Abstract
Genome-wide association studies have identified SNPs within the human FTO gene that display a strong association with obesity. Individuals homozygous for the at-risk rs9939609 A allele weigh ~3kg more. Loss of function and/or expression of FTO in mice leads to increased energy expenditure and a lean phenotype. We show here that ubiquitous overexpression of Fto leads to a dose-dependent increase in body and fat mass, irrespective of whether mice are fed a standard or high fat diet. The increased body mass results primarily from increased food intake. Glucose intolerance develops with increased Fto expression on a high fat diet. This study provides the first direct evidence that increased Fto expression causes obesity in mice.
Introduction
There is currently a worldwide epidemic of obesity and up to 58% of the world’s adult population are predicted to be overweight or obese by 20301. Obesity predisposes to numerous diseases, including heart disease, type 2 diabetes and cancer: thus understanding how body weight is regulated is of major scientific, health and economic importance. Genome-wide association studies have revealed that single-nucleotide polymorphisms (SNPs; including rs9939609, rs17817449, rs3751812, rs1421085 and rs9930506) in intron-1 of the fat mass and obesity-associated (FTO) gene are associated with an increased risk of obesity2-5: approximately 16% of European descent population’s are homozygous for the at-risk allele, weighing on average ~3 kg more than controls4,5. Evidence suggests that these individuals also exhibit increased food intake6-13.
FTO is an AlkB-like 2-oxoglutarate dependent nucleic acid demethylase14-16 with a strong preference for 3-methylthymidine and 3-methyluracil in single-stranded DNA and RNA, respectively, due to unique structural features17. A human FTO mutation (R316Q), that inhibits catalytic activity, results in an autosomal-recessive lethal syndrome15.
Mice lacking FTO show increased postnatal lethality, postnatal growth retardation, reduced adipose tissue and spontaneous locomotor activity, increased energy expenditure and relative hyperphagia18. A dominant mutation (I367F) in the mouse Fto gene that reduces its DNA demethylation activity also results in reduced fat mass19. Taken together, these studies in the mouse suggest loss of expression and/or function of Fto protects against obesity.
FTO is ubiquitously expressed. High mRNA levels are observed in the brain including the cerebellum, hippocampus and hypothalamus14,20,21. In humans, they are higher in males and are positively correlated with BMI in adipose tissue22 (although see ref23 for an exception). They are also greater in subcutaneous adipose tissue (SAT)24-26 of obese individuals.
The association between brain FTO levels and food intake is somewhat controversial, with both increases (in rat27 in the hypothalamus) and decreases (in mice14,28 in the arcuate nucleus) on fasting being reported. Direct manipulation of FTO levels selectively in the arcuate nucleus of rats by adenoviral infection of Fto, or siRNA against Fto, influences food intake, with a reduction in Fto expression increasing food intake and enhanced expression decreasing food intake29. Interestingly, I367F mutant mice did not show detectable altered food intake19.
Taken together, these data demonstrate that there is no clear consensus on the role of FTO in food intake in either man or mouse. Many6,8,9,11, but not all12,30,31, studies suggest that food intake is greater in humans carrying at-risk SNPs. A problem with obesity studies is determining cause and effect because, for example, increased body mass requires increased energy expenditure for locomotion and potentially increased energy intake to match. The effects of loss of FTO function in rodents also remains unclear as deletion of the gene is reported to cause relative hyperphagia18 whereas reduced demethylation activity had no effect on food intake19. This raises the question of whether increased FTO expression alters food intake and body weight. Interestingly, there is now the first evidence that primary transcripts containing an at-risk A allele at SNP rs9939609 are more abundant than those with the T allele in blood and fibroblast RNA samples from several individuals, suggesting that increased expression may be correlated with obesity32. To test this hypothesis, we compared the effects of different copy numbers of Fto using mouse models. Our data provide evidence that enhanced expression of FTO causes obesity.
Results
To test the hypothesis that upregulation of FTO expression causes obesity, we generated mice globally expressing either one or two additional copies of the Fto gene (Supplementary Fig. 1; see Methods). We refer to these as FTO-3 and FTO-4 mice (as they have either 3 or 4 copies of Fto in total), and we refer to wild-type mice as FTO-2 mice.
Fto Overexpression
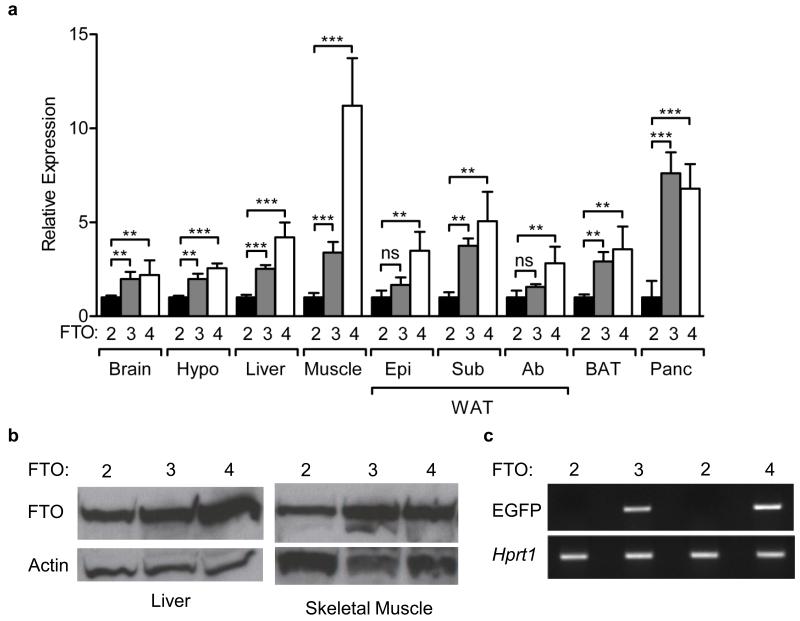
An increase in Fto mRNA expression in multiple tissues of mice carrying additional copies of the Fto gene was shown by qRT-PCR (Fig. 1a). In FTO-3 mice, this increase was largest in the pancreas (~8-fold), whereas in FTO-4 mice the greatest increase in expression (~11-fold) was found in skeletal muscle, followed by the pancreas. FTO protein overexpression was confirmed in skeletal muscle and liver by Western blot analysis (Fig. 1b): the increase ranged from 1.9-fold in skeletal muscle of FTO-3 mice to 2.3-fold in FTO-4 mouse liver. Expression of the EGFP reporter was confirmed by RT-PCR (Fig. 1c)
Figure 1. Generation of a mouse model overexpressing Fto.
(a) Relative Fto expression in the indicated tissues of FTO-2 (n=10) FTO-3 (n=10) and FTO-4 (n=10) mice. Epi, epigonadal. Ab, abdominal. Sub, subcutaneous. WAT, white adipose tissue. Data are expressed as mean±standard error (s.e.m.). *P<0.05, **P<0.01, ***P<0.0001. ns, non-significant.
(b) Representative Western blots of FTO and actin (loading control) from skeletal muscle and liver from FTO-2, FTO-3 and FTO-4 mice.
(c) RT-PCR of EGFP using brain cDNA prepared from FTO-2, FTO-3 and FTO-4 mice. Hypoxanthine-guanine phosphoribosyltransferase (Hprt1) is included as a control.
Dose-dependent Fto expression affects body weight
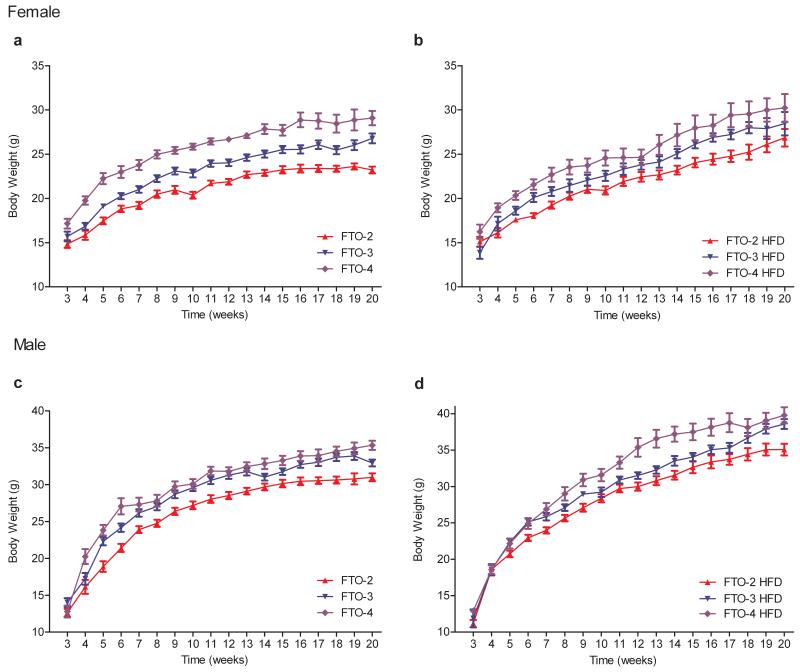
Mice carrying additional copies of the Fto gene exhibited increased body weights (Fig. 2). On a standard diet, female FTO-3 mice diverged from wild-type at ~5 weeks, becoming 11±3% heavier than FTO-2 littermates by 20 weeks of age (Fig. 2a, 3a). Female FTO-4 mice showed earlier divergence (4 weeks of age; Fig. 2a) and were 22±4% heavier at 20 weeks (Fig. 3a). Similar results were found for male FTO-3 and FTO-4 mice, although the increase in weight was slightly less dramatic than for female mice: by 20 weeks of age, male FTO-3 and FTO-4 mice were 7±2% and 10±1%, respectively, heavier than their FTO-2 littermates (Fig. 2c, 3a).
Figure 2. Fto dose-dependent increases in body weight are observed in male and female mice on standard (SD) and high fat (HFD) diets.
(a) Females SD, FTO-2 (wild-type, n=16), FTO-3 (n=28, P=0.0003) and FTO-4 (n=16, P<0.0001)
(b) Females HFD, FTO-2 (n=15), FTO-3 (n=14, P=0.04) and FTO-4 (n=15, P=0.002)
(c) Males SD, FTO-2 (n=16), FTO-3 (n=31, P=0.001) and FTO-4 (n=16, P=0.0001).
(d) Males HFD, FTO-2 (n=18), FTO-3 (n=15, P=0.01) and FTO-4 (n=16, P<0.0001).
Data are expressed as mean±s.e.m. Statistical analysis was performed using a repeated measures ANOVA. All P values are against FTO-2.
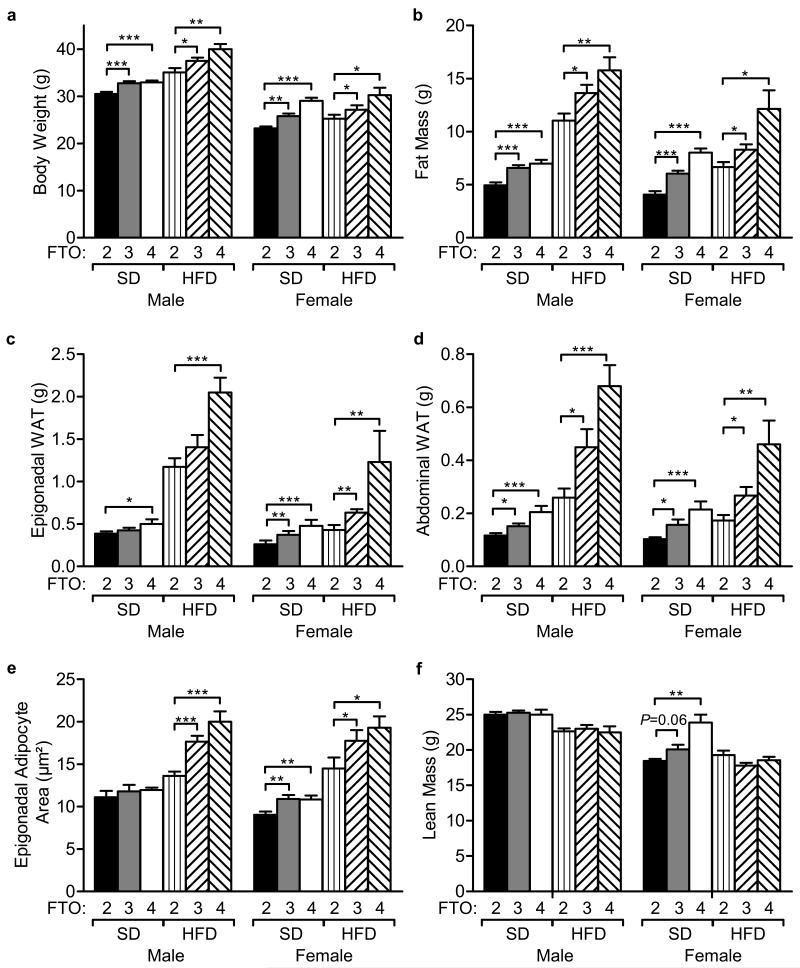
Figure 3. Body Composition varies with Fto copy number.
(a) Body weight of 20-week old male and female mice on a standard (SD) and high fat diet (HFD). Males: SD, FTO-2 (n=16), FTO-3 (n=31), FTO-4 (n=15); HFD, FTO-2 (n=18), FTO-3 (n=15), FTO-4 (n=16). Females: SD, FTO-2 (n=16), FTO-3 (n=28), FTO-4 (n=16); HFD, FTO-2 (n=15), FTO-3 (n=14), FTO-4 (n=15).
(b) Total fat mass measured by DEXA scanning in male and female mice on standard (SD) and high fat diets (HFD). Males: SD, FTO-2 (n=16), FTO-3 (n=30), FTO-4 (n=15); HFD, FTO-2 (n=17), FTO-3 (n=15), FTO-4 (n=16). Females: SD, FTO-2 (n=16), FTO-3 (n=28), FTO-4 (n=16); HFD, FTO-2 (n=16), FTO-3 (n=14), FTO-4 (n=15).
(c,d) Weights of epigonadal WAT (c) and abdominal WAT (d) in mice overexpressing Fto. Males: SD, FTO-2 (n=16), FTO-3 (n=21), FTO-4 (n=15); HFD, FTO-2 (n=15), FTO-3 (n=14), FTO-4 (n=14). Females: SD, FTO-2 (n=15), FTO-3 (n=28), FTO-4 (n=15); HFD, FTO-2 (n=12), FTO-3 (n=14), FTO-4 (n=14).
(e) Epigonadal adipocyte area is increased in female mice on both standard and high fat diets and in males on a HFD (n=5 in each case).
(f) Lean body mass in male and female mice on SD and HFD. Same mouse numbers as in (b).
Data (a-f) are expressed as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.0001.
Feeding a high fat diet (HFD) increased body weight in all mouse genotypes, but to a greater extent in mice overexpressing FTO (Fig. 2b, d). After 20 weeks, female FTO-3 and FTO-4 mice were 9±2% and 18±6% heavier, respectively, than FTO-2 mice (Fig. 2b, 3a). Male mice also showed an increased body weight (7±2% for FTO-3 and 13±3% for FTO-4) (Fig. 2d, 3a).
Body length was not different from wild-type in FTO-3 and FTO-4 mice (data not shown).
Dose-dependent expression of Fto affects fat mass
Mice overexpressing Fto showed a significant increase in fat mass (Fig. 3b, Supplementary Fig. 2). On a standard diet, this amounted to 42±8% for 20-week old female FTO-3 mice and 85±9.5% for FTO-4 mice (Fig. 3b). Male FTO-3 and FTO-4 mice showed similar increases in fat mass (Fig. 3b). A greater increase in fat mass was seen on a high fat diet, with similar increases in FTO-3 and FTO-4 mice relative to wild-type mice. This was again particularly pronounced in female mice, where fat mass was 18±7% (FTO-3) and 68±21% (FTO-4) greater than wild-type at 20 weeks (Fig. 3b). In male mice fat mass was also increased, being 24±7% (FTO-3) and 43±10% (FTO-4) greater than wild-type (Fig. 3b).
Similar correlations were observed between Fto copy number and the weight of dissected fat pads. Thus, epigonadal and abdominal white adipose tissues (WAT), were heavier in female FTO-3 and FTO-4 mice than in FTO-2 mice (Fig. 3c,d). Similar results were also found in male mice, except that epigonadal WAT was not increased in FTO-3 mice. High fat feeding further exacerbated the dose-dependent effect of Fto overexpression on epigondal and abdominal WAT in both male and female mice (Fig 3c,d). Haematoxylin and eosin histological analysis of epigonadal WAT (Supplementary Fig. 3a) showed a significant increase in adipocyte size at 20 weeks of age on both standard and high-fat diets in females, and on a high fat diet in males, when assessed quantitatively through adipocyte area (Fig. 3e).
Lean mass was affected to a lesser extent when Fto was overexpressed, but female FTO-4 mice showed a 21±9% increase on a standard diet (Fig. 3f). No significant difference in lean mass between FTO-2 and FTO-3 or FTO-4 mice was found on a high fat diet (Fig. 3f and Supplementary Fig. 2b,d).
Regression analysis showed that mice overexpressing FTO have a different body composition to wild-type mice, showing a higher fat-to-lean tissue mass ratio (Supplementary Fig. 2).
Fto Overexpression increases food intake
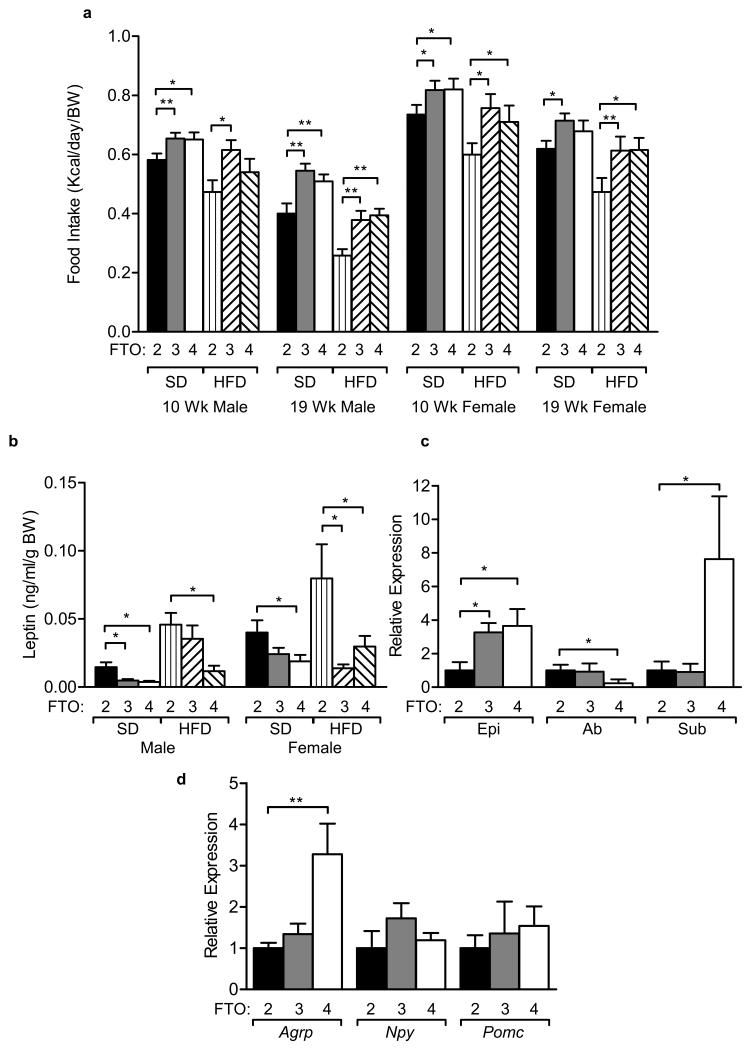
Many human studies have suggested that individuals carrying the at-risk FTO allele exhibit increased energy intake6-11,13. FTO-3 and FTO-4 mice consumed more than FTO-2 mice on both standard and high fat diets at 10 and 19 weeks of age (Supplementary Fig. 3b). The relative increase in food intake for FTO-3 and FTO-4 mice compared to their wild-type littermates was even greater on a high fat diet (Supplementary Fig. 3b). This was maintained when food intake was normalised to body weight for FTO-3 and FTO-4 mice, with the exception of FTO-4 female mice on a standard diet where this was reduced to a trend (Fig. 4a).
Figure 4. Effects of FTO on energy intake and plasma leptin.
(a) Food intake over 24 hours normalised to body weight (BW), measured in 10 and 19-week old mice. Males: SD, FTO-2 (n=16) FTO-3 (n=31) and FTO-4 (n=16); HFD, FTO-2 (n=18) FTO-3 (n=15) and FTO-4 (n=16). Females: SD, FTO-2 (n=16), FTO-3 (n=28) and FTO-4 (n=16); HFD, FTO-2 (n=15) FTO-3 (n=14) and FTO-4 (n=15).
(b) Plasma leptin levels at 8 weeks of age adjusted for body weight (BW) following an overnight 16-hour fast. Males: SD, FTO-2 (n=14), FTO-3 (n=25), FTO-4 (n=14); HFD, FTO-2 (n=12), FTO-3 (n=12), FTO-4 (n=12). Females: SD, FTO-2 (n=16), FTO-3 (n=25), FTO-4 (n=14); HFD, FTO-2 (n=13), FTO-3 (n=10), FTO-4 (n=10).
(c) Relative Leptin (Lep) gene expression in 20 week-old female epigonadal (Epi), abdominal (Ab) and subcutaneous (sub) WAT. FTO-2 (n=10), FTO-3 (n=10), FTO-4 (n=10).
(d) Relative gene expression of hypothalamic neuropeptides. FTO-2 (n=10) FTO-3 (n=10) and FTO-4 (n=10) mice.
Data (a-d) are expressed as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.0001.
These differences were confirmed by regression analysis against body weight at 10 weeks, which showed increased food intake at all body weights for FTO-3 and FTO-4 mice (Supplementary Fig. 4). At 20 weeks a similar pattern was evident for males (Supplementary Fig. 5a,b). Further, for FTO-4 males at 20 weeks food intake was elevated regardless of lean mass, fat mass (a trend on a standard diet, significant on a high fat diet) or body composition (defined as the Fat mass/Lean Mass ratio; Supplementary Fig. 5). These trends were less clear in females (Supplementary Fig. 6).
As food intake is under endocrine and neuronal control, and is influenced by leptin, we measured plasma leptin levels. At 8 weeks of age circulating leptin levels following a 16-hour overnight fast, either as raw data (data not shown) or corrected for body weight, were significantly lower in male and female FTO-4 mice than in FTO-2 mice, whether mice were maintained on a standard or high fat diet (Fig. 4b). This was largely confirmed by regression analysis against body weight (Supplementary Fig. 7a,b and 8a,b).
There was no significant difference in circulating leptin at 20 weeks of age either as raw data (data not shown) or corrected for fat mass with the exception of FTO-3 males on a standard diet showing a significant increase in relative leptin levels (with a trend for an increase, P=0.07, observed for FTO-4 males, Supplementary Fig. 3c, 7c and 8c). Regression analysis showed a clear positive correlation of leptin levels with fat mass for mice on a high fat diet, explaining 76% (P=0.0009) and 44% (P=0.0018) of the variation in leptin for FTO-4 females and males, respectively (Supplementary Fig. 7d and 8d). Consistent with the elevated circulating leptin, leptin mRNA expression was higher in epigonadal and subcutaneous WAT but was reduced in abdominal WAT of 20 week-old FTO-4 mice (Fig. 4c).
Hypothalamic expression of Agrp was elevated on fasting in 20 week FTO-4 male mice but Npy and Pomc levels were unaffected (Fig. 4d).
Fto and alterations in energy expenditure
Previous studies with knock-out18 and FtoI367F19 mice suggested that an increased metabolic rate might underlie their lean phenotype. We therefore used indirect calorimetry to assess metabolic rate in 18-week old mice.
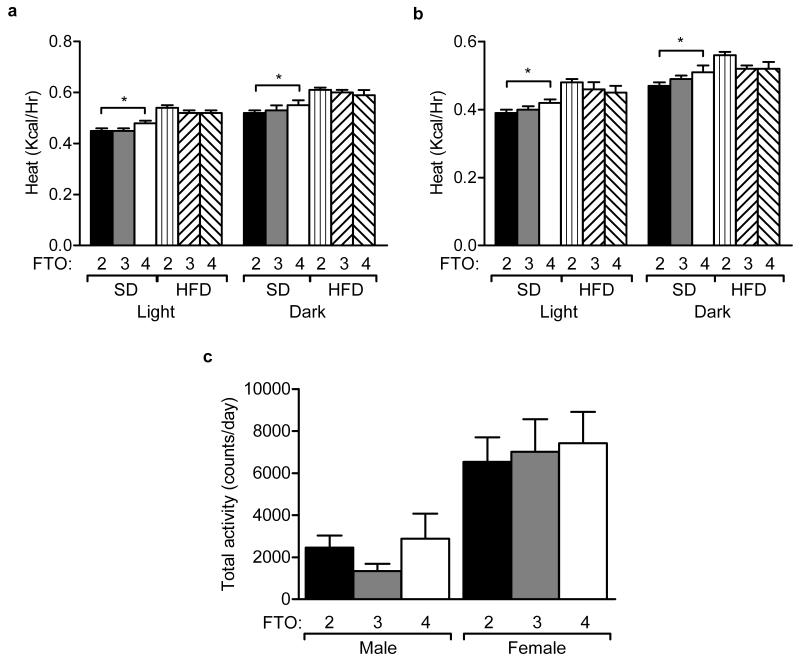
FTO-4 mice showed a significant increase in energy expenditure (Heat, Kcal/h) on a standard diet during both light and dark periods, no other differences were observed (Fig. 5a,b). We then carried out multiple regression analysis for energy expenditure to assess the effect of potential explanatory variables (weight, lean mass, fat mass, sex, genotype and diet) using an additive linear model (Supplementary Table 1). Lean mass (P=0.0446) and high fat diet (P=4.2 ×10−7) were identified as predictors. We then used a model that automatically selected relevant variables identifying weight (P=0.0077), lean mass (P=0.0003) and high fat diet (P=6.05×10−8) as the main predictor of energy expenditure (adjusted R-squared 0.441, P=<2.2×10−16). These results were confirmed using a robust fit linear model (data not shown). When tested alone, genotype was not a predictor of energy expenditure (data not shown).
Figure 5. Effects of Fto on energy expenditure and physical activity.
(a) Male and (b) Female heat production over a 22-hour period during the light and dark phases for 18-week old male (a,b) and female (c,d) mice on a standard (SD) or high fat diet (HFD). Males: SD, FTO-2 (n=15), FTO-3 (n=25), FTO-4 (n=16); HFD, FTO-2 (n=12), FTO-3 (n=12), FTO-4 (n=15). Females: SD, FTO-2 (n=16), FTO-3 (n=22), FTO-4 (n=15); HFD, FTO-2 (n=12), FTO-3 (n=13), FTO-4 (n=14).
(c) Physical activity measured as the number of rotations of an activity wheel in a 7-day period, following a 3-day entrainment period. Males and females: FTO-2 (n=7), FTO-3 (n=7), FTO-4 (n=6).
Data (a-c) are expressed as mean±s.e.m. *P<0.05, **P<0.01
There was no significant change in respiratory exchange ratio (RER) for FTO-3 or FTO-4 mice on either a standard or high fat diet (data not shown).
Overexpression of Fto has no effect on activity or circadian period
To assess if reduced locomotor activity contributes to the obese phenotype of mice overexpressing Fto, we measured activity at 12 weeks of age by wheel-running. No significant difference in wheel-running was observed during a 7-day period in either female or male FTO-3 or FTO-4 mice compared to FTO-2 mice (Fig. 5c). The length of wheel-running time during circadian challenges, including a shift to constant darkness or constant light, also showed no significant differences when compared with wild-type littermates (Supplementary Fig. 9a-d).
An open-field anxiety paradigm was used to test a combination of locomotor activity, exploratory drive and other aspects of anxiety at 10 weeks of age. Female FTO-3 and FTO-4 mice showed no difference in total activity as determined by distance moved during the period (Supplementary Fig. 9e), but female FTO-4 mice spent less time in the centre of the open field, suggesting they may be more anxious (Supplementary Fig. 9 f-h). No significant phenotype was observed in male FTO-3 and FTO-4 mice (data not shown). Following 4 weeks access to running-wheels, and the same light/dark regime, FTO-3 and FTO-4 mice showed increased body mass and fat mass at 12 weeks of age consistent with the phenotype observed at 20 weeks of age (Supplementary Table 2).
Dose-dependent effect of Fto expression on glucose tolerance
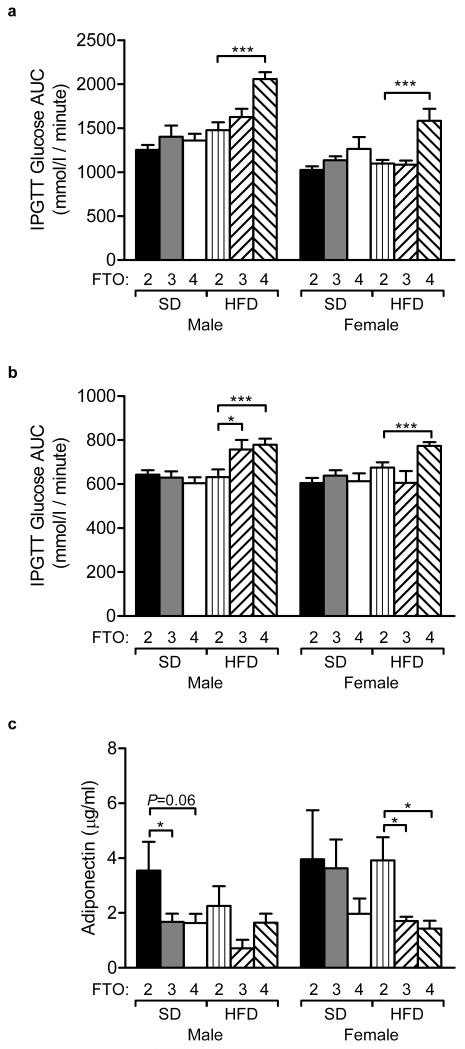
Glucose homeostasis was assessed at 12 weeks of age with a 120-minute intraperitoneal glucose tolerance test (IPGTT) and examined again at 16 weeks of age with a 30 minute IPGTT (data not shown). Glucose tolerance was quantified by measuring the area under the curve (AUC) relating plasma glucose levels to time. No significance difference in AUC was observed at 12 weeks between mice expressing different numbers of the Fto gene fed a standard diet (Fig. 6a). Interestingly, when challenged with a high fat diet, FTO-4 mice exhibited a reduction in glucose tolerance compared to wild-type mice, as shown by an increase in glucose AUC at 12 (Fig. 6a) and 16 weeks (Fig. 6b).
Figure 6. Glucose homeostasis and Fto overexpression.
(a) Area under the curve (AUC) during a 120-minute IPGTT in 12-week old mice. Males: SD, FTO-2 (n=15), FTO-3 (n=25), FTO-4 (n=16); HFD, FTO-2 (n=12), FTO-3 (n=13), FTO-4 (n=12). Females: SD, FTO-2 (n=16), FTO-3 (n=22), FTO-4 (n=15); HFD, FTO-2 (n=12), FTO-3 (n=12), FTO-4 (n=13).
(b) AUC analysis for glucose during a 30-minute IPGTT in 16-week old mice. Males: SD, FTO-2 (n=15), FTO-3 (n=23), FTO-4 (n=15); HFD, FTO-2 (n=12), FTO-3 (n=12), FTO-4 (n=14). Females: SD, FTO-2 (n=16), FTO-3 (n=22), FTO-4 (n=15); HFD, FTO-2 (n=12), FTO-3 (n=11), FTO-4 (n=15).
(c) Adiponectin levels at 20-weeks of age following a 6-hour light phase fast. Males: SD, FTO-2 (n=12), FTO-3 (n=22), FTO-4 (n=10); HFD, FTO-2 (n=12), FTO-3 (n=10), FTO-4 (n=10). Females: SD, FTO-2 (n=11), FTO-3 (n=22), FTO-4 (n=9); HFD, FTO-2 (n=10), FTO-3 (n=9), FTO-4 (n=10).
Data (a-c) are expressed as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.0001.
Plasma insulin levels were unchanged at 8 (fasting), 16 (over a 30min IPGTT) and 20 (fasting) weeks of age in male and female FTO-3 and FTO-4 mice on a standard diet (data not shown). However, male FTO-4 mice on a high fat diet had elevated fasting insulin at 16 weeks of age (1.05±0.22μg/l compared to 0.64±0.09μg/l for wild-type, P=0.025). Female FTO-3 and FTO-4 mice had lower adiponectin levels than wild-type mice on a high fat diet, but not a standard diet (Fig. 6c), whereas the reverse was found for male mice (Fig. 6c).
Fto overexpression alters plasma biochemistry
Plasma biochemistry measured in 20-week old female mice is shown in Table 1. Circulating plasma levels of triglycerides, free fatty acids (FFA) and HDL cholesterol were significantly greater in FTO-3 and FTO-4 mice than FTO-2 mice, but LDL cholesterol was reduced. Liver function was assessed by alkaline phosphatase (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST). FTO-4 mice exhibited significantly increased ALT and lower albumin levels. Creatine kinase was increased in FTO-3 and FTO-4 mice on standard and high fat diets. No significant differences were found in males (data not shown).
Table 1. 20 week plasma biochemistry in female mice.
20-week plasma biochemistry in female FTO-2, FTO-3 and FTO-4 mice on standard diet.
All data are given as mean ±s.e.m. ALP, alkaline Phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FFA, free fatty acids; LDH, lactate dehydrogenase; CK, creatine kinase. Cholesterol, total cholesterol; HDL-C, HDL-cholesterol; LDL, LDL-Cholesterol. Bold text P<0.05
| FTO-2 | FTO-3 | FTO-4 | t test | |||
|---|---|---|---|---|---|---|
| Units | n=16 | n=28 | n=17 | FTO-2 vs FTO-3 (P=) | FTO-2 vs FTO-4(P=) | |
| ALP | U/l | 84.7 ± 6 | 86 ± 4 | 87 ± 8. | 0.8696 | 0.8173 |
| ALT | U/l | 42 ± 5. | 42 ± 4 | 70 ± 17 | 0.8913 | 0.0473 |
| AST | U/l | 134 ± 20 | 166 ± 15 | 192 ± 41 | 0.2198 | 0.1680 |
| Albumin | g/l | 30 ± 1.6 | 27 ± 0.4 | 26 ± 0.6 | 0.0229 | 0.0785 |
| Cholesterol | mmol/l | 2.4 ± 0.19 | 2.6 ± 0.13 | 2.1 ± 0.14 | 0.3435 | 0.6101 |
| HDL-C | mmol/l | 1.2 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 0.0320 | 0.0460 |
| LDL-C | mmol/l | 0.68 ± 0.17 | 0.39 ± 0.02 | 0.33 ± 0.22 | 0.0347 | 0.0657 |
| Glucose | mmol/l | 16.8 ± 0.7 | 19.6 ± 0.8 | 15.9 ± 1.7 | 0.0137 | 0.5247 |
| Triglycerides | mmol/l | 0.69 ± 0.04 | 0.94 ± 0.06 | 0.80 ± 0.03 | 0.0074 | 0.0472 |
| Glycerol | μmol/l | 562 ± 25 | 577 ± 24 | 543 ± 19 | 0.7683 | 0.5745 |
| FFA | mmol/l | 0.82 ± 0.06 | 1.07 ± 0.06 | 1.11 ± 0.08 | 0.0273 | 0.0320 |
| LDH | U/l | 680 ± 72 | 743 ± 54 | 879 ± 122 | 0.5240 | 0.2366 |
| Amylase | U/l | 580 ± 22 | 632 ± 78 | 743 ± 109 | 0.1420 | 0.1819 |
| CK | U/l | 207 ± 38 | 367 ± 43 | 450 ± 73 | 0.0308 | 0.0147 |
DISCUSSION
Our data show that FTO is directly involved in the regulation of energy intake and metabolism in mice, and that enhanced expression of FTO leads to increased food intake and obesity.
Body weight and composition
Overexpression of FTO caused a dose-dependent increase in body weight and fat mass. The increase in weight of mice overexpressing FTO appears to be largely due to an increase in fat mass, as it is for humans carrying the at-risk allele4,33. On a high fat diet, female FTO-4 mice were 18%, and FTO-3 mice 9% heavier than controls; and on a standard diet they were 22 and 11% heavier, respectively. In comparison, humans carrying two at-risk alleles (e.g. rs9939609 A allele) are on average ~3.4% heavier (assuming an average adult body weight of 90kg) than those carrying the low-risk alleles. Thus, FTO activity/expression in our mice is likely to be greater than in at-risk humans, enabling the phenotype to be dissected more easily.
Frayling et al. (2007) showed that the increased body mass manifests from childhood4. Likewise, in FTO-4 mice the increase in body weight was present early, being significantly different from wild-type at 4 weeks of age on a standard diet.
Energy intake
Overexpression of FTO led to a marked increase in food intake. This was observed on both standard and high fat diets and at both 10 and 19 weeks of age. We conclude that the increase in food intake contributes to the increased fat mass and body weight of FTO-3 and FTO-4 mice. Regression analysis at 20 weeks showed that food intake in male FTO-4 mice was elevated regardless of body weight, lean mass, fat mass or body composition (FM/LM). Mice overexpressing FTO reduced their food intake on a high fat diet, relative to standard diet, indicating that their energy intake was still regulated, but that increased FTO expression had shifted the set point to a higher level. Most studies in humans report that at-risk SNPs enhance energy intake6-9,11, with some others finding no evidence of any effect on food intake12,30,31. Manipulation of FTO levels in the arcuate nucleus using adenoviral technology decreased food intake in contrast to our observations29. However, in our mice other tissues may also influence food intake, such as additional brain regions, the gut and adipose tissue.
Leptin, a hormone released from adipocytes that acts in the brain, is a potent regulator of food intake. We observed a reduction in circulating leptin levels at 8 weeks of age in mice over-expressing FTO that varied with Fto copy number, being greatest for FTO-4 mice. It is therefore possible that the hyperphagia of FTO-3 and FTO-4 mice is due to an FTO-dependent reduction in leptin concentration. However, at 20 weeks these differences were not observed and leptin levels correlated well with the increased fat mass. In human population studies, individuals with the FTO at-risk allele showed increased plasma leptin31,34, but this effect disappeared when adjusted for BMI34, suggesting this is a result of increased adiposity.
Energy expenditure - metabolism
Multivariate regression analysis in our mice indicates that the most significant predictor of energy expenditure is a high fat diet, relative to standard diet, probably due to the diet increasing body weight which subsequently requires greater energy expenditure for physical activity (Fig. 5a,b). Other significant determinants of energy expenditure were lean mass and weight. Importantly, genotype or sex effects were not detected in this global analysis of energy expenditure determinants. We conclude that in our mouse models energy expenditure is not a major determinant of the obese phenotype. Although humans carrying the FTO at-risk allele exhibited increased resting energy expenditure31,35,36, when adjusted for fat mass (or lean body mass) this difference was abolished31,35,36.
Energy expenditure - Physical activity
We found no effect of Fto copy number on spontaneous locomotor activity in mice. Similarly, in humans, there is no association between FTO risk-alleles and the extent of leisure time physical activity35,37 (although physical activity may modify the effect of the FTO risk-allele because BMI was attenuated in high physical activity groups38).
Glucose tolerance
Obesity strongly increases the risk of type 2 diabetes. Consistent with this, FTO-4 mice showed marked glucose intolerance on a high fat diet.
Limitations of this study
The ubiquitous unregulated over-expression in our mice does not necessarily recapitulate normal expression patterns, although FTO is expressed ubiquitously4,14. The functional consequences of the human risk alleles are likely to be more restricted than our promiscuous allele. Analysis was carried out after the body weight had significantly diverged and potentially may be confounded by these changes.
Conclusion
We show that increased expression of FTO leads to increased fat mass and obesity via hyperphagia. These data suggest that the at-risk SNPs in the human FTO gene may enhance the expression and/or activity of FTO. Our data further suggest that anti-obesity drugs targeted to FTO should be designed to reduce FTO expression/activity and predict that their effects in vivo will largely act by reducing appetite.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Medical Research Council (LM, LT, SW, PMN, GTB, RDC and studentships to CC and FM) and the Royal Society (Research Professorship to FMA) for personnel support, and the MRC and Wellcome Trust for financing the research (RDC, FMA). We thank Dr Chris Holmes, Dr George Nicholson and Mr Octavio Espinosa for help with multiple regression analysis using R.
Appendix
ONLINE METHODS
Gene targeting and genotyping
The targeting construct for generation of FTO overexpressing mice was generated by insertion of the Fto cDNA into a pCAGGs-STOP-EGFP-ROSA-TV plasmid (downstream of the STOP cassette, see Supplementary Fig. 1a). Further details are available on request. The linearised targeting vectors were electroporated into R1 embryonic stem (ES) cells39. Targeted ES cells were injected into C57BL/6J blastocysts to generate chimeras that transmitted the targeted allele when crossed to C57BL/6J mice. F1 mice were crossed to a line carrying the β-actin-Cre recombinase (Jackson Laboratory (Stock name Tg(ACTA1-cre)79Jme/J) on a C57BL/6J background and offspring backcrossed again to C57BL/6J to remove Cre recombinase. These mice were then intercrossed in multiple different matings to generate the test populations. Genotyping was performed on DNA extracted by QIAGEN DNeasy blood and tissue kit (Qiagen, USA), (Supplementary Table 3)
Animal Experiments
All animal studies were carried out in accordance with UK Home Office legislation and local ethical guidelines issued by the Medical Research Council (Responsibility in the Use of Animals for Medical Research, July 1993). Mice were kept under controlled light (light 7am-7pm, dark 7pm-7am), temperature (21±2 °C) and humidity (55±10%) conditions. They had free access to water (25 ppm chlorine). They were fed ad libitum on a commercial diet (SDS Rat and Mouse No.3 Breeding diet (RM3) containing 11.5 kcal% fat, 23.93 kcal% protein and 61.57 kcal% carbohydrate. Where indicated, mice were maintained on a high fat diet (D12451, Research Diets, New Brunswick, NJ, USA) containing 45 kcal% fat, 20 kcal% protein and 35 kcal% carbohydrate. Phenotyping tests were performed according to EMPReSS (European Phenotyping Resource for Standardised Screens from EUMORPHIA) standardized protocols as described at (http://empress.har.mrc.ac.uk).
RNA extraction and qPCR
Total ribonucleic acid (RNA) was prepared from brain, hypothalamus, gastrocnemius muscle, pancreas, brown adipose tissue (BAT) and epigondadal white adipose tissue (WAT) of free-fed female mice using RNeasy fibrous Mini Kit, RNeasy for skeletal muscle, a Lipid Tissue Mini Kit for BAT and WAT (Qiagen, USA), and a RNeasy Plus Mini Kit (Qiagen, USA for hypothalamus, according to the manufacturer’s protocol. RNA concentration was assessed using a NanoDrop ND-1000 spectrophotometer (Thermo-Fischer Scientific, USA). Extracted RNA was stored at −80°C.
cDNA was prepared using superscript III reverse transcriptase (Invitrogen, USA) according to the manufacturer’s instructions. For each tissue, quantitative PCR was performed using TaqMan Gene Expression Assay reagents and TaqMan FAM dye-labelled probes (Applied Biosystems Inc, USA), using an ABIPRISM 7700 Fast Real-Time PCR System (Applied Biosystems Inc, USA). All data were normalized to expression of the endogenous housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and analyzed by the Comparative ΔΔCT method to determine the difference in sample groups relative to control animals. Hrpt and EGFP were tested by semiquantitative PCR using the primers described18,40.
Protein Extraction and Immunoblotting
Mouse skeletal muscle and liver tissue samples were homogenized and Western blotting performed as previously described19. Western blots were performed on 40 μg of total proteins using a custom rabbit anti recombinant mFTO antibody.
Body Composition analysis
Body composition was anaysed by dual energy X-ray absorptiometry (DEXA) using the Lunar PIXImus Mouse Densitometer (Wipro GE Healthcare, WI, USA).
Histology
Mice were killed by exsanguniation. Epigonadal WAT was dissected and fixed in neutral buffered formaldehyde (Surgipath Europe Ltd, United Kingdom). Paraffin-embedded sections of epigonadal WAT were stained with hematoxylin and eosin. Photomicrographs were captured by optic microscopy (Zeiss Axiostar Plus, Göttingen, Germany) with the ALTRA20 Soft Imaging System (Olympus, Muenster, Germany) Adipocyte area was measured under the same microscope at x40 with the aid of computerized image analysis (Soft Imaging System cell*, Olympus, Muenster, Germany).
Metabolic and endocrine testing
Mice (10 or 19 weeks old) were placed in metabolic cages (Techniplast) for measurement of food, water and urine. Plasma leptin, insulin, and adiponectin levels were measured using a mouse endocrine MILLIPLEX kit (MILLIPLEX MAP, Millipore, Billerica, MA, USA), and a Bio-Plex 200 system (Bio-Rad, Hemel Hempstead, UK), according to the manufacturer’s instructions. Plasma insulin was measured at 8, 16 and 20 weeks of age, and leptin was measured at 8 and 20 weeks of age. At 20 weeks of age, fasted mice were anaesthetized, killed by exsanguination and blood was collected by cardiac puncture. Plasma concentrations of albumin, glucose, triglycerides, ALP, alkaline Phosphatase; ALT, alanine aminotransferase; AST; aspartate aminotransferase; FFA, free fatty acids; LDH, lactate dehydrogenase; CK, creatine kinase; Cholesterol (total cholesterol), HDL-C, HDL-cholesterol; LDL; LDL-Cholesterol were measured on an AU400 (Olympus UK), as described41.
Metabolic Rate and Activity Measurements
Metabolic rate was measured at 18 weeks of age using indirect calorimetry (Oxymax; Columbus Instruments) to determine oxygen consumption, carbon dioxide production, respiratory exchange ratio (RER) and heat production19. Oxygen consumption and carbon dioxide production were normalised to body weight, fat and lean tissue mass. Heat production (energy expenditure) was calculated using; Heat = CV×VO2, CV = 3.815 +1.232 × RER (CV, calorific value based on the observed respiratory exchange ratio; Oxymax; Columbus Instruments). Physical activity was assessed by circadian wheel running activity42.
Intraperitoneal Glucose Tolerance Test
Mice were fasted overnight (16 hours) to establish a baseline glucose level “T0” (time zero). Mice were weighed, and a blood sample collected from the tail vein after administration of local anesthestic (EMLA cream, Eutectic Mixture of Local Anesthetics, Lidocaine/Prilocaine, AstraZeneca, UK)using Lithium-Heparin microvette tubes (Sarstedt, Germany). They were then injected intraperitoneally with 2 g glucose/kg body weight (20% glucose in 0.9% NaCl). Blood samples were taken at 60 and 120 min (or 10, 20 and 30 min) after injection. Plasma glucose was measured using an Analox Glucose Analyser GM9 (Analox, UK). Plasma insulin was measured using a Mercodia ultrasensitive mouse ELISA kit (Mercodia, Sweden). AUC analysis was performed using GraphPad Prism version 5.02 for Windows.
Statistical methods
Results are expressed as mean±s.e.m. Comparisons between two groups were made by unpaired two-tailed Student’s t-test and one-way analysis of variance (ANOVA) with repeated measures, as appropriate (GraphPad Prism). AUC analysis performed using GraphPad Prism. The relationship between body compositions, including body weight, lean mass, fat mass and the fat mass/lean mass ratio (FM/LM), with food intake, leptin, oxygen consumption, and carbon dioxide production were evaluated by linear regression analysis (GraphPad Prism). P<0.05 was considered to be statistically significant. Multiple regression analysis was carried out using R (http://www.r-project.org/).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors have no competing financial interests.
References
- 1.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 2.Scuteri A, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinney A, et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS One. 2007;2:e1361. doi: 10.1371/journal.pone.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dina C, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–6. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 6.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–66. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 7.Haupt A, et al. Variation in the FTO gene influences food intake but not energy expenditure. Exp Clin Endocrinol Diabetes. 2009;117:194–7. doi: 10.1055/s-0028-1087176. [DOI] [PubMed] [Google Scholar]
- 8.Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 2008;16:1961–5. doi: 10.1038/oby.2008.318. [DOI] [PubMed] [Google Scholar]
- 9.Timpson NJ, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88:971–8. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardle J, et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 11.Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes (Lond) 2009;33:42–5. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- 12.Tanofsky-Kraff M, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr. 2009;90:1483–8. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonestedt E, et al. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90:1418–25. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- 14.Gerken T, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–72. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boissel S, et al. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet. 2009;85:106–11. doi: 10.1016/j.ajhg.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia G, et al. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008;582:3313–9. doi: 10.1016/j.febslet.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han Z, et al. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature. 2010;464:1205–9. doi: 10.1038/nature08921. [DOI] [PubMed] [Google Scholar]
- 18.Fischer J, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–8. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 19.Church C, et al. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet. 2009;5:e1000599. doi: 10.1371/journal.pgen.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 21.Olszewski PK, et al. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci. 2009;10:129. doi: 10.1186/1471-2202-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunnet LG, et al. Regulation and function of FTO mRNA expression in human skeletal muscle and subcutaneous adipose tissue. Diabetes. 2009;58:2402–8. doi: 10.2337/db09-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloting N, et al. Inverse relationship between obesity and FTO gene expression in visceral adipose tissue in humans. Diabetologia. 2008;51:641–7. doi: 10.1007/s00125-008-0928-9. [DOI] [PubMed] [Google Scholar]
- 24.Villalobos-Comparan M, et al. The FTO gene is associated with adulthood obesity in the Mexican population. Obesity (Silver Spring) 2008;16:2296–301. doi: 10.1038/oby.2008.367. [DOI] [PubMed] [Google Scholar]
- 25.Wahlen K, Sjolin E, Hoffstedt J. The common rs9939609 gene variant of the fat mass- and obesity-associated gene FTO is related to fat cell lipolysis. J Lipid Res. 2008;49:607–11. doi: 10.1194/jlr.M700448-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Zabena C, et al. The FTO obesity gene. Genotyping and gene expression analysis in morbidly obese patients. Obes Surg. 2009;19:87–95. doi: 10.1007/s11695-008-9727-0. [DOI] [PubMed] [Google Scholar]
- 27.Fredriksson R, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149:2062–71. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- 28.Stratigopoulos G, et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1185–96. doi: 10.1152/ajpregu.00839.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tung YC, et al. Hypothalamic-specific manipulation of Fto, the ortholog of the human obesity gene FTO, affects food intake in rats. PLoS One. 2010;5:e8771. doi: 10.1371/journal.pone.0008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakanen M, et al. FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity. J Clin Endocrinol Metab. 2009;94:1281–7. doi: 10.1210/jc.2008-1199. [DOI] [PubMed] [Google Scholar]
- 31.Do R, et al. Genetic variants of FTO influence adiposity, insulin sensitivity, leptin levels, and resting metabolic rate in the Quebec Family Study. Diabetes. 2008;57:1147–50. doi: 10.2337/db07-1267. [DOI] [PubMed] [Google Scholar]
- 32.Berulava T, Horsthemke B. The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet. 2010;18:1054–6. doi: 10.1038/ejhg.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haupt A, et al. Impact of variation in the FTO gene on whole body fat distribution, ectopic fat, and weight loss. Obesity (Silver Spring) 2008;16:1969–72. doi: 10.1038/oby.2008.283. [DOI] [PubMed] [Google Scholar]
- 34.Qi L, et al. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes. 2008;57:3145–51. doi: 10.2337/db08-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berentzen T, et al. Lack of association of fatness-related FTO gene variants with energy expenditure or physical activity. J Clin Endocrinol Metab. 2008;93:2904–8. doi: 10.1210/jc.2008-0007. [DOI] [PubMed] [Google Scholar]
- 36.Goossens GH, et al. Several obesity- and nutrient-related gene polymorphisms but not FTO and UCP variants modulate postabsorptive resting energy expenditure and fat-induced thermogenesis in obese individuals: the NUGENOB study. Int J Obes (Lond) 2009;33:669–79. doi: 10.1038/ijo.2009.59. [DOI] [PubMed] [Google Scholar]
- 37.Jonsson A, et al. Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults. Diabetologia. 2009;52:1334–8. doi: 10.1007/s00125-009-1355-2. [DOI] [PubMed] [Google Scholar]
- 38.Vimaleswaran KS, et al. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. Am J Clin Nutr. 2009;90:425–8. doi: 10.3945/ajcn.2009.27652. [DOI] [PubMed] [Google Scholar]
- 39.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girard CA, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hough TA, et al. Novel phenotypes identified by plasma biochemical screening in the mouse. Mamm Genome. 2002;13:595–602. doi: 10.1007/s00335-002-2188-1. [DOI] [PubMed] [Google Scholar]
- 42.Bacon Y, et al. Screening for novel ENU-induced rhythm, entrainment and activity mutants. Genes Brain Behav. 2004;3:196–205. doi: 10.1111/j.1601-183X.2004.00070.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.