Abstract
Glatiramer acetate (GA, copolymer-1, Copaxone®) is a Food and Drug Administration-approved drug for the treatment of relapsing-remitting multiple sclerosis (MS). However, its mechanism of action remains ill-defined. The available evidence indicates that GA induces antigen-presenting cells with anti-inflammatory properties and promotes the generation of immunoregulatory T cells that suppress pathogenic T cells. A new study by Kala et al. (Exp. Neurol. 2010. 221, 136–145) now shows that B lymphocytes, which are best known for their antibody-secreting properties, contribute to the beneficial effects of GA against experimental autoimmune encephalomyelitis (EAE), the animal model of MS. This commentary discusses these new findings in the context of the pathogenesis of MS and EAE, the emerging immunoregulatory role of B cells in autoimmunity, and the relevance of B cells as targets for immunotherapy in MS.
Keywords: Antigen-presenting cells, Experimental autoimmune encephalomyelitis, Glatiramer acetate/copolymer 1/Copaxone®, Immunomodulation, Multiple sclerosis, Pathogenic T cells, Regulatory B cells, Regulatory T cells, Treatment
Introduction
Multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE), are primarily mediated by T lymphocytes that produce pro-inflammatory cytokines such as IFN-γ, TNF-α and IL-17 in response to autoantigens expressed in the central nervous system (CNS) (Bhat and Steinman, 2009; El-behi et al., 2010; Goverman, 2009). In healthy individuals, autoantigen-specific T cells are kept in check by a variety of regulatory mechanisms, including immunosuppressive antigen-presenting cells (APCs) and regulatory T cells (Tregs) (Goverman, 2009; McFarland and Martin, 2007). Multiple subsets of Tregs have been implicated in suppressing pathogenic T cells in MS and EAE (Cvetanovich and Hafler, 2010; Zozulya and Wiendl, 2008): IL-4- and IL-10-producing T helper 2 (Th2) cells, TGF-β-producing Th3 cells, CD4+CD25+ T cells expressing the forkhead transcription factor Foxp3, CD8+ suppressor T cells, and natural killer T cells. Emerging evidence indicates that B lymphocytes, which are best known for their capacity to produce antibodies, can impact the pathogenesis of MS and EAE as well (Franciotta et al., 2008; McLaughlin and Wucherpfennig, 2008).
A better understanding of the immunoregulatory circuits that normally protect against the development of CNS autoimmunity should guide the development of improved immunotherapies for MS and other autoimmune disorders. The disease modifying drug glatiramer acetate (GA, copolymer-1, Copaxone®) interrupts the pathogenic process in MS by reinforcing these immunoregulatory networks (Arnon and Aharoni, 2009; Blanchette and Neuhaus, 2008; Liblau, 2009; Schrempf and Ziemssen, 2007; Weber et al., 2007a). Prior studies have shown that GA promotes the immunoregulatory functions of both innate and adaptive components of the immune system, including dendritic cells, monocytes and Tregs. The new study by Kala et al. (2010) shows that GA also promotes regulatory properties in B lymphocytes. This commentary will first review the current knowledge of the mechanism of action of GA for treatment of MS and the role of B cells in the development of autoimmunity in the CNS. It will then discuss the new findings of Kala et al. (2010) that provide evidence for a contribution of regulatory B cells (Bregs) in the protective effects of GA in EAE and possibly MS.
GA and its effects on MS
GA was first synthesized nearly forty years ago as a research tool to facilitate the reproducible induction of EAE in rodents (Arnon, 1996; Teitelbaum et al., 1971). At the time, EAE was usually induced by immunization of animals with crude myelin preparations derived from the spinal cord of guinea pigs or other animals. Thus, with the goal of standardizing methods to induce EAE, efforts were made to synthesize molecular mimetics of myelin basic protein (MBP), a major product of oligodendrocytes that has been posited to function as an autoantigen in MS. GA is a standardized mixture of polypeptides with an average length of 40 to 100 residues, synthesized from four amino acids, namely glutamic acid, lysine, alanine and tyrosine (G-L-A-T), in a random order and at a defined molar ratio of approximately 1.5:3.6:4.6:1.0, as found in MBP. However, instead of inducing EAE, GA protected against EAE induced in response to crude myelin preparations (Teitelbaum et al., 1971). These serendipitous findings prompted a pilot trial, which provided evidence for a beneficial effect of GA in MS (Bornstein et al., 1987). The results from this trial were confirmed in a large randomized clinical trial (Johnson et al., 1995), which led to the regulatory approval of GA for treatment of relapsing-remitting MS in 1996.
Mechanism of action of GA in MS
Multiple mechanisms have been proposed for the protective effects of GA in MS and EAE (Arnon and Aharoni, 2009; Blanchette and Neuhaus, 2008; Liblau, 2009; Schrempf and Ziemssen, 2007; Weber et al., 2007a). Initial studies focused on the capacity of GA to bind promiscuously with major histocompatibility complex (MHC) class II molecules on APCs, without the need for intracellular processing (Fridkis-Hareli and Strominger, 1998). Thus, binding of GA with MHC class II was shown to compete with binding of MBP-derived peptides and antagonize T cell responses in vitro (Aharoni et al., 1999). Furthermore, in addition to its function as an antagonist of MBP-specific T cell responses, it has been suggested that GA can function as an altered peptide ligand to induce regulatory cytokine production in T cells (Gran et al., 2000).
There is ample evidence that GA induces the generation of GA-specific Th2 cells that produce IL-4 and IL-10, and possibly Th3 cells that produce TGF-β (Duda et al., 2000; Neuhaus et al., 2000). These Th2 and Th3 cells can suppress the pathogenic effects of autoantigen-specific Th1 and Th17 cells. However, the precise mechanism of this suppression remains unclear. While studies with EAE have shown that GA-specific Th2 cells can enter the CNS (Aharoni et al., 2000), it is unlikely that sufficient amounts of GA are available in the CNS to activate these cells in situ. Although some GA-specific T cell lines can cross-react with MBP, this does not appear to be the norm (Aharoni et al., 2000; Chen et al., 2001). Nevertheless, it has been hypothesized that GA-specific Th2 cells exhibit broad cross-reactivity with myelin-derived antigens and possibly other autoantigens (Liblau, 2009). Secretion of anti-inflammatory cytokines by these GA-reactive Th2 cells subsequently leads to suppression of pathogenic, autoantigen-specific Th1 and Th17 cells in the CNS through “bystander suppression.” Furthermore, more recent studies have shown that these GA-specific T cells can produce neurotrophic factors such as BDNF (brain-derived neurotrophic factor) or induce production of these factors in other cell types of the CNS (Arnon and Aharoni, 2009; Blanchette and Neuhaus, 2008). These neurotrophic factors can promote neuronal protection and repair, without impinging on the inflammatory process (Kerschensteiner et al., 1999; Linker et al., 2010), which likely contributes to the capacity of GA to halt or reverse some of the neuronal damage inflicted during EAE and MS.
The idea that Th2 cells are required for the suppressive effects of GA in MS has been challenged by studies investigating the role of Th2 cell-derived cytokines in the capacity of GA to suppress EAE induced in C57BL/6 mice following immunization with a myelin oligodendrocyte glycoprotein (MOG) peptide (Jee et al., 2006). GA moderately suppressed EAE but failed to induce Th2-polarized responses in these animals. Furthermore, GA was also protective in IL-4-deficient, IL-10-deficient and IL-4/IL-10 double-deficient mice. Therefore, it is likely that mechanisms other than Th2 cells contribute to the therapeutic effects of GA. Indeed, several research groups showed that GA expands or promotes the activity of Foxp3-expressing Tregs in vitro and in vivo (Aharoni et al., 2010; Hong et al., 2005; Jee et al., 2007; Putheti et al., 2003). Furthermore, Tregs from GA-treated mice were more effective than Tregs from untreated mice in preventing EAE upon adoptive transfer (Jee et al., 2007).
It has also been reported that GA induces GA-specific CD8+ T cell responses in MS patients, which was associated with an improved clinical response (Farina et al., 2001; Karandikar et al., 2002). These cytotoxic CD8+ T cells might exhibit regulatory properties similar to those of Foxp3-expressing Tregs and/or directly lyse the pathogenic CD4+ T cells that are activated in MS.
GA also provides significant protection against diseases other than MS and EAE, including arthritis, uveoretinitis, inflammatory bowel disease, and graft rejection in mice (Arnon and Aharoni, 2004; Gur et al., 2006). These findings suggested that some of the beneficial effects of GA are due to mechanisms independent of the direct recognition of GA by antigen-specific receptors of the adaptive immune system. Indeed, there is strong evidence that GA directly affects APCs, including dendritic cells and monocytes (Vieira et al., 2003; Weber et al., 2007b). Dendritic cells exposed to GA became impaired for IL-12 production and promoted the induction of IL-4-secreting Th2 cells (Vieira et al., 2003). Similarly, GA promoted the development of anti-inflammatory, type 2 monocytes, which are characterized by reduced secretion of IL-12 and increased secretion of IL-10 and TGF-β (Weber et al., 2007b). This type 2 phenotype in monocytes was induced without the need for GA binding with MHC class II molecules. In turn, these type 2 monocytes promoted the differentiation of Th2 cells and Foxp3-expressing Tregs. Adoptive transfer of GA-induced type 2 monocytes was able to reverse EAE (Weber et al., 2007b). Therefore, these findings provided evidence that cross-reactivity of GA-specific T cells with myelin antigens is not required for the protective effects of GA in EAE.
GA also induced antibody responses in treated MS patients, which were initially predominantly of the IgG1 subclass but then switched towards the IgG4 subclass, suggesting interaction with Th2 cells (Basile et al., 2006; Schrempf and Ziemssen, 2007). These antibodies did not appear to interfere with the clinical efficacy of GA and, in fact, higher titers were detected in relapse-free patients (Brenner et al., 2001). Although these findings suggested that GA-specific antibodies might be beneficial to the mechanism of action of GA, possibly by facilitating neuronal repair, the new study from Kala et al. (2010) indicates that the contribution of B cells in the therapeutic effects of GA in EAE is largely due the acquisition of a regulatory phenotype in these cells. Before discussing the work of Kala et al. (2010) in more depth, it is worthwhile to briefly review the role of B cells in MS and EAE.
B cells and CNS autoimmunity
B cells can play opposing roles in the development of CNS autoimmunity (Kurosaki, 2008). While these cells are best known for their capacity to produce antibodies, they can also function as APCs for T lymphocytes and modulate various immune responses via cytokine and chemokine production. In a seminal study, Janeway and colleagues showed that mice deficient in B cells (due to a targeted mutation in the IgM heavy chain) developed a more severe and chronic course of EAE disease than wild-type animals (Wolf et al., 1996). These findings suggested a suppressive role of B cells in the development of EAE. Subsequent studies provided evidence for a critical role of IL-10 production by B cells in suppressing EAE (Fillatreau et al., 2002). Consistent with these findings, depletion of B cells prior to induction of EAE resulted in disease exacerbation (Matsushita et al., 2008). Importantly, adoptive transfer of an IL-10-producing CD1dhiCD5+ B cell subset was able to prevent this disease exacerbation (Matsushita et al., 2008). B cells expressing CD5 and high levels of CD1d have been shown to exhibit potent immunoregulatory activities by producing high levels of IL-10 that can modulate T cell responses and the antigen-presenting functions of dendritic cells (Bouaziz et al., 2008; Lund and Randall, 2010). The available evidence suggests that the Bregs that suppress EAE are likely specific for myelin antigens (Fillatreau et al., 2002).
In sharp contrast with the results obtained for early B cell depletion, depletion of B cells during progression of EAE potently ameliorated disease (Matsushita et al., 2008). Although pathogenic antibodies might contribute to these effects, it is more likely that pathogenic B cells functioned as APCs to promote the expansion of pathogenic Th1 and Th17 cells (Kurosaki, 2008). The latter findings in mice are consistent with results from clinical studies with the B cell-depleting antibody rituximab (an engineered monoclonal antibody that reacts with human CD20), which showed reduced inflammatory brain lesions and clinical relapses in patients with relapsing-remitting MS (Hauser et al., 2008). Interestingly, it has been shown that, following depletion of B cells with rituximab, B cells that initially repopulated the hosts exhibited a naïve and tolerogenic phenotype (Duddy et al., 2007; Hu et al., 2007). Similarly, it has been suggested that treatment with natalizumab (a humanized monoclonal antibody directed against CD49, the α4 subunit of the VLA-4 receptor, which blocks entry of immune cells into the CNS) or alemtuzumab (a humanized monoclonal antibody directed against CD52 that depletes multiple cell types, including B cells) results in the increased release of immature B cells from the bone marrow, which might provide a tolerance-enhancing environment (Bielekova and Becker, 2010). Thus, mobilization of Bregs might contribute to the therapeutic effects of these antibodies against MS.
Regulatory role of B cells in the therapeutic effects of GA in EAE
Based on the evidence that B cells play an important role in the development and pathogenesis of EAE and MS, Kala et al. (2010) tested the hypothesis that B cells contribute to the protective effects of GA in EAE, independently of the capacity of these cells to produce antibodies. These investigators treated mice for seven consecutive days with GA and studied the phenotype of B cells, which demonstrated reduced expression of the co-stimulatory molecules CD80 and CD86, and enhanced production of IL-10 but not IL-4 or TGF-β by these cells. In an independent study, Begum-Haque et al. (2010) showed that GA-treated mice exhibited an increase in the prevalence of CD19+ B cells expressing CD5, a marker for Bregs (see above). These investigators further showed that B cells from GA-treated and MOG peptide-challenged mice expressed increased levels of IL-4, IL-10 and IL-13, decreased levels of IL-6, IL-12 and TNF-α, and decreased surface levels of the BAFF (B cell-activating factor of the TNF family) receptor, which promotes the survival of B cells by interacting with BAFF and APRIL (a proliferation-inducing ligand) (Begum-Haque et al., 2010). These findings suggested that GA promotes the induction of Bregs while suppressing pathogenic B cells. Although the significance of BAFF receptor signaling remains unclear, Bregs might have a lower requirement for BAFF receptor-mediated survival signals than pathogenic B cells. Consistent with the conclusion that GA induces a regulatory phenotype in B cells, Kala et al. (2010) found that B cells from GA-treated animals had reduced capacity to stimulate the expansion of MOG peptide-reactive T cells ex vivo.
Kala et al. (2010) further showed that adoptive transfer of B cells from GA-treated animals was able to prevent the induction of EAE, suggesting that these cells play an important role in the therapeutic activities of GA. Consistent with this conclusion, they found that GA was unable to protect B cell-deficient mice against the development of EAE. Mechanistic studies further showed that adoptive transfer of B cells from GA-treated mice caused a reduction in T cells (both CD4+ and CD8+ T cells) and CD11b+ macrophages in the recipient animals that were challenged with MOG peptide. Mononuclear cells from the spleen and lymph nodes of these recipient animals generated reduced proliferative responses when stimulated ex vivo with MOG peptide. Further, this T cell response was characterized by decreased IFN-γ and IL-17 production and a concomitant increase in IL-10 production, whereas TGF-β remained unchanged. These findings suggested that B cells from GA-treated animals inhibited pathogenic Th1 and Th17 responses while promoting anti-inflammatory T cell responses. Consistent with this conclusion, recipient animals had increased levels of MOG-specific antibodies of the IgG1 subclass (associated with Th2 responses) and reduced levels of MOG-specific antibodies of the IgG2b subclass (associated with Th1 responses). Based on these findings, the authors suggested that Th2 cells are likely the relevant regulatory T cell subset induced by the Bregs. They failed to observe an expansion of Foxp3-expressing Tregs, but did not investigate the functions of these cells or look for these cells in the CNS.
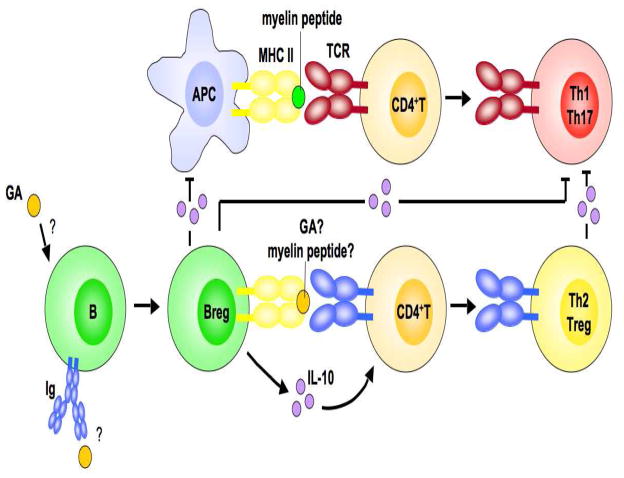
In summary, the findings of Kala et al. (2010), which are supported by the studies of Begum-Haque et al. (2010), indicate that IL-10-producing Bregs play an important role in the therapeutic effects of GA in EAE (and possibly MS) by dampening pathogenic Th1 and Th17 cell responses and enhancing immunosuppressive Th2 cell responses (Fig. 1).
Fig. 1.
Proposed role for B cells in the protective effects of GA in CNS autoimmunity. Based on the studies of Kala et al. (2010), a model is proposed for the role of IL-10-producing Bregs in the mechanism of action of GA in EAE and MS. GA induces a regulatory phenotype in B cells that is likely independent of their specificity for GA. These IL-10-producing Bregs might suppress autoimmunity in multiple ways, by (1) inhibiting the pathogenic activity of myelin antigen-specific Th1 and Th17 cells, (2) promoting the generation of Th2 cells (and possibly Foxp3-expressing Tregs), which, in turn, suppress pathogenic Th1 and Th17 cells through “bystander suppression,” and (3) suppressing the capacity of APCs to induce pathogenic T cells. It is unclear whether these proposed mechanisms are restricted to the periphery or occur in the CNS as well. Abbreviations: Ig, immunoglobulin; MHC II, MHC class II; TCR, T cell receptor.
Implications for treatment of MS
GA is only partially effective for treatment of MS. Therefore, a better understanding of its mechanism of action might provide important information for the development of new and improved therapies of MS. The studies by Kala et al. (2010) and Begum-Haque et al. (2010) highlight the role of Bregs in the mechanism of action of GA and, therefore, support efforts to target B cells for immunotherapy of MS and other autoimmune diseases. This possibility is supported by studies that have identified Bregs in humans (Bouaziz et al., 2008; Lund and Randall, 2010). Furthermore, B cells from patients with MS produced decreased amounts of IL-10 as compared with B cells from healthy subjects (Duddy et al., 2007), suggesting that restoration of Breg cell function in MS patients might be beneficial. As already noted, treatment of MS patients with antibodies such as rituximab, natalizumab or alemtuzumab results in the mobilization of immature B cells from the bone marrow, which might have suppressive activities (Bielekova and Becker, 2010). Subsequent treatment with GA might provide an opportunity to expand and promote the tolerogenic activities of immature B cells.
Perspectives and outstanding questions
Many questions regarding the role of Bregs in the therapeutic effects of GA in EAE and MS remain. The precise phenotype of the Bregs remains unclear. Similar to the Bregs that influenced the natural progression of EAE and other autoimmune disorders, the B cells that contributed to the therapeutic effects of GA produced copious amounts of IL-10 and other anti-inflammatory cytokines, and produced sharply reduced amounts of pro-inflammatory cytokines (Begum-Haque et al., 2010; Kala et al., 2010). These cells also expressed the CD5 marker (Begum-Haque et al., 2010), which has been associated with a regulatory phenotype in B cells. It will be important to determine whether these cells also express high levels of CD1d and can be enriched based on their expression of CD5 and CD1d markers. The antigen-specificity of these Bregs also remains unclear. Although B cells can generate GA-specific antibodies, it is unclear whether GA-specificity of the B cell antigen receptor is a requirement for the generation of Bregs. Such GA-specific Bregs might be able to cross-react with myelin antigens, present the myelin antigens to T cells and, by producing IL-10 and other immunosuppressive cytokines, inhibit pathogenic T cell responses. While this is one possible scenario, it should be noted that the effects of GA on the generation of type 2 monocytes appeared to be independent of class II expression and the presence of T cells and, hence, antigen non-specific (Weber et al., 2007b). It is therefore equally plausible that the regulatory phenotype induced in B cells by GA is independent of the antigen-specificity of these cells. Clearly, GA is protective in diseases other than EAE and MS (Arnon and Aharoni, 2004; Gur et al., 2006), indicating that antigen-specificity is not a prerequisite for its efficacy. In the case of B cells, it will be revealing to determine whether adoptive transfer of B cells from GA-treated animals can suppress diseases other than EAE.
Kala et al. (2010) found that the GA-induced, IL-10-producing B cells suppressed pathogenic Th1 and Th17 cell responses and promoted suppressive Th2 cell responses in the periphery, but they did not observe any alterations in Foxp3-expressing Tregs. A previous study showed that B cell-deficiency delays the emergence of Foxp3-expressing Tregs in the CNS during recovery from EAE but does not influence the prevalence of these cells in the periphery (Mann et al., 2007). Therefore, it remains possible that the Bregs induced by GA promote the generation of Foxp3-expressing Tregs in the periphery, which subsequently enter the CNS to suppress inflammation.
It also remains unclear whether the Bregs induced by GA function exclusively in the periphery or also enter the CNS to suppress inflammation. In this context, it will be interesting to determine whether adoptive transfer of B cells from GA-treated animals can modulate active EAE disease in mice. This type of experiment will be directly relevant to treatment of MS patients, as GA treatment is initiated during active disease.
Because GA impacts multiple different cell types, it will also be important to determine the relative roles of distinct APCs, including dendritic cells, monocytes and B cells, in the effects of GA on EAE and MS. Specifically, it will be important to determine whether these cells play redundant or required roles in mediating the effects of GA.
Finally, a cautionary note regarding studies such as those performed by Kala et al. (2010). That is, results obtained with EAE models do not always recapitulate mechanisms observed in human patients. Thus, findings obtained with the animal models will need to be confirmed in MS patients treated with GA. Nevertheless, future studies to unravel the immunomodulatory circuits induced by GA in animal models will provide invaluable information to guide the development of improved treatments of MS and other neurodegenerative and autoimmune diseases.
Acknowledgments
Work in the author’s laboratory is supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharoni R, Eilam R, Stock A, Vainshtein A, Shezen E, Gal H, et al. Glatiramer acetate reduces Th-17 inflammation and induces regulatory T-cells in the CNS of mice with relapsing-remitting or chronic EAE. J Neuroimmunol. 2010;225:100–111. doi: 10.1016/j.jneuroim.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Aharoni R, Teitelbaum D, Arnon R, Sela M. Copolymer 1 acts against the immunodominant epitope 82–100 of myelin basic protein by T cell receptor antagonism in addition to major histocompatibility complex blocking. Proc Natl Acad Sci USA. 1999;96:634–639. doi: 10.1073/pnas.96.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni R, Teitelbaum D, Leitner O, Meshorer A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci USA. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R. The development of Cop 1 (Copaxone), an innovative drug for the treatment of multiple sclerosis: personal reflections. Immunol Lett. 1996;50:1–15. doi: 10.1016/0165-2478(96)02506-0. [DOI] [PubMed] [Google Scholar]
- Arnon R, Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14593–14598. doi: 10.1073/pnas.0404887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R, Aharoni R. Neuroprotection and neurogeneration in MS and its animal model EAE effected by glatiramer acetate. J Neural Transm. 2009;116:1443–1449. doi: 10.1007/s00702-009-0272-3. [DOI] [PubMed] [Google Scholar]
- Basile E, Gibbs E, Aziz T, Oger J. During 3 years treatment of primary progressive multiple sclerosis with glatiramer acetate, specific antibodies switch from IgG1 to IgG4. J Neuroimmunol. 2006;177:161–166. doi: 10.1016/j.jneuroim.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Begum-Haque S, Sharma A, Christy M, Lentini T, Ochoa-Reparaz J, Fayed IF, et al. Increased expression of B cell-associated regulatory cytokines by glatiramer acetate in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;219:47–53. doi: 10.1016/j.jneuroim.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64:123–132. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Becker BL. Monoclonal antibodies in MS: mechanisms of action. Neurology. 2010;74(Suppl 1):S31–40. doi: 10.1212/WNL.0b013e3181c97ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette F, Neuhaus O. Glatiramer acetate: evidence for a dual mechanism of action. J Neurol. 2008;255(Suppl 1):26–36. doi: 10.1007/s00415-008-1005-5. [DOI] [PubMed] [Google Scholar]
- Bornstein MB, Miller A, Slagle S, Weitzman M, Crystal H, Drexler E, et al. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med. 1987;317:408–414. doi: 10.1056/NEJM198708133170703. [DOI] [PubMed] [Google Scholar]
- Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Brenner T, Arnon R, Sela M, Abramsky O, Meiner Z, Riven-Kreitman R, et al. Humoral and cellular immune responses to Copolymer 1 in multiple sclerosis patients treated with Copaxone. J Neuroimmunol. 2001;115:152–160. doi: 10.1016/s0165-5728(01)00250-8. [DOI] [PubMed] [Google Scholar]
- Chen M, Gran B, Costello K, Johnson K, Martin R, Dhib-Jalbut S. Glatiramer acetate induces a Th2-biased response and crossreactivity with myelin basic protein in patients with MS. Mult Scler. 2001;7:209–219. doi: 10.1177/135245850100700401. [DOI] [PubMed] [Google Scholar]
- Cvetanovich GL, Hafler DA. Human regulatory T cells in autoimmune diseases. Curr Opin Immunol. 2010 doi: 10.1016/j.coi.2010.08.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda PW, Schmied MC, Cook SL, Krieger JI, Hafler DA. Glatiramer acetate (Copaxone) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J Clin Invest. 2000;105:967–976. doi: 10.1172/JCI8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–197. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Then Bergh F, Albrecht H, Meinl E, Yassouridis A, Neuhaus O, et al. Treatment of multiple sclerosis with Copaxone (COP): Elispot assay detects COP-induced interleukin-4 and interferon-gamma response in blood cells. Brain. 2001;124:705–719. doi: 10.1093/brain/124.4.705. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- Fridkis-Hareli M, Strominger JL. Promiscuous binding of synthetic copolymer 1 to purified HLA-DR molecules. J Immunol. 1998;160:4386–4397. [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gran B, Tranquill LR, Chen M, Bielekova B, Zhou W, Dhib-Jalbut S, et al. Mechanisms of immunomodulation by glatiramer acetate. Neurology. 2000;55:1704–1714. doi: 10.1212/wnl.55.11.1704. [DOI] [PubMed] [Google Scholar]
- Gur C, Karussis D, Golden E, Doron S, Ilan Y, Safadi R. Amelioration of experimental colitis by Copaxone is associated with class-II-restricted CD4 immune blocking. Clin Immunol. 2006;118:307–316. doi: 10.1016/j.clim.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci USA. 2005;102:6449–6454. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee Y, Liu R, Bai XF, Campagnolo DI, Shi FD, Vollmer TL. Do Th2 cells mediate the effects of glatiramer acetate in experimental autoimmune encephalomyelitis? Int Immunol. 2006;18:537–544. doi: 10.1093/intimm/dxh394. [DOI] [PubMed] [Google Scholar]
- Jee Y, Piao WH, Liu R, Bai XF, Rhodes S, Rodebaugh R, et al. CD4+CD25+ regulatory T cells contribute to the therapeutic effects of glatiramer acetate in experimental autoimmune encephalomyelitis. Clin Immunol. 2007;125:34–42. doi: 10.1016/j.clim.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- Kala M, Rhodes SN, Piao WH, Shi FD, Campagnolo DI, Vollmer TL. B cells from glatiramer acetate-treated mice suppress experimental autoimmune encephalomyelitis. Exp Neurol. 2010;221:136–145. doi: 10.1016/j.expneurol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR, et al. Glatiramer acetate (Copaxone) therapy induces CD8+ T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109:641–649. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T. Paradox of B cell-targeted therapies. J Clin Invest. 2008;118:3260–3263. doi: 10.1172/JCI37099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liblau R. Glatiramer acetate for the treatment of multiple sclerosis: evidence for a dual anti-inflammatory and neuroprotective role. J Neurol Sci. 2009;287(Suppl 1):S17–23. doi: 10.1016/S0022-510X(09)71296-1. [DOI] [PubMed] [Google Scholar]
- Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. 2010;133:2248–2263. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Wucherpfennig KW. B cells and autoantibodies in the pathogenesis of multiple sclerosis and related inflammatory demyelinating diseases. Adv Immunol. 2008;98:121–149. doi: 10.1016/S0065-2776(08)00404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus O, Farina C, Yassouridis A, Wiendl H, Then Bergh F, Dose T, et al. Multiple sclerosis: comparison of copolymer-1- reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proc Natl Acad Sci USA. 2000;97:7452–7457. doi: 10.1073/pnas.97.13.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putheti P, Soderstrom M, Link H, Huang YM. Effect of glatiramer acetate (Copaxone) on CD4+CD25high T regulatory cells and their IL-10 production in multiple sclerosis. J Neuroimmunol. 2003;144:125–131. doi: 10.1016/j.jneuroim.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Schrempf W, Ziemssen T. Glatiramer acetate: mechanisms of action in multiple sclerosis. Autoimmun Rev. 2007;6:469–475. doi: 10.1016/j.autrev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- Vieira PL, Heystek HC, Wormmeester J, Wierenga EA, Kapsenberg ML. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J Immunol. 2003;170:4483–4488. doi: 10.4049/jimmunol.170.9.4483. [DOI] [PubMed] [Google Scholar]
- Weber MS, Hohlfeld R, Zamvil SS. Mechanism of action of glatiramer acetate in treatment of multiple sclerosis. Neurotherapeutics. 2007a;4:647–653. doi: 10.1016/j.nurt.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007b;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol. 2008;4:384–398. doi: 10.1038/ncpneuro0832. [DOI] [PubMed] [Google Scholar]