Abstract
Biomarkers are gaining increasing interest to predict risk but also to aid in diagnostics. Tissue-specific biomarkers are of utmost importance to detect diseases of respective organs. As of yet there are no atriumspecific biomarkers for risk stratification of atrial disease, such as atrial fibrillation. Bioinformatics such as mRNA microarrays can help to detect tissue-enriched and possibly tissue-specific expressed genes that can be targets for biomarkers. We describe an approach to identify genes preferably expressed in atrial cardiomyocytes compared with ventricular cardiomyocytes by RNA microarray and confirmed by quantitative real-time polymerase chain reaction. By this approach we identified several atrium-enriched genes but also ventricle-enriched genes. As expected atrial natriuretic peptide (ANP) mRNA showed higher expression in atrial cardiomyocytes while with adrenergic stimulation expression was almost as high in ventricular as in atrial cells. Brain-type natriuretic peptide (BNP), however, was not different between atrial and ventricular cells giving a possible explanation for increased levels of NT-proBNP in atrial fibrillation patients. Interesting identified candidates are serpine1 and ltbp2 as atrium-enriched genes whereas alpha-adrenergic receptor subtype 1b and S100A1 expression was significantly higher in ventricular cells. The identified genes need to be confirmed in human tissue and might ultimately be tested as potential biomarkers for atrial stress. (Neth Heart J 2010;18:610–4.)
Keywords: Atrial, Ventricular; Cardiac Myocytes; Fibroblasts; Atrial Fibrillation; Microarray
Biomarkers are increasingly used to predict the risk for certain diseases but also to monitor disease progression. For heart diseases, biomarkers have long been used to measure the degree of myocardial damage with creatine kinase and its isoforms. In recent years, troponin T and troponin I as more sensitive markers for myocardial damage and n-terminal pro-brain type natriuretic peptide (NT-proBNP) are routinely used for risk stratification and monitoring of the clinical situation and success of therapy. Heart failure seems to be a prototypical target for biomarkers as decompensation is difficult to predict with clinical parameters only.1
Atrial fibrillation (AF) is often a consequence of underlying heart disease such as hypertension and heart failure.2 Not all patients with these diseases, however, develop atrial arrhythmias.3 The occurrence of AF is of prognostic relevance. Once AF occurs treatment is often difficult as structural remodelling such as fibrosis has progressed so far that it is irreversible.4–6 To identify (a set of) biomarkers measuring atrial stress could help to predict patients at risk for atrial arrhythmias and could aid in initiating more aggressive therapy and to test efficacy of new or known treatment regimens. 7
Methods
Neonatal rat atrial and ventricular myocytes (NRAMs, NRVMs) and fibroblasts were isolated from one- to two-day-old Sprague Dawley rats by trypsin digestion, as described previously.8 For microarray analysis total RNA was isolated using TRIzol reagent (Invitrogen Corporation), and cleaned using the Nucleospin II kit (Macherey-Nagel). Processing of RNA and microarray analysis was performed in the core lab (Department of Genetics, University Medical Center Groningen, University of Groningen, the Netherlands). RNA was processed using the Ambion RNA kit (Applied Biosystems) and Illumina Rat Ref12 Expression Platform was used for the microarray. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to confirm microarray data of selected genes and to investigate effects of stimulation with 20 μM of the α-adrenergic agonist phenylephrine for 24 hours on both NRAM and NRVM.9 Total RNA was isolated using the Nucleospin II kit (Macherey-Nagel) and converted to cDNA by QuantiTect Reverse Transcription (Qiagen). Gene expression was measured with ABsolute QPCR SYBR Green ROX Mix (Abgene) in the presence of 7.5 ng cDNA and 200 nM forward and reverse primers. qRTPCR was conducted on the Biorad CFX384 (Biorad). Gene expression levels were corrected for 36b4 reference gene expression, and values were expressed relative to NRVM control levels. Primers used were ANP forward atgggctccttctccatcac, ANP reverse tctaccggcatcttctcctc, BNP forward acaatccacgatgcagaagct, BNP reverse gggccttggtcctttgaga, α-myosin heavy chain (αMHC) forward gacaactcctcccgctttgg, αMHC reverse aagatcacccgggacttctc, Adra1b forward aaccttgggcattgtagtcg, Adra1b reverse tgaggcagctgttgaagtag, serpine1 forward ccggcagcagatccaagatg, serpine1 reverse ggtcccgctggacaaagatg, S100a1 forward ggagaccctcatcaatgtg, S100a1 reverse cagcatctgcatccttctg, Wisp2 forward ttctggccacttccttcctc, Wisp2 reverse ttacagcagccacagccatc, pln forward ttgtcttcctggcatcatgg, pln reverse cagcttgtcacagaagcatcac, 36b4 forward gttgcctcagtgcctcactc, 36b4 reverse gcagccgcaaatgcagatgg. Microarray analysis was performed using GeneSpring GX (Version 10.0, Agilent Technologies). Statistical significance was tested using unpaired t-test with Benjamini-Hochberg correction. qRT-PCR results are expressed as mean values ± standard error of the mean (SEM) and statistical analysis was performed using Mann-Whitney u-test, using SPSS (Version 16, SPSS Inc). Values of p<0.05 were considered statistically significant.
Results
Atrial and ventricular cells showed no significant morphological difference besides a slightly larger cell size of NRAM (figure 1).
Figure 1.
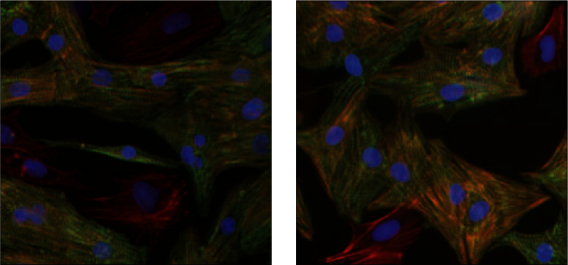
Primary neonatal rat atrial (1A) and ventricular (1B) myocytes and fibroblasts were stained for α-actinin with specific antibodies (green) and total actin was stained with Texas red-phalloidin. Cardiac myocytes stain both red and green, whereas fibroblasts stain red for actin exclusively. Nuclei are stained blue with DAPI.
Tabel 1A.
Atrium-enriched genes.
| Gene symbol | Fold A/V microarray | P value | Fold A/V pcr | P value | Protein |
|---|---|---|---|---|---|
| Myl7-pred | 7.9 | 0.005 | nd | Regulatory myosin light chain7 | |
| Ltb2 | 4.5 | 0.03 | 2.92 | 0.01 | Latent TGF-b binding protein2 |
| Serpine1 | 4.37 | 0.01 | 4.76 | <0.01 | Serine peptidase inhibitor |
| Nppa | 3.33 | 0.03 | 5.98 | <0.01 | Atrial natriuretic peptide |
| Wisp2 | 2.52 | 0.08 | 2.47 | 0.07 | WNT1 inducible signalling pathway |
| Myh6 | 2.02 | 0.03 | 1.63 | 0.02 | α-myosin heavy chain |
A/v= ratio atrial vs. ventricular expression, nd= not determined.
Tabel 1B.
Ventricle-enriched genes.
| Gene symbol | Fold V/A microarray | P value | Fold V/A pcr | P value | Protein |
|---|---|---|---|---|---|
| Adra1b | 6.19 | 0.01 | 4.68 | 0.03 | Adrenergic α-1b receptor |
| Ckm | 3.62 | 0.03 | nd | Muscle creatine kinase | |
| Pln | 2.49 | 0.06 | 1.59 | 0.01 | Phospholamban |
| S100A1 | 2.43 | 0.02 | 2.31 | 0.02 | S100 calcium binding protA1 |
V/A= ratio ventricular vs. atrial expression, nd= not determined.
We excluded genes with low abundance and the ones that showed less than twofold difference from our further analysis. This yielded 80 differentially expressed genes with statistical significance. Furthermore, we focused on genes with a known function and possible physiological or pathophysiological roles in cardiac diseases. Not surprisingly, atrial natriuretic peptide (ANP) mRNA showed a sixfold higher expression in NRAM. Table 1 lists the most important differentially expressed genes. In table 1A atrial-enriched genes are shown whereas table 1B lists important ventricle-enriched genes. There were some differences in known cardiac structural genes such as MHC, creatine kinase, phospholamban, and regulatory myosin light chain. As further interesting candidates, we investigated serpine1 and wisp2, which showed fivefold and 2.5-fold higher expression in NRAMs, respectively. The alpha-adrenergic receptor subtype 1b and S100A1 showed fivefold and 2.3-fold higher expression in NRVMs, respectively. Latent transforming growth factor beta-binding protein 2 (ltb2) was expressed threefold higher in atrial cells, but further analysis showed that it is exclusively expressed in cardiac fibroblasts. Even though our analysis was done in isolated cardiac myocytes the cultures are always contaminated with 10 to 20% non-myocytes, whereas in the heart 90% of cells are non-myocytes.
To determine if there were differences between natriuretic peptides under basal conditions and stress, we treated the cells with the strong hypertrophic stimulus phenylephrine. This led to a fivefold increase of ANP and BNP in NRVM. Levels of ANP under these pathological conditions were almost as high as in NRAM (figure 2).
Figure 2.
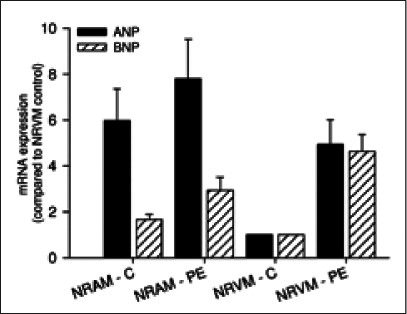
Comparison of mRNA expression of atrial (ANP) and brain-type (BNP) natriuretic peptides in primary neonatal rat atrial (NRAM) and ventricular (NRVM) myocytes. Cells were also treated with 20 μM phenylephrine (PE) to induce hypertrophy which leads to increases in both ANP and BNP in NRVM only to levels comparable with NRAM at baseline.
Discussion
We demonstrate differentially expressed genes in atrial and ventricular cardiac myocytes via RNA microarray that are confirmed via qRT-PCR. These results must clearly be confirmed in human tissue before we think about using them as biomarkers of atrial stress or disease. We identified, however, several interesting candidates including serpine1 and ltb2.
Serpine1, also known as plasminogen activator inhibitor 1 (PAI1), is a pleiotropic factor involved in multiple processes, such as inflammation, fibrinolysis, and insulin resistance. 10 So far atrial-enriched expression had not been described. PAI-1 has been implicated in the development of atrial fibrillation. Elevated levels of PAI-1 independently predict development of AF after cardiac surgery.11
Ltbp2 is a fibroblast-specific factor and the differences we demonstrate with the microarrays are caused by differences in atrial and ventricular fibroblasts (data not shown). It has been suggested that cardiac fibroblasts can also produce natriuretic peptides.12 In our hands, however, expression in fibroblasts was 100-fold lower than in cardiac myocytes (data not shown). There seems to be a profound difference between atrial and ventricular fibroblasts. Atrial fibroblasts seem to respond stronger to transforming growth factor β(TGF-β).13 Ltbp2 could be involved in this mechanism, as it has been suggested to enhance TGF-β function.
There are obviously no optimal biomarkers. Individual markers or panels for certain situations can be envisioned. If a biomarker for tissue damage is to be developed, this protein needs to be tissue-specific and once it is released into the circulation, half-life should not be too short. To detect stress instead of damage, the biomarker needs to be a secreted protein, again with a decent half-life in plasma or serum.
A problem with biomarkers might be regulation through pathological signals leading to ‘aberrant’ expression. A good example is the increase in ANP mRNA in hypertrophic NRVM as shown in our study. Levels can reach the levels in NRAM with adrenergic stimulation. NT-proBNP is a prototypical marker for ventricular stress. We show here that NRAM express as much BNP mRNA as NRVM. Stress can lead to an increase in BNP mRNA in NRAM. This might add to the high levels of NT-proBNP in patients with AF. NT-proBNP has been shown to be an independent predictor of the development of AF.14 In advanced heart failure, however, we showed that AF affects (NT-pro)ANP levels, but not (NT-pro)BNP levels, although NT-proBNP was an independent determinant of prognosis in advanced heart failure, irrespective of the rhythm, AF or sinus rhythm.15 Nevertheless, the previous concept of ventricular stress only leading to increase in serum NT-pro-BNP needs to be revised.
Outlook
Further experiments are aimed at utilising human cardiac tissue to look for differentially expressed genes in patients. This is troubled by cell composition but our approach has shown that this approach does not preclude identification of differentially expressed genes from certain cell types as we detected cardiomyocyte-specific as well as fibroblastspecific genes. We thus envision finding structural markers for atrial myocytes but also genes that are specific for atrial versus ventricular fibroblasts. Microarray experiments can obviously be combined with the candidate approach. As an example for this approach, a recent publication has suggested endothelin-1 as an important player in atrial fibrillation in patients with structural heart disease.16
A novel approach will be to look for atrial specific microRNAs (miRNAs). Pinto’s group has recently demonstrated that circulating miRNAs can be detected in patient blood and that certain miRNAs are enriched in patients with heart failure.17 It is possible that there are atriumspecific miRNAs that can also be detected in blood.
To identify biomarkers for atrial stress, we will use a secretion trap screen with human atrial cDNA expressed in secretion-deficient yeast to identify known or novel secreted proteins.18 We hope to find a secreted factor with sufficient atrial enrichment that is abundant and stable enough to be measured in human serum or plasma.
Treatment of patients with AF is currently undergoing a shift of paradigm. The most relevant outcomes are related to patient well being and objective measures of cardiovascular morbidity. In symptomatic patients this includes abolishment of AF. To improve outcome in patients with AF, patient-tailored therapy could be aided by biomarkers. In addition to identifying patients at risk of AF they could help in stratifying therapy. We still do not know which patients will fail rhythm control therapy. On the other hand, in patients where we chose for rate control it might be necessary to identify patients with an adverse outcome who need more aggressive therapy.
References
- 1.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation. Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 3.Buck S, Rienstra M, Maass AH, Nieuwland W, Van Veldhuisen DJ, Van Gelder IC. Cardiac resynchronization therapy in patients with heart failure and atrial fibrillation: importance of new-onset atrial fibrillation and total atrial conduction time. Europace. 2008;10:558–565. doi: 10.1093/europace/eun064. [DOI] [PubMed] [Google Scholar]
- 4.Cosio FG, Aliot E, Botto GL, Heidbuchel H, Geller CJ, Kirchhof P, et al. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10:21–27. doi: 10.1093/europace/eum276. [DOI] [PubMed] [Google Scholar]
- 5.Van Gelder IC, Crijns HJ, Tieleman RG, Brugemann J, De Kam PJ, Gosselink AT, et al. Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation. Arch Intern Med. 1996;156:2585–2592. doi: 10.1001/archinte.156.22.2585. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S, Rienstra M, Crijns HJ, Links TP, Wiesfeld AC, Hillege HL, et al. Continuous vs episodic prophylactic treatment with amiodarone for the prevention of atrial fibrillation: a randomized trial. JAMA. 2008;300:1784–1792. doi: 10.1001/jama.300.15.1784. [DOI] [PubMed] [Google Scholar]
- 7.Smit MD, Van Gelder IC. Will we be able to predict in which atrial fibrillation patients a rhythm control strategy will be successful? Europace. 2009;11:846–847. doi: 10.1093/europace/eup148. [DOI] [PubMed] [Google Scholar]
- 8.Maass A, Langer SJ, Oberdorf-Maass S, Bauer S, Neyses L, Leinwand LA. Rational promoter selection for gene transfer into cardiac cells. J Mol Cell Cardiol. 2003;35:823–831. doi: 10.1016/S0022-2828(03)00140-8. [DOI] [PubMed] [Google Scholar]
- 9.Maass AH, Ikeda K, Oberdorf-Maass S, Maier SK, Leinwand LA. Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation. 2004;110:2102–2109. doi: 10.1161/01.CIR.0000144460.84795.E3. [DOI] [PubMed] [Google Scholar]
- 10.Cesari M, Pahor M, Incalzi RA. Plasminogen Activator Inhibitor-1 (PAI-1): A Key Factor Linking Fibrinolysis and Age-Related Subclinical and Clinical Conditions. Cardiovasc Ther. 2010;28:e72–e79. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pretorius M, Donahue BS, Yu C, Greelish JP, Roden DM, Brown NJ. Plasminogen activator inhibitor-1 as a predictor of postoperative atrial fibrillation after cardiopulmonary bypass. Circulation. 2007;116(11 Suppl):I1–I7. doi: 10.1161/CIRCULATIONAHA.106.677906. [DOI] [PubMed] [Google Scholar]
- 12.Jarai R, Kaun C, Weiss TW, Speidl WS, Rychli K, Maurer G, et al. Human cardiac fibroblasts express B-type natriuretic peptide: fluvastatin ameliorates its up-regulation by interleukin-1alpha, tumour necrosis factor-alpha and transforming growth factor-beta. J Cell Mol Med. 2009;13:4415–4421. doi: 10.1111/j.1582-4934.2009.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 14.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rienstra M, Van Gelder IC, Van den Berg MP, Boomsma F, Van Veldhuisen DJ. Natriuretic peptides in patients with atrial fibrillation and advanced chronic heart failure: determinants and prognostic value of (NT-) ANP and (NT-pro)BNP. Europace. 2006;8:482–487. doi: 10.1093/europace/eul060. [DOI] [PubMed] [Google Scholar]
- 16.Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, et al. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. 2010;3:369–379. doi: 10.1161/CIRCEP.109.924985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, et al. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106:1035–1039. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 18.Frost RJ, Engelhardt S. A secretion trap screen in yeast identifies protease inhibitor 16 as a novel antihypertrophic protein secreted from the heart. Circulation. 2007;116:1768–1775. doi: 10.1161/CIRCULATIONAHA.107.696468. [DOI] [PubMed] [Google Scholar]


