Abstract
The cardiac neural crest (arising from the level of hindbrain rhombomeres 6–8) contributes to the septation of the cardiac outflow tract and the formation of aortic arches. Removal of this population after neural tube closure results in severe septation defects in the chick, reminiscent of human birth defects. Because neural crest cells from other axial levels have regenerative capacity, we asked whether the cardiac neural crest might also regenerate at early stages in a manner that declines with time. Accordingly, we find that ablation of presumptive cardiac crest at stage 7, as the neural folds elevate, results in reformation of migrating cardiac neural crest by stage 13. Fate mapping reveals that the new population derives largely from the neuroepithelium ventral and rostral to the ablation. The stage of ablation dictates the competence of residual tissue to regulate and regenerate, as this capacity is lost by stage 9, consistent with previous reports. These findings suggest that there is a temporal window during which the presumptive cardiac neural crest has the capacity to regulate and regenerate, but this regenerative ability is lost earlier than in other neural crest populations.
Keywords: Regeneration, ablation, cardiac neural crest, cardiac outflow tract, aorticopulmonary septum, regulation
INTRODUCTION
The contribution of cardiac neural crest cells to the heart and outflow tract is crucial for normal cardiovascular development and function. The term “cardiac neural crest” was coined almost three decades ago in response to findings suggesting that a limited subregion of the neural crest is key for cardiovascular development: bilateral ablation of the neural crest and neural folds at the caudal hindbrain level in the stage 9 chick (neurula stage) resulted in abnormal remodeling of the great vessels and a failure of the cardiac outflow tract to become septated into the aorta and the pulmonary trunk (Kirby et al., 1983). These conotruncal and septal defects mimic important aspects of DiGeorge and CHARGE syndromes seen in human birth defects, which are widely accepted to involve aberrant development of the cardiac neural crest (Creazzo et al., 1998; Jerome and Papaioannou, 2001; Wurdak et al., 2006). Thus, attention has been focused on the special qualities of this apparently unique neural crest sub-population.
Prior to migration, both ablation studies and fate-mapping studies have revealed that cardiac neural crest cells occupy the dorsal neural tube of the hindbrain from the mid-otic level through the level of somite 3 (rhombomeres 6–8). The neural crest emigrates from the hindbrain as it becomes segmented into eight rhombomeres, with the migrating neural crest cells forming three separate streams adjacent to rhombomeres 2, 4 and 6. The rhombomeres and the streams provide convenient landmarks for studying the regional differences of the neural crest, and may predict distinct fates. For example the neural crest arising from rhombomere 6 (r6), migrates in the r6 stream, coursing caudal to the otic vesicle to populate the third branchial arch (Birgbauer et al., 1995; Kulesa et al., 2000; Le Douarin and Kalcheim, 1999). By performing inter-species grafts from quail to chick, it has been possible to map the fate of the r6 stream and establish that it is the most rostral set of cardiac neural crest cells (Miyagawa-Tomita et al., 1991). After delamination from the neural tube, the cardiac crest from the r6 stream and somites 1–3 level take a circumpharyngeal route (Kuratani and Kirby, 1991) and populate branchial arches 3, 4 and 6. Within the branchial arches, cardiac neural crest cells help form the tunica media of persisting arch arteries and help regulate their regression (Waldo et al., 1996). Further ventrally, cardiac neural crest cells partition the outflow tract of the forming heart into aorta and pulmonary trunk (Kirby et al., 1983), thereby helping to establish oxygenated and de-oxygenated circulatory systems.
Interestingly, the origin of the cardiac neural crest overlaps significantly with the origin of the vagal neural crest, which has distinct fates and regenerative abilities. The vagal neural crest emigrates from the neural tube adjacent to somites 1–7 and gives rise to the enteric nervous system that innervates the gut (Le Douarin and Teillet, 1973). The overlap of these two distinct populations at the level of somites 1–3 raises questions about the mechanisms that assign fate to the cardiac and vagal neural crest. These two populations of neural crest also differ in their ability to regenerate or regulate after ablation. In contrast to the regeneration seen at vagal levels (Barlow et al., 2008), studies of the cardiac neural crest show that it cannot reform after ablation at neurula stages (stage 9/10) (Kirby et al., 1983; Kirby et al., 1985). This might be stage-dependent, as there is some indication of regeneration if the cardiac neural crest is ablated earlier in development (Sechrist et al., 1995; Suzuki and Kirby, 1997). This incomplete regeneration following ablation might reflect a redirection of adjacent neural crest cells to become cardiac neural crest and/or a cell fate change of non-neural crest cells (i.e. ventromedial neuroepithelium or lateral ectoderm) to become cardiac neural crest cells. Either redirected migration or change of prospective cell fate after cardiac neural crest ablation would minimize the potential abnormalities following cardiac neural crest ablation. Any such regulation would have implications for understanding the development of the neural crest and the etiology of cardiac birth defects.
Dynamic fate mapping of the dorsal neural tube and neural crest (Ezin et al., 2009) makes it possible to identify the origins of the vagal and cardiac neural crest, offering a means to better understand the difference between these two populations. The dynamic fate map reveals that the presumptive neural crest cells of a wide range of axial levels originate from an intermixed crescent-shaped domain at early gastrula stages. Through cell rearrangements and shearing motions, the cells in this progenitor region reorganize to take up predictable positions along the neural axis by the early neurula (stage 7). Cardiac neural crest precursors are initially located in the neural folds at the level forming somites 1–3 (Ezin et al., 2009). From stage 7 to stage 10, this region expands rostrally to include r6. This dynamic fate map enables experimental tests of the development of the cardiac neural crest at earlier stages than previously possible.
To better understand the timing, spatial location and nature of the cardiac neural crest’s development, we tested whether these cells might have the capacity to regenerate at early stages, as they first take up their positions as a recognizable and distinct population. To this end, we ablated the cardiac neural crest population at chick stage 7 (1–2 somite stage) and examined the subsequent effects on cardiac development. The results show that the presumptive cardiac neural crest can be replaced when ablated at this stage. Lipophilic dye labeling reveals that the new cardiac crest cells are formed from neuroepithelial cells ventromedial to the ablation, as well as neural crest cells rostral to the ablation (particularly from the r5 level), which are not known to normally contribute cardiac descendants. The aorticopulmonary septum forms normally in these embryos, albeit in a delayed fashion. The branchial arch arteries undergo appropriate maintenance, although their regression seems delayed. The enteric nervous system forms normally with little delay. The profound regulative ability to form cardiac neural crest diminishes with time and is lost by stage 9.
MATERIALS AND METHODS
Embryos
Fertilized Colorado Red and White Leghorn chicken eggs, obtained from local farms, were incubated horizontally at 38°C for 26 to 31 hours to obtain stage 7 embryos, or 36 to 42 hours to obtain stage 9–10 embryos (Hamburger and Hamilton, 1951). The eggs were sprayed with 70% ethanol, and 3mL of egg white was extracted from each egg with a syringe and reserved. The top of the egg was windowed with a small pair of sharp scissors and a small amount of freshly made solution of organic blue food dye (8mg/mL in Ringer’s solution containing 1% Penicillin and Streptomycin) was injected sub-blastodermally. Embryos at stage 7 (1 or 2 somites) or stage 10 (9–11 somites) had their vitelline membrane opened with a glass needle after 20µL of sterile Ringer’s solution was applied. To faciliate experimental manipulation, the embryo was raised to the top of the egg by adding 3–5 mL of the egg white reserved earlier. After the experimental manipulation and/or dye labeling, a few drops of Ringer’s solution were deposited carefully around the embryo, the egg was sealed with clear adhesive tape and re-incubated in a humidified incubator. In this study, control embryos were un-operated embryos collected at the same time as the ablated embryos. The dye labeled controls were injected but not ablated.
Presumptive Cardiac Neural Crest Ablations
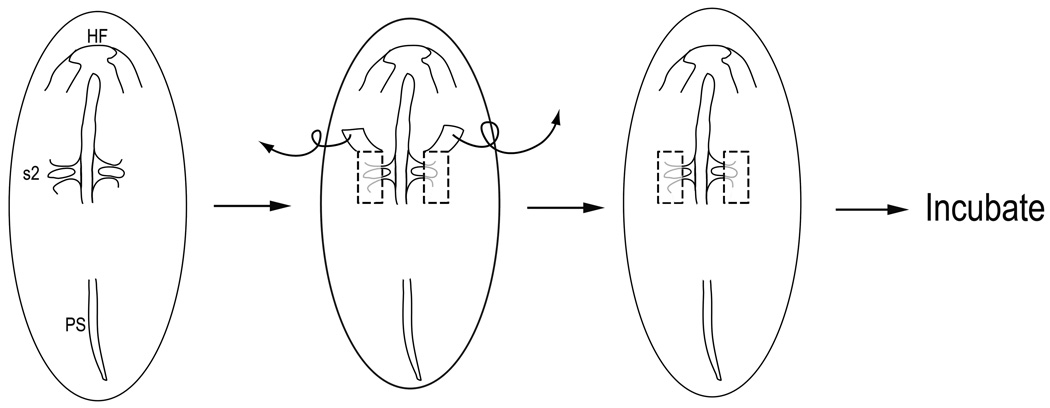
The presumptive cardiac neural crest tissue was removed from stage 7 chick embryos using glass needles, guided by our dynamic fate map (Ezin et al., 2009). Visualizing the embryo under a Nikon SMZ-ZT microscope and illuminating it with a fiber-optic lamp, a pair of mediolateral incisions were made one somite’s length rostral to and caudal to the first formed somite (somite number 2); a rostrocaudal cut was made along the middle of the somites (being careful to not damage the forming somites), and another rostrocaudal cut was made more laterally at the edge of the neural fold and non-neural ectoderm (Fig. 1). The tissue was peeled back and removed. This region removes the forming cardiac neural crest, which will later extend from the level of rostral r6 and/or r5 (mid-otic level) to the caudal edge of somite 3 (Fig. 2A–C). In a few embryos, we performed a more extensive rostral ablation, removing the neural folds as far forward as the caudal midbrain. Eggs were sealed and re-incubated at 38°C for 24–36 hours (to stage 12+-15), or until Embryonic Day 4 (E4) or E6. Any deformed embryos were discarded. Cardiac neural crest ablations at stage 10 (9–11 somites) were performed by making incisions perpendicular to the neural tube at the r5/r6 boundary and immediately caudal to somite 3; the region of dorsal neural tube from rostral r6 to somite 4 was then removed.
Figure 1.
To remove presumptive cardiac neural crest at stage 7 in ovo, bilateral cuts were made with a glass needle to remove the neural folds of chick embryos having 1 or 2 somites. Incisions extended one-somite’s length rostral and caudal to the first formed somite (presumptive somite 2). The medial edge of the incision ran along the middle of the somites without damaging the underlying somitic tissue. The lateral edge of the cut was just beyond the border of the neural fold and non-neural ectoderm. The ablated tissue was removed and eggs were sealed and re-incubated until the desired stage.
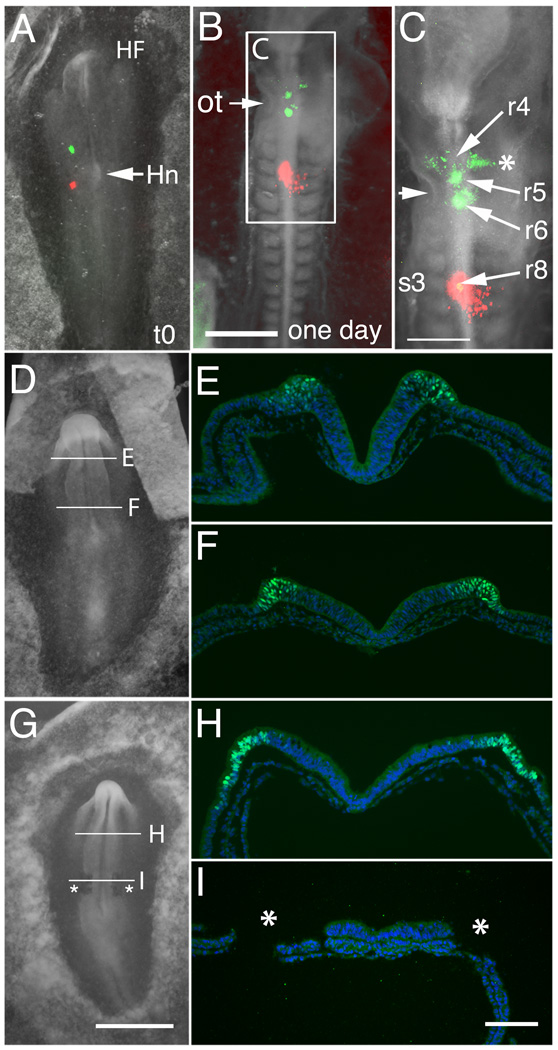
Figure 2.
Fate map analysis of normal and ablated embryos. (A–C) Dye-labeling of control embryos reveals that presumptive cardiac neural crest lies in the neural folds at the rostral margin of forming somite 1 (DiO, green) and caudal margin of forming somite 3 (DiI, red) in a representative embryo at stage 7 (A, t=0min). (B) Twenty-four hours after labeling. (C) Enlargement of “B”. By stage 12/13, the green neural fold injection originally placed at somite 1 level now labels the neural tube/neural crest at the mid-otic level (ot, arrow; rhombomeres 5/6 area); the red neural fold injection spot originally placed at somite 3 level remains at the same somite 3 level (rhombomere 8). The neural fold cells at the level of somite 1 at stage 7 translocate rostrally and the neural fold undergoes extension, resulting in a forward shift of the neural folds of ~100 mm. The DiO-positive cells in the r4 stream (asterisk) come from r5. The two midline DiO spots are at the level of r5 and r6. (D–I) Expression of Pax7, a marker of the forming dorsal neural folds, at stage 7 in control and ablated embryos. (D) Whole mount of a stage 7 control embryo. (E) Representative section showing Pax7 expression (green) in the neural folds at the level of the midbrain. DAPI (blue) stains cell nuclei. (F) Pax7 expression in the forming neural folds at the level of the caudal hindbrain (which contains the presumptive cardiac neural crest). (G) Whole mount of a stage 7 embryo after bilateral ablation of the cardiac neural folds from level of somite 1–3 (asterisks). (H) Pax7 expression in a similar embryo, as in (G), in the neural folds at the level of the intact midbrain. (I) Absence of Pax7 expression confirms the removal of the cardiac neural folds at the level of ablation (asterisks; region of ablated folds).
Abbreviations: HF- head fold; Hn-Hensen’s node; ot-otic cup; r4, r5 r6 and r8-Rhombomeres 4, 5, 6 and 8.
Scale bars. G: 1mm, in panels A, D, G. B: 500µm. C: 300µm. I: 100µm, in panels E, F, H, I.
Lipophilic Dye Labeling
We used focal lipophilic dye injections to validate the fate map of the cardiac neural crest at stage 7, as well as to determine the derivatives of the labeled cell populations in control and in ablated embryos. We used paired injections of DiI (red, CM-DiI or Vybrant DiI, Molecular Probes, 1 mM) and DiO (green, Vybrant DiO, Molecular Probes, 1 mM) to differentially label two cell populations in each embryo. CM-DiI was resuspended in 10 µL of absolute ethanol, diluted with 90µL of 30% sucrose for a final concentration of 0.5 µg/µL. Focal injections were carried out using a fine-tipped glass micropipette with a Picospritzer III (Parker Instrumentation). Some non-operated embryos were DiI-labeled at stage 10 to examine pathways of cardiac neural crest migration. After labeling, the embryos were imaged using a Leica MZFLIII microscope, an X-cite series 120 fluorescent lamp and an RT Color Spot camera (Diagnostic Instruments, Inc).
Immunohistochemistry
Embryos were fixed in 4% paraformaldehyde for 20 min at room temperature for stage 7 embryos or for 24 hours at 4°C for older embryos, extensively washed in buffer (PBS + 0.1% Tween) and blocked in antibody buffer (PBS + 0.1% Tween + 5% (by volume) goat serum + 0.2% BSA) for one hour before the primary antibody Pax7 (Aviva System Biology; diluted 1:1 in antibody buffer) was used to recognize the dorsal neural tube, pre-migratory and migrating neural crest and somitic tissue. Large volumes of wash buffer were used to wash the embryos 8–10 times before the incubation with the fluorescently labeled (alexa-488 or alexa 594) secondary antibody for 12 hours at 4°C (1:200 or 1:1000 dilution). After a minimum of 8 washes, stage 13 embryos were imaged in whole mount on a Zeiss Discovery M2 Bid microscope using the Axiovision (Rel 4.2) software. They were then embedded in gelatin, sectioned at 10µm on a Microm HM 550 cryostat and stained with Human Natural Killer 1 (HNK1) antibody (hybridoma culture media diluted 1:50 in antibody buffer).
Older embryos (E4 or E6) were sectioned and processed for section antibody staining with the Tuj1 antibody (recognizing beta tubulin in nerve cells; 1:250 dilution; from Covance) overnight at 4°C and the SMA antibody (recognizing smooth muscle actin; 1:250 dilution; from Sigma-Aldrich). Sections were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) to identify cell nuclei before the slides were coverslipped in Permafluor (Thermo Scientific) and imaged on a Zeiss Axioskop Plus, using the Axiovision (Rel 4.6) software. Imaging was performed using Zeiss 10× Fluar (NA 0.5), 20× plan apochromat (NA 0.75) and 40× plan apochromat (NA 0.95 Korr) objectives. Image processing was done using Adobe Photoshop CS4 Extended Version 11.0.
Data Analysis
Control and cardiac crest ablated embryos were categorized by the presence or absence of a septated outflow tract and presence or remodeling of branchial arteries at selected developmental stages (E4 and E6). A two-tailed Fisher’s exact test was used to determine the significance of the differences (p=0.05) in the presence or absence of a septated outflow tract.
RESULTS
Fate map of the presumptive cardiac neural crest at stage 7
Our previous dynamic fate map of the neural crest (Ezin et al., 2009) showed that it is possible to identify progenitors of particular neural crest subpopulations by stage 7. To validate these predictions and focus on the presumptive cardiac neural crest, we labeled regions of the neural folds with lipophilic green (DiO) and red (DiI) dyes at stage 7. Dye injections of the forming neural folds at the level of somite 1 demonstrated that this region extends forward and contributes to the dorsal neural tube at the mid-otic level by stages 10–13 (Fig. 2A–C). Labeling of this axial level marks both rostral rhombomere 6 (r6) and caudal r5, including the neural crest they generate. As previously observed, the neural crest cells often migrate bilaterally even when only one side is labeled (Scherson et al., 1993; Sechrist et al., 1995; Couly et al, 1996).
DiI injections into the neural folds at the segmental plate level of future somite 3 demonstrated that this region contributes to the neural tube adjacent to somite 3 by stages 10–13 (Fig. 2A–C). This is the caudal limit of the cardiac neural crest (Suzuki and Kirby, 1997). Thus, at stage 7, the progenitors of the cardiac neural crest largely reside in the neural folds adjacent to future somite 1 through 3.
DiI labeling of dorsal r5 or r6 at stage 10 demonstrated an important contribution from both rhombomeres to the cardiac neural crest. From caudal r5, neural crest cells take a path between the dorsal neural tube and the otic cup, forming bilateral tracts, which are continuous with the r6 streams more caudally (Suppl. Fig. 1A–E). This is consistent with previous findings suggesting that caudal r5 contributes to caudally migrating neural crest cells (Birgbauer et al., 1995). Upon delamination, neural crest cells from r6 exit the neural tube as distinct, bilateral streams of migrating cells. The remainder of the cardiac neural crest arises from the vagal region, at the level of somites 1–3.
Ablation of the neural folds at stage 7 removes the presumptive cardiac neural crest
To test the regenerative capacity of the cardiac neural crest, we microsurgically ablated the cardiac neural folds at stage 7 (Fig. 1). As schematized in Figure 1, bilateral ablation requires cutting a rectangular region (400 × 200 × 40 µm) of the forming neural fold and carefully peeling off the tissue without damaging the underlying somites or segmental plate. To confirm the efficacy of the ablations, we fixed several embryos immediately after microsurgical dissection of the presumptive cardiac neural crest and stained them for Pax7, the earliest marker for neural crest precursors within the dorsal neural folds, and later of early migrating neural crest cells and somites. Because the early ablated embryos are extremely fragile, we could only perform antibody staining at these stages and not in situ hybridization. In un-operated stage 7 embryos, the forming neural folds are Pax7-positive, directly above the somites and segmental plate, as seen in transverse section (Fig. 2D–F). At the level of surgery, sections reveal that the full thickness of the neural folds has been extirpated, leaving no Pax7 expression in the ablated region (Fig. 2G, I). As an internal control, we find that Pax7 is retained in the neural folds rostral (Fig. 2H) and caudal to the site of surgery.
Cardiac neural crest cells are partially restored by stage 13 in embryos ablated at stage 7 but not at stage 9–10
To examine whether dorsal neural tube and migrating neural crest cells were present at the level of the cardiac neural crest after neural fold ablation at stage 7, embryos were incubated to stages 13–14 (Fig 3A and 3F) when neural crest cells should normally be actively migrating. We used two markers to identify migrating neural crest cells, Pax7, which marks the dorsal neural tube, migrating neural crest cells as well as the somites, and HNK1, a marker for migrating neural crest cells. A return of Pax7 in the dorsal neural tube at the original site of ablation would indicate that the dorsal neural tube fate is restored.
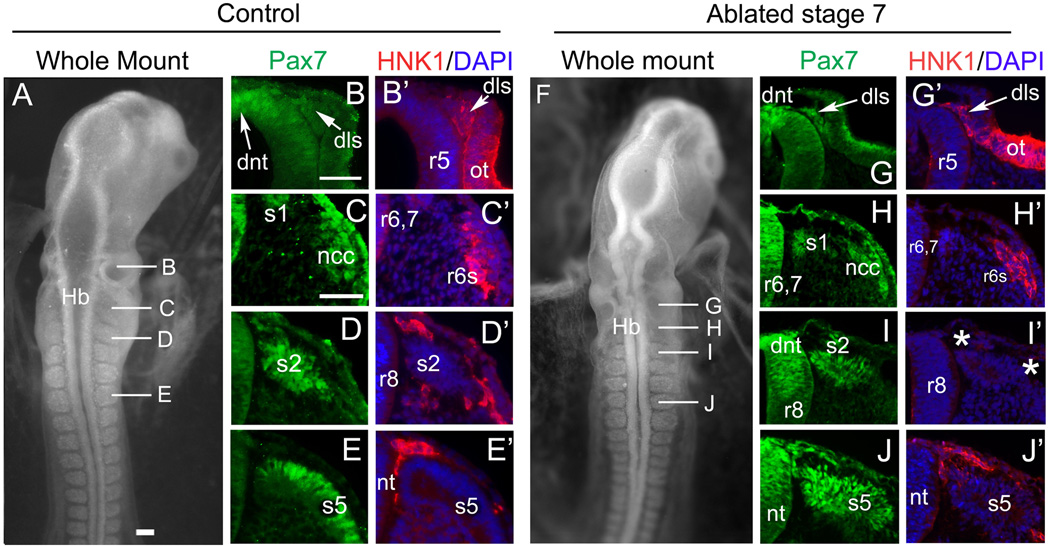
Figure 3.
The dorsal neural tube at caudal hindbrain level and the cardiac neural crest re-forms by stage 13 in embryos ablated at stage 7. Pax7 (green) marks the dorsal neural tube, the somites and migrating neural crest cells, HNK1 (red) marks migrating neural crest cells, DAPI (blue) stains all nuclei. (A–E) represent a control embryo at stage 13, (F–J) show a stage 13 embryo that was subjected to cardiac neural crest ablation at stage 7. (A) Whole mount control embryo at stage 13 with representative sections in B–E. Lines indicate level of corresponding sections. (B–B’) Mid-otic (caudal r5) level. (B) Pax7 expression is seen in the dorsal neural tube (dnt) and dorsolateral stream (dls) of neural crest migrating between otic vesicle and dorsal neural tube. (B’) Dorsolateral stream cells express HNK1, as does the otic vesicle (ot). (C–C’) r6/7 level. (C) The dorsal neural tube, somite 1 and migrating neural crest cells (ncc) express Pax7. (C’) The caudo-lateral r6 stream (r6s) of migrating neural crest cells co-expresses HNK1 and Pax7. (D–D’) r8 level at somite 2 (s2). (D) Pax7 positive somite and neural crest cells. (D’) HNK1 positive neural crest cells migrate on the dorsal and ventral aspects of somite 2. (E–E’) Vagal level at somite 5. (E) Pax7 expression in somite 5. (E’) HNK1 positive neural crest cells migrating at the level of somite 5. (F) Whole mount of a stage 13 embryo that was subjected to cardiac neural crest ablation at stage 7. Lines indicate level of corresponding sections. (G–G’) Level of r5. (G) Pax7 expression is visible in the dorsal neural tube and dorsolateral stream. (G’) HNK1 expression in the migrating cells of the dorsolateral stream and the otic cup. (H–H’) Level of r6,7. (H’) The dorsal neural tube, somite 1 and neural crest cells express Pax7. (H’) The migrating neural crest cells at the r6 stream express HNK1. (I–I’) Level of r8 at somite 2. (I) Pax 7 positive dorsal neural tube and somite 2. (I’) Neural crest cells at this level are missing (asterisks), or at least significantly reduced, as evident by the lack of HNK1 staining around the somite. (J–J’) Caudal to the level of ablation, Pax7- and HNK1-positive neural crest cells are present around somite 5, which also expresses Pax7.
Abbreviations: dls- dorsolateral stream; Hb- hindbrain; nt- neural tube; ot-otic cup; r5, r6, r8- rhombomere 5, 6, 8; s1, s2, s5- somite 1, 2, 5.
Scale bars in A = 100mm, applies to panel F; B= 50mm, applies to all other panels.
Rostral and caudal to the ablation site, stage 7 ablated embryos (n=17) appeared very similar to unoperated controls. For example, at the r4 level of the hindbrain, the ablated embryos showed normal expression of Pax7 in the dorsal neural tube and a normal, large stream of Pax7- and HNK1-positive cells (the r4 stream; Suppl. Fig. 2B,H). Just rostral to the ablation site (caudal r5), the otic vesicle is closely apposed to the neural tube, with an accumulation of neural crest cells called the “dorsolateral stream” in the open space between the dorsal neural tube and otic vesicle, similar to controls (compare Fig 3B,B’ to 3G,G’; see also Suppl. Fig. 1A–C). In some embryos, the otic vesicle of ablated embryos appeared abnormal and the dorsal ectoderm was either torn or mis-apposed. Caudal to the ablation, the dorsal neural tube, somites and the neural crest cells all appeared intact, with normal morphology, migration and Pax7 and HNK1 expression (Fig. 3E,E’ and 3J,J’).
At the level of the ablation (r6–r8), some differences were noted between the dorsal neural tube and neural crest cells of ablated versus control embryos. As shown previously (Fig. 2I), immediately after ablation at stage 7, there is a clear absence of Pax7 staining in the neural folds at the level of ablation (presumptive cardiac crest level). When embryos ablated at stage 7 are incubated to stage 13–14, the dorsal aspect of the neural tube re-expresses Pax7 by stage 13–14. The presence of Pax7 staining indicates a re-acquisition of a normal dorsal neural tube fate (Fig. 3H and I). The r6 stream was also prominent and positive for HNK1 and Pax7 (compare Fig. 3C,C’ with 3H,H’; n=17). In contrast, in embryos ablated at stages 9–10, Pax7 expression was absent in the neural tube within the ablated region by stage 13–14 (data not shown), and the r6 stream either did not form at all or contained only a few scattered neural crest cells (Suppl. Fig. 2P).
By stage 13–14, at the level of somites 1–3 (r7 and r8), there was an absence of migrating neural crest cells in embryos ablated at both stage 7 and stage 9–10. Although the dorsal neural tube and somites expressed Pax7, few if any HNK1-positive neural crest cells migrated from the neural tube at this level by stage 13 (asterisks in Fig. 3I’ and Suppl. Fig. 2Q). In contrast, in control embryos, HNK1-positive neural crest cells migrated both ventral to and over somites 1–3 (Fig. 3D,D’). The absence of neural crest cells results from a delay, since embryos ablated at stage 7 but incubated to older stages (i.e. stage 15) exhibited neural crest migration at the somite 1–3 level (data not shown). In embryos ablated at stage 9–10, the neural tube at the level of somites 1–3 failed to generate cardiac crest cells (Suppl. Fig. 2P,Q) even when incubated to older stages.
Formation of the aorticopulmonary septum occurs in embryos with cardiac crest ablation at stage 7 but not at stage 9–10
The cardiac neural crest plays a seminal role in the septation of the cardiac outflow tract, and the absence of cardiac neural crest leads to the absence of septation. Thus, the presence of a septum would indicate the reconstitution of the cardiac neural crest after its ablation at stage 7. To determine whether regeneration of cardiac neural crest observed at stage 13–14 (Fig. 3) subsequently led to formation of a septum in the cardiac outflow tract, some ablated embryos were incubated until Embryonic Day 4 (E4), by which time septation has occurred in control embryos (Fig. 4A). At E4, the hearts of the ablated embryos (n=4) appeared immature relative to control embryos (n=8) and did not have a septated outflow tract (Fig 4C; Table 1, p=0.002). Table 1 indicates the presence and absence of septation for control and ablated embryos at both E4 and E6.
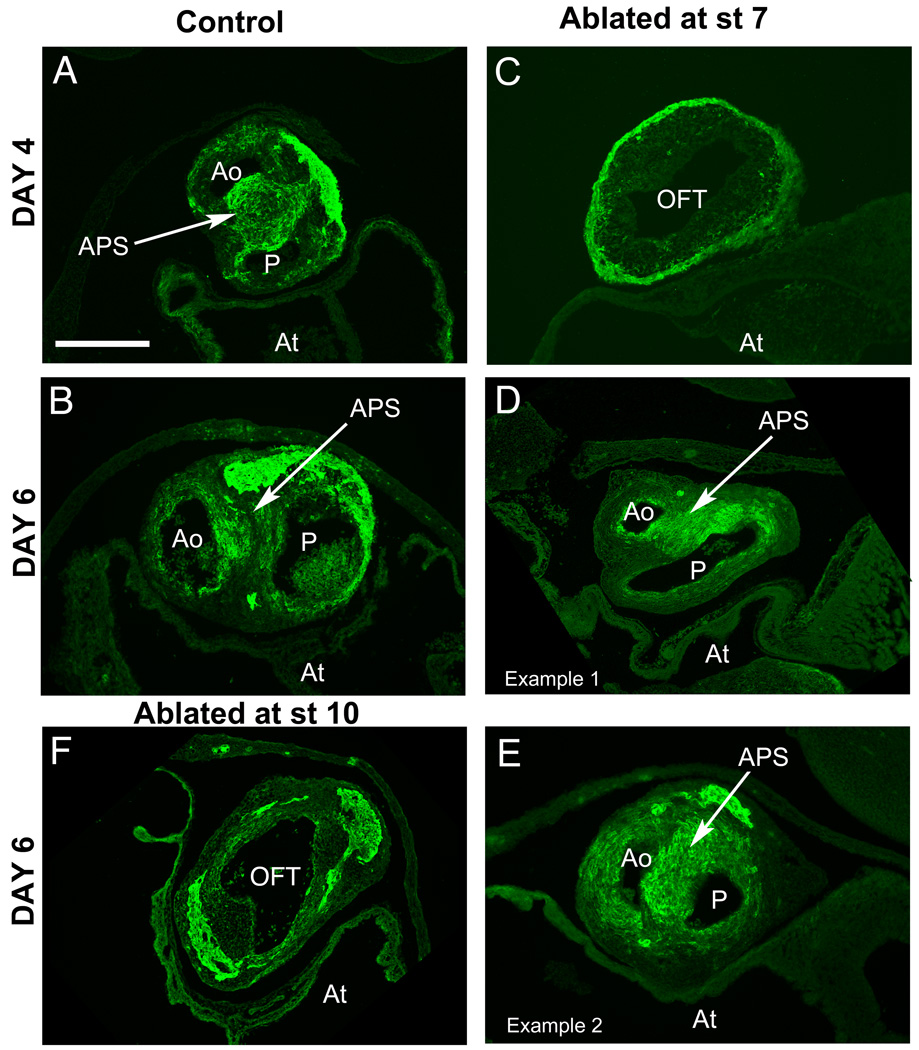
Figure 4.
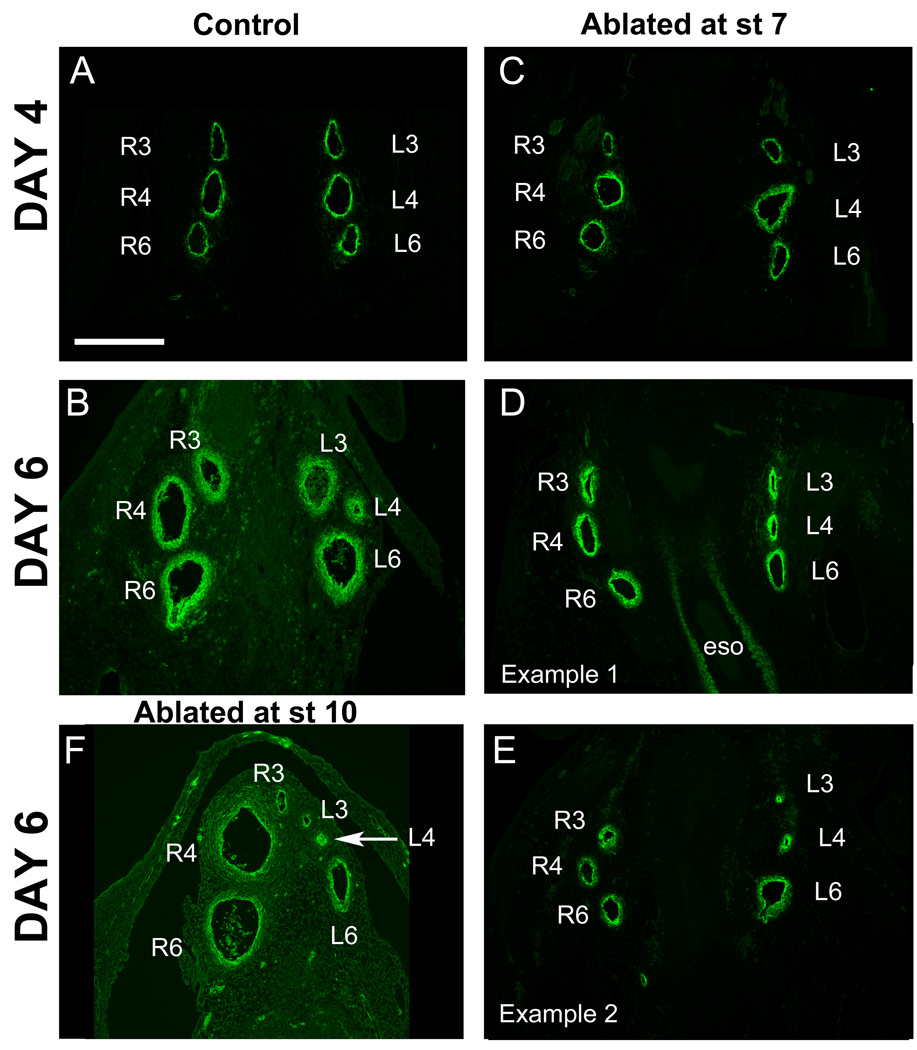
The outflow tract septum forms in embryos subjected to ablation of the presumptive cardiac neural crest at stage 7. (A, B) Sections through control Day 4 and Day 6 outflow tract show the presence of the aorticopulmonary septum (APS) with heavy smooth muscle actin (SMA) staining (green). (C) A section through the outflow tract of a Day 4 embryo after cardiac neural crest ablation at stage 7 lacks the septum. (D,E) By Day 6, septation has occurred in embryos subjected to cardiac neural crest ablation at stage 7. (D) An embryo in which outflow tract septation occurred albeit in a delayed manner, with strong SMA staining in the aorticopulmonary septum. (E) Another Day 6 embryo after ablation illustrating septum formation with more advanced ourflow tract rotation than “E”. (F) In contrast, an embryo after cardiac neural crest ablation at stage 10 (10–11 somite stage) has an unseptated outflow tract even at Day 6.
Abbreviations: Ao- aorta; APS – aorticopulmonary septum; At- atrium; OFT- outflow tract; P- pulmonary trunk.
Scale bar: 300mm.
Table 1.
Presence or Absence of Septum Formation in Control and Cardiac Crest Ablated Embryos at E4 and E6.
Fisher’s Exact Test, p = 0.003
Septation occurred, but the septum is variable in appearance.
The absence of a septum at E4 appears to represent a developmental delay rather than a permanent failure to septate. Ablated embryos allowed to develop for an additional two days, to E6, displayed a muscularized outflow tract septum that expressed smooth muscle actin (n=8; compare Fig. 4B to 4D,E; Table 1). Although the septum formed by E6 in all ablated cases, the degree of maturation of the septum in these embryos varied between that observed in E4 (n=8; Fig. 4A) and E6 (n=8; Fig. 4B) controls. After formation of the septum at E4, the outflow tract usually rotates 90° between E4 and E6. By E6, some ablated embryos showed no rotation or only partial rotation, again reflecting a developmental delay (Suppl. Table 1).
In contrast, embryos (n=3) in which the cardiac neural crest was ablated at stage 9/10 lacked septation even by E6 (Fig. 4F). This is in agreement with numerous previous reports in which the cardiac neural crest contributions to the heart are absent after neural crest ablation at stage 9/10.
The upper membranous portion of the interventricular septum in the heart derives from cardiac neural crest cells. At E6 in controls, the interventricular septum is not yet fully formed (Suppl. Fig. 3A,B). Septum formation in the stage 7 ablated embryos was at least as developed as in the E6 controls (Suppl. Fig. 3C,D). In contrast, the stage 9/10 ablated embryos displayed a large opening between ventricles at the upper membranous level (Suppl. Fig. 3E,F). Thus, there seems to be adequate cardiac crest regeneration in stage 7 ablated embryos since they have a nearly closed interventricular septum similar to controls.
To ensure that the apparent regeneration was not due to incomplete ablation of the cardiac neural crest, we performed larger ablations at stage 7, removing the neural folds of more rostral rhombomeres in addition to the cardiac neural crest. With ablation up to the level of r4, HNK1+ neural crest cells were observed migrating in the r6 stream at stage 15 (data not shown). At E6, in an embryo ablated from r2 through r8, an abnormal misaligned septum expressing detectable smooth muscle actin formed. The 3rd and 4th but not 6th arch arteries were present and the septum appeared to abnormally separate the left 4th from the other fused arches (Left 3rd and Right 3rd and 4th; data not shown). With the pulmonary trunk absent and the pulmonary arteries connecting to the 4th arch arteries, there is inadequate separation of oxygenated and de-oxygenated blood circuits.
Ablation of the presumptive cardiac neural crest at stage 7 does not inhibit morphogenesis of branchial arch arteries
The cardiac neural crest regulates the maintenance and remodeling of the paired branchial arch arteries 3, 4 and 6 (presumptive aorta, pulmonary arteries, paired ductus arteriosus, brachiocephalic arteries and carotid arteries) and helps form their tunica media (Suppl. Fig. 1G). To determine if embryos subjected to cardiac neural crest ablation at stage 7 could maintain these three paired branchial arch arteries, ablated embryos were incubated until E4 (n=4). At this stage, all six arch arteries are patent in both ablated (n=4) and control (n=7) embryos (Fig. 5A and 5C, Suppl. Table 2), suggesting that the cardiac neural crest had been reconstituted. The ablated embryos display slight abnormalities including asymmetries of the arteries across the midline and uneven spacing between individual vessels (Bockman et al., 1989).
Figure 5.
The branchial arch arteries persist in embryos subjected to ablation of the presumptive cardiac neural crest at stage 7. (A) All 3 pairs of branchial arch arteries are equally patent at Day 4 in control embryos. (B) By Day 6 in control embryos, the left 4th artery is undergoing regression. By Day 6, the left 4th artery is undergoing regression. (C) In embryos ablated at stage 7, the arch arteries also remain patent by Day 4 (C) but remodel by Day 6 in ablated embryos (D, E). The left 3rd and 4th arteries undergo variable regression in some cases (exemplified in D). In other cases, the left 4th alone regresses or the 6th arteries may regress. (F) In an embryo subjected to cardiac neural crest ablation at stage 10, the arch arteries regress unpredictably, as seen here. The right 3rd, as well as the left 3rd and 4th (arrow), are regressed while the right 4th and 6th are enlarged.
Abbreviations: eso- esophagus; L3, L4, L6- left third, fourth and sixth arch arteries; R3, R4, R6- right third, fourth and sixth arch arteries.
Scale bar: 300mm.
By Day 6, most of the embryos ablated at stage 7 (n=6 of 8) successfully accomplished the next developmental phase of remodeling, in which arch arteries 3, 4 and 6 are reshaped to establish separate pulmonary and systemic blood circuits. Remodeling includes: a) the fusion of the 6th arch arteries to form the pulmonary trunk, b) the reduction and elimination of the left 4th arch artery (see Suppl. Table 2), c) the retention (in the chick) of the right 4th aortic arch artery to form the aorta (see Suppl. Table 2) and d) the branching of both 3rd arch arteries from the aorta to form the brachiocephalic arteries supplying blood to the wings and the head.
Three embryos ablated at stage 7 and allowed to develop to E6 were analyzed in depth using serial sections to track the pattern of vessel remodeling. In all three cases, the paired 6th arteries were patent and fused to give rise to the pulmonary trunk, establishing the pulmonary blood system as in controls. Vessels were further remodeled to give rise to a functional systemic vessel network, but the manner in which this was generated seemed variable. The left 4th aortic arch artery regressed in 1 of 3 cases only; in the other two, it merged into the right 4th arch (aorta) in one instance and into the left 3rd arch in the other. The aorta arose normally from the right 4th artery in two cases; however, it arose from the 3rd branchial arch artery in one case. The 3rd arch arteries connected normally to the right 4th artery in the cases in which they did not give rise to the aorta. Thus, although there was significant variability, remodeling events were sufficiently complete to give rise to functional and separate systemic and pulmonary circulatory systems.
In contrast, embryos that undergo cardiac neural crest ablation at stages 9–10 exhibit abnormal vessel remodeling (Bockman et al., 1989). To replicate these results, we performed cardiac neural crest ablation experiments at stage 9–10, removing the neural folds from the level of caudal r5 to somite 3–4. In a typical E6 example (Fig. 5F), the 6th arch arteries formed but, while the left 6th arch artery was normal in size, the right 6th and right 4th arteries were overly large. The left 4th arch artery began to regress but the other arch arteries regressed unpredictably and appeared smaller in size than controls (both right and left 3rd arch arteries).
Cells from ventromedial r5 and r6 in embryos ablated at stage 7 regulate to form cardiac crest cells by stage 14
To ascertain the source of the regenerated population, we labeled either the medial or lateral edges of the control and ablated neural folds with lipophilic dyes (DiI and DiO) at stage 7. Normally (without ablation), DiI labeled cells from this medial position of the neural plate give rise to the ventral or intermediate regions of the neural tube (Fig. 6A–D, F, G). In contrast to marking the neural tube, dye-labeled cells at a lateral position contributed to the lateral ectoderm (Fig. 6E).
Figure 6.
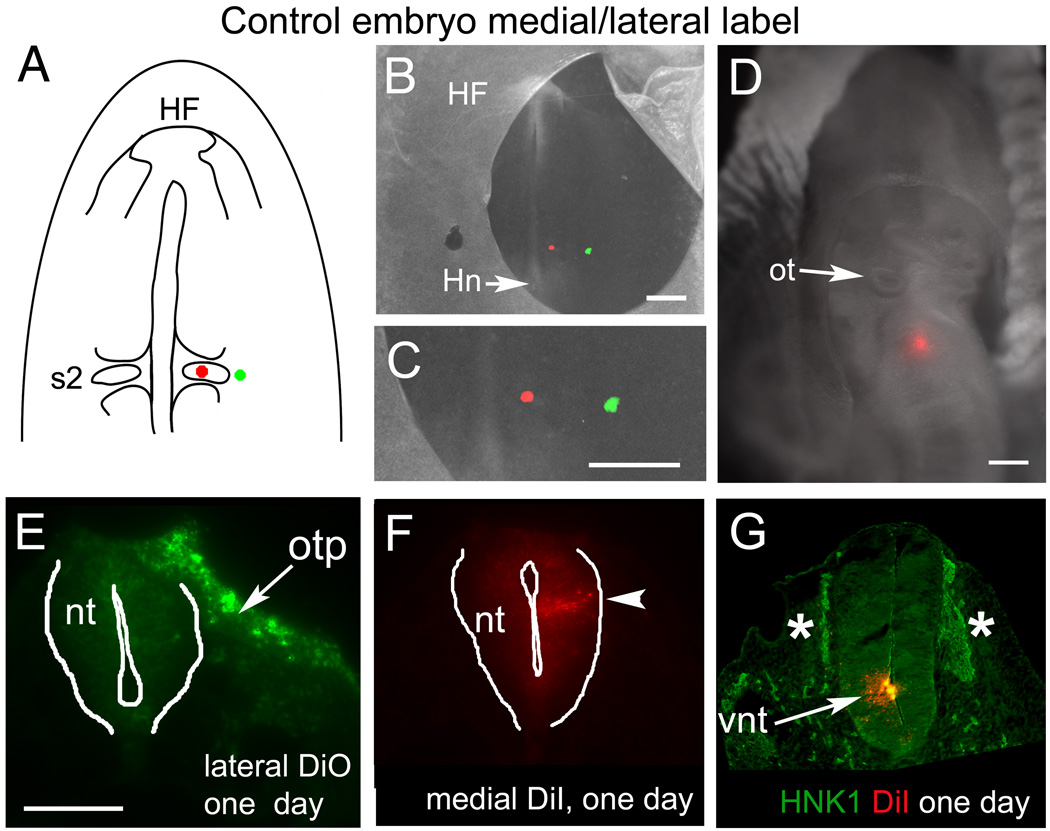
Fate of the ventromedial neuroepithelium and lateral ectoderm in control embryos. (A) Schematic diagram showing the placement of dye injection spots—medial DiI and lateral DiO at somite 2 level. (B–C) Whole mount image of an embryo immediately after injection at stage 7. (C) Close-up of the region labeled in “B”. (D) Whole mount image of another embryo, labeled with a single, ventromedial post-otic injection of DiI at stage 7, and re-incubated to stage 13. (E) Representative section at stage 12 through the lateral, DiO-labeled region showing that those cells (in “C”) gave rise to ectoderm; in this case, to the caudal otic placode (arrow, otp) and lateral ectoderm. (F) The medial, DiI-labeled population is confined to an intermediate region in the post-otic neural tube at stage 12. (G) Section through the embryo in “D”, showing that the DiI-labeled cells (arrow) are confined to the ventral neural tube (vnt) and do not co-localize with dorsal neural tube cells or HNK1 positive migrating neural crest (asterisks).
Abbreviations: HF- head fold; Hn- Hensen’s node; nt- neural tube; ot- otic cup; otp- otic placode; s2- somite 2; vnt- ventral neural tube.
Scale bars. B, C, D: 200mm; E, F, G: 100mm.
Embryos ablated and labeled at stage 7 (Fig. 7A) were examined at stages 12–14 for potential contribution of the labeled cell populations to the cardiac neural crest cells. In ablated embryos, the medial edge of the ablation site at the level of presumptive r5/r6 contributed to the dorsal neural tube and reconstituted a population of migrating cardiac neural crest in the r6 streams (Fig. 7B,C). For example, the ablated embryo in figure 7C was labeled with multiple DiI spots at the medial edge of the ablated neural folds. After one day of incubation, the rostral labeled cells adjacent to somite 1 in this same embryo populated the dorsal neural tube at r5 and 6, as well as contributed to both the preotic (r4/5) and cardiac (r5/6) neural crest streams (Fig. 7C’–F). At the level of r8, medial DiI labeled cells populated the dorsal neural tube or fold, but expressed little or no Pax7 protein, and gave rise to very few or no HNK1-positive migrating neural crest cells by stage 13 (Fig. 7 C’). Caudal to the ablation, migrating HNK1+ neural crest cells were visible (data not shown but see Fig. 3E’, J’ and Suppl. Fig. 2L).
Figure 7.
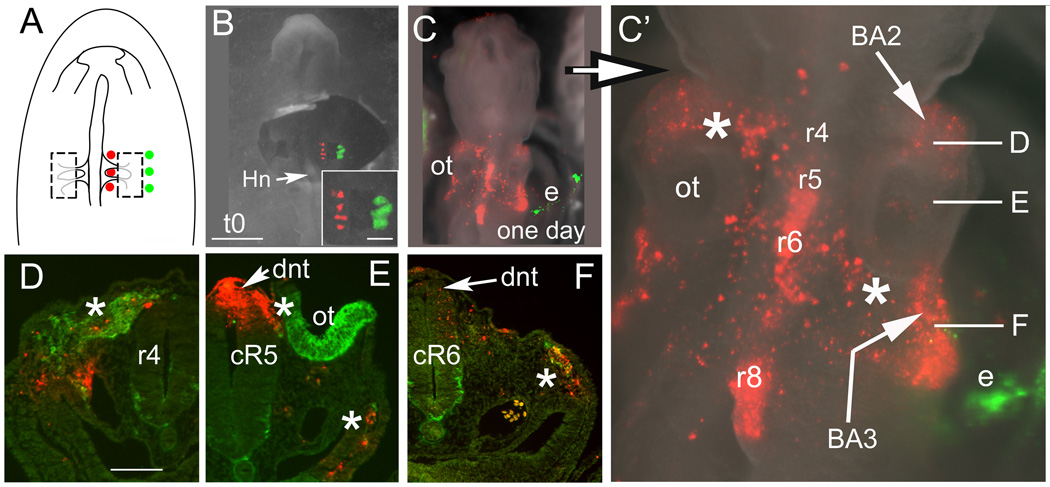
After bilateral neural fold ablation at stage 7, the ventromedial neuroepithelial cells change fate to form dorsal neural folds and subsequently migrating neural crest cells by stage 13. (A) Schematic diagram showing the multiple placement of dye injection spots medial and lateral to the ablation site on the right side. (B, inset) Whole mount images of the embryo immediately after ablation and labeling at stage 7. Ventromedial cells are DiI-labeled and lateral ectoderm cells are DiO-labeled. Inset shows an enlargement of “B”. (C, C’) Whole mount images of the same ablated embryo shown in “B” after re-incubation to stage 13. The dorsal hindbrain (r4, r5, r6, r8) neural tube cells labeled with DiI were previously ventromedial cells at stage 7. (C’) Enlargement of “C”. DiI-labeled neural crest cells migrate bilaterally to the 2nd and 3rd branchial arches (BA2, BA3) by way of r4 (upper left asterisk) and r6 (lower right asterisk) streams. Caudal DiI spot in the dorsal neural tube at occipital somite level (r8) generates few neural crest cells by this stage. The DiO–labeled cells (green, e) in the lateral ectoderm do not contribute to the neural tube or neural crest population, labeling the lateral ectoderm and extra-embryonic membrane instead. Axial level of sections “D–F” are indicated. (D) Cross section through r4 revealing the r4 stream (asterisk), rostral to the ablation site. DiI-labeled cells (red) are within the HNK1 expressing region (green). The dorsal neural tube is not labeled, indicating that the neural crest cells originate from the more caudal r5 level. (E) Section at the level of caudal r5. The dorsal neural tube (dnt, arrow) is heavily labeled. Labeled, dorsolateral stream cells (left of top asterisk) are migrating between the otic cup (ot, green, HNK1 staining of caudal otic cup) and neural tube. Ventrolateral to the foregut, dye labeled cells co-localize with HNK1+ staining of the neural crest (bottom asterisk). (F) Level of caudal r6 (cR6). The dorsal neural tube (arrowhead) and a few proximal migrating neural crest cells are lightly labeled with DiI. Some labeled neural crest cells are migrating and approaching the circumpharyngeal neural crest. The DiI/HNK1 staining (asterisk) just rostral to somite 1 is the caudal aspect of the r6 stream.
Abbreviations: BA2, BA3- branchial arch 2, 3; cR5, cR6- caudal rhombomeres 5, 6; dnt-dorsal neural tube; e- lateral ectoderm or extraembryonic membrane; Hn- Hensen’s node; ot- otic cup; r4, r5, r6, r8- rhombomere 4, 5, 6, 8.
Scale bars. B, C: 200mm; inset, D–F: 100mm.
Cells lateral to the ablation site did not contribute to the reconstituted cardiac crest cells. Instead, DiO labeled lateral cells remained in the lateral ectoderm, similar to controls (Fig. C and C’). In one case, a few labeled cells were observed in the dorsal neural tube as well (n=1 of 4 embryos).
Neural folds rostral and caudal to the cut regulate to form cardiac neural crest
In addition to the ventral neural tube, it is possible that neural crest populations adjacent to the ablation site also contribute to the reconstituted cardiac neural crest. To test this, we labeled the rostral and caudal edges of the cut with lipophilic dye and re-incubated embryos to stages 13–14 (Fig. 8). In unoperated control embryos, the rostral axial level normally contributes to the r4 and/or r5 streams (n=5 control embryos, green label; Fig. 8A–C; see also 2A–C). In ablated embryos (n=6), the original dye injection spot rostral to the cut site labels the dorsal neural tube at r5 (strongest green label in Fig. 8D–F’ and Fig 8H). Lighter label at the level of r3 and r4 suggests that the neural tube stretched in the r3 and r4 (Fig. 8G) dorsal neural tube regions from the original injection at r5. Neural crest cells from the labeled dorsal neural tube migrated in both the r2 and r4 streams (rostral two asterisks in Fig. 8 F’ and G). Interestingly, the r4 stream had an extra branch that extended caudally (arrow in Fig. 8F’). Labeled neural crest cells also began migrating caudally at r6 level (Fig. 8I).
Figure 8.
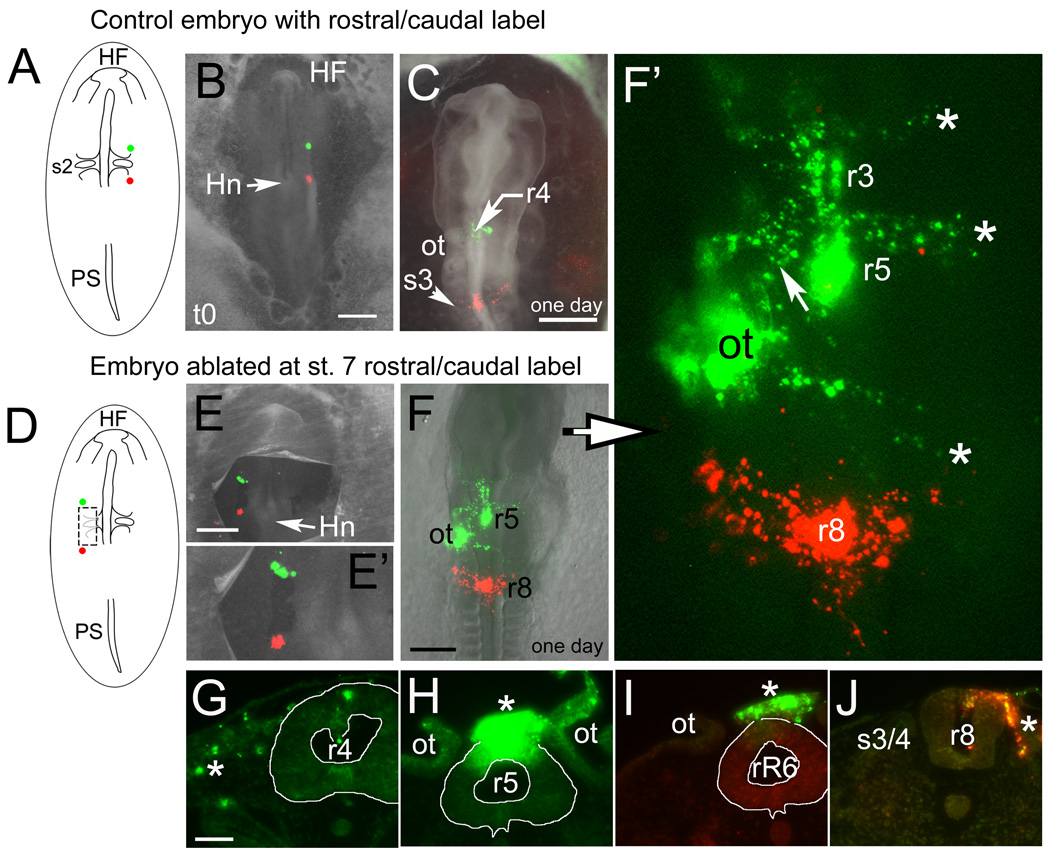
The neural fold cells rostral and caudal to the ablation site also contribute to neural crest cells in the ablated region by stage 12/13. (A–C) control embryos and (D–J) ablated embryos. (A) Schematic showing the rostrocaudal placement of dye injection spots. (B) Whole mount image of an embryo immediately after injection at stage 7. (C) Whole mount image of a similar embryo incubated to stage 12 after labeling at stage 7. The rostral DiO labeled cells populate the dorsal neural tube and neural crest stream at r4 (just rostral to otic level) and the caudal DiI labeled cells are in the dorsal tube and neural crest from the level of somite 3/4 (s3). (D) Schematic diagram showing the rostrocaudal placement of dye injection spots on the left side in an embryo ablated at stage 7. (E) Whole mount image of an embryo after ablation and rostro-caudal dye labeling at stage 7. (E’) Enlargement of dye injection shown in “E”. (F and F’) Same embryo shown in “E” after incubation to stage 13 and shown enlarged and without bright field in “F’ ”. The rostral DiO labeled cells populate the dorsal neural tube at r3, r4, r5 and r7 as well as the r2, r4 and r7 migrating neural crest streams (asterisks, from the top-r2, r4 and r7 streams). Neural crest cells that will contribute to the r5/r6 streams appear delayed in their dorsomedial exit from the tube (see “I”). The large DiO patch on the left side of the embryo is ectoderm label overlapping the otic vesicle (ot). Between r4 and the otic vesicle is an extra, DiO-labeled stream of cells (arrow) on the left side. The caudal, DiI labeled cells are in the dorsal tube (r8 level) and in neural crest from the level of somite 3 migrating laterally and forward as in control embryos. (G–J) Sections through the dye-labeled regions of the embryo shown in “E–F”. (G) Rhombomere 4 level. DiO labels migrating neural crest cells (asterisk) and to a lesser extent the dorsal neural tube. (H) Rhombomere 5, near mid-otic level. The dorsal neural tube and overlying neural crest (asterisk) are very heavily labeled, indicating this region as the probable original site of injection. (I) Rostral r6 level. Labeled neural crest cells (asterisk) from r5 are found in merged dorsolateral streams above the neural tube but have not yet extended ventrolaterally as the r6 stream. (J) Somite 3/4 level. DiI-labeled neural crest cells are seen in the dorsal tube and migrating (asterisk) medial to the somite.
Abbreviations: HF- head fold; Hn- Hensen’s node; ot- otic cup; PS- primitive streak; r3, r4, r5, r6, r7, r8-rhombomere 3, 4, 5, 7, 8; rR6- Rostral rhombomere 6; s3/4- somite 3/4.
Scale bars. B, C, E, F: 500mm; G: 300mm; G–J: 50mm.
Neural crest cells at the level of somite 4 normally make a limited contribution to the caudal cardiac neural crest (Suzuki and Kirby, 1997; Fig. 8A–C; red label). In embryos ablated at stage 7, labeling the forming neural folds caudal to the cut site (n=6; red label) gave rise to dorsal neural tube cells and migrating neural crest cells (Fig. 8D–F’, 8J) at stages 13–14, similar to controls. As in controls, the contribution to circumpharyngeal neural crest seemed lower than from r5 and r6 levels.
Ablation of the presumptive cardiac neural crest at stage 7 does not disturb innervation of the gut
The enteric nervous system derives from the vagal neural crest (Anderson et al., 2006; Le Douarin, 1982), which partially overlaps with cardiac neural crest. As a result, we asked whether the reformation of the cardiac neural crest after ablation at stage 7 occurs at the expense of the contribution from the same region to the enteric nervous system.
The enteric nervous system has an extrinsic component (the vagal nerve) and an intrinsic component (the neuronal cells embedded within the gut itself), both of which derive from the vagal region of the neural tube. The intrinsic neurons arise from neural crest cells that migrate ventrolaterally (Suppl. Fig. 1F). Staining with the neuronal marker, Tuj1, revealed that both components of the enteric nervous system formed normally in ablated embryos (n=9). The vagal nerve was seen outside the gut sending projections into the gut at E4 and E6, as in controls (Suppl. Fig. 4A and data not shown). Neural crest-derived Tuj1-positive cell bodies clustered in ganglia embedded within the gut itself included bipolar and unipolar cells with extended nerve processes (Suppl. Fig. 4A’, B and B’). Moreover, the intrinsic enteric nervous system was organized into a normal myenteric plexus within and just outside the forming outer muscle layer (Suppl. Fig. 4B and B’). Thus, the enteric nervous system formed normally and without major developmental delay in embryos whose cardiac neural crest cells were removed at stage 7.
DISCUSSION
Understanding the progression of embryonic events that give cells and tissues positional identity and defined cell fates is key to both developmental biology and regenerative medicine. Ablation studies have played pivotal roles in our understanding of regulative events by offering insights into the fates of the missing cells (Chabry, 1887; Roux, 1974), as well as informing on the stages at which tissue patterning becomes fixed (De Bernardi, 2009; Reverberi and Minganti, 1946). Studies of the regeneration of tissues after ablation have served as important means for revealing the range of mechanisms by which cells and tissues are patterned. At one extreme, regeneration takes place by a regrowth of the missing pieces from regions neighboring the ablation (French et al., 1976); alternatively, regeneration of the body plan can take place by the repatterning of the remaining portions to reconstitute a complete tissue (Gierer, 1981). In tissues sufficiently mature to have a fully elaborated pattern, regeneration is often accompanied by a pronounced dedifferentiation of the organized cells; in embryos, regeneration of ablated regions can take place without apparent dedifferentiation (Vaglia and Hall, 1999).
Ablation studies of the cardiac neural crest have produced much of our current understanding of this important subpopulation of the neural crest. By ablating parts of the neural tube or neural folds (Kirby et al., 1983; Kirby et al., 1985) together with cell marking techniques like quail-chick chimerae (Phillips et al., 1987), the neural crest cells that contribute to the heart were shown to originate from the post-otic region, overlapping with the regions that give rise to the enteric nervous system (Le Douarin and Teillet, 1973). Mammalian cardiac neural crest originates from a similar region as chick (Chan et al., 2004; Jiang et al., 2000) and genetic ablation of the cardiac neural crest gives rise to similar cardiovascular defects as those observed in the chick embryo (Ivanova et al., 2005; Porras and Brown, 2008). Interestingly, the cardiac neural crest is set aside earlier and is more specialized than neural crest from other axial levels. For example, other regions of neural fold grafted in place of the cardiac neural crest are incapable of substituting for the ablated cells (Kirby, 1989) and vice versa (Lwigale et al., 2004). Moreover, regeneration of the cardiac neural crest appears to be reduced and nearly absent (Suzuki and Kirby, 1997) at stages when other axial levels including r6 maintain regenerative ability (Scherson et al., 1993; Sechrist et al., 1995; Suzuki and Kirby, 1997). Together, these findings suggest that the fates of the cardiac neural crest are specified by interactions that occur earlier than for other axial levels.
Our recent dynamic fate map of the neural crest (Ezin et al., 2009) shows that the precursors of different axial regions of the neural crest are intermixed until shortly before neural fold stages. Accordingly, the early specification of the cardiac neural crest must involve either a segregation of the cardiac population from the rest of the neural crest before neurulation or a patterning in response to signals present in a restricted region for a limited time at the beginning of neurulation.
The early neurula can regulate to reconstitute cardiac neural crest cells
To better define the stages at which key events in cardiac neural crest specification take place, we have extended previous ablation analyses to earlier stages. Guided by the dynamic fate map of the neural crest (Ezin et al., 2009), we performed ablations at stage 7 at the somite 1–3 level (early neurula) and found that the embryos had relatively normal cardiac crest derivatives. A new or re-directed population of migratory cells emerged from the neural tubes of these early ablated embryos by stages 13–15. The cardiovascular ectomesenchymal derivatives of the cardiac neural crest, specifically the aorticopulmonary septum and the tunica media (muscular layer) of the branchial arch arteries, were delayed but consistently present by E6. We noted smooth muscle actin staining in the aorticopulmonary septum, suggesting that it had undergone myocardialization (Boot et al., 2004; van den Hoff et al., 1999). The reconstituted cardiac neural crest in embryos ablated at stage 7 appeared to be functional, based on the persistence and remodeling of aortic arch arteries 3, 4 and 6 through E6 (Waldo et al., 1996). Thus, some cell populations must have regulated to take on the fate of the cardiac neural crest.
Our results from stage 7 ablations are consistent with previous work showing that the neural tube has a higher capacity to regulate for ablation at earlier stages than at later ones (Sechrist et al., 1995). Suzuki and Kirby (1997) noted some limited regeneration after stage 8 (3–5 somite stage) ablations, “without reconstitution of the entirety of ablated crest population”. Our ablations performed at stage 9–10 agree with the extensive experiments of Kirby and colleagues that showed a failure to regenerate following ablation of the cardiac neural crest in late-neurula stage (stages 9–10) chick embryos. In their previous experiments, ablation of the neural folds above somites 1–3 or from mid-otic level to somite 3 results in the absence of an aorticopulmonary septum (persistent truncus arteriosus; (Kirby et al., 1983; Kirby et al., 1985)), in abnormal vessel patterning (Bockman et al., 1987), and in abnormal immune and endocrine glands (Bockman and Kirby, 1984, 1985).
The ventral neuroepithelium of r5 through r8 and neural crest from r5 can regenerate cardiac neural crest
To better understand the contribution of the residual neural tube after ablation at stage 7, we labeled the ventromedial cut edge with lipophilic dye. Twenty-four hours later, at stage 13, dye-labeled cells were observed in dorsal r5 and rostral r6 (as well as dorsal r8) due to wound healing and morphogenetic movements (Fig. 7). Labeled cells were seen in migrating neural crest in r6 streams heading toward branchial arches 3 and 4 as well as in the r4 stream to branchial arch 2. The absence of labeled migrating neural crest cells from r8 is consistent with a similar absence of HNK1 staining at stage 13–14 (Fig 3I, Suppl. Fig. 2K). This represents only a delay, however; by stage 15–16, the dorsal neural tube expresses Pax7, and gives rise to HNK1-positive crest cells that migrate at these more caudal cardiac neural crest levels (data not shown). In contrast, labeled cells in the lateral non-neural ectoderm did not contribute to the reconstitution of the cardiac neural crest.
Taken together, these results indicate that ventromedial neuroepithelial cells have become re-specified as dorsal neural tube and neural crest cells. This change in fate might involve a down-regulation of Sonic Hedgehog (SHH) signaling, as shown in a recent paper where blocking of SHH signaling allowed ventromedial neuroepithelial cells at stage 10 to become respecificed as dorsal neural tube and neural crest cells (Hutson et al., 2009). Our experiments show that the ventral neural tube cells move dorsally to close the wound and are competent at stage 7 to respond to signals that induce the dorsal neural tube and cardiac neural crest cell fate.
To evaluate whether ventral neural tube cells alone, without input from r5 neural crest, can reconstitute the cardiac crest, we also performed larger ablations that extended rostrally to the level of r2. By E6, an r2–r8 ablation at stage 7 resulted in the formation of an abnormal and delayed septum that was misoriented; examination of vascular patterning showed that both branchial arch 6 arteries failed to form. These results suggest that ventromedial neuroepithelial cells alone partially regenerate cardiac neural crest.
Dye labeling rostral to the ablation in stage 7 embryos (Fig. 8E–J) and r5 control DiI injections (Suppl. Fig. 1A–C) show that r5 neural crest can contribute to the r6 post-otic stream. In ablated embryos, the descendants of labeled cells rostral to the cut site are found to contribute to labeled cells in the dorsal neural tube at r5 and in the neural crest cells from r5 migrating toward the r6 post-otic stream. This suggests a role for intact r5 in the reconstitution of the ablated cardiac neural crest. Neural crest cells from caudal r5 can normally contribute to the r6 stream (Birgbauer et al., 1995; Kulesa et al., 2000; Nieto et al., 1995; Saldivar et al., 1996) and neural crest cells from rostral and caudal to sites of neural crest ablation have been shown to alter their migration and fill in for the missing population (Couly et al., 1996). Furthermore, it has been shown that ablation of r4 neural folds can alter the production of neural crest by r5 (Ellies et al., 2002). This raises the intriguing possibility that a similar up-regulation of r5 neural crest production may result from r6 ablation.
Having a dynamic fate map (Ezin et al., 2009) of the presumptive neural crest at stage 7 was key in accurately determining the location of precursors of the cardiac neural crest and other neural crest. The difference between the present results demonstrating efficient regeneration of the cardiac neural crest, and those of Kirby and colleagues likely lie in both in the extent of the ablation and in the age of the embryo. The lack of full regeneration seen at stage 8 in the work of Kirby and colleagues (Suzuki and Kirby, 1997) may have been due to the embryos being a stage older than in our study and/or from removing all or part of r5. At stage 7, we find partial reconstitution of the cardiac neural crest when r5 is removed and complete functional regeneration if r5 neural fold is left intact, resulting in a substantial r6 stream. Our results show that, together with ventromedial neuroeptithelium, r5-derived neural crest cells help make a functional contribution to cardiac crest at early stages.
Our studies show that ventromedial neural tube cells make a significant contribution to the regenerated cardiac neural crest; in addition, we find that rostral and caudal neural crest populations also regulate to replace portions of the regenerated cardiac crest. The cells rostral to the ablation, particularly at the level of r5, are the major contributor to the regulative process. The lateral non-neural ectodermal cells contribute little, if at all, to the regeneration.
Regulative mechanisms to replace cardiac neural crest cells
Three events would be expected in the functional replacement of cardiac neural crest cells after ablation at stage 7: a) respecification of ventromedial neuroepithelial cells, b) respecification of flanking rostral and caudal neural crest cells, c) enhanced cell proliferation. This work offers direct evidence of the first two.
The fact that there remains delays and some abnormalities at E6 suggests that the ventral neuroepithelium: a) takes time to heal the wound and establish new cell-cell contacts with the lateral ectoderm and flanking folds, b) must synergize with r5 neural crest to regenerate the missing cells and/or c) must undergo additional cell division to generate the appropriate number of cardiac crest cells required for septation of the heart’s outflow tract and ventricles and remodeling of vessels. Although our work shows that the ventromedial neuroepithelium regulates to give rise to cardiac neural crest after ablation at stage 7, it is not yet clear whether the residual neuroepithelial cells that regulate after ablation represent an undifferentiated population of “neural stem cells” or cells that must de-differentiate to transfate into dorsal neural tube and neural crest.
The rostral and caudal flanking neural folds also help to compensate for cardiac neural crest ablation. By leaving r5 intact in our ablation, we noted that r5 contributed far more significantly to the regeneration of the cardiac neural crest than the neural fold at somite 4. Rhombomere 5 normally makes a small contribution to the r6 stream. In ablated embryos, an increased number of cells seem to be recruited and deployed from r5 to help form the r6 streams.
Given that a normal appearing enteric plexus formed in our ablated embryos, as observed in previous ablation studies (Barlow et al., 2008), the reconstitution of cardiac neural crest does not appear to occur at the expense of the enteric nervous system. Therefore, increased cell proliferation must accompany the regulation. Studies in mouse have shown that a threshold number of cardiac neural crest cells must be present for normal septation (Conway et al., 2000; Morgan et al., 2008). The relatively normal size of the aorticopulmonary septum, the interventricular septum and the remodeled aortic arch arteries at E6 suggest that the regulation has yielded a sufficient number of neural crest cells to reach this threshold after ablation at stage 7.
Innervation of the gut occurs normally
The enteric nervous system (ENS) is the subdivision of the peripheral nervous system that innervates the digestive tract (reviewed in (Anderson et al., 2006; Laranjeira and Pachnis, 2009; Le Douarin, 1982). The ENS derives from the vagal neural crest, which encompasses cells originating from the neural tube at the level of somites 1 to 7. The ENS we observe after ablation of the cardiac neural crest agrees with previous work (Peters-van der Sanden et al., 1993), showing that vagal neural crest cells from the level of somites 1–3 are not required for proper formation of the enteric nervous system. Recent studies suggest that the dorsal neural tube at the level of somites 3 to 6 is adequate for enteric nervous system formation (Barlow et al., 2008). Thus, the level of somite 4 to 7 together with any regenerated neural crest from somites 1–3 level are sufficient to colonize the gut and form the myenteric and submucosal plexuses. This shows that the replacement of the ablated tissues must be a true regulation rather than a diversion of ENS precursors from their normal fates.
In summary, the cardiac neural crest is a unique cell population critical for establishing an efficient circulatory system that can meet the oxygen and homeostatic needs of larger organisms. These special roles of the cardiac neural crest seem to be specified early in neurulation, perhaps beginning as early the stages when the progenitors are intermixed extensively at gastrula stages (~ stage 4) and grafting other levels of neural crest into the site of the cardiac neural crest at stage 9 results in an absence of a cardiac contribution (Kirby, 1989). The apparent limited regeneration after cardiac neural crest ablation at stage 8 and the lack of regeneration after ablation at stage 9 were surprising given the considerable regulative ability of more rostral cranial crest populations. This failure might have indicated a very early segregation of the cells that could make the key contributions of the cardiac neural crest. The results presented here show that, like other neural crest populations, cardiac neural crest cells can fully regenerate after early ablation, by a combination of 1) a replacement of the neural crest from remaining cells in the ventromedial neuroepithelium and 2) robust contribution from r5 neural crest rostral to the ablation when that region of the neural tube is left intact. Respecification of adjacent neural crest rostral to the ablation and replacement of neural crest from cells in the ventromedial epithelium demonstrated that signals capable of specifying the cardiac neural crest are still operational at stage 7. The loss of regeneration in chick by stage 9 makes the cardiac neural crest one of the earliest specified fates of the neural crest.
Supplementary Material
Supplementary Figure 1. DiI labeling of the hindbrain (mid-otic to somite 3 level) dorsal neural tube to mark migrating chick cardiac neural crest cells. (A–B) Whole mount images of HH12+ (18 somite) embryo after focal DiI injection into mid rhombomere 5 (r5) roof at HH10 (11 somite). Combined bright field (A) and fluorescent (B) images show initial central injection dye spot near caudal r5–r6 junction; labeled cells migrate both rostrally (to 2nd branchial arch) and caudally (to 3rd branchial arch) in a dorsolateral stream (dls) between the dorsal part of the otic vesicle (ot) and r5–r6 hindbrain. (C) Transverse section through rostral r6 and the caudal portion of the right otic vesicle (ot) of same embryo in (A) showing both DiI positive dorsolateral streams (dls) migrating caudally and soon ventrally toward the circumpharyngeal neural crest region (not shown); they will join neural crest cells from rostral r6 as shown below. (D–E) Whole mount images of HH12 (16 somite) embryo after localized DiI r6 tube fill at HH 10 (11 somite). (E) Migrating dye labeled neural crest cells at rostral r6 level are blocked by the otic vesicle (ot). Together with caudal migrating r5 cells, they contribute the anterior tract (caudal extension of dls) of the circumpharyngeal crest.
(F) Transverse section at the level of somite 3 (r8) of a HH14 (23 somite) 2 day embryo after focal DiI injection into roof of neural tube between 3rd somite pair at HH10+ (12 somite). The dye labeled neural crest (nc) cells have migrated along the ventrolateral pathway medial to the dermomyotome of somite 3 (s3). (G) Horizontal section through ventral branchial arches (BA 1–4) of a HH18 (35 somite) 3 day embryo after focal DiI injection into the roof of rostral r6 at HH 10 (10 somite). The majority of dye labeled cardiac neural crest cells (asterisk) is in the 3rd branchial arch with some in the 4th arch. Many cells surround the 3rd branchial artery with the leading ones (arrows) about to enter the wall of the aortic sac (AS). By embryonic day 4 (E4), as shown in the body of this study, some of these cells contribute to aortico-pulmonary septum formation in the heart outflow tract in unoperated embryos (DiI image available but not shown).
Abbreviations: AS- aortic sac; BA- branchial arch; dls- dorsolateral stream; nc- neural crest; ot- otic vesicle; r5, 6, 8- rhombomere 5, 6, 8; s3- somite 3.
Scale bars. 100 µm in all panels.
Supplementary Figure 2. The dorsal neural tube at caudal hindbrain level and the cardiac neural crest re-forms by stage 13 in embryos ablated at stage 7. HNK1 marks migrating neural crest cells. (A–F) represent a control embryo at stage 13, (G–L) show a stage 13 embryo that was subjected to cardiac neural crest ablation at stage 7, (M–R) are of a stage 13 embryo that was subjected to cardiac neural crest ablation at stage 9–10. (A) Whole mount control embryo at stage 13 with representative sections in B–F. (B) Rhombomere 4 (r4) just rostral to the otic vesicle. HNK1 labels the neural crest cells in the r4 stream. (C) Mid-otic level, caudal r5, showing HNK1 positive dorsolateral tract (dls; arrow) of neural crest migrating between otic vesicle and dorsal neural tube. The otic vesicle (ot) also expresses HNK1. (D) r6 level, with the caudo-lateral r5/r6 stream of migrating HNK1 positive neural crest cells. (E) r8 level at somite 2 (s2). HNK1 positive neural crest cells migrate on the dorsal and ventral aspects of somite 2. (F) HNK1 positive neural crest cells migrating at the level of somite 5. (G) Whole mount of a stage 13 embryo that was subjected to cardiac neural crest ablation at stage 7. Arrows in H–L indicate level of corresponding section described below. (H) Level of rhombomere 4, rostral to the ablated level, showing neural crest migration in the r4 stream. (I) Level of rostral r5 and otic vesicle, showing HNK1-positive cells in the dorsolateral stream, between the otic cup and the neural tube. (J) At the level of caudal rhombomere 6, the lateral stream is present, although migration is delayed: the leading edge of r5/r6 stream is located more dorsally than in the controls. (K) At the occipital somite level (s2 here), neural crest cells are missing (asterisk), as evident by the lack of HNK1 staining around the somite. (L) Caudal to the level of ablation, HNK1 positive neural crest cells are present around somite 5. (M) Whole mount of a stage 13 embryo previously subjected to cardiac neural crest ablation at stage 9/10. (N) r4 stream rostral to the ablation site. (O) Dorsolateral stream at the otic level adjacent to rostral r5. (P) The r6 neural crest cell stream is missing (asterisk) at the level of caudal medial otic placode (mo) fusion in front of somite 1. The HNK1 staining labels the medial otic placode. (Q) At the occipital somites (s2 here), there is no HNK1 staining reflecting the absence of neural crest cells (asterisk). (R) Caudal to the ablated region, HNK1-positive neural crest cells are seen migrating around somite 5.
Abbreviations: dls- dorsolateral stream; mo- medial otic placode; nt- neural tube; ot-otic cup; r4, r5, r6, r8- rhombomere 4, 5, 6, 8; s2, s5- somite 2, 5.
Scale bars in A, F, L = 400mm; All other panels: 60mm.
Supplementary Figure 3. Paired images of three sectioned embryonic day 6 chick hearts to show the extent of interventricular septum formation comparing control and cardiac crest ablated (HH stage 7 and 10) embryos. The atria (At), ventricles (V), and interventricular septa (IVS) are labeled and an incomplete interventricular septum is shown as double arrowheads. (A–B) By E6 some control sections (A) still show continuity (double arrowheads) between ventricles whereas others (B) appear to have a complete IVS partition with no continuity. (C–D) The stage 7 ablated embryo’s heart was slightly smaller but septum formation between the ventricles was as developed or more so than the control; only a thin slit-like continuity (C, double arrowheads) was detected in several sections. As shown in (D) the IVS was complete in other sections by E6. (E–F) The stage 10 ablated embryos had the largest opening (defect) between ventricles at the upper membranous level (E, double arrowheads) even though the lower muscular septum (IVS) had formed as shown in (F). The plane of section in (E–F) is perpendicular to those in (A–D) to better illustrate the larger size of the defect in the IVS by E6 following stage 10 ablation. The sections are unstained except for (D) in which smooth muscle actin antibody stain better shows the forming aorticopulmonary septum in the outflow tract included in the upper left of the figure.
Scale bar: 400mm, applies to all panels.
Supplementary Figure 4. The enteric nervous system forms normally after ablation of the presumptive cardiac neural crest at stage 7. All panels show sections at stomach level. (A) Neuronal tissue (Tuj1, red), including vagal nerve input (upper left) and intrinsic neural crest derived neurons (arrows), is present in the gut at Day 6 in control embryos. The thick muscle layer expresses smooth muscle actin (green). (A’) Higher magnification view of intrinsic neurons (red, asterisk) in the outer stomach wall. DAPI staining labels nuclei in neurons and other stomach tissue. (B) In an embryo ablated at stage 7, the gut and enteric nervous system develops similarly to controls at Day 6. Neurons express Tuj1 (asterisks, red), DAPI stained nuclei are visible in a background of lightly stained smooth muscle actin staining (green tinge). (B’) Intrinsic neurons of the enteric nervous system (same section) showing nerve cells (asterisks) and nerve fibers (Tuj1 only) of the outer myenteric plexus.
Scale bars. A: 200mm; A, B, B’: 100mm.
ACKNOWLEDGEMENTS
We would like to thank all members of the Bronner-Fraser and Fraser labs. Thanks is extended to Aura Keeter for sectioning, antibody staining and lab support. We would also like to thank Peter Lwigale for training in microdissection. Katrin Wunnenberg-Stapleton and Simone Lutolf provided excellent assistance with DiI labeling of control embryos. We thank Tamira Elul for productive discussions about data quantification. Finally, special thanks to Kathryn McCabe for assistance troubleshooting antibody staining procedures and Nicolas Plachta and Jennifer Yang for help with live and static imaging, respectively. This work was funded by NIH grants HD037105 and NS36585 to MB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Advances in experimental medicine and biology. 2006;589:181–196. doi: 10.1007/978-0-387-46954-6_11. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development (Cambridge, England) 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Sechrist J, Bronner-Fraser M, Fraser S. Rhombomeric origin and rostrocaudal reassortment of neural crest cells revealed by intravital microscopy. Development. 1995;121:935–945. doi: 10.1242/dev.121.4.935. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Kirby ML. Dependence of thymus development on derivatives of the neural crest. Science. 1984;223:498–500. doi: 10.1126/science.6606851. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Kirby ML. Neural crest interactions in the development of the immune system. J Immunol. 1985;135:766s–768s. [PubMed] [Google Scholar]
- Bockman DE, Redmond ME, Kirby ML. Alteration of early vascular development after ablation of cranial neural crest. Anat Rec. 1989;225:209–217. doi: 10.1002/ar.1092250306. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Redmond ME, Waldo K, Davis H, Kirby ML. Effect of neural crest ablation on development of the heart and arch arteries in the chick. Am J Anat. 1987;180:332–341. doi: 10.1002/aja.1001800403. [DOI] [PubMed] [Google Scholar]
- Boot MJ, Steegers-Theunissen RP, Poelmann RE, van Iperen L, Gittenberger-de Groot AC. Cardiac outflow tract malformations in chick embryos exposed to homocysteine. Cardiovascular research. 2004;64:365–373. doi: 10.1016/j.cardiores.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Chabry LM. Contribution a l'embryologie normale teratologique des ascidies simples. J Anat Physiol Norm Pathol. 1887;23:167–321. [Google Scholar]
- Chan WY, Cheung CS, Yung KM, Copp AJ. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development (Cambridge, England) 2004;131:3367–3379. doi: 10.1242/dev.01197. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM. Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the splotch (Sp(2H))/Pax3 mouse mutant. Cardiovascular research. 2000;47:314–328. doi: 10.1016/s0008-6363(00)00098-5. [DOI] [PubMed] [Google Scholar]
- Couly G, Grapin-Botton A, Coltey P, Le Douarin NM. The regeneration of the cephalic neural crest, a problem revisited: the regenerating cells originate from the contralateral or from the anterior and posterior neural fold. Development. 1996;122:3393–3407. doi: 10.1242/dev.122.11.3393. [DOI] [PubMed] [Google Scholar]
- Creazzo TL, Godt RE, Leatherbury L, Conway SJ, Kirby ML. Role of cardiac neural crest cells in cardiovascular development. Annu Rev Physiol. 1998;60:267–286. doi: 10.1146/annurev.physiol.60.1.267. [DOI] [PubMed] [Google Scholar]
- De Bernardi F. Professor Giuseppe Reverberi and the ascidian school in Palermo. Invertebrate Survival Journal. 2009;6:S3–S8. [Google Scholar]
- Ellies DL, Tucker AS, Lumsden A. Apoptosis of premigratory neural crest cells in rhombomeres 3 and 5: consequences for patterning of the branchial region. Dev Biol. 2002;251:118–128. doi: 10.1006/dbio.2002.0815. [DOI] [PubMed] [Google Scholar]
- Ezin AM, Fraser SE, Bronner-Fraser M. Fate map and morphogenesis of presumptive neural crest and dorsal neural tube. Dev Biol. 2009;330:221–236. doi: 10.1016/j.ydbio.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French V, Bryant PJ, Bryant SV. Pattern regulation in epimorphic fields. Science. 1976;193:969–981. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- Gierer A. Some physical, mathematical and evolutionary aspects of biological pattern formation. Philosophical transactions of the Royal Society of London. 1981;295:429–440. doi: 10.1098/rstb.1981.0151. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hutson MR, Sackey FN, Lunney K, Kirby ML. Blocking hedgehog signaling after ablation of the dorsal neural tube allows regeneration of the cardiac neural crest and rescue of outflow tract septation. Dev Biol. 2009;335:367–373. doi: 10.1016/j.ydbio.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, Martinez-Barbera JP. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 2005;43:129–135. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development (Cambridge, England) 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Plasticity and predetermination of mesencephalic and trunk neural crest transplanted into the region of the cardiac neural crest. Developmental biology. 1989;134:402–412. doi: 10.1016/0012-1606(89)90112-7. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Turnage KLr, Hays BM. Characterization of conotruncal malformations following ablation of "cardiac" neural crest. Anat Rec. 1985;213:87–93. doi: 10.1002/ar.1092130112. [DOI] [PubMed] [Google Scholar]
- Kulesa P, Bronner-Fraser M, Fraser S. In ovo time-lapse analysis after dorsal neural tube ablation shows rerouting of chick hindbrain neural crest. Development (Cambridge, England) 2000;127:2843–2852. doi: 10.1242/dev.127.13.2843. [DOI] [PubMed] [Google Scholar]
- Kuratani SC, Kirby ML. Initial migration and distribution of the cardiac neural crest in the avian embryo: an introduction to the concept of the circumpharyngeal crest. Am J Anat. 1991;191:215–227. doi: 10.1002/aja.1001910302. [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci. 2009;151:61–69. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. The Neural Crest. New York: Cambridge University Press; 1982. [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. second edn. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- Lwigale PY, Conrad GW, Bronner-Fraser M. Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development (Cambridge, England) 2004;131:1979–1991. doi: 10.1242/dev.01106. [DOI] [PubMed] [Google Scholar]
- Miyagawa-Tomita S, Waldo K, Tomita H, Kirby ML. Temporospatial study of the migration and distribution of cardiac neural crest in quail-chick chimeras. Am J Anat. 1991;192:79–88. doi: 10.1002/aja.1001920109. [DOI] [PubMed] [Google Scholar]
- Morgan SC, Lee HY, Relaix F, Sandell LL, Levorse JM, Loeken MR. Cardiac outflow tract septation failure in Pax3-deficient embryos is due to p53-dependent regulation of migrating cardiac neural crest. Mech Dev. 2008;125:757–767. doi: 10.1016/j.mod.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Sechrist J, Wilkinson DG, Bronner-Fraser M. Relationship between spatially restricted Krox-20 gene expression in branchial neural crest and segmentation in the chick embryo hindbrain. EMBO J. 1995;14:1697–1710. doi: 10.1002/j.1460-2075.1995.tb07159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-van der Sanden MJ, Kirby ML, Gittenberger-de Groot A, Tibboel D, Mulder MP, Meijers C. Ablation of various regions within the avian vagal neural crest has differential effects on ganglion formation in the fore-, mid- and hindgut. Dev Dyn. 1993;196:183–194. doi: 10.1002/aja.1001960305. [DOI] [PubMed] [Google Scholar]
- Phillips MT, Kirby ML, Forbes G. Analysis of cranial neural crest distribution in the developing heart using quail-chick chimeras. Circulation research. 1987;60:27–30. doi: 10.1161/01.res.60.1.27. [DOI] [PubMed] [Google Scholar]
- Porras D, Brown CB. Temporal-spatial ablation of neural crest in the mouse results in cardiovascular defects. Dev Dyn. 2008;237:153–162. doi: 10.1002/dvdy.21382. [DOI] [PubMed] [Google Scholar]
- Reverberi G, Minganti A. Fenomeni di evocazione nello sviluppo dell’uovo di ascidie. Pubbl Staz Zool Napoli. 1946;20:201–252. [Google Scholar]
- Roux W. Contributions to the developmental mechanics of the embryo. On the artificial production of half-embryos by destruction of one of the first two blastomeres and the later development (postgeneration) of the missing half of the body. In: Willier BH, Oppenheimer JM, editors. Foundations of Experimental Embryology. New York: Hafner; 1974. pp. 2–37. [Google Scholar]
- Saldivar JR, Krull CE, Krumlauf R, Ariza-McNaughton L, Bronner-Fraser M. Rhombomere of origin determines autonomous versus environmentally regulated expression of Hoxa-3 in the avian embryo. Development (Cambridge, England) 1996;122:895–904. doi: 10.1242/dev.122.3.895. [DOI] [PubMed] [Google Scholar]
- Scherson T, Serbedzija G, Fraser S, Bronner-Fraser M. Regulative capacity of the cranial neural tube to form neural crest. Development (Cambridge, England) 1993;118:1049–1062. doi: 10.1242/dev.118.4.1049. [DOI] [PubMed] [Google Scholar]
- Sechrist J, Nieto MA, Zamanian RT, Bronner-Fraser M. Regulative response of the cranial neural tube after neural fold ablation: spatiotemporal nature of neural crest regeneration and up-regulation of Slug. Development. 1995;121:4103–4115. doi: 10.1242/dev.121.12.4103. [DOI] [PubMed] [Google Scholar]
- Suzuki HR, Kirby ML. Absence of neural crest cell regeneration from the postotic neural tube. Dev Biol. 1997;184:222–233. doi: 10.1006/dbio.1997.8529. [DOI] [PubMed] [Google Scholar]
- Vaglia JL, Hall BK. Regulation of neural crest cell populations: occurrence, distribution and underlying mechanisms. Int J Dev Biol. 1999;43:95–110. [PubMed] [Google Scholar]
- van den Hoff MJ, Moorman AF, Ruijter JM, Lamers WH, Bennington RW, Markwald RR, Wessels A. Myocardialization of the cardiac outflow tract. Developmental biology. 1999;212:477–490. doi: 10.1006/dbio.1999.9366. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski D, Kirby ML. Cardiac neural crest is essential for the persistence rather than the formation of an arch artery. Dev Dyn. 1996;205:281–292. doi: 10.1002/(SICI)1097-0177(199603)205:3<281::AID-AJA8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Wurdak H, Ittner LM, Sommer L. DiGeorge syndrome and pharyngeal apparatus development. Bioessays. 2006;28:1078–1086. doi: 10.1002/bies.20484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. DiI labeling of the hindbrain (mid-otic to somite 3 level) dorsal neural tube to mark migrating chick cardiac neural crest cells. (A–B) Whole mount images of HH12+ (18 somite) embryo after focal DiI injection into mid rhombomere 5 (r5) roof at HH10 (11 somite). Combined bright field (A) and fluorescent (B) images show initial central injection dye spot near caudal r5–r6 junction; labeled cells migrate both rostrally (to 2nd branchial arch) and caudally (to 3rd branchial arch) in a dorsolateral stream (dls) between the dorsal part of the otic vesicle (ot) and r5–r6 hindbrain. (C) Transverse section through rostral r6 and the caudal portion of the right otic vesicle (ot) of same embryo in (A) showing both DiI positive dorsolateral streams (dls) migrating caudally and soon ventrally toward the circumpharyngeal neural crest region (not shown); they will join neural crest cells from rostral r6 as shown below. (D–E) Whole mount images of HH12 (16 somite) embryo after localized DiI r6 tube fill at HH 10 (11 somite). (E) Migrating dye labeled neural crest cells at rostral r6 level are blocked by the otic vesicle (ot). Together with caudal migrating r5 cells, they contribute the anterior tract (caudal extension of dls) of the circumpharyngeal crest.
(F) Transverse section at the level of somite 3 (r8) of a HH14 (23 somite) 2 day embryo after focal DiI injection into roof of neural tube between 3rd somite pair at HH10+ (12 somite). The dye labeled neural crest (nc) cells have migrated along the ventrolateral pathway medial to the dermomyotome of somite 3 (s3). (G) Horizontal section through ventral branchial arches (BA 1–4) of a HH18 (35 somite) 3 day embryo after focal DiI injection into the roof of rostral r6 at HH 10 (10 somite). The majority of dye labeled cardiac neural crest cells (asterisk) is in the 3rd branchial arch with some in the 4th arch. Many cells surround the 3rd branchial artery with the leading ones (arrows) about to enter the wall of the aortic sac (AS). By embryonic day 4 (E4), as shown in the body of this study, some of these cells contribute to aortico-pulmonary septum formation in the heart outflow tract in unoperated embryos (DiI image available but not shown).
Abbreviations: AS- aortic sac; BA- branchial arch; dls- dorsolateral stream; nc- neural crest; ot- otic vesicle; r5, 6, 8- rhombomere 5, 6, 8; s3- somite 3.
Scale bars. 100 µm in all panels.
Supplementary Figure 2. The dorsal neural tube at caudal hindbrain level and the cardiac neural crest re-forms by stage 13 in embryos ablated at stage 7. HNK1 marks migrating neural crest cells. (A–F) represent a control embryo at stage 13, (G–L) show a stage 13 embryo that was subjected to cardiac neural crest ablation at stage 7, (M–R) are of a stage 13 embryo that was subjected to cardiac neural crest ablation at stage 9–10. (A) Whole mount control embryo at stage 13 with representative sections in B–F. (B) Rhombomere 4 (r4) just rostral to the otic vesicle. HNK1 labels the neural crest cells in the r4 stream. (C) Mid-otic level, caudal r5, showing HNK1 positive dorsolateral tract (dls; arrow) of neural crest migrating between otic vesicle and dorsal neural tube. The otic vesicle (ot) also expresses HNK1. (D) r6 level, with the caudo-lateral r5/r6 stream of migrating HNK1 positive neural crest cells. (E) r8 level at somite 2 (s2). HNK1 positive neural crest cells migrate on the dorsal and ventral aspects of somite 2. (F) HNK1 positive neural crest cells migrating at the level of somite 5. (G) Whole mount of a stage 13 embryo that was subjected to cardiac neural crest ablation at stage 7. Arrows in H–L indicate level of corresponding section described below. (H) Level of rhombomere 4, rostral to the ablated level, showing neural crest migration in the r4 stream. (I) Level of rostral r5 and otic vesicle, showing HNK1-positive cells in the dorsolateral stream, between the otic cup and the neural tube. (J) At the level of caudal rhombomere 6, the lateral stream is present, although migration is delayed: the leading edge of r5/r6 stream is located more dorsally than in the controls. (K) At the occipital somite level (s2 here), neural crest cells are missing (asterisk), as evident by the lack of HNK1 staining around the somite. (L) Caudal to the level of ablation, HNK1 positive neural crest cells are present around somite 5. (M) Whole mount of a stage 13 embryo previously subjected to cardiac neural crest ablation at stage 9/10. (N) r4 stream rostral to the ablation site. (O) Dorsolateral stream at the otic level adjacent to rostral r5. (P) The r6 neural crest cell stream is missing (asterisk) at the level of caudal medial otic placode (mo) fusion in front of somite 1. The HNK1 staining labels the medial otic placode. (Q) At the occipital somites (s2 here), there is no HNK1 staining reflecting the absence of neural crest cells (asterisk). (R) Caudal to the ablated region, HNK1-positive neural crest cells are seen migrating around somite 5.
Abbreviations: dls- dorsolateral stream; mo- medial otic placode; nt- neural tube; ot-otic cup; r4, r5, r6, r8- rhombomere 4, 5, 6, 8; s2, s5- somite 2, 5.
Scale bars in A, F, L = 400mm; All other panels: 60mm.
Supplementary Figure 3. Paired images of three sectioned embryonic day 6 chick hearts to show the extent of interventricular septum formation comparing control and cardiac crest ablated (HH stage 7 and 10) embryos. The atria (At), ventricles (V), and interventricular septa (IVS) are labeled and an incomplete interventricular septum is shown as double arrowheads. (A–B) By E6 some control sections (A) still show continuity (double arrowheads) between ventricles whereas others (B) appear to have a complete IVS partition with no continuity. (C–D) The stage 7 ablated embryo’s heart was slightly smaller but septum formation between the ventricles was as developed or more so than the control; only a thin slit-like continuity (C, double arrowheads) was detected in several sections. As shown in (D) the IVS was complete in other sections by E6. (E–F) The stage 10 ablated embryos had the largest opening (defect) between ventricles at the upper membranous level (E, double arrowheads) even though the lower muscular septum (IVS) had formed as shown in (F). The plane of section in (E–F) is perpendicular to those in (A–D) to better illustrate the larger size of the defect in the IVS by E6 following stage 10 ablation. The sections are unstained except for (D) in which smooth muscle actin antibody stain better shows the forming aorticopulmonary septum in the outflow tract included in the upper left of the figure.
Scale bar: 400mm, applies to all panels.
Supplementary Figure 4. The enteric nervous system forms normally after ablation of the presumptive cardiac neural crest at stage 7. All panels show sections at stomach level. (A) Neuronal tissue (Tuj1, red), including vagal nerve input (upper left) and intrinsic neural crest derived neurons (arrows), is present in the gut at Day 6 in control embryos. The thick muscle layer expresses smooth muscle actin (green). (A’) Higher magnification view of intrinsic neurons (red, asterisk) in the outer stomach wall. DAPI staining labels nuclei in neurons and other stomach tissue. (B) In an embryo ablated at stage 7, the gut and enteric nervous system develops similarly to controls at Day 6. Neurons express Tuj1 (asterisks, red), DAPI stained nuclei are visible in a background of lightly stained smooth muscle actin staining (green tinge). (B’) Intrinsic neurons of the enteric nervous system (same section) showing nerve cells (asterisks) and nerve fibers (Tuj1 only) of the outer myenteric plexus.
Scale bars. A: 200mm; A, B, B’: 100mm.