Abstract
Mongolian gerbils and Wistar rats were inoculated orally with 240 and 2,500 Toxocara cati embryonated eggs, respectively, to evaluate the larval recovery in different tissues and organs, such as the liver, lungs, heart, kidney, and skeletal muscles after 5, 30, 49, 70, and 92 days post-infection (PI). Larval recovery rates were 1.7-30.0% in Mongolian gerbils on days 5-92 PI and 0.2-3.8% in rats on the same days. These results indicate that Mongolian gerbils and Wistar rats are suitable experimental paratenic hosts for the study of neurological toxocariasis as well as visceral toxocariasis.
Keywords: Toxocara cati, Mongolian gerbil, Wistar rat, experimental infection
Toxocara cati is the common round worm of cats and its second stage larvae are possible causes of visceral larva migrans (VLM) or ocular larvae migrans (OLM) [1]. Human infection can occur by accidental ingestion of embryonated eggs of Toxocara species. The embryonated Toxocara eggs, when fed, hatch in the gut contents of human and the larvae migrate to the soft tissue of the body such as liver, heart, lungs and other organs [2,3].
Experimentally, mice, rats, monkeys, golden hamsters, Japanese quail pigs, rabbits, chicken, and gerbils were infected with Toxocara canis and encapsulated larvae were found in the tissues of internal organs of these animals. In the experiments, however, the rate of larvae recovery was not determined in some animals [4-13].
Several studies have reported that cats can be infected by the ingestion of T. cati embryonated eggs with contaminated soil or by eating larvae within paratenic hosts, including birds and small rodents in their tissue. Transmammary migration of larvae is another means of transmission in cats [14-16]. There are few little findings on the location of T. cati larvae in mentioned animals as causative agents of visceral larva migrans or ocular larva migrans [14,17-20].
Information on behavioral changes of T. cati larvae in rodents is scanty. Dubey [17] showed larval migration patterns of T. cati in mice. In other previous studies, it was proved that Mongolian gerbils could be a paratenic host for T. cati, which may result in ocular larva migrans and neurotoxocariasis [18,21]. The present study presents further information on the experimental infection of Mongolian gerbils and Wistar rats with T. cati and larval recovery at different times of infection.
Ten Mongolian gerbils, Meriones unguiculatus (1-month-old males), and 10 Wistar rats (1-month-old males) were divided into 2 groups. The animals were maintained under standard conditions, in an environment with controlled temperature and humidity. Each animal in the experimental groups was intubated with approximately 240 and 2,500 embryonated T. cati eggs, respectively [18,21,22]. The eggs were derived from adult female worms and were embryonated according to our previous paper [23]. In brief, adult T. cati worms were collected from the intestines of infected stray cats. T. cati eggs were isolated from uteri of female worms and were decorticated by sodium hypochlorite (7% w/v) and embryonated in 2.5% formalin/ringer solution while incubated in an atmosphere of 5% CO2 in 37℃ for 3 weeks. The inoculated animals were euthanized, 2 from each group, on days 5, 30, 49, 70, and 92 post-infection (PI) and their different organs were carefully inspected for larvae. The visceral organs (liver, heart, lungs, kidneys, and brain) and skeletal muscles of the infected animals were fragmented with pointed forceps and examined by dissecting microscope. Thereafter, the tissues were put into digestive solution (pepsin, 5 g; Hcl 37%, 10 ml in 1,000 ml/water) and kept overnight at 37℃ to recover the remaining larvae. Sedimental liquid was poured into a centrifuge tube and centrifuged for 2 min at 1,500 rpm, 2 ml sediments were collected thoroughly mixed, and 0.1 samples were viewed for larvae counts [24].
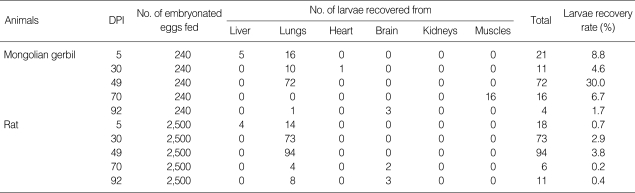
Larvae were recovered in organs of the experimentally infected animals on different days PI (Fig. 1). The numbers of larvae recovered from the experimentally infected animals are shown in Table 1. Larval recovery rates in Mongolian gerbils were 1.7-30.0% (15.9% on average) on days 5-92 PI. Similarly, the average rate of larval recovery in rats was 2.0% (0.2-3.8%). At day 5 PI, all larval recovery was observed in the liver and the lungs whether they were still alive. In the positive animals, many larvae were found in the lungs at day 49 PI, although at day 30 PI, a few were present in the lungs.
Fig. 1.
A larva of Toxocara cati in the brain of an experimentally infected animal.
Table 1.
Experimental infection of Mongolian gerbil and Wistar rat with Toxocara cati embryonated eggs
DPI, Days Post Infection.
Recently, using a dose of 1,000 T. cati embryonated eggs, Cardillo et al. [19] obtained a larger concentration of larvae in the liver by the 6th day PI. On the other hand, analyzing T. cati larval migration in a previous study showed a higher concentration of larvae in the lungs on day 60 after inoculation [20]. In an earlier report, after analyzing T. cati larvae migration in mice (C57BL6), it was stated that higher concentration in the liver, lungs, and kidney was found at day 5 PI, although a study noted encapsulated larvae in the liver tissue of experimentally infected chicken at day 14 PI [3,25]. It seems that the transmission of larvae was from the intestine to the liver and lungs then to the heart, kidneys, muscles, and brain. According to a previous study, during the first 24 hr, the hatched T. cati larvae in the intestine migrated to the liver and lungs and they presented in other organs, such as muscles [17]. Larvae were recovered from muscles of Mongolian gerbils on day 70 PI. The results suggest a likeness to that observed by Havasiva et al. [13].
In the present study, a more detailed distribution of larvae in the brain was determined. After day 70 PI, an increase in larval recovery from the brain of animals was found, lasting up to the 92th day of observation. Hrckova et al. [25] reported that the most of larvae were recovered from mice on day 84 PI. Lauthenslager et al. [27] showed that T. cati larvae migrated to the brain without getting together and most larvae obtained from rabbits on day 40 PI, when embryonated eggs were fed. Subsequently, among infective inoculums of 500 embryonated eggs to mice (C57BL/J), 22.8% of the larvae were recovered [13], so that 1.08% of the larvae migrated to the brain. Prokopic and Figallova [27] obtained a persistent recovery of larvae between days 2 and 28 PI and Hrckova et al. [25] between days 2 and 84 PI. Similar to that already observed in mice, larvae in Mongolian gerbils and rats were restricted to the brain, whereas in those animals larvae were prominent in the liver, lungs, and the muscles [8,28].
In conclusion, the present study is the first report of experimental infection of Mongolian gerbils with embryonated eggs of T. cati that showed persistence of the larvae in the brain for at least 92 days PI. Moreover, the high recovery rate of larvae in experimental infected Mongolian gerbils and Wistar rats suggests that they are suitable experimental paratenic hosts for T. cati. In addition, the experimental infection of Mongolian gerbils using T. cati is though to be very useful in studying migration patterns and pathogenesis (visceral larva migrans) in man and animals.
ACKNOWLEDGEMENTS
We gratefully acknowledge Dr. Nobuaki Akao for his valuable suggestions during the course of this investigation, Mr. Sakura of the Department of Parasitology at Kobe University Graduate School of Health Science for technical supporting this work.
References
- 1.Dubinsky P. New approaches to control larval toxocariasis. Helminthologia. 1999;36:159–165. [Google Scholar]
- 2.Schantz PM. Toxocara larva migrans now. Am J Trop Med Hyg. 1989;41:21–34. doi: 10.4269/ajtmh.1989.41.21. [DOI] [PubMed] [Google Scholar]
- 3.Azizi S, Oryan A, Sadjjadi SM, Zibaei M. Histopathologic change and larval recovery of Toxocara cati in experimentally infected chickens. Parasitol Res. 2007;102:47–52. doi: 10.1007/s00436-007-0722-5. [DOI] [PubMed] [Google Scholar]
- 4.Olson LJ, Rose JE. Effect of Toxocara canis infection on the ability of white rats to solve maze problems. Exp Parasitol. 1966;19:77–84. doi: 10.1016/0014-4894(66)90055-5. [DOI] [PubMed] [Google Scholar]
- 5.Tomimura T, Yokota M, Takiguch H. Experimental larva migrans in monkeys. 1. Clinical, haematological, biochemical, and gross pathological observation on monkeys inoculated with embryonated eggs of the dog ascarid, Toxocara canis. Nippon Juigaku Zasshi. 1976;38:533–548. doi: 10.1292/jvms1939.38.533. [DOI] [PubMed] [Google Scholar]
- 6.Dunsmore JD, Thompson RCA, Bates IA. The accumulation of Toxocara canis larvae in the brains of mice. Int J Parasitol. 1983;13:517–521. doi: 10.1016/s0020-7519(83)80017-4. [DOI] [PubMed] [Google Scholar]
- 7.Glickman LT, Summer BA. Experimental Toxocara canis infection in cynomolgus macaques (Macaca fascicularis) Am J Vet Res. 1983;44:2347–2354. [PubMed] [Google Scholar]
- 8.Abo-Shehada MN, Herbert IV. The migration of larval Toxocara canis in mice. Vet Parasitol. 1984;17:75–83. doi: 10.1016/0304-4017(84)90066-9. [DOI] [PubMed] [Google Scholar]
- 9.Agnithotri RK, Bhatia BB, Kumar D. Visceral larva migrans. 1. Migratory behavior of Toxocara canis larvae in golden hamster and chicken. Indian J Anim Sci. 1987;57:853–855. [Google Scholar]
- 10.Nakamura S, Sotoyama T, Hayasaka S, Kameyama Y, Maruyama S, Katsube Y. Parasitism of Toxocara canis larvae in Japanse quails by inoculation of the ascarid eggs. J Vet Med Sci. 1991;53:865–872. doi: 10.1292/jvms.53.865. [DOI] [PubMed] [Google Scholar]
- 11.Buijs J, Egbers MW, Nijkamp FP. Toxocara canis induced airway eosinophilia and tracheal hyporeactivity in guinea pigs and mice. Eur J Pharmacol. 1995;293:207–215. doi: 10.1016/0926-6917(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama S, Nino T, Yamamoto K, Katsube Y. Parasitism of Toxocara canis larvae in chickens inoculated with the ascarid eggs. J Vet Med Sci. 1994;56:139–141. doi: 10.1292/jvms.56.139. [DOI] [PubMed] [Google Scholar]
- 13.Havasiova-Reiterova K, Tomasovicova O, Dubinsky P. Effect of various doses of infective Toxocara canis and Toxocara cati eggs on the humoral response and distribution of larvae in mice. Parasitol Res. 1995;81:13–17. doi: 10.1007/BF00932411. [DOI] [PubMed] [Google Scholar]
- 14.Sprent JF. The life history and development of Toxocara cati (Schrank 1788) in the domestic cat. Parasitology. 1956;46:54–78. [PubMed] [Google Scholar]
- 15.Swerczek TW, Nielsen SW, Helmboldt CF. Transmammary passage of Toxocara cati in the cat. Am J Vet Res. 1971;32:89–92. [PubMed] [Google Scholar]
- 16.Coati N, Schnieder T, Epe C. Vertical transmission of Toxocara cati Schrank 1788 (Anisakidae) in the cat. Parasitol Res. 2004;92:142–146. doi: 10.1007/s00436-003-1019-y. [DOI] [PubMed] [Google Scholar]
- 17.Dubey JP. Migration of Toxocara cati larvae in mice. Trop Geogr Med. 1968;20:172–176. [PubMed] [Google Scholar]
- 18.Akao N, Takayanagi TH, Suzuki R, Tsukidate S, Fujita K. Ocular larva migrans caused by Toxocara cati in Mongolian gerbils and a comparison of ophthalmologic findings with those produced by T. canis. J Parasitol. 2000;86:1133–1135. doi: 10.1645/0022-3395(2000)086[1133:OLMCBT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Cardillo N, Rosa A, Ribicich M, Lopez C, Sommerfelt I. Experimental infection with Toxocara cati in BALB/c mice, migratory behaviour and pathological changes. Zoonoses Public Health. 2009;56:198–205. doi: 10.1111/j.1863-2378.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 20.Santos SV, Lescano SZ, Castro JM, Chieffi PP. Larval recovery of Toxocara cati in experimentally infected Rattus norvegicus and analysis of the rat as potential reservoir for this ascarid. Mem Inst Oswaldo Cruz. 2009;104:933–934. doi: 10.1590/s0074-02762009000600020. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi E, Akao N, Fujita K. Evidence for the involvement of the optic nerve as a migration route for larvae in ocular toxocariasis of Mongolian gerbils. J Helminthol. 2003;77:311–315. doi: 10.1079/joh2003186. [DOI] [PubMed] [Google Scholar]
- 22.Lescano SA, Queiroz ML, Chieffi PP. Larval recovery of Toxocara canis in organs and tissues of experimentally infected Rattus norvegicus. Mem Inst Oswaldo Cruz. 2004;99:627–628. doi: 10.1590/s0074-02762004000600016. [DOI] [PubMed] [Google Scholar]
- 23.Zibaei M, Sadjjadi SM, Jahadi-Hosseini SH, Sarkari B. A method for accelerating the maturation of Toxocara cati eggs. Iranian J Parasitol. 2007;2:39–42. [Google Scholar]
- 24.Xi WG, Jin LZ. A novel method for the recovery of Toxocara canis in mice. J Helminthol. 1998;72:183–184. doi: 10.1017/s0022149x00016382. [DOI] [PubMed] [Google Scholar]
- 25.Hrckova G, Velebny S, Tomasovicova O, Medvedova M, Pajersky A. Pathological changes in mice infected with Toxocara cati following administration of fenbendazole and glucan. Acta Parasitol. 2001;46:313–320. [Google Scholar]
- 26.Lautenslager JP. Toxocara visceral larva migrans in rabbits. Diss Abstr Int. 1972;32:4705. [Google Scholar]
- 27.Prokopic J, Figallova V. Migration of some roundworm species in experimentally infected white mice. Folia Parasitol. 1982;29:309–313. [PubMed] [Google Scholar]
- 28.Bardón R, Cuellar C, Guillen JL. Larval distribution of Toxocara canis in BALB/c mice at nine weeks and one year post-inoculation. J Helminthol. 1994;68:359–360. doi: 10.1017/s0022149x00001644. [DOI] [PubMed] [Google Scholar]