, i.e. number of vesicles per 10 μmt3 volume of DA neuron (TH immunofluorescence) was calculated (Figure 3). As shown in Figure 3, the concentrations of EEA.1- and Rab5-positive early endosomes were, respectively, 50% and 75% lower in the axonal processes and growth cones than in the somatodendritic regions of DA neurons. In contrast, TfR- and Syntaxin13-positive recycling endosomes had approximately twofold higher concentration in the axonal processes and growth cones than in the soma and dendrites. On the other hand, Rab7-positive late endosomes were found in similar densities in all regions of the neurons except in the growth cones where the densities were significantly lower compared to other regions of the neuron. The apparent concentrations of all endosomal markers were similar in the cell body and proximal dendritic regions. Essentially similar patterns of endosome distribution were observed in cultured postnatal DA neurons (data not shown). Together, the data presented in Figures 1–3 suggest that recycling is the major pathway of post-endocytic trafficking of cargo proteins in axons whereas multiple endocytic trafficking pathways occur in the somatodendritic compartment of DA neurons.
Figure 3. Quantification of the relative concentrations of endocytic compartments in different regions of embryonic DA neurons.
A number of endocytic structures (endosomes and clathrin-coated pits/vesicles) identified by various endocytic markers present per 10 μmt3 volume of the DA neuron was calculated as described in “Methods” in a large number of images exemplified in Figure 1, 2 and Supplemental Figure 2. The concentration of endocytic structures was calculated for soma (light purple), dendrites (dark purple), growth cones (yellow) and axonal processes (blue). The bars represent mean values and the error bars are the standard errors of the mean.
Subcellular localization of DAT in different regions of DA neurons
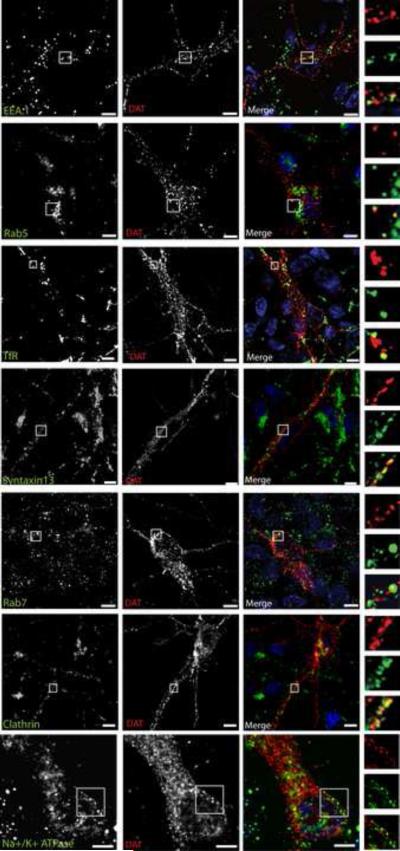
Following the characterization of sub-neuronal distribution of endosomes, we next examined whether DAT is targeted to these endosomes. DAT was found to be present in early, recycling and late endosomes in non-neuronal cells (Loder and Melikian, 2003; Sorkina et al., 2005; Miranda et al., 2007). To study the localization of endogenous DAT in endosomes, rat embryonic DA neurons were co-stained with the DAT antibody and antibodies to resident endosomal proteins. Analysis of colocalization of DAT with endosomal markers showed that a small pool of DAT is associated with clathrin-positive structures, EEA.1- and Rab5-containing early endosomes, TfR- and Syntaxin13-positive recycling endosomes, and Rab7-containing late endosomes in the somatodendritic region of DA neurons (Figure 4). A small fraction of DAT was also associated with diffusely distributed and clustered Na+/K+ ATPase, a marker of the plasma membrane.
Figure 4. DAT is present in early, recycling and late endosomal compartments, and in the plasma membrane in the soma of embryonic dopaminergic neurons.
Embryonic ventral mesencephalic cultures were co-stained with rat monoclonal antibodies to DAT (red) and antibodies to different endocytic or plasma membrane markers as indicated (green). The cell nuclei are stained with DAPI (blue). Individual optical sections of deconvoluted 3-D images are shown. The images depict the cell body and dendritic regions of DAT-positive neurons. Z-stacks of images were acquired through Cy3, FITC and DAPI filter channels and the merged images are presented with the “Yellow” indicating overlap of red (Cy3) and green (FITC) fluorescence. Insets (on the right) represent high magnification of the regions of the images indicated by white rectangles with examples of structures positive for DAT and respective endocytic markers. Scale bars, 5 μm.
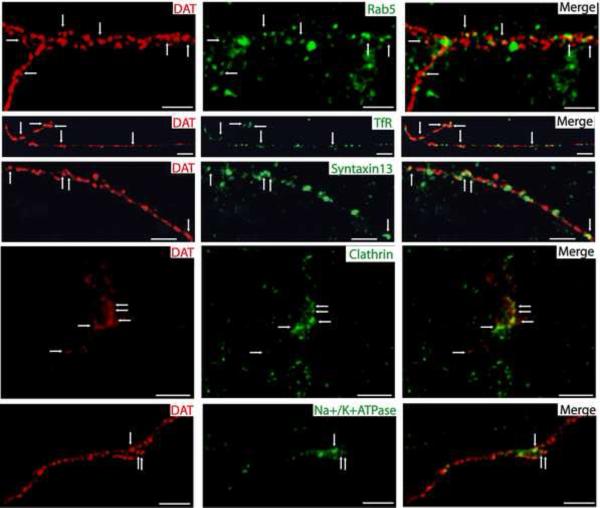
In contrast, only a small amount of DAT was co-localized with the markers of endosomes in axonal processes of embryonic and postnatal cultured DA neurons. Since postnatal DA neurons are more differentiated and express higher amounts of DAT, the frequency of detection of DAT in endocytic compartments was higher as compared to the embryonic neurons. DAT colocalized with Rab5-postive early endosomes more frequently in postnatal DA cultures than embryonic cultures (Figure 5). However, in both embryonic and postnatal DA neurons, DAT was rarely co-localized with EEA.1 or Rab7 endosomes (data not shown), whereas DAT co-localization with TfR- and Syntaxin13-positive recycling endosomes was substantially higher (Figure 5 and Supplemental Figure S4). Axonal DAT was also co-localized with clathrin punctae and plasma membrane (Fig. 5 and Supplemental Fig. S4). It should be noted that synapses of DA neurons are very small and hence individual endosomes and other organelles within one synapse cannot be resolved by diffraction-limited conventional fluorescence microscopy. Therefore, apparent co-localization of DAT with an endosomal marker within one synapse or small varicosity (typically containing two active zones) does not necessarily indicate the presence of DAT within the same endocytic structure as the marker. Overall, immunofluorescence analysis (Figure 5 and Supplemental Figure S4) shows that only a small pool of axonal DAT is located in endosomes and that internalized DAT preferentially traffics to the recycling endosomal compartments directly or via an intermediate Rab5-positive compartments.
Figure 5. DAT co-localizes with TfR, Syntaxin13, clathrin and Na+/K+ ATPase positive structures in axons of postnatal DA neurons.
Postnatal mesencephalic cultures were co-stained with rat monoclonal antibody to DAT (red) and antibodies to TfR, Syntaxin13, clathrin or Na+/K+ ATPase (all in green). Z-stacks of images were acquired through Cy3 and FITC filter channels and individual optical sections of deconvoluted 3-D images are shown. The images depict the axonal processes and varicosities of DAT-positive neurons. The arrows show examples of endocytic structures that contain DAT in axonal varicosities and extensions. Scale bars, 2.5 μm.
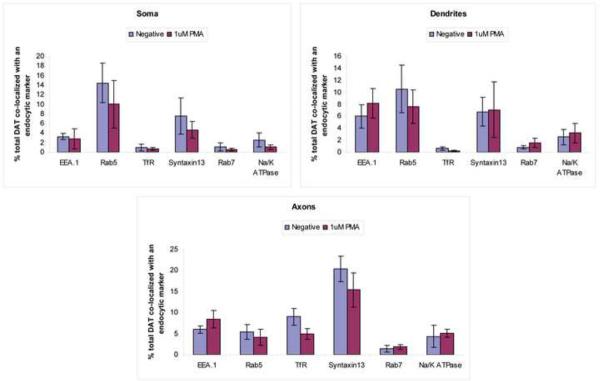
We further analyzed if the distribution pattern of DAT changes as a result of activation of PKC by PMA, which in non-neuronal cells results in accumulation of DAT in endosomes. The percent of total DAT fluorescence co-localized with an endocytic marker was calculated after treatment of postnatal DA neurons with 1 μM PMA or DMSO (Fig. 6). We found that activation of PKC did not cause any significant changes in association of DAT with endocytic compartments or the plasma membrane in different regions of the DA neurons.
Figure 6. Treatment of postnatal DA neurons with PMA does not change the subcellular distribution of DAT.
Postnatal DA neurons were treated with 1 μM PMA or DMSO for 30 minutes at 37°C, and stained for DAT and different endocytic markers. The percent of DAT fluorescent intensity co-localized with a particular endocytic or plasma membrane marker was calculated and plotted. These values for the soma, dendrites and axonal processes of the DA neurons are shown.
DAT co-fractionates with the plasma membrane and recycling endosomes in striatal synaptosome preparations
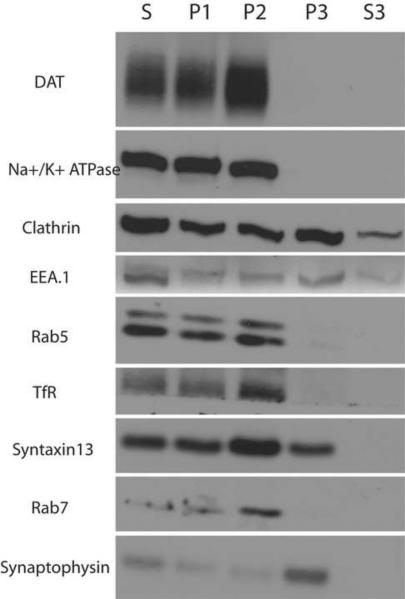
The fluorescence microscopy data strongly suggest that in the axonal processes of cultured dopaminergic neurons DAT is distributed between the plasma membrane and recycling endosomes. In the adult brain, DAT is mostly concentrated in dopaminergic axons that largely innervate the striatum (Tashiro et al., 1989; Mura et al., 1995). Therefore, to confirm our findings in embryonic and postnatal DA neurons, we investigated the subcellular distribution of DAT in adult rat striatal synaptosomes using differential centrifugation. Synaptosomes were lysed by hypo-osmotic shock and the resulting membrane compartments were separated to obtain the following fractions as shown in Figure 7: plasma membrane fraction enriched in Na+/K+ ATPase (P1); endosomal fraction enriched for Rab5, TfR, Syntaxin13 and Rab7 (P2); synaptic vesicle and coated vesicle fraction enriched in synaptophysin and clathrin, respectively, (P3), and a cytoplasmic fraction (S3). DAT was found to be concentrated in the P1 and P2 fractions that are enriched in plasma membrane and endocytic vesicles, respectively. DAT was absent in the P3 fraction strongly suggesting that it does not associate with synaptic vesicles.
Figure 7. DAT is enriched in the plasma membrane and endosomal fractions of adult rat striatal synaptosomes.
Striatal synaptosomes were mildly homogenized and the homogenates were fractionated by differential centrifugation as described in “Methods”. Crude striatal synaptosomes (S) and fractions P1, P2, P3 and S3 were probed by Western blotting with different antibodies to plasma membrane, endosomes, synaptic vesicles and DAT as indicated on the left.
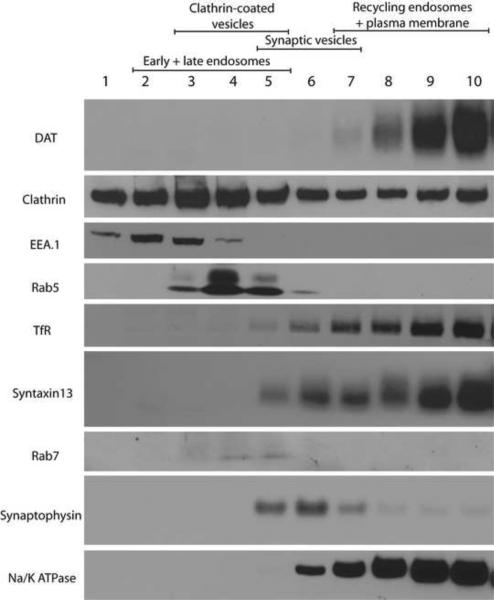
The “endosomal” fraction obtained by differential centrifugation consisted of the plasma membrane and all endosomal subtypes. To better separate different populations of endosomes, striatal synaptosome homogenates were subjected to sucrose density gradient centrifugation. This technique allowed us to better resolve different endocytic compartments. As shown in Figure 8, fractions #2–5 contained Rab5- and EEA.1-positive early endosomes and Rab7-positive late endosomes. The lighter fraction # 1 likely corresponded to synaptosomal cytosol. Fractions #5–7 were enriched in synaptophysin, a marker specific for synaptic vesicles, while fractions # 7–10 were enriched in TfR- and Syntaxin13-positive recycling compartments and the plasma membrane. DAT primarily co-fractionated with TfR, Syntaxin13 and Na+/K+ ATPase. Consistent with the fluorescence imaging of axons in DA neuronal cultures described in previous sections, subcellular fractionation analysis suggested that axonal DAT in adult rat brain is restricted to the plasma membrane and recycling endosomal compartments.
Figure 8. Sucrose gradient centrifugation of adult rat striatal synaptosomes demonstrates co-fractionation of DAT with recycling endosomes and plasma membrane.
Striatal synaptosomes were mildly homogenized and the homogenates were fractionated by sucrose density gradient as described in “Methods”. The fractions were electrophoresed and probed by Western blotting with antibodies to DAT and several organelle markers as indicated.
Discussion
The main objective of this study was to characterize different populations of endocytic vesicles in different regions of DA neurons and analyze the association of DAT with these endocytic organelles. The basic endosome/lysosome membrane system is well characterized in non-neuronal cultured cells (Maxfield and McGraw, 2004). Limited information about the distribution of the endosomal compartments in several types of cultured neurons is also available (Horton and Ehlers, 2003; Wu et al., 2009). DA neurons have highly complex axon morphology and they do not form terminal synapses but contain active zones in multiple small varicosities along the processes (Nirenberg et al., 1996; Nirenberg et al., 1997a). The endosomal compartments within these axonal varicosities have not been characterized. Labeling of endosomes with fluorescent markers in DA neurons has been problematic because heterologous expression of proteins is notoriously difficult in these neurons. Also, overexpression of resident endosomal proteins may affect the morphology and distribution of endosomes. Therefore, we analyzed the organization of the endosomal compartment and DAT localization in DA neurons by immunofluorescence and immunoblotting of endogenous endosomal markers. It should be noted that these markers have not been visualized in DA neurons with the exception of the TfR (Eriksen et al., 2009).
Visual inspection of numerous images of primary mesostriatal cultures revealed that DA neurons (TH- or DAT-positive) generally have fewer endosomes compared to the surrounding neurons and glia. Endosomes were particularly scarce in axonal processes. Quantitative analysis showed that recycling endosomes (containing Syntaxin13 or TfR) were more frequently seen in axons while early/intermediate (EEA.1 positive) endosomes were mainly restricted to the soma and dendrites. Interestingly, this is in contrast to the findings in hippocampal and motor neurons where Rab5 and Syntaxin13 vesicles are present in all regions while TfR and EEA.1 vesicles are restricted to the somatodendritic region of neurons (Parton et al., 1992; de Hoop et al., 1994; Prekeris et al., 1998; Wilson et al., 2000; Deinhardt et al., 2006).
Only a small pool of axonal DAT was found in endosomes, suggesting that most of the DAT immunoreactivity in axons is associated with the plasma membrane. Indeed, a substantial amount of DAT was co-localized with the clusters of Na+/K+ ATPase, a protein concentrated at the cell surface. Our data are consistent with the electron microscopy analysis of adult rat striatal DA neurons, which demonstrated predominant surface localization of DAT in axons (Ciliax et al., 1995; Nirenberg et al., 1996; Nirenberg et al., 1997a; Nirenberg et al., 1997b). Therefore, it can be proposed that only a small pool of DAT is constitutively internalized or the rate of DAT recycling is very high in axons.
Importantly, DAT was co-localized mainly with recycling endosome markers in axons of cultured neurons (Figs. 5 and 6). Subcellular fractionation studies also demonstrated the lack of co-localization of axonal DAT with early/intermediate endosomes (Rab5 and EEA.1) in adult striatum (Figs. 7 and 8). The small number of Rab5-containing endosomes and presence of only a very small fraction of DAT in these endosomes in axons is surprising. The general model of endocytosis presumes that the endocytic vesicles first deliver cargo to early endosomes, which contain Rab5, and that recycling can occur either from these early endosomes and “intermediate” endosomes (containing Rab5 and EEA.1) or after subsequent sorting of the cargo, such as TfR, to the “late” recycling compartment. It can be speculated that in axonal varicosities and growth cones of DA neurons, internalized cargo like DAT or TfR undergoes a more simplified routing involving shuttling between the cell surface and “early/recycling” endosomes (containing the recycling endosome SNARE – Syntaxin 13).
Certainly, light microscopy cannot resolve individual organelles within the small varicosities of DA axons. For instance, it could be possible that after internalization, DAT is sorted to synaptic vesicles, which are located in close proximity to endosomes and plasma membrane. Recent studies have demonstrated the interaction of DAT with the synaptic vesicle protein, synaptogyrin-3, and proposed an association of DAT with synaptic vesicles (Egana et al., 2009). GABA transporter, another closely-related neurotransmitter transporter (Deken et al., 2003), as well as choline transporter (Ferguson et al., 2003) have been reported to be present in vesicles that are enriched in synaptic vesicle proteins. Although in the latter studies sucrose velocity gradients demonstrated co-fractionation of the choline transporter with synaptophysin, DAT was completely excluded from these fractions. Earlier, Melikian and Buckley (1999) reported that in pheochromocytoma (PC12) cells, DAT is excluded from the synaptic vesicles. Our experimental data (Figures 7 and 8) are coherent with these observations strongly suggesting that DAT is unlikely to associate with synaptic vesicles.
In contrast to axons, all types of endosomes were detected in the soma of DA neurons and proximal dendrites. This is consistent with the study that used fluorescently-labeled cocaine analogues to demonstrate association of DAT with vesicles containing internalized transferrin, transfected heterologous Rab5 and Rab7 (Eriksen et al., 2009; Eriksen et al., 2010). Previously, electron microscopic immunolabeling demonstrated the localization of DAT in multivesicular endosomes in the soma of DA neurons of SN and VTA (Ciliax et al., 1995; Nirenberg et al., 1997a; Nirenberg et al., 1997b). Thus, we propose that trafficking pathways of internalized DAT and other cargo in somaptodendritic compartment are similar to the classical pathways of internalization, recycling and degradation described in non-neuronal cultured cells.
In addition, we found that treatment of postnatal DA neurons with PMA did not alter the distribution pattern of DAT. These findings are consistent with the recent studies using fluorescent cocaine analog where no visible re-distribution of DAT was observed in response to PKC activation in postnatal DA neurons (Eriksen et al., 2009). However, since the resolution of light microscopy is limited, the quantitative changes in DAT localization in DA neurons upon PMA treatment might be difficult to detect. Studies using DAT with an extracellular epitope (Sorkina et al., 2009) may allow clear separation between intracellular and extracellular DAT and provide a more accurate measurement for the constitutive and PKC-dependent endocytic activity in DA neurons.
In summary, while the basic organizations of endocytic pathways in neurons and nonneuronal cells are thought to be similar, the uniqueness of DA neurons lies in the extraordinary distances they cover from the ventral mesencephalon to different regions of the brain including the striatum where they form synapses with a very large number of striatal neurons (Matsuda et al., 2009). It can be hypothesized that this complex and specialized organization of the axonal compartment of DA neurons exists at the expense of the basic endocytic machinery which is reduced to limited quantities and types of endosomes, and therefore to a limited amount of endocytic trafficking of plasma membrane proteins in dopaminergic axons.
Materials and Methods
Reagents
Antibodies were purchased from the following sources: monoclonal rat antibody against DAT and mouse monoclonal TH antibody were purchased from Millipore (Bedford, MA). Polyclonal rabbit antibody against TH was obtained from Pel-Freez Biologicals (Rogers, AR), mouse anti-TfR was from Zymed (San Francisco, CA), rabbit anti-clathrin antibody was from Abcam (Cambridge, MA), mouse anti-Na+/K+ ATPase was from Affinity Bioreagents (Rockford, IL), and mouse anti-MAP2 was from Millipore (Bedford, MA). Mouse anti-EEA.1, rabbit anti-Rab5, rabbit anti-Rab7 and mouse anti-Tau1 were purchased from B.D. Transduction laboratories (San Jose, CA). Rabbit polyclonal anti-Syntaxin13 antibody was a kind gift from Dr. Rytis Prekeris (University of Colorado Denver, CO). FITC and Cy3 conjugated secondary antibodies to mouse or rabbit and Cy3 conjugated secondary antibody against rat (adsorbed against mouse serum) were purchased from Jackson Immunoresearch Laboratories (West Grove, PA). All other chemical reagents were from Fisher Scientific (Pittsburgh, PA), unless otherwise noted.
Cell cultures
Primary ventral mesencephalic cultures were obtained from E15 embryos of Sprague Dawley rats as described previously (Sorkina et al., 2006). Pregnant dams were purchased from Sasco Charles River (Wilmington, MA). Animal handling and procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use committee at the University of Colorado Health Sciences Center, Aurora, CO. Ventral mesencephalic cells (480,000 viable cells/cm2) were co-cultured with striatal cells (120,000 cells/cm2) on 12 mm glass coverslips pretreated with polyethylenimine. Cells were fed with 1 ml Neurobasal media supplemented with 10 ng/ml GDNF (glial derived neurotrophic factor) and FDU (2-fluorodeoxyuridine/uridine) the following day to prevent mitotic cell growth.
For postnatal mesencephalic cultures, neonatal pups aged P0–P3 were used as described previously (Mani and Ryan, 2009). Briefly, pups were euthanized and decapitated. Brains were quickly removed and rinsed in ice cold HBSS (Hank's buffered saline solution). Coronal sections were taken and the mesencephalic dopamine region was dissected and placed in ice cold HBSS until enzymatic degradation of connective tissue could be performed. Papain (Worthington; Lakewood, NJ) (20U/ml EBSS per 4–5 brains) with a final concentration of 0.5 μM kynurenic acid was used. Tissue was incubated in papain solution for 12 minutes at 37°C in a water bath with constant carbogen application to maintain O2/CO2 and pH levels. Tissue was carefully rinsed in previously warmed neurobasal A solution and then triturated in supplemented neurobasal A solution (10% heat inactivated fetal bovine solution, 1× B27, and glutamate (Invitrogen; Carlsbad, CA). Neurons were then filtered through a 0.45 um filter (BD Biosciences) and viable cell numbers were determined using the tryphan blue exclusion method. 140,000 cells were plated onto 12mm diameter glass coverslips coated with poly-d-lysine (Millipore) in 1 ml of supplemented neurobasal A. Neurons were allowed to settle and attach overnight and then treated with FDU the following day. 10 ng/ml GDNF was also added to support DA neuron survival.
Immunofluorescence microscopy
All staining of embryonic and postnatal neurons was carried out after 7–10 days in vitro (DIV). For the PMA treatment experiment, neurons were treated with 1 μM PMA (Sigma-Aldrich) or DMSO for 30 minutes at 37°C.
Neurons were fixed using freshly-prepared 4% paraformaldehyde (PFA) for 10 min followed by permeabilization with 0.1% saponin in PBS containing 0.5% BSA. Cultures were incubated with primary antibodies for 1 hr followed by washes with PBS and then incubated with secondary antibody for 30 min. Coverslips were mounted using Prolong gold with DAPI (Invitrogen). Syntaxin13, TfR and clathrin immunostaining was performed after permeabilization with 0.1% Triton X-100 for 3 minutes. Because DAT staining was highly inefficient in Triton X-100 permeabilized cells, sequential staining was carried out for co-localization studies where saponin-permeabilized neurons were first stained with the DAT antibody, then permeabilized with Triton X-100 and incubated with antibodies to clathrin/syntaxin13/TfR. Rab5 staining in axons of postnatal neurons was also more efficient with Triton X-100 permeabilization.
Fluorescent microscopy
Images were acquired through FITC, Cy3 and DAPI filter channels using Marianas™ epi-fluorescence workstation and SlideBook 4.2 software (Intelligent Imaging Innovation) as described previously (Sorkina et al., 2006). Z-stacks of 20–30 images were acquired at 300 nm intervals and deconvoluted using the constrained iteration method (with Gaussian noise smoothing). Images were acquired using a 63X oil immersion objective lens with a 2 × 2 binning mode. Under these imaging acquisition conditions, one pixel in the image corresponded to ~200 × 200 nm. To quantify the number of endocytic vesicles in different regions of the DA neuron, several 3-dimensional images were acquired for each of the endocytic markers. Areas positive for TH were selected as the area of interest using the “mask selection” feature of Slidebook. The number of vesicles positive for an endocytic marker within the masked TH positive region of DA neurons was calculated. The density of positive vesicles per 10 μmt3 volume of neuron was calculated using the Slidebook program. Images containing only the somatodendritic region or the axonal processes were used for calculations. The soma of the neurons was selected using a manual mask tool and the fluorescence intensity values were subtracted from those values obtained for the entire somatodendritic region to calculate the density of vesicles in dendrites. For the PMA-treated neurons, the amount of DAT fluorescent intensity co-localized with an endocytic marker fluorescent intensity was calculated using SlideBook statistical module as described (Huang et al., 2006). These values were presented as percent of DAT colocalized with the marker of total DAT intensity in a particular region of DA neurons. The data were collected from at least three different sets of DA neuronal cultures and obtained on different days of the culture.
Subcellular fractionation
Adult male Sprague-Dawley rats were used for a modified method of preparation of synaptosomes as described previously (Hoover et al., 2007). Briefly, following decapitation, striatum was dissected out and homogenized in PBS containing 0.32 M sucrose and 20 mM HEPES. The homogenate was centrifuged at 1000 ×g for 10 min and the pellet discarded. The supernatant was spun at 12,500 ×g for 20 min. The resulting synaptosomal pellet was fractionated using differential fractionation (Lim et al., 2001). Synaptosomes were lysed in hypotonic media on ice for 45 min and centrifuged at 2000 × g for 20 min. The supernatant was sequentially centrifuged at 32,000 × g for 1 hr and 100,000 × g for 2 hrs to obtain P2 and P3 pellets, respectively. The remaining supernatant was termed S3. All the above steps of centrifugation were carried out using a TLA100.4 Beckman rotor. Protein concentrations of all the fractions were measured using Bradford assay and equal amounts of total protein were loaded on a 7.5% SDS PAGE gel and immunoblotted for specific antigens. These experiments were repeated at least 3 times.
Sucrose gradients were used for fractionation of membranes of striatal synaptosomes as described (Ferguson et al., 2003). Briefly, synaptosomes were prepared using the above method and lysed using hypotonic shock. The resulting vesicles were layered on a 9 ml 50–1000 mM sucrose gradient and centrifuged in a SW40Ti Beckman rotor at 65,000 × g for 3 hrs. After centrifugation, 10 equal fractions were collected from the top of the gradient, and protein precipitation was performed using 6% trichloroacetic acid (TCA) and 0.02% deoxycholate. Total protein precipitated from each fraction was loaded on a 7.5% SDS PAGE gel and resolved. Fractionation using sucrose gradients were carried out in at least 3 independent experiments.
Supplementary Material
Acknowledgments
We thank Dr. Prekeris for a kind gift of antibodies. This work was supported by NIH/NIDA grant DA014204 (A.S. and A. R.) and NIAAA Postdoctoral training grant (D.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Joh TH, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in rat nucleus accumbens. J Comp Neurol. 1984;227:92–103. doi: 10.1002/cne.902270110. [DOI] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- de Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, Dotti CG. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Deken SL, Wang D, Quick MW. Plasma membrane GABA transporters reside on distinct vesicles and undergo rapid regulated recycling. J Neurosci. 2003;23:1563–1568. doi: 10.1523/JNEUROSCI.23-05-01563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Bosler O, Doucet G. Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: a quantitative autoradiographic and immunocytochemical analysis. J Comp Neurol. 1996;375:167–186. doi: 10.1002/(SICI)1096-9861(19961111)375:2<167::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Egana LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, Pena K, Quiroz M, Hong WC, Dorostkar MM, Janz R, Sitte HH, Torres GE. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci. 2009;29:4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Bjorn-Yoshimoto WE, Jorgensen TN, Newman AH, Gether U. Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons. J Biol Chem. 2010 doi: 10.1074/jbc.M110.131003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Rasmussen SG, Rasmussen TN, Vaegter CB, Cha JH, Zou MF, Newman AH, Gether U. Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J Neurosci. 2009;29:6794–6808. doi: 10.1523/JNEUROSCI.4177-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci. 2003;23:9697–9709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Caron MG. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psychiatry. 1999;46:303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Everett CV, Sorkin A, Zahniser NR. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem. 2007;101:1258–1271. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J Neurosci. 1996;16:5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SN, Bonzelius F, Low SH, Wille H, Weimbs T, Herman GA. Identification of discrete classes of endosome-derived small vesicles as a major cellular pool for recycling membrane proteins. Mol Biol Cell. 2001;12:981–995. doi: 10.1091/mbc.12.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani M, Ryan TA. Live imaging of synaptic vesicle release and retrieval in dopaminergic neurons. Front Neural Circuits. 2009;3:3. doi: 10.3389/neuro.04.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Dionne KR, Sorkina T, Sorkin A. Three ubiquitin conjugation sites in the amino terminus of the dopamine transporter mediate protein kinase C-dependent endocytosis of the transporter. Mol Biol Cell. 2007;18:313–323. doi: 10.1091/mbc.E06-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Mura A, Jackson D, Manley MS, Young SJ, Groves PM. Aromatic L-amino acid decarboxylase immunoreactive cells in the rat striatum: a possible site for the conversion of exogenous L-DOPA to dopamine. Brain Res. 1995;704:51–60. doi: 10.1016/0006-8993(95)01104-8. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Pohorille A, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter: comparative ultrastructure of dopaminergic axons in limbic and motor compartments of the nucleus accumbens. J Neurosci. 1997a;17:6899–6907. doi: 10.1523/JNEUROSCI.17-18-06899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Immunogold localization of the dopamine transporter: an ultrastructural study of the rat ventral tegmental area. J Neurosci. 1997b;17:5255–5262. doi: 10.1523/JNEUROSCI.17-14-05255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Beckley SC, Joh TH, Reis DJ. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225:373–385. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Joh TH, Field PM, Becker CG, Reis DJ. Cellular localization of tyrosine hydroxylase by immunohistochemistry. J Histochem Cytochem. 1975;23:1–12. doi: 10.1177/23.1.234988. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJ, Wang YT, Niznik HB. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26:8195–8205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkina T, Richards TL, Rao A, Zahniser NR, Sorkin A. Negative regulation of dopamine transporter endocytosis by membrane-proximal N-terminal residues. J Neurosci. 2009;29:1361–1374. doi: 10.1523/JNEUROSCI.3250-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro Y, Sugimoto T, Hattori T, Uemura Y, Nagatsu I, Kikuchi H, Mizuno N. Tyrosine hydroxylase-like immunoreactive neurons in the striatum of the rat. Neurosci Lett. 1989;97:6–10. doi: 10.1016/0304-3940(89)90130-4. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Vandenbergh DJ, Miner LL. Knockout mice and dirty drugs. Drug addiction. Curr Biol. 1996;6:935–936. doi: 10.1016/s0960-9822(02)00630-9. [DOI] [PubMed] [Google Scholar]
- Wilson JM, de Hoop M, Zorzi N, Toh BH, Dotti CG, Parton RG. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol Biol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics. 2009;72:46–55. doi: 10.1016/j.jprot.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Semin Cell Dev Biol. 2009;20:411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR. Activation of protein kinase C inhibits uptake, currents and binding associated with the human dopamine transporter expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;282:1358–1365. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.