Abstract
Predation is a major selective force for the evolution of behavioural characteristics of prey. Predation among consumers competing for food is termed intraguild predation (IGP). From the perspective of individual prey, IGP differs from classical predation in the likelihood of occurrence because IG prey is usually more rarely encountered and less profitable because it is more difficult to handle than classical prey. It is not known whether IGP is a sufficiently strong force to evolve interspecific threat sensitivity in antipredation behaviours, as is known from classical predation, and if so whether such behaviours are innate or learned. We examined interspecific threat sensitivity in antipredation in a guild of predatory mite species differing in adaptation to the shared spider mite prey (i.e. Phytoseiulus persimilis, Neoseiulus californicus and Amblyseius andersoni). We first ranked the players in this guild according to the IGP risk posed to each other: A. andersoni was the strongest IG predator; P. persimilis was the weakest. Then, we assessed the influence of relative IGP risk and experience on maternal strategies to reduce offspring IGP risk: A. andersoni was insensitive to IGP risk. Threat sensitivity in oviposition site selection was induced by experience in P. persimilis but occurred independently of experience in N. californicus. Irrespective of experience, P. persimilis laid fewer eggs in choice situations with the high- rather than low-risk IG predator. Our study suggests that, similar to classical predation, IGP may select for sophisticated innate and learned interspecific threat-sensitive antipredation responses. We argue that such responses may promote the coexistence of IG predators and prey.
Keywords: Amblyseius andersoni, antipredation behaviour, innate predator recognition, learned predator recognition, learning, Neoseiulus californicus, oviposition habitat selection, Phytoseiulus persimilis, predator–predator interaction
Predation risk is a major selective force for the evolution of behavioural characteristics of prey (Lima & Dill 1990; Kats & Dill 1998). During their life, most prey species are faced with multiple predator species posing different levels of predation risk (Sih et al. 1998). Additionally, there can be large temporal and spatial variation in predator species composition in a given predator–prey community (Lima & Dill 1990; Lima & Bednekoff 1999). Consequently, predation risk is a highly variable component in the life of prey. Prey that overreacts by responding to each predator encounter irrespective of the risk posed by the predator would incur a fitness decrease, because antipredation behaviour is commonly traded off against foraging and/or mating and/or reproduction. Conversely, underestimation of predation risk may have dramatic consequences for prey (its death) or lead to a fitness decrease of prey by lowering its reproductive success. Hence, prey should be able to assess the magnitude of predator threat and adjust its behaviour accordingly (Sih 1982, 1986; Helfman 1989). The degree of predation risk may be determined by numerous interrelated but hierarchically structured factors such as predator species, sex or life stage. On top of this hierarchy is predator species recognition, because it allows discrimination of predatory from nonpredatory species and high-risk from low-risk species. Interspecific threat-sensitive prey responses are well documented in both aquatic (Kiesecker et al. 1996; Botham et al. 2008) and terrestrial (Stapley 2003; Edelaar & Wright 2006; Blumstein et al. 2008; Monclus et al. 2009) communities in classical predator–prey interactions.
Predation among species competing for shared resources is called intraguild predation (IGP; Polis et al. 1989). IGP differs from classical predation in various aspects. Like classical predation, intraguild (IG) predators may gain energy by food intake, but unlike classical predation they also gain from eliminating a competitor and potential predator of themselves and/or their offspring. For many predators IG prey has a lower profitability, defined as energy content divided by handling time (e.g. Charnov 1976), than extraguild prey, as measured in predator survival, growth, development or oviposition (ladybirds: Kagata & Katayama 2006; mirid bugs: Provost et al. 2006; predatory mites: Schausberger & Croft 2000a; spiders: Matsumura et al. 2004). Although the nutrient composition of IG prey per se seems favourable for IG predators (Denno & Fagan 2003; Matsumura et al. 2004), subduing, capturing and killing IG prey is energetically costly because IG prey are predators themselves and in mutual IGP may counterattack their predators. In extreme cases, the energetic costs of IGP may even exceed the benefits obtained (predatory mites: Lawson-Balagbo et al. 2008). In addition, true predators (carnivores and omnivores), and consequently potential IG prey, are usually less abundant than classical prey such as herbivores (Begon et al. 1996), reducing the predator–prey encounter frequency and making predator adaptations that help exploit IG prey more efficiently less likely than adaptations that improve exploitation of classical prey. Hence, although omnipresent in natural and artificial food webs (Polis et al. 1989; Arim & Marquet 2004) IGP events should be rarer than classical predation events. It is not known whether IGP is enough of a selective force to evolve interspecific threat-sensitive antipredation behaviours in IG prey, as is common in classical predation.
A precondition for displaying effective threat-sensitive antipredation responses is the ability to recognize a given predator species by direct and/or indirect cues. In general, recognition of predator species can be innate or learned, or a combination of both, ranging from strictly innate recognition unaffected by experience (e.g. tadpoles: Gallie et al. 2001) to innate recognition modifiable by experience (e.g. fish: Hawkins et al. 2008) to recognition only after experience (e.g. rodents: Kindermann et al. 2009). However, most studies on interspecific threat-sensitive antipredation behaviour do not allow discrimination between innate and learned predator recognition because of studying wild animals in the field (e.g. Edelaar & Wright 2006; Blumstein et al. 2008) or using either wild-caught or experienced experimental animals (Kiesecker et al. 1996; Stapley 2003; Botham et al. 2008; Monclus et al. 2009). Unambiguous evidence for learned threat sensitivity is rare and mostly relates to intraspecific threat sensitivity in aquatic or semiaquatic animals such as larval mosquitoes, tadpoles, fishes and water striders in classical predator–prey interactions (Ferrari et al. 2005, 2008; Ferrari & Chivers 2009; Hirayama & Kasuya 2009). Evidence for learned interspecific threat sensitivity in IGP and terrestrial animals is lacking.
We studied the influence of predation risk and experience on antipredation behaviour within a guild of three predatory mite species: Phytoseiulus persimilis, Neoseiulus californicus and Amblyseius andersoni (Acari: Phytoseiidae). All three species are plant-inhabiting predators of phytophagous mites (e.g. McMurtry & Croft 1997; De Moraes et al. 2004). They naturally co-occur in the Mediterranean basin (De Moraes et al. 2004; A. Walzer, personal observation), presumably share a long coevolutionary history and interact with each other via competition for shared prey, such as the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae), and mutual IGP. They differ in diet breadth and adaptation to, and strength in competition for, spider mites. Phytoseiulus persimilis is a highly specialized predator of spider mites and the strongest competitor; N. californicus is a generalist predator with a ranked preference for spider mites and an intermediate competitor while A. andersoni is a generalist predator poorly adapted to utilize spider mites as prey and therefore the weakest competitor for spider mites (McMurtry & Croft 1997). Regarding the propensity to engage in IGP, the ranking is reversed, with A. andersoni being a highly aggressive IG predator, followed by the intermediate N. californicus. Phytoseiulus persimilis is a comparably weak IG predator, only occasionally preying on other predatory mites (Walzer & Schausberger 1999; Schausberger & Croft 2000b). Thus, owing to a joint natural history and presumable differences in the IGP risk posed to each other, the three predatory mites are perfectly suitable animals to test for threat-sensitive anti-IGP behaviours.
As with many other animals (Polis et al. 1989), IGP among phytoseiid mites is mutual but asymmetric with respect to size. Small/younger juveniles are usually preyed upon by larger/older juveniles and/or adult females, whereas adult females and eggs are relatively invulnerable to IGP (Croft et al. 1996; Schausberger 1997; Walzer & Schausberger 1999; Schausberger & Croft 2000b). The larva is the smallest and least mobile juvenile stage and most in danger of falling victim to larger IG predators (Walzer & Schausberger 1999; Schausberger & Croft 2000b). The IGP risk for larvae may be reduced by antipredation behaviours of the larvae themselves (e.g. Schausberger 2003) or by maternal investment in reducing larval predation risk (Faraji et al. 2001; Walzer et al. 2006). Here, we focused on the latter. Foraging gravid phytoseiid females facing IG predators have several possibilities for decreasing the predation risk of their offspring. They may avoid prey patches with IG predators and choose predator-free patches for oviposition (e.g. Walzer et al. 2006); they may kill potential IG predators of their offspring (e.g. Schausberger & Croft 2000b); or they may reduce/postpone oviposition in the presence of IG predators (e.g. Montserrat et al. 2007; Abad-Moyano et al. 2010a) and resume oviposition when conditions improve.
We investigated the influence of IGP risk and IG predator experience of IG prey on the above-mentioned three maternal strategies to reduce offspring IGP risk. In the first experiment, we assessed the relative risk that P. persimilis, N. californicus and A. andersoni posed to each other in IGP, allowing categorization of each species as a low- or high-risk IG predator of another species. In the second experiment, we scrutinized prey patch choice and oviposition behaviour of IG predator-naïve and predator-experienced females of P. persimilis, N. californicus and A. andersoni when confronted with low- and high-risk IG predators.
Methods
Species Origin and Rearing
Phytoseiulus persimilis, N. californicus and A. andersoni are indigenous, co-occurring species in Sicily (De Moraes et al. 2004). All three species were sampled from herbs and trees in the state of Trapani in 2007. About 20–30 specimens of each species were used to initiate populations reared in the laboratory. Rearing arenas consisted of plastic tiles resting on water-saturated foam cubes in plastic boxes half-filled with water. The edges of the tiles were covered with moist tissue paper to confine the predators to the rearing arenas. Cotton wool fibres under coverslips served as shelter and oviposition sites for A. andersoni. To prevent contamination of the predator populations, an adhesive (Raupenleim, Avenarius Agro, Wels, Austria) was applied to the rim of the plastic boxes and the boxes were placed in a tray containing water with dishwashing detergent. The predators were fed in 2–3-day intervals with T. urticae, reared on whole bean plants (Phaseolus vulgaris), by adding bean leaves infested with spider mites (for P. persimilis, N. californicus) or by brushing spider mites from infested leaves (for A. andersoni) onto arenas.
Categorization of IG Predators
In the first experiment, we assessed the IGP risk posed by adult females to larvae to categorize each species as a low- or high-risk predator of another species (Schausberger & Croft 2000b). We measured the attack probability and attack latency of IG predator females on IG prey larvae confined in closed acrylic cages. Each cage consisted of a cylindrical cell (15 mm in diameter and 3 mm in height) with a fine-mesh screen at the bottom and closed on the upper side with a microscope slide (Schausberger 1997). Treatments were all possible combinations of P. persimilis, A. andersoni and N. californicus as IG predators and prey. Gravid females randomly taken from the rearing units were singly placed into closed cages and starved for 12 h. Subsequently, a single heterospecific larva was added to each cage. Each female and each larva was used only once. Each cage was checked for the first successful attack of the predator female on larval prey (killed and sucked out) every 10–15 min for 6 h at the longest. Each treatment (predator–prey combination) was replicated 19–20 times.
Prey Patch Choice and Oviposition Behaviour
In the second experiment, we assessed prey patch choice and oviposition behaviour of IG predator-naïve and predator-experienced females of P. persimilis, N. californicus and A. andersoni given a choice between a spider mite patch with and without cues (traces of foraging females and their eggs) from a low- or high-risk IG predator. We used a full 3 (IG prey female species) × 2 (naïve and experienced) × 2 (cues of high- and low-risk IG predator) factorial design. Each choice situation was replicated 20–31 times.
Generating Naïve and Experienced Prey Females
To obtain naïve and experienced prey females for experiments, three groups of 13–15 eggs each of A. andersoni, N. californicus and P. persimilis were randomly taken from the rearing units and placed on separate leaf arenas for development. Each leaf arena consisted of a detached bean leaf (5 × 5 cm) placed upside down on a water-saturated foam cube in a plastic box half-filled with water. Water-saturated cellulose strips (1 cm height) at the edge of the leaf confined the arena and prevented the mites from escaping. For each species, one group of eggs was placed on a leaf with only spider mites (to be used as naïve IG prey females in experiments), and the second and third groups of eggs were placed on separate leaves with spider mites and either five low- or high-risk IG predator females, respectively (to be used as experienced IG prey females in experiments). The developmental progress of IG prey was observed and spider mite prey replenished daily to exclude food competition. The IG predator females mainly preyed on spider mites but also killed a few IG prey individuals and produced eggs. Mortality of IG prey was about 10–15% higher in rearing units with IG predators than without, but the set-up provided for random IGP (A. Walzer & P. Schausberger, unpublished data). Therefore, during development IG prey to be used as experienced females in experiments were exposed to all direct (predators, their eggs, chemical footprints and/or metabolic waste products) and indirect (killed conspecifics and spider mites) volatile and/or tactile chemosensory cues possibly indicating IG predator presence (Walzer et al. 2006; Montserrat et al. 2007). Gravid IG prey females appeared after 10 days and were then subjected to choice experiments.
Choice Experiments
Each experimental choice unit consisted of two similarly sized leaflets (5–7 cm2) taken from trifoliate bean leaves placed upside down on a foam cube in a plastic box (15 × 10 cm and 4 cm high) half-filled with water. The leaflets were connected by a wax bridge (1 × 0.5 cm; Walzer et al. 2006). One leaflet only harboured spider mites and the other harboured spider mites plus cues (traces of foraging females, such as metabolic waste products and/or chemical footprints, and their eggs) of either the low- or the high-risk IG predator. The set-up was designed to simulate a natural scenario in which a gravid female searches for a prey patch and oviposition site among leaves on a branch within a plant. During preparation of the prey patches before the choice experiment took place, we blocked the bridge with a strip of moist tissue paper. Prey patches were created by placing 30 juvenile and four to seven adult T. urticae females on each leaflet. After 24 h either no or five low-risk or five high-risk IG predator females were added and allowed to produce eggs. After a further 24 h the T. urticae females and the IG predator females were removed and spider mite densities (juveniles and eggs) adjusted, by adding or removing individuals using a brush, to identical predetermined levels on leaflets with and without IG predator cues. IG predator eggs were reduced to five per leaflet and their position was marked by a tiny watercolour dot on the leaf surface to ease identification of eggs produced by the experimental IG prey females. To account for species-specific prey stage preferences (eggs versus mobile prey; Blackwood et al. 2001) and prey needs (Vanas et al. 2006) we adjusted the density and composition of each spider mite patch to 60 eggs and 20 juveniles for P. persimilis, 30 eggs and 10 juveniles for N. californicus and 30 eggs and 30 juveniles for A. andersoni. Each patch allowed the IG prey female to reach the maximum oviposition rate during the 24 h experimental period and leave enough prey for her offspring to reach adulthood (Vanas et al. 2006). Therefore, differences in prey patch choice and/or oviposition behaviour of IG prey females can be delimited to the presence or absence of IG predator cues. Through the above-mentioned pre-experimental procedure, the presence of an IG predator in a given spider mite patch was indicated by traces left by the foraging and ovipositing IG predator (e.g. metabolic waste products and/or chemical footprints), eggs laid and killed spider mites (Grostal & Dicke 1999).
Before we ran the choice experiments, each experimental IG prey female was singly placed into a closed acrylic cage (described above) and starved for 12 h. Only females producing at least one egg during the starvation period were used for experiments. Subsequently, each female was singly released in the middle of the wax bridge and given a choice between a prey patch with only spider mites and a prey patch with spider mites and cues of either a low-risk or a high-risk IG predator (determined for each species in experiment 1). Each choice unit and each IG prey female was used only once. The position of the IG prey female was checked eight times during the experiment (immediately after release (first choice), and then after 1, 2, 3, 4, 5, 6 and 24 h). After 24 h we recorded eggs deposited in each prey patch and predation by the IG prey female on IG predator eggs.
Statistical Analyses
Statistical analyses were carried out for each species separately using SPSS 15.0.1 (SPSS, Chicago, IL, U.S.A.). In experiment 1, larval survival functions (combination of cumulative survival and survival time), used as indicators of the relative IGP risk posed by the other two species, were analysed by the Kaplan–Meier procedure and pairwise Breslow tests (Bühl 2008). In experiment 2, the influence of experience (naïve versus experienced) and predation risk (low versus high risk) on the residence frequency of IG prey females in prey patches with IG predator cues during the experiment (presence in the prey patch with IG predator cues out of eight observation points) was analysed using generalized linear models (GLM; counts of events; binomial distribution with logit link function; Bühl 2008). Within each choice situation, the numbers of eggs laid in the prey patch with and without predator cues were compared by Wilcoxon signed-ranks tests owing to non-normality of data. The influence of experience and predation risk on total egg production (eggs laid in both prey patches combined; log-transformed before analysis to meet variance homogeneity and improve normality) and the number of IG eggs preyed upon by the IG prey females were analysed using two-factorial (experience and predation risk) ANOVAs. The proportion of eggs laid by the IG prey females in prey patches with IG predator cues (eggs in patch with IG predator cues out of total egg production; females producing no eggs were removed from the analysis) were compared among treatments using GLMs (counts of events; binomial distribution with logit link function; Bühl 2008).
Results
Categorization of IG Predators
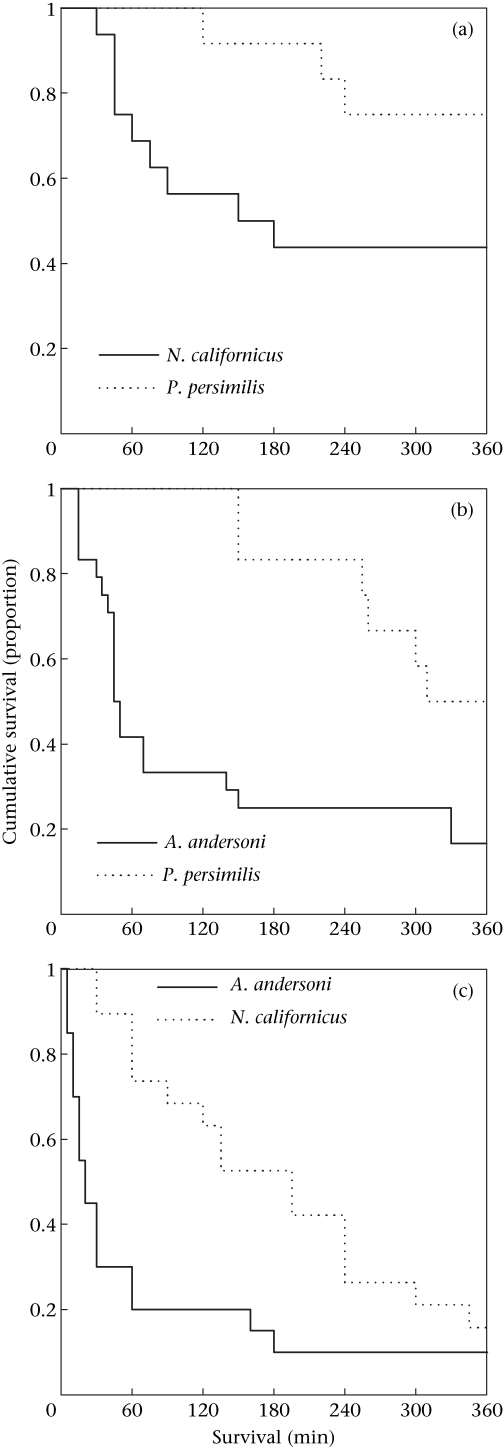
For each species, the survival functions of larvae differed significantly between the two IG predator species (Fig. 1; Breslow tests within Kaplan–Meier analyses). Based on these differences we categorized the two IG predators of a given species as low- and high-risk predators: for A. andersoni, low-risk P. persimilis and high-risk N. californicus (chi-square test: χ12 = 4.507, P = 0.034); for N. californicus, low-risk P. persimilis and high-risk A. andersoni (χ12 = 9.743, P = 0.002); for P. persimilis, low-risk N. californicus and high-risk A. andersoni (χ12 = 13.875, P < 0.001).
Figure 1.
Survival of singly caged IG prey larvae of (a) A. andersoni, (b) N. californicus and (c) P. persimilis when confronted with a high-risk (solid line) or low-risk (dotted line) IG predator female for 360 min.
Prey Patch Choice and Oviposition Behaviour
Amblyseius andersoni
Prey patch choice by A. andersoni females was influenced by experience but not by predation risk (Table 1). Experienced females resided in prey patches with IG predator cues less often than naïve females (Fig. 2).
Table 1.
Generalized linear model for the influence of predation risk (low versus high) and experience (naïve versus experienced) on prey patch choice (residence frequency in the prey patch with IG predator cues out of eight observation points) by IG prey females of A. andersoni, N. californicus and P. persimilis
| Species | Variables | df | Wald χ2 | P |
|---|---|---|---|---|
| Amblyseius andersoni | Experience | 1 | 14.083 | <0.001 |
| Predation risk | 1 | 0.413 | 0.091 | |
| Experience*predation risk | 1 | 0.246 | 0.620 | |
| Neoseiulus californicus | Experience | 1 | 4.080 | 0.043 |
| Predation risk | 1 | 0.792 | 0.373 | |
| Experience*predation risk | 1 | 5.043 | 0.015 | |
| Phytoseiulus persimilis | Experience | 1 | 2.415 | 0.120 |
| Predation risk | 1 | 4.087 | 0.043 | |
| Experience*predation risk | 1 | 8.025 | 0.005 | |
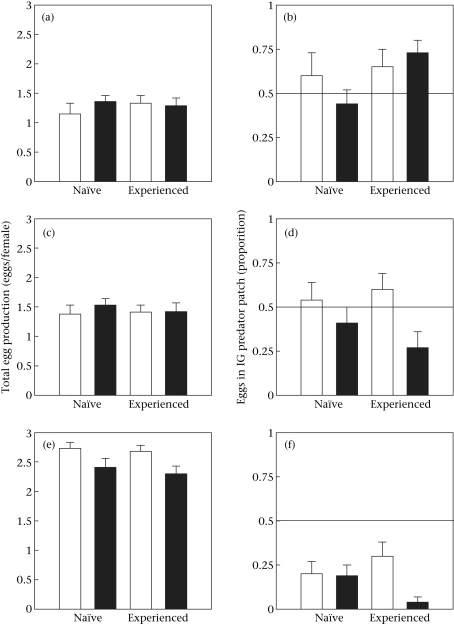
Figure 2.
The influence of experience and IGP risk on residence of (a) A. andersoni, (b) N. californicus and (c) P. persimilis females in the prey patches with spider mites and IG predator cues (eggs and traces such as metabolic waste products and/or chemical footprints of an IG predator female; mean proportion ± SE; calculated from eight observations during 24 h). Each female was given a choice between a prey patch with only spider mites and a prey patch with cues of a low-risk (white bars) or high-risk (black bars) IG predator.
Within choice situations, experienced females confronted with cues of the high-risk predator deposited more eggs in the patch with predator cues (Wilcoxon signed-ranks exact test: Z = −2.822, N = 28, P = 0.005). In the other choice situations the numbers of deposited eggs did not differ between patches with and without predator cues (P > 0.05; Fig. 3). Total egg production was not affected by experience, predation risk or the interaction of the two sources of variation (ANOVA experience: F1,96 = 0.159, P = 0.691; predation risk: F1,96 = 0.353, P = 0.554; experience*predation risk: F1,96 = 0.889, P = 0.348). The proportion of eggs laid in the prey patch with IG predator cues was marginally influenced by experience (GLM: Wald χ12 = 3.446, P = 0.063) but not by predation risk (Wald χ12 = 0.001, P = 0.995) or the interaction between experience and predation risk (Wald χ12 = 1.057, P = 0.304). Experienced females laid a slightly higher proportion of eggs in prey patches with IG predator cues than naïve females (Fig. 3).
Figure 3.
Total egg production (both patches combined) and proportion of eggs (mean ± SE) laid by (a, b) A. andersoni, (c, d) N. californicus and (e, f) P. persimilis in the prey patches with spider mites and IG predator cues (eggs and traces such as metabolic waste products and/or chemical footprints of an IG predator female). Each female was given a choice between a prey patch with only spider mites and a prey patch with spider mites and cues of a low-risk (white bars) or high-risk (black bars) IG predator. Horizontal lines indicate random choice for proportion of eggs laid (b, d, f).
The IGP rates of A. andersoni were not influenced by experience (ANOVA: F1,96 = 1.999, P = 0.161) and predation risk (F1,96 = 0.317, P = 0.575) as main effects but were influenced by the interaction of the two sources of variation (F1,96 = 3.955, P = 0.050). Naïve females killed similar numbers of eggs of the low- and high-risk IG predator (mean ± SE = 0.9 ± 0.3, N = 20 versus 1.2 ± 0.3, N = 25), whereas experienced females killed more eggs of the low- than of the high-risk IG predator (1.0 ± 0.2, N = 28 versus 0.4 ± 0.1, N = 28).
Neoseiulus californicus
The influence of experience on prey patch choice by N. californicus females was dependent on predation risk (Table 1, Fig. 2). The females were similarly distributed between prey patches with or without IG predator cues, except experienced females subjected to the choice situation with the high-risk IG predator A. andersoni, which were more often found in the prey patch with only spider mites (Table 1, Fig. 2).
Within choice situations, experienced females confronted with cues of the high-risk predator deposited more eggs in the prey patch with only spider mites (Wilcoxon signed-ranks exact test; Z = −2.658, N = 31, P = 0.008). In the other choice situations, the numbers of deposited eggs did not differ between patches with and without predator cues (P > 0.05; Fig. 3). Total egg production was unaffected by experience and predation risk (ANOVA: experience: F1,110 = 0.112, P = 0.738; predation risk: F1,110 = 0.349, P = 0.556; experience*predation risk: F1,110 = 0.253, P = 0.616; Fig. 3). Irrespective of experience, the proportion of eggs laid in prey patches with predator cues was higher in choice situations with the low-risk IG predator than in those with the high-risk IG predator (GLMs: experience: Wald χ12 = 0.121, P = 0.728; predation risk: Wald χ12 = 8.928, P = 0.003; experience*predation risk: Wald χ12 = 2.710, P = 0.100; Fig. 3).
The IGP rates of N. californicus were not influenced by experience (ANOVA: F1,111 = 0.002, P = 0.951) and predation risk (F1,111 = 0.612, P = 0.330) as main factors but were influenced by the interaction of these two sources of variation (F1,111 = 3.010, P = 0.032). Irrespective of predation risk, experienced females killed similar numbers of eggs of the low- and high-risk IG predator (mean ± SE = 0.6 ± 0.2, N = 27 versus 0.5 ± 0.1, N = 30), whereas naïve females killed more eggs of the high- than low-risk IG predator (0.8 ± 0.2, N = 27 versus 0.3 ± 0.1, N = 31).
Phytoseiulus persimilis
The influence of predation risk on prey patch choice by P. persimilis females was dependent on experience (Table 1, Fig. 2). Experienced females resided more often in the predator-free prey patch in choice situations with the high-risk IG predator compared with the other choice situations (Fig. 2).
Within each choice situation P. persimilis deposited more eggs in the prey patch without predator cues than in the prey patch with predator cues (Wilcoxon signed-ranks exact test: naïve female/harmless predator: Z = −3.106, N = 26, P = 0.002; naïve female/harmful predator: Z = −3.382, N = 22, P = 0.001; experienced female/harmless predator: Z = −2.519, N = 27, P = 0.012; experienced female/harmful predator: Z = −4.193, N = 23, P < 0.001; Fig. 3). Total egg production was affected by predation risk but not by experience (ANOVA: experience: F1,94 = 0.354, P = 0.533; predation risk: F1,94 = 7.533, P = 0.007; experience*predation risk: F1,94 = 0.045, P = 0.833). Irrespective of experience, P. persimilis females produced fewer eggs in choice situations with the high-risk IG predator than in choice situations with the low-risk IG predator (Fig. 3). Predation risk (GLM: Wald χ12 = 7.377, P = 0.007) but not experience (GLM: Wald χ12 = 2.647, P = 0.104) influenced the proportion of eggs laid by P. persimilis in prey patches with IG predator cues. However, the significant interaction between predation risk and experience (GLM: Wald χ12 = 6.554, P = 0.010) indicates that experience decreased the proportion of eggs laid in the patch with predator cues in choice situations with the high-risk IG predator but not in choice situations with the low-risk IG predator (Fig. 3).
The IGP rates of P. persimilis (mean ± SE = 0.11 ± 0.06, N = 26 versus 0.12 ± 0.06, N = 29 and 0.04 ± 0.04, N = 22 versus 0.09 ± 0.06, N = 23 eggs of the high- and low-risk IG predator for naïve and experienced females, respectively) were unaffected by experience and predation risk (ANOVA: experience: F1,94 = 0.599, P = 0.441; predation risk: F1,94 = 0.189, P = 0.665; experience*predation risk: F1,94 = 0.132, P = 0.718).
Discussion
Our study shows that IGP is a sufficiently strong force to select for interspecific threat sensitivity in antipredation behaviours. It documents innate and learned interspecific threat-sensitive anti-IGP responses and provides experimental evidence for learned threat-sensitive antipredation in a strictly terrestrial animal (e.g. Kats & Dill 1998). We observed three maternal strategies to reduce predation risk of offspring. These strategies are common in classical predator–prey interactions but are less well documented for IGP, that is, killing predators (for classical predation: Saito 1986; for IGP: Walzer & Schausberger 2009), decreasing and postponing oviposition (for classical predation: Skaloudova et al. 2007; for IGP: Montserrat et al. 2007) and oviposition site avoidance (for classical predation: Grostal & Dicke 1999; Murphy 2003; for IGP: Walzer et al. 2006). However, the strategies adopted, threat sensitivity and the influence of predator experience differed between species. First, IG prey females of all three species killed IG predator eggs. IG egg predation was most pronounced in A. andersoni and negligible in P. persimilis. Only naïve N. californicus females behaved in a threat-sensitive manner in IG egg predation. Second, both naïve and experienced P. persimilis females laid fewer eggs in the presence of the high-risk IG predator than in the presence of the low-risk IG predator, indicating innate threat sensitivity. Egg production by A. andersoni and N. californicus was unaffected by predation risk and experience. Third, both N. californicus and P. persimilis were threat sensitive in oviposition site selection. Both laid a lower proportion of eggs in prey patches with the high-risk predator than in those with the low-risk IG predator. However, threat sensitivity in oviposition site selection was induced by experience in P. persimilis but not in N. californicus.
Species-specific Threat Sensitivity
Amblyseius andersoni females were threat insensitive in prey patch selection and oviposition behaviour. A possible interpretation is that A. andersoni is not able to discriminate between low- and high-risk IG predators. More likely, the lack of threat sensitivity in A. andersoni was specific to the IGP risks posed by P. persimilis and N. californicus, respectively. The risks posed by P. persimilis and N. californicus to A. andersoni were lower and their difference smaller than those in the other IG predator–prey combinations. Therefore, it could be that the overall predation risk was too low and the difference between risks too small to trigger a threat-sensitive response in A. andersoni. Alternatively or additionally, experiment 1 indicates that larvae of A. andersoni are better able to escape from or defend themselves against IGP than are the larvae of N. californicus and P. persimilis (see also Zhang & Croft 1995). Experienced A. andersoni females preferred to deposit their eggs in prey patches with cues of the high-risk IG predator. At first glance such behaviour seems maladaptive. However, IG predator eggs are not only potential future predators and competitors of offspring but also an alternative prey for A. andersoni. Phytoseiid mites are a higher quality prey for A. andersoni than are T. urticae, allowing rapid juvenile development and sustained oviposition (Schausberger & Croft 2000a). Therefore, experience may have enhanced acceptance and utilization of IG predator eggs as food by A. andersoni females. Higher predation by experienced females on eggs of P. persimilis than N. californicus may be explained by differing nutritional quantity of single eggs. Eggs of P. persimilis are about one-third larger than those of N. californicus (Croft et al. 1999). Whether the eggs of N. californicus and P. persimilis also differ in nutritional quality is unknown.
Both N. californicus and P. persimilis females responded in a threat-sensitive manner in prey patch selection and oviposition behaviour. However, the relative contribution of innate and learned components to threat sensitivity differed between the two species. Only N. californicus experienced with the high-risk IG predator avoided residence in the patch with IG predator cues. By contrast, both naïve and experienced P. persimilis females avoided patches with IG predator cues, but only experienced females were threat sensitive in patch selection. In P. persimilis, oviposition site selection matched prey patch selection. Irrespective of predation risk and experience, P. persimilis laid more eggs in predator-free patches, but experience induced threat sensitivity and fine-tuned oviposition site selection. To our knowledge, only one previous study dealt with threat-sensitive oviposition site selection influenced by experience in a classical predator–prey interaction. Water striders, Aquarius paludum insularis learned to adjust the oviposition depth to the risk of egg parasitism (Hirayama & Kasuya 2009). The total number of eggs laid by P. persimilis was lower in choice situations with the high-risk IG predator than in those with the low-risk IG predator. Proximate explanations for decreased egg production in the presence of a high-risk IG predator include reduced feeding and/or egg retention (Montserrat et al. 2007; Abad-Moyano et al. 2010a). In N. californicus, oviposition site selection and prey patch selection did not exactly match. Experience induced threat sensitivity in prey patch selection, whereas threat sensitivity in oviposition site selection was innate and unmodified by experience. A likely explanation is that offspring are much more vulnerable to IGP than are the adult females themselves (Croft et al. 1996), rendering oviposition site selection a much stronger selective force than prey patch selection.
Threat-sensitivity and Mutual IGP Risk
The species-specific strategies to reduce IGP on offspring and their complexities and magnitudes reflect the species ranking in mutual predation (experiment 1; Schausberger & Croft 2000b). They further indicate that IGP, which contains elements of both predation and competition, and not merely competition for spider mites, has been the selective force driving the evolution of these behaviours. If competition for spider mites was the driving force, the weakest competitor A. andersoni would have had the strongest response, and the strongest competitor P. persimilis the least, respectively, to the presence of the other species. However, the opposite was true. The lack of threat-sensitive behaviours in A. andersoni may indicate that their juveniles are the least endangered IG prey within the guild studied. Moreover, killing potential IG predator eggs by A. andersoni IG prey females both yields nutritional benefits and relaxes offspring predation and competition risks. Cues from killed conspecifics may prevent further IG predators from entering prey patches, as for example shown for the predatory mite Iphiseius degenerans (Faraji et al. 2001). Neoseiulus californicus females were threat sensitive in IGP and oviposition site selection. However, the latter was only evident in prey patches with the high-risk IG predator A. andersoni, indicating that P. persimilis represents a negligible risk for N. californicus offspring. Phytoseiulus persimilis is the most vulnerable to IGP within the guild studied. The females responded to both IG predators under all circumstances, indicating that both predators constitute a considerable risk for their offspring. Experience drastically increased threat sensitivity of P. persimilis, which was reflected in almost complete oviposition avoidance in prey patches with cues of the high-risk IG predator (only two of 51 eggs were placed in the patch with IG predator cues).
Implications for Population and Community Dynamics
Threat sensitivity in IGP may have important implications for population and community dynamics. For example, graded oviposition avoidance may result in graded direct trait-mediated IG interactions (Luttbeg & Kerby 2005; Preisser et al. 2005; Abad-Moyano et al. 2010b). Oviposition site selection affects spatial distribution of IG prey independent of the level of IGP risk, which in turn may trigger indirect trait-mediated effects on the shared prey (e.g. Werner & Peacor 2003; Walzer et al. 2009). The ability to perform threat-sensitive anti-IGP behaviours should have stabilizing effects on the coexistence of IG predators and prey. Currently, the general theoretical prediction deduced from IGP models is that coexistence of IG predators and prey within communities at ecological timescales is unlikely at high productivity levels (Janssen et al. 2006), but there is sparse empirical support for this assumption (e.g. Amarasekare 2008). Threat sensitivity in prey patch and oviposition site selection could be a mechanism promoting the coexistence of IG predators and prey (Heithaus 2001; Amarasekare 2008).
Acknowledgments
This work was funded by the Austrian Science Fund (project P19824-B17). We thank D. Hoffmann, S. Peneder and M. Strodl for comments on the manuscript. A.W. dedicates this manuscript to his wife Masha.
MS. number: 10-00259R
References
- Abad-Moyano R., Urbaneja A., Schausberger P. Intraguild interactions between Euseius stipulatus and the candidate biological control agents of Tetranychus urticae in Spanish clementine orchards: Phytoseiulus persimilis and Neoseiulus californicus. Experimental and Applied Acarology. 2010;50:23–34. doi: 10.1007/s10493-009-9278-7. [DOI] [PubMed] [Google Scholar]
- Abad-Moyano R., Urbaneja A., Hoffmann D., Schausberger P. Effects of Euseius stipulatus on establishment and efficacy in spider mite suppression of Neoseiulus californicus and Phytoseiulus persimilis in clementine. Experimental and Applied Acarology. 2010;50:329–341. doi: 10.1007/s10493-009-9320-9. [DOI] [PubMed] [Google Scholar]
- Amarasekare P. Coexistence of intraguild predators and prey in resource-rich environments. Ecology. 2008;89:2786–2797. doi: 10.1890/07-1508.1. [DOI] [PubMed] [Google Scholar]
- Arim M., Marquet P.A. Intraguild predation: a widespread interaction related to species biology. Ecology Letters. 2004;7:557–564. [Google Scholar]
- Begon M., Harper J.L., Townsend C.R. Blackwell Science; Oxford: 1996. Ecology: Individuals, Populations and Communities. [Google Scholar]
- Blackwood J.S., Schausberger P., Croft B.A. Prey-stage preference in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environmental Entomology. 2001;30:1103–1111. [Google Scholar]
- Blumstein D.T., Barrow L., Luterra M. Olfactory predator discrimination in yellow-bellied marmots. Ethology. 2008;114:1135–1143. [Google Scholar]
- Botham M.S., Hayward R.K., Morrell L.J., Croft D.P., Ward J.R., Ramnarine I., Krause J. Risk-sensitive antipredator behavior in the Trinidadian guppy, Poecilia reticulata. Ecology. 2008;89:3174–3185. doi: 10.1890/07-0490.1. [DOI] [PubMed] [Google Scholar]
- Bühl, A. 2008. SPSS 16. Einführung in die Moderne Datenanalyse. Munich: Pearson Studium.
- Charnov E.L. Optimal foraging: attack strategy of a mantid. American Naturalist. 1976;110:141–151. [Google Scholar]
- Croft B.A., Kim S.S., Kim D.I. Intra- and interspecific predation on four life stage groups by the adult females of Metaseiulus occidentalis, Typhlodromus pyri, Neoseiulus fallacis and Amblyseius andersoni. Experimental and Applied Acarology. 1996;20:435–444. [Google Scholar]
- Croft B.A., Luh H.K., Schausberger P. Larval size relative to larval feeding, cannibalism of larvae, egg or adult female size and larval–adult setal patterns among 13 phytoseiid mite species. Experimental and Applied Acarology. 1999;23:599–610. [Google Scholar]
- De Moraes G.J., McMurtry J.A., Denmark H.A., Campos C.B. Magnolia Press; Auckland: 2004. A Revised Catalog of the Mite Family Phytoseiidae. [Google Scholar]
- Denno R.F., Fagan W.F. Might nitrogen limitation promote omnivory among carnivorous arthropods? Ecology. 2003;84:2522–2531. doi: 10.1890/09-2080.1. [DOI] [PubMed] [Google Scholar]
- Edelaar P., Wright J. Potential prey make excellent ornithologists: adaptive, flexible responses towards avian predation threat by Arabian babblers Turdoides squamiceps living at a migratory hotspot. Ibis. 2006;148:664–671. [Google Scholar]
- Faraji F., Janssen A., Sabelis M.W. Predatory mites avoid ovipositing near counterattacking prey. Experimental and Applied Acarology. 2001;25:613–623. doi: 10.1023/a:1016100212909. [DOI] [PubMed] [Google Scholar]
- Ferrari M.C.O., Chivers D.P. Temporal variability, threat sensitivity and conflicting information about the nature of risk: understanding the dynamics of tadpole antipredation behaviour. Animal Behaviour. 2009;78:11–16. [Google Scholar]
- Ferrari M.C.O., Trowell J.J., Brown G.E., Chivers D.P. The role of learning in the development of threat-sensitive predator avoidance in fathead minnows. Animal Behaviour. 2005;70:777–784. [Google Scholar]
- Ferrari M.C.O., Messier F., Chivers D.P. Threat-sensitive learning of predators by larval mosquitoes Culex restuans. Behavioral Ecology and Sociobiology. 2008;62:1079–1083. [Google Scholar]
- Gallie J.A., Mumme R.L., Wissinger S.A. Experience has no effect on the development of chemosensory recognition of predators by tadpoles of the American toad, Bufo americanus. Herpetologica. 2001;57:376–383. [Google Scholar]
- Grostal P., Dicke M. Direct and indirect cues of predation risk influence behavior and reproduction of prey: a case for acarine interactions. Behavioral Ecology. 1999;10:422–427. [Google Scholar]
- Hawkins L.A., Magurran A.E., Armstrong J.D. Ontogenetic learning of predator recognition in hatchery-reared Atlantic salmon, Salmo salar. Animal Behaviour. 2008;75:1663–1671. [Google Scholar]
- Heithaus M.R. Habitat selection by predators and prey in communities with asymmetric intraguild predation. Oikos. 2001;92:542–554. [Google Scholar]
- Helfman G.S. Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behavioral Ecology and Sociobiology. 1989;24:47–58. [Google Scholar]
- Hirayama H., Kasuya E. Oviposition depth in response to egg parasitism in the water strider: high-risk experience promotes deeper oviposition. Animal Behaviour. 2009;78:935–941. [Google Scholar]
- Janssen A., Montserrat M., HilleRisLambers R., de Ross M.A., Pallini A., Sabelis M.W. Intraguild predation usually does not disrupt biological control. In: Brodeur J., Boivin G., editors. Trophic and Guild Interactions in Biological Control. Springer; Heidelberg: 2006. pp. 21–44. [Google Scholar]
- Kagata H., Katayama N. Does nitrogen limitation promote intraguild predation in an aphidophagous ladybird? Entomologia Experimentalis et Applicata. 2006;119:239–246. [Google Scholar]
- Kats L.B., Dill L.M. The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience. 1998;5:361–394. [Google Scholar]
- Kiesecker J.M., Chivers D.P., Blaustein A.R. The use of chemical cues in predator recognition by western toad tadpoles. Animal Behaviour. 1996;52:1237–1245. [Google Scholar]
- Kindermann T., Siemers B.M., Fendt M. Innate or learned acoustic recognition of avian predators in rodents? Journal of Experimental Biology. 2009;212:506–513. doi: 10.1242/jeb.024174. [DOI] [PubMed] [Google Scholar]
- Lawson-Balagbo L.M., Gondim M.G.C., Jr., de Moraes G.J., Hanna R., Schausberger P. Compatibility of Neoseiulus paspalivorus and Proctolaelaps bickleyi, candidate biocontrol agents of the coconut mite Aceria guerreronis: spatial niche use and intraguild predation. Experimental and Applied Acarology. 2008;45:1–13. doi: 10.1007/s10493-008-9156-8. [DOI] [PubMed] [Google Scholar]
- Lima S.L., Bednekoff P.A. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. American Naturalist. 1999;141:675–686. doi: 10.1086/303202. [DOI] [PubMed] [Google Scholar]
- Lima S.L., Dill L.M. Behavioral decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology. 1990;68:619–659. [Google Scholar]
- Luttbeg B., Kerby J.L. Are scared prey as good as dead? Trends in Ecology & Evolution. 2005;20:416–418. doi: 10.1016/j.tree.2005.05.006. [DOI] [PubMed] [Google Scholar]
- McMurtry J.A., Croft B.A. Life-styles of phytoseiid mites and their roles in biological control. Annual Review of Entomology. 1997;42:291–321. doi: 10.1146/annurev.ento.42.1.291. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Trafelet-Smith G.M., Gratton C., Finke D.L., Fagan W.F., Denno R.F. Does intraguild predation enhance predator performance? A stoichiometric perspective. Ecology. 2004;85:2601–2615. [Google Scholar]
- Monclus R., Palomares F., Tablado Z., Martınez-Fonturbel A., Palme R. Testing the threat-sensitive predator avoidance hypothesis: physiological responses and predator pressure in wild rabbits. Oecologia. 2009;158:615–623. doi: 10.1007/s00442-008-1201-0. [DOI] [PubMed] [Google Scholar]
- Montserrat M., Bas C., Magalhães S., Sabelis M.W., de Roos A.M., Janssen A. Predators induce egg retention in prey. Oecologia. 2007;150:699–705. doi: 10.1007/s00442-006-0527-8. [DOI] [PubMed] [Google Scholar]
- Murphy P.J. Context-dependent reproductive site choice in a neotropical frog. Behavioral Ecology. 2003;14:626–633. [Google Scholar]
- Polis G.A., Myers C.A., Holt R.D. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annual Review of Ecology and Systematics. 1989;20:297–330. [Google Scholar]
- Preisser E.L., Bolnick D.I., Benard M.F. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- Provost C., Lucas E., Coderre D. Prey preference of Hyaliodes vitripennis as an intraguild predator: active predator choice or passive selection? Biological Control. 2006;37:148–154. [Google Scholar]
- Saito Y. Prey kills predator: counter-attack success of a spider mite against its specific phytoseiid predator. Experimental and Applied Acarology. 1986;2:47–62. [Google Scholar]
- Schausberger P. Inter- and intraspecific predation on immatures by adult females in Euseius finlandicus, Typhlodromus pyri and Kampimodromus aberrans (Acari: Phytoseiidae) Experimental and Applied Acarology. 1997;21:131–150. [Google Scholar]
- Schausberger P. Cannibalism among phytoseiid mites: a review. Experimental and Applied Acarology. 2003;29:173–191. doi: 10.1023/a:1025839206394. [DOI] [PubMed] [Google Scholar]
- Schausberger P., Croft B.A. Nutritional benefits of intraguild predation and cannibalism among generalist and specialist phytoseiid mites. Ecological Entomology. 2000;25:473–480. [Google Scholar]
- Schausberger P., Croft B.A. Cannibalism and intraguild predation among phytoseiid mites: are aggressiveness and prey preference related to diet specialization? Experimental and Applied Acarology. 2000;24:709–725. doi: 10.1023/a:1010747208519. [DOI] [PubMed] [Google Scholar]
- Sih A. Foraging strategies and the avoidance of predation by an aquatic insect, Notonecta hoffmanni. Ecology. 1982;63:786–796. [Google Scholar]
- Sih A. Antipredator responses and the perception of danger by mosquito larvae. Ecology. 1986;67:434–441. [Google Scholar]
- Sih A., Englund G., Wooster D. Emergent impacts of multiple predators on prey. Trends in Ecology & Evolution. 1998;13:350–355. doi: 10.1016/s0169-5347(98)01437-2. [DOI] [PubMed] [Google Scholar]
- Skaloudova B., Zemek R., Krivan V. The effect of predation risk on an acarine system. Animal Behaviour. 2007;74:813–821. [Google Scholar]
- Stapley J. Differential avoidance of snake odours by a lizard: evidence for prioritized avoidance based on risk. Ethology. 2003;109:785–796. [Google Scholar]
- Vanas V., Enigl M., Walzer A., Schausberger P. The predatory mite Phytoseiulus persimilis adjusts patch-leaving to own and progeny needs. Experimental and Applied Acarology. 2006;39:1–11. doi: 10.1007/s10493-006-0024-0. [DOI] [PubMed] [Google Scholar]
- Walzer A., Schausberger P. Cannibalism and interspecific predation in the phytoseiid mites Phytoseiulus persimilis and Neoseiulus californicus: predation rates and effects on reproduction and juvenile development. BioControl. 1999;43:457–468. [Google Scholar]
- Walzer A., Schausberger P. Non-consumptive effects of predatory mites on thrips and its host plant. Oikos. 2009;118:934–940. [Google Scholar]
- Walzer A., Paulus H.F., Schausberger P. Oviposition behavior of predatory mites: response to the presence of con- and heterospecific eggs. Journal of Insect Behavior. 2006;19:305–320. [Google Scholar]
- Walzer A., Moder K., Schausberger P. Spatiotemporal within-plant distribution of the spider mite Tetranychus urticae and associated specialist and generalist predators. Bulletin of Entomological Research. 2009;99:457–466. doi: 10.1017/S0007485308006494. [DOI] [PubMed] [Google Scholar]
- Werner E.E., Peacor S.D. A review of trait-mediated indirect interactions in ecological communities. Ecology. 2003;84:1083–1100. [Google Scholar]
- Zhang Z.-Q., Croft B.A. Interspecific competition and predation between immature Amblyseius fallacis, Amblyseius andersoni, Typhlodromus occidentalis and Typhlodromus pyri (Acari: Phytoseiidae) Experimental and Applied Acarology. 1995;19:247–257. [Google Scholar]