Abstract
Study Aims
Cardiac arrest mortality is significantly affected by failure to obtain return of spontaneous circulation (ROSC) despite cardiopulmonary resuscitation (CPR). Severe myocardial dysfunction and cardiovascular collapse further affects mortality within hours of initial ROSC. Recent work suggests that enhancement of nitric oxide (NO) signaling within minutes of CPR can improve myocardial function and survival. We studied the role of NO signaling on cardiovascular outcomes following cardiac arrest and resuscitation using endothelial NO synthase knockout (NOS3-/-) mice.
Methods
Adult female wild-type (WT) and NOS3-/- mice were anesthetized, intubated, and instrumented with left-ventricular pressure-volume catheters. Cardiac arrest was induced with intravenous potassium chloride. CPR was performed after 8 min of untreated arrest. ROSC rate, cardiac function, whole-blood nitrosylhemoglobin (HbNO) concentrations, heart NOS3 content and phosphorylation (p-NOS3), cyclic guanosine monophosphate (cGMP), and phospho-troponin I (p-TnI) were measured.
Results
Despite equal quality CPR, NOS3-/- mice displayed lower rates of ROSC compared to WT (47.6% [10/21] vs. 82.4% [14/17], p<0.005). Among ROSC animals, NOS3-/- versus WT mice exhibited increased left-ventricular dysfunction and 120 min mortality. Prior to ROSC, myocardial effectors of NO signaling including cGMP and p-TnI were decreased in NOS3-/- vs. WT mice (p<0.05). Following ROSC in WT mice, significant NOS3-dependent increases in circulating HbNO were seen by 120 min. Significant increases in cardiac p-NOS3 occurred between end-arrest and 15 min post-ROSC, while total NOS3 content was increased by 120 min post-ROSC (p<0.05).
Conclusions
Genetic deletion of NOS3 decreases ROSC rate and worsens post-ROSC left-ventricular function. Poor cardiovascular outcomes are associated with differences in NOS3-dependent myocardial cGMP signaling and circulating NO metabolites.
Keywords: sudden cardiac arrest, cardiopulmonary arrest, cardiopulmonary resuscitation, nitric oxide, nitric oxide synthase 3, cyclic guanosine monophosphate, troponin I
1. INTRODUCTION
Despite advances in cardiopulmonary resuscitation (CPR), less than 30% of out-of-hospital cardiac arrest victims achieve return of spontaneous circulation (ROSC).1 Profound hemodynamic instability, characterized by myocardial dysfunction, is a major cause of early death during the first few hours following initial ROSC.2 Such severe myocardial dysfunction, which can persist in the absence of underlying focal coronary artery occlusion,3-5 thus contributes substantially to overall hospital discharge rates of only 5-7%.1
Recent reports from preclinical cardiac arrest models suggest that nitric oxide (NO) plays a cardioprotective role following cardiac arrest.6-8 In the heart, the NO is involved in subcellular signaling events that regulate calcium handling and myofibrillar contraction.9-11 Tightly-controlled NO signaling occurs through the oxidation of L-arginine via the constitutive nitric oxide synthase (NOS) isoforms neuronal NOS (nNOS or NOS1) and endothelial NOS (eNOS or NOS3) localized within the cardiomyocyte.12 In the heart, NOS3-derived NO directly binds to soluble guanylyl cyclase to increase cGMP-mediated second messenger signaling.13 While cGMP signaling has been found to be cardioprotective in models of focal ischemia/reperfusion (I/R) injury14 its role has not been studied in cardiac arrest. In the current study, we examine the impact of NOS3 on cGMP signaling, circulating NO production, and post-cardiac arrest myocardial dysfunction following cardiac arrest.
2. METHODS
Animal Preparation
Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Chicago in accordance with National Institutes of Health guidelines. Adult (age 6 - 8 months) female mice homozygous for the targeted disruption of the NOS3 gene (NOS3-/-, B6.129P2-Nos3tm1Unc/J, The Jackson Laboratory, Bar Harbor, ME), which were initially generated on a 129SVJ/C57Bl/6J mixed background and subsequently backcrossed to C57Bl/6J for twelve generations, were matched with wild-type (WT) female C57BL/6 (Taconic Farms, Germantown, NY) controls. Mice were allowed free access to food and water prior to studies.
Randomized WT (n=24) and NOS3-/- (n=24) mice were anesthetized, orally-intubated, and ventilated as previously described for this model.15-17 Next, animals were instrumented with a pressure-volume (P-V) micro-catheter (SPR-839, P/N 840-8111, Millar Instruments, Houston, TX) advanced from the carotid artery into the left ventricle and a heparinized micro-catheter (BioTime Inc., Berkeley, CA) placed in the left jugular vein for fluid administration. All physiologic signals, including end-tidal CO2 (Columbus Instruments, Columbus, OH) and 3-lead ECG were acquired using PowerLab Chart (ADInstruments, Colorado Springs, CO).
Cardiac Arrest Protocol
Following 50 min of mechanical ventilation, the P-V catheter was withdrawn into the descending aorta for baseline arterial blood pressure measurement. Mice were excluded for mean arterial pressures (MAP) less than 80 mmHg, PETCO2 less than 35 mmHg, surgical complications, or prolonged surgical time (>30 min). Cardiac arrest was then induced by an intravenous bolus of 0.08 mg/g KCl . After 8 min of untreated arrest, chest compressions and mechanical ventilation were initiated along with intravenous administration of 50μL of 0.9% saline at 60 sec intervals. CPR was terminated after 5 min or upon ROSC, which was defined as the return of sinus rhythm with a MAP greater than 40 mmHg lasting at least 5 min. Following ROSC, the P-V catheter was advanced back into the left ventricle. Resuscitated animals received intravenous 0.9% saline at a rate of 100 μL/h and were monitored on mechanical ventilation for up to 120 min. The protocol was terminated if an animal exhibited a MAP less than 40 mmHg for at least 5 min.
Reagents
Unless otherwise specified, chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA). Mouse monoclonal antibodies to total NOS3 and phosphorylated NOS3 (Ser1177 and Thr495) were obtained from BD Biosciences (San Jose, CA, USA). Rabbit polyclonal antibodies to TnI and phospho-TnI (Ser23/24) were purchased from Cell Signaling (Danvers, MA, USA). Mouse monoclonal antibody to α-tubulin was purchased from Lab Vision (Fremont, CA).
Whole Blood Nitrosylhemoglobin
Nitrosylhemoglobin (HbNO), a product of NO and deoxygenated hemoglobin18 was measured by electron paramagnetic resonance spectroscopy (EPR). Additional WT (n = 4 per time point) and NOS3-/- (n = 4 per time point) mice underwent surgical instrumentation and were then sacrificed at i) baseline prior to arrest (BL), ii) following 8 min of cardiac arrest without resuscitation (CA8), or iii) 120 min after ROSC (R120). Venous blood was aspirated from the right ventricle into a heparinized 1ml syringe, centrifuged at 3000 RPM for 5 min at 4 °C, flash frozen in liquid nitrogen, and stored at −80 °C. HbNO calibration solutions were created from deoxygenated naïve mouse erythrocytes re-suspended in a solution of Krebs-Hepes buffer, sodium dithionate, and graded concentrations (1, 5, and 10 μM) of sodium nitrite (NaNO2). X-band spectra were acquired under liquid nitrogen conditions (77 K) from calibration solution and experimental whole blood samples using a Magnettech MiniScope MS200 bench-top EPR device (Berlin, Germany). HbNO concentration was linearly correlated to the peak-to-peak amplitude of the first peak of the characteristic HbNO triplet.
Tissue Western Blots
In a separate group of WT mice (n = 3 per time point), whole hearts were collected at BL, CA8, 15 min following ROSC (R15), 60 min following ROSC (R60), and R120 for Western blot analysis. Total NOS3 and phosphorylation at the serine residue in the reductase domain (Ser1177), which has been shown to increase NOS3 activity two- to threefold above basal levels,19 were measured. In addition, phosphorylation at the p-NOS3(Thr495) site located within the CaM binding domain, which is associated with decreased enzyme activity19 was measured. In additional WT and NOS3-/- animals (n = 5 per strain per time point), whole hearts were harvested and snap frozen at BL and after 1 minute of CPR (prior to ROSC) for measurement of total TnI and p-TnI (Ser23/24). Frozen hearts were pulverized under liquid nitrogen into a fine powder, and proteins were extracted in ice-cold lysis buffer (Cell Signaling, Danvers, MA) on ice for 10 min. Protein concentrations were determined using the Bradford protein assay (Bio-Rad, Hercules, CA). Proteins (25 μg) were then separated on 7.5% gels by SDS-PAGE, transferred to nitrocellulose membranes, and subjected to immunoblotting with primary antibodies overnight at 4 °C. After washing and incubation with appropriate secondary antibodies, bands were visualized using the Supersignal chemiluminescent detection system (Pierce, Rockford, IL). Densitometry was performed using Quantity One Software (Bio-Rad, Richmond, CA).
Cyclic guanosine monophosphate
Heart cGMP content was measured as a marker of functional NO signaling. Whole hearts were collected and lysed as described above. Next, lysates were purified by serial extraction in trichloroacetic acid and water-saturated diethyl ether. Following extraction, samples were heated to 70 °C for 5 min and then acetylated. Heart cyclic guanosine monophosphate (cGMP) was subsequently measured by commercially available competitive enzyme immunoassay using the recommended technique (Cayman Chemical, Ann Arbor, MI).
Statistical Methods
Statistical computations were performed with GraphPad Prism 5 (GraphPad Software Inc, La Jolla, CA) with significance levels of p<0.05 except where noted. Continuous data were described by group means and standard error measures. Mann-Whitney U testing was utilized to compare strain group differences at BL and during CPR (Table 1). Strain group differences in ROSC rate were determined by Fisher’s exact testing. Kaplan-Meier survival analysis was performed using log-rank (Mantel-Cox) testing. Time-related and between-strain differences were identified by repeated measures (mixed model) two-way analysis of variance (ANOVA) with post-hoc testing by the Bonferroni method (p<0.05). For WT Western blot analysis experiments, time-related changes were identified by Kruskal-Wallis ANOVA by ranks with post-hoc Dunn’s testing for multiple comparisons.
Table 1.
Wild-type (WT) and NOS3 deficient (NOS3-/-) group hemodynamic characteristics (mean ± S.D.) during baseline and CPR resuscitation periods.
| WT (n = 17) |
NOS3-/- (n = 21) |
||
|---|---|---|---|
| Baseline | Heart Rate, bpm | 298 ± 50 | 265 ± 72 |
| LVPmax, mmHg | 90.1 ± 12.8 | 111.3 ± 14.6a | |
| dP/dtmax, mmHg/ms | 5.1 ± 0.83 | 5.9 ± 1.1a | |
| τ, ms | 13.7 ± 1.5 | 14.9 ± 2.76 | |
| Stroke Volume, μL | 11.9 ± 2.2 | 12.7 ± 3.8 | |
| Ejection Fraction, % | 39.7 ± 14.4 | 39.6 ± 16.4 | |
| PETCO2, mmHg | 39.7 ± 3.7 | 39.0 ± 3.2 | |
|
| |||
| CPR Period | ROSC, n (%) | 14 (82.4) | 10 (47.6)b |
| Time to ROSCc, s | 168 ± 58.8 | 140 ± 61.8 | |
| CC rate, bpm | 311 ± 26.1 | 312 ± 27.4 | |
| DBP, mmHg | 16.6 ± 7.3 | 18.0 ± 4.2 | |
| PETCO2, mmHg | 26.8 ± 5.0 | 27.0 ± 4.8 | |
p<0.05 compared to WT group (Mann-Whitney U test). Values displayed as mean ± standard deviation.
p<0.05 compared to WT group (Fisher’s exact test)Mann-Whitney U test).
ROSC animals only.
3. RESULTS
Survival and hemodynamics
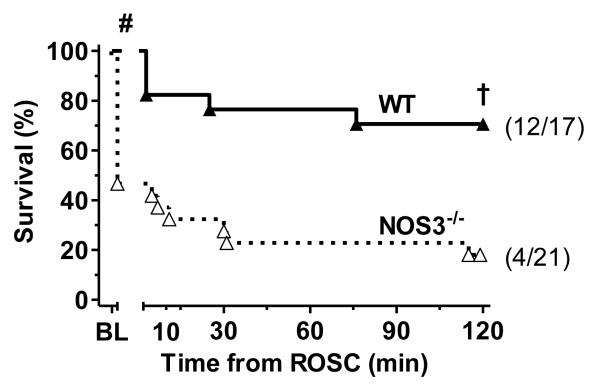
Arrest was induced in 17 of 24 (71 %) WT and 21 of 24 (87.5%) NOS3-/- mice. Consistent with previous reports, NOS3-/- mice had lower body weights (24.8 ± 2.3 g vs. 27.7 ± 2.5 g, p<0.001), higher baseline left-ventricular maximum pressure (LVPmax, Table 1), and higher maximum rate of pressure change dP/dtmax as compared to WT animals (p<0.05).20 Baseline heart rates, while similar to previous reports in this model17 and others,6 were relatively bradycardic in both groups due to the cardiovascular effects of anesthesia.21 All other baseline P-V indices were statistically similar between strain groups. CPR metrics including chest compression rate, aortic diastolic blood pressure, PETCO2, and time to ROSC were similar between strain groups (Table 1). NOS3-/- mice also exhibited lower overall 120 min survival rates than the WT comparison group (19.0% [4/21] vs. 70.6% [12/17], p<0.05).
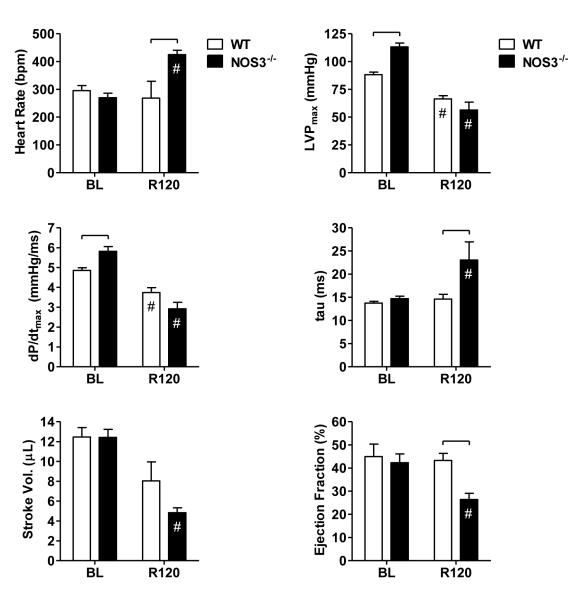
Comparing the subset of animals achieving ROSC, 120 min survival rates (Figure 1) were lower in NOS3-/- versus WT animals (40.0% [4/10] vs. 85.7% [12/14], p<0.05), suggesting greater post-ROSC cardiac dysfunction in the former group. As depicted in Figure 2, NOS3-/- mice, as compared with WT mice, exhibited higher heart rates, longer left-ventricular relaxation time constants (tau), and lower ejection fractions (p<0.05) by 120 min post-ROSC. In addition, elevations in LVPmax, noted at baseline in NOS3-/- mice, were no longer evident by 120 min. Contractility (dP/dtmax) and stroke volume also tended to be lower in NOS3-/- animals by 120 min. However, these latter differences were not statistically significant when adjusted for multiple comparisons.
Figure 1.
Kaplan-Meier plot of WT and NOS3-/- strain 120 min survival following cardiac arrest and resuscitation. # p<0.05 ROSC rate vs. WT (Fisher’s exact test). † p<0.05 vs. WT (Mantel-Cox log-rank). Survival in the subset of NOS3-/- animals achieving ROSC (data not shown) was also significantly lower than WT (p<0.05, Mantel-Cox log-rank).
Figure 2.
Left-ventricular pressure-volume indices at baseline (BL) and 120 min following ROSC (R120), including heart rate (HR), peak left-ventricular pressure (LVPmax), maximum rate of pressure change (dP/dtmax), isovolumic relaxation time constant (τ), stroke volume (SV), and ejection fraction (EF). Significant differences between strain groups (brackets,  ) or relative to BL (#) are indicated (p<0.05, two-way ANOVA).
) or relative to BL (#) are indicated (p<0.05, two-way ANOVA).
Whole blood nitrosylhemoglobin
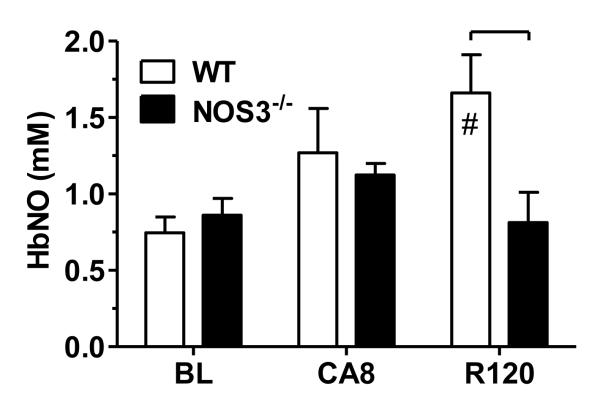
At baseline, whole blood HbNO concentrations (Figure 3) were similar in WT and NOS3-/- (0.75 ± 0.10 μM vs. 0.86 ± 0.11 μM, p=0.46). In NOS3-/- animals, HbNO concentration increased over baseline values after 8 min of untreated cardiac arrest (1.12 ± 0.08 μM, p<0.05). A similar trend was noted in WT animals by CA8 (1.42 ± 0.19 μM, p = 0.071) that did not reach significance. Following 120 min post-ROSC, HbNO concentrations were elevated in WT but not NOS3-/- mice relative to baseline (p<0.05).
Figure 3.
Whole blood nitrosylhemoglobin (HbNO) concentration at baseline (BL), following 8 min of untreated cardiac arrest (CA8), and 120 min post-ROSC (R120). Significant differences between strain groups (brackets,  ) or relative to BL (#) are indicated (p<0.05, two-way ANOVA).
) or relative to BL (#) are indicated (p<0.05, two-way ANOVA).
Myocardial NOS3 protein content and phosphorylation
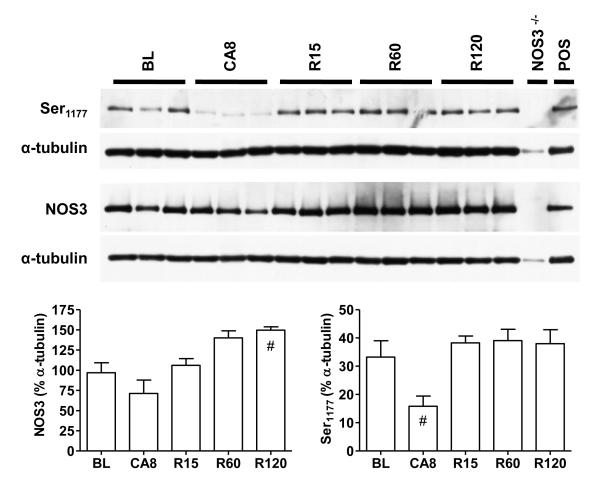
NOS3 protein was constitutively expressed in WT but not NOS3-/- hearts (Figure 3). By 120 min following resuscitation, myocardial NOS3 content increased above baseline values (p<0.05). As expected, BL animals exhibited phosphorylation of NOS3 at the Ser1177 site.19 After 8 min of arrest, NOS3 phosphorylation at this site dramatically decreased but then returned to baseline levels following ROSC. By contrast, phosphorylation of NOS3 at Thr495 was not significantly modulated by arrest or resuscitation (data not shown). WT and NOS3-/- hearts exhibited similar amounts of NOS1 and did not express measurable amounts of NOS2 protein at BL or R120 (see online Appendix A for details).
Myocardial cGMP content and TnI phosphorylation
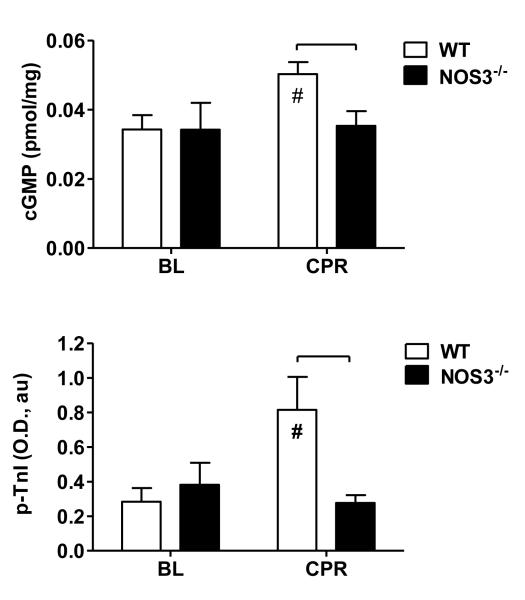
Given the observed NOS3-dependent differences in ROSC rate and observed changes in NOS3 phosphorylation during CPR we measured cyclic guanosine monophosphate (cGMP) and troponin-I phosphorylation (p-TnI) as mediators of myocardial NO signaling central to cardiac function.22 Baseline cGMP and p-TnI levels were similar between strains (Figure 5). However, following 1 minute of CPR, cGMP and p-TnI levels were significantly higher in WT than NOS3-/- mice (p<0.05).
Figure 5.
Whole heart cyclic guanosine monophosphate (cGMP, upper panel) and (B) phospho-troponin-I (p-TnI, lower panel) at baseline (BL) and following 1 min of CPR, prior to ROSC. Significant differences between strain groups (brackets,  ) or relative to BL (#) are indicated (p<0.05, two-way ANOVA).
) or relative to BL (#) are indicated (p<0.05, two-way ANOVA).
4. DISCUSSION
In this study, we report for the first time the detrimental effects of NOS3 genetic deletion on ROSC rate and cardiac function following normothermic cardiac arrest. This work extends reports of severe post-ROSC cardiac stunning from our lab and others in models utilizing WT mice15-17, 23 and swine24-28 by demonstrating that NOS3-/- mice exhibit lower ROSC rates and increased post-ROSC cardiac dysfunction when compared to WT. Poor outcomes were associated with lower myocardial cGMP and p-TnI levels during CPR and lower circulating NO following ROSC, suggesting potential roles for both subcellular and circulating NO signaling in determining outcomes following cardiac arrest.
Nitric oxide synthase 3 and post-arrest cardioprotection
The link between enzyme-mediated NO signaling and post-ROSC cardioprotection established by the current study is consistent with results from our laboratory’s in vitro model of simulated I/R29, 30 and work by others in cardiac arrest models in mouse6 and swine.8 Our results also extend recent work in this mouse model demonstrating that the ROS-sensitive serine/threonine kinase Akt (PKB), which closely regulates NOS3 via phosphorylation of its Ser1177 site,19, 31 is essential to post-ROSC cardiac function.17 Of particular relevance to the current work, Nishida and colleagues demonstrated worse myocardial function in NOS3-/- versus WT mice, including higher heart rates, smaller ejection fractions, and longer post-ROSC diastolic relaxation times, which is consistent with our results.6 It is important to note that the prolongation in diastolic relaxation time (τ) that we noted in NOS3-/- mice likely underestimates the true strain-related difference in post-ROSC diastolic dysfunction given the known dependence of τ on heart rate.32 Our data extend Nishida’s findings by demonstrating that such NOS3-related differences in cardiovascular dysfunction persist under normothermic conditions and lead to significantly higher rates of cardiovascular collapse and death within the first 120 min following ROSC. Our results are also consistent with work by others in a swine model of electrically-induced VF arrest, which demonstrated that nonselective pharmacologic inhibition of NOS, 30 min prior to arrest induction, significantly reduced 3 h survival.8 Here, we add to the literature by demonstrating that selective loss of NOS3 is sufficient to potentiate post-ROSC cardiac dysfunction.
Our novel finding that NOS3 deletion worsens ROSC rate suggests an important role for NOS3-mediated signaling during CPR. In the study by Nishida and colleagues, NOS3-/- mice exhibited a trend towards longer CPR time to ROSC, but no difference in ROSC rate relative to WT.6 The lack of effect on ROSC rate noted in the Nishida study can possibly be explained by their use of profound intra-arrest hypothermia (28 °C) in their protocol that resulted in ROSC rates of greater than 95% in both groups and a lack of significant post-ROSC cardiac dysfunction in WT animals.6 Our findings conflict with reports from others in porcine models of VF arrest suggesting that nonselective NOS inhibition has either no effect,8, 33 or positive impact34 on initial ROSC rates. This apparent lack of agreement on the role of NOS following arrest, can be explained by noting that selective inhibition of inducible NOS (i.e. NOS2) has been shown to improve outcomes following VF arrest in a swine model.8 Thus, their results reflect the net positive effect of inhibiting both protective (i.e. NOS3) and harmful (i.e. NOS2) NOS isoforms.
cGMP signaling following cardiac arrest
Evidence from a variety of models suggests an important role for NO signaling in the regulation of cardiac function during both normal and pathophysiologic states.11 Within the cardiomyocyte, NOS3-derived NO directly binds to soluble guanylyl cyclase to increase signaling through cGMP-mediated second messenger pathways.13 The elevation in myocardial cGMP levels noted in WT mice during CPR is consistent with work by others in an isolated rat heart suggesting that cGMP levels increase during ischemia.35 Furthermore, the association between elevated cGMP and increased ROSC rates in WT versus NOS3-/- mice, is consistent with the idea that cGMP participates in cardioprotective signaling following I/R.14
While several putative pathways of cGMP cardioprotection have been suggested, of particular relevance to our study is the role of cGMP-dependent protein kinase G (PKG) in reducing myofibril Ca2+ sensitivity during reperfusion through phosphorylation of TnI, 14, 22 which could improve lusitropy and serve to limit myocardial contracture during the partial reperfusion state induced by CPR. The post-translational modification of myofibrillar proteins, such as TnI, is an important mechanism of cardiac contractile function regulation36 that has previously been implicated in post-ischemic myocardial stunning and heart failure.37, 38 Thus, the reported association between increased cardiac cGMP content and TnI phosphorylation in WT, versus NOS3-/-, mice suggests a novel link between cGMP-NO signaling and effective CPR.
Circulating HbNO following cardiac arrest
While EPR spectroscopy has been used by others to quantitate whole blood HbNO concentrations in animal models of cardiac I/R injury and hemorrhagic shock, 39-41 the present study represents the first reported attempts to apply to profile this bioavailable NO metabolite following cardiac arrest and resuscitation. It is worthwhile to note that the reported baseline HbNO concentrations are similar to basal levels previously measured by others in naïve WT and NOS3-/- mice.42 Following ROSC, we observed an increase in circulating NO in WT, but not NOS3-/- mice, suggesting NOS3-dependent NO production. This finding is consistent with work by others demonstrating NOS-dependent increases in NO following reperfusion in the coronary effluent of an isolated heart model of focal I/R.43 It should be noted that the avidity of NO binding to hemoglobin increases in the deoxygenated state, which could contribute to the observed increase in HbNO concentrations following arrest.42 Our results also suggest an association between such elevations in circulating NO and improved post-ROSC cardiovascular outcomes. This association is consistent with work by Dezfulian et al. in a mouse model of cardiac arrest demonstrating the benefit of intravenous nitrite, a putative NO donor, during CPR on improved post-ROSC cardiac function, long-term survival, and neurologic outcomes.23 However, while exogenous nitrite therapy shortened CPR time to ROSC in that study, it did not improve ROSC rate.23 The current study extends this work by suggesting that cardioprotective NO signaling may also be supported by enzyme-mediated production of NO.
Cardiac arrest alters myocardial NOS3 content and phosphorylation
Our results suggest that cardiac arrest and resuscitation significantly alter myocardial NOS3 protein content and are consistent with work by others in a swine model of ventricular fibrillation (VF) arrest showing significant decreases in heart NOS3 expression during VF followed by increased expression after ROSC.25 Post-ROSC elevations in myocardial NOS3 could lead to increased capacity for NO biosynthesis from L-arginine as well as increased reduction of cardioprotective nitrite to NO.44 While NOS3 protein content is regulated by both transcriptional and posttranscriptional mechanisms triggered by a variety of physiological and pathophysiological stimuli,45 the relative contribution of these mechanisms following cardiac arrest is not known. However, given the long half-life of NOS3 mRNA (10-35 h) within the cytosol, we hypothesize that posttranscriptional regulation, which could rapidly impact mRNA stability and half-life of existing transcripts, may be more rapidly efficient than transcriptional regulation of protein expression.45
While changes in cardiac NOS3 phosphorylation have been previously reported following focal coronary artery ischemia, to our knowledge this is the first study to examine changes in NOS phosphorylation in the setting of cardiac arrest.46 The decrease in myocardial p-NOS3(Ser1177) demonstrated in Figure 4 suggests a decrease in NOS3 activity during arrest followed by resumption of activity following resuscitation.19 However, it should be noted that, NOS3 mediated NO production is also regulated by a variety of post-translational factors including the availability of substrate, intracellular Ca2+, and cofactors such as tetrahydrobiopterin (BH4).47 The dramatic shifts in the cellular micro-environment following cardiac arrest have the potential to deplete and oxidize L-arginine and BH4 resulting in uncoupling of mitochondrial electron transfer and ROS (e.g. superoxide) generation instead of NO signaling.47, 48 Whether modulation of NOS3 expression and phosphorylation ultimately results in significant differences in tissue NO or ROS production remains to be determined.
Figure 4.
Whole heart total NOS3 content, p-NOS(Ser1177) levels in WT at baseline (BL), following 8 min of untreated cardiac arrest (CA8); and at 15 (R15), 60 (R60), and 120 min(R120) post ROSC. Densitometry values displayed as a mean percentage of α-tubulin + standard deviation. Differences relative to BL ( ) or CA8 (#) are indicated (p<0.05, Kruskal-Wallis ANOVA).
) or CA8 (#) are indicated (p<0.05, Kruskal-Wallis ANOVA).
Limitations
Though the current study establishes a causal link between NOS3 deletion and cardiac dysfunction following cardiac arrest, the precise mechanisms of NO cardioprotection remain to be established. For example, NOS3-derived NO plays an important role in maintaining microvascular barrier integrity,49 which may be an important determinant of myocardial contractility following I/R. In addition to altering cGMP-dependent signaling, NOS3-derived NO has the potential to regulate cardiac function via reversible modification of cysteine thiol moieties (i.e. nitrosylation) within a variety of intracellular protein targets involved in beta-adrenergic receptor signaling, excitation-contraction coupling, mitochondrial respiration, oxidant stress, and apoptosis.11, 50-52 The role of nitrosylation, which is not addressed in our study, was recently explored by Dezfulian et al. in a related mouse model of cardiac arrest. They suggested an association between nitrite-induced elevations in heart s-nitrosothiols and improved ROSC and post-ROSC cardiac outcomes.23 Further work is necessary to better understand how these two NO signaling pathways may interact during arrest and resuscitation. Finally, we utilized retired breeder female mice. Similar studies are necessary to confirm the role of NOS3-NO signaling in male mice as estradiol is known to upregulate NOS3 in aortic in vitro preparations.53
5. CONCLUSIONS
Our results demonstrate that NOS3 plays an important role in determining short-term outcomes following cardiac arrest, possibly via cGMP-dependent signaling pathways within the myocardium. While circulating NO metabolites increase in a NOS3-independent fashion following arrest, their production following ROSC relies on NOS3. Resuscitation increases myocardial NOS3 content and restores NOS3 phosphorylation, which may help sustain NO survival signaling following ROSC.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge EPR technical assistance from Karel Chalupsky and editorial assistance from Kimberly Wojcik and Michael Retzer.
Financial Support: Supported by grants 5K08HL091184 (DGB), HL68951 (TVH), HL079641 (KJH) HL084643 (KJH), HL61322 (EMM), and HL78926 (EMM) from the National Heart, Lung, And Blood Institute.
Footnotes
6. CONFLICT OF INTEREST The authors have no relevant financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nichol G, Stiell IG, Laupacis A, Pham B, De Maio VJ, Wells GA. A cumulative meta-analysis of the effectiveness of defibrillator-capable emergency medical services for victims of out-of-hospital cardiac arrest. Ann Emerg Med. 1999;34:517–525. [PubMed] [Google Scholar]
- 2.Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Schoenenberger RA, von Planta M, von Planta I. Survival after failed out-of-hospital resuscitation. Are further therapeutic efforts in the emergency department futile? Arch Intern Med. 1994;154:2433–2437. [PubMed] [Google Scholar]
- 4.Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez MM, Berg RA, Nadkarni VM, et al. Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation. 2008;117:1864–1872. doi: 10.1161/CIRCULATIONAHA.107.740167. [DOI] [PubMed] [Google Scholar]
- 6.Nishida T, Yu JD, Minamishima S, et al. Protective effects of nitric oxide synthase 3 and soluble guanylate cyclase on the outcome of cardiac arrest and cardiopulmonary resuscitation in mice. Crit Care Med. 2009;37:256–262. doi: 10.1097/CCM.0b013e318192face. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dezfulian C, Shiva S, Alekseyenko A, et al. Nitrite therapy after cardiac arrest reduces ROS generation, improves cardiac and neurological function and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JA, Wu D, Bassuk J, et al. Nitric oxide synthase isoform inhibition before whole body ischemia reperfusion in pigs: vital or protective? Resuscitation. 2007;74:516–525. doi: 10.1016/j.resuscitation.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Oess S, Icking A, Fulton D, Govers R, Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J. 2006;396:401–409. doi: 10.1042/BJ20060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadei B. The emerging role of neuronal nitric oxide synthase in the regulation of myocardial function. Exp Physiol. 2006;91:943–955. doi: 10.1113/expphysiol.2006.035493. [DOI] [PubMed] [Google Scholar]
- 11.Saraiva RM, Hare JM. Nitric oxide signaling in the cardiovascular system: implications for heart failure. Curr Opin Cardiol. 2006;21:221–228. doi: 10.1097/01.hco.0000221584.56372.dc. [DOI] [PubMed] [Google Scholar]
- 12.Liaudet L, Soriano FG, Szabo C. Biology of nitric oxide signaling. Crit Care Med. 2000;28 doi: 10.1097/00003246-200004001-00005. [DOI] [PubMed] [Google Scholar]
- 13.Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res. 2003;93:907–916. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- 14.Piper HM, Abdallah Y, Schafer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004;61:365–371. doi: 10.1016/j.cardiores.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Abella B, Zhao D, Alvarado J, Hamann K, Hoek TL Vanden, Becker L. Intra-Arrest Cooling Improves Outcomes in a Murine Cardiac Arrest Model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 16.Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation. 2008;77:242–249. doi: 10.1016/j.resuscitation.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beiser DG, Wojcik KR, Zhao D, Orbelyan GA, Hamann KJ, Hoek TL Vanden. Akt1 genetic deficiency limits hypothermia cardioprotection following murine cardiac arrest. Am J Physiol Heart Circ Physiol. 2010;298:H1761–1768. doi: 10.1152/ajpheart.00187.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink F, Sergey D, Fink N. ESR techniques for the detection of nitric oxide in vivo as an index of endothelial function. Pharmacological Reports. 2006;58:8–15. [PubMed] [Google Scholar]
- 19.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 20.Shesely EG, Maeda N, Kim HS, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satoh S, Makino N. Intracellular mechanisms of cGMP-mediated regulation of myocardial contraction. Basic Res Cardiol. 2001;96:652–658. doi: 10.1007/s003950170018. [DOI] [PubMed] [Google Scholar]
- 23.Dezfulian C, Shiva S, Alekseyenko A, et al. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazmuri RJ, Weil MH, Bisera J, Tang W, Fukui M, McKee D. Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med. 1996;24:992–1000. doi: 10.1097/00003246-199606000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Kern KB, Berg RA, Hilwig RW, Larson DF, Gaballa MA. Myocardial cytokine IL-8 and nitric oxide synthase activity during and after resuscitation: Preliminary observations in regards to post-resuscitation myocardial dysfunction. Resuscitation. 2008;77:401–409. doi: 10.1016/j.resuscitation.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kern KB, Hilwig RW, Berg RA, et al. Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation. 1997;95:2610–2613. doi: 10.1161/01.cir.95.12.2610. [DOI] [PubMed] [Google Scholar]
- 27.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 28.Niemann J, Garner D, Lewis R. Tumor necrosis factor-[alpha] is associated with early postresuscitation myocardial dysfunction. Crit Care Med. 2004;32:1753–1758. doi: 10.1097/01.ccm.0000132899.15242.d3. [DOI] [PubMed] [Google Scholar]
- 29.Shao ZH, Chang WT, Chan KC, et al. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol Heart Circ Physiol. 2007;292:H1995–2003. doi: 10.1152/ajpheart.01312.2005. [DOI] [PubMed] [Google Scholar]
- 30.Shao ZH, Sharp WW, Wojcik KR, et al. Therapeutic hypothermia cardioprotection via Akt- and nitric oxide-mediated attenuation of mitochondrial oxidants. Am J Physiol Heart Circ Physiol. 2010;298:H2164–2173. doi: 10.1152/ajpheart.00994.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Baines CP, Zong C, et al. Functional proteomic analysis of a three-tier PKCepsilon-Akt-eNOS signaling module in cardiac protection. Am J Physiol Heart Circ Physiol. 2005;288:H954–961. doi: 10.1152/ajpheart.00756.2004. [DOI] [PubMed] [Google Scholar]
- 32.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. Journal of Clinical Investigation. 1976;58:751–760. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Boddicker KA, Rhee BJ, Davies LR, Kerber RE. Effect of nitric oxide synthase modulation on resuscitation success in a swine ventricular fibrillation cardiac arrest model. Resuscitation. 2005;67:127–134. doi: 10.1016/j.resuscitation.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Krismer AC, Lindner KH, Wenzel V, Rainer B, Mueller G, Lingnau W. Inhibition of nitric oxide improves coronary perfusion pressure and return of spontaneous circulation in a porcine cardiopulmonary resuscitation model. Crit Care Med. 2001;29:482–486. doi: 10.1097/00003246-200103000-00003. [see comment] [DOI] [PubMed] [Google Scholar]
- 35.Depre C, Hue L. Cyclic GMP in the perfused rat heart. Effect of ischaemia, anoxia and nitric oxide synthase inhibitor. FEBS Lett. 1994;345:241–245. doi: 10.1016/0014-5793(94)00459-5. [DOI] [PubMed] [Google Scholar]
- 36.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Van Eyk JE, Murphy AM. The role of troponin abnormalities as a cause for stunned myocardium. Coron Artery Dis. 2001;12:343–347. doi: 10.1097/00019501-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Murphy AM. Heart failure, myocardial stunning, and troponin: a key regulator of the cardiac myofilament. Congest Heart Fail. 2006;12:32–38. doi: 10.1111/j.1527-5299.2006.04320.x. quiz 39-40. [DOI] [PubMed] [Google Scholar]
- 39.Kuppusamy P, Shankar RA, Roubaud VM, Zweier JL. Whole body detection and imaging of nitric oxide generation in mice following cardiopulmonary arrest: detection of intrinsic nitrosoheme complexes. Magnetic Resonance in Medicine. 2001;45:700–707. doi: 10.1002/mrm.1093. [DOI] [PubMed] [Google Scholar]
- 40.Tiravanti E, Samouilov A, Zweier JL. Nitrosyl-heme complexes are formed in the ischemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signaling in post-ischemic tissues. J Biol Chem. 2004;279:11065–11073. doi: 10.1074/jbc.M311908200. [DOI] [PubMed] [Google Scholar]
- 41.Atkins JL, Day BW, Handrigan MT, Zhang Z, Pamnani MB, Gorbunov NV. Brisk production of nitric oxide and associated formation of S-nitrosothiols in early hemorrhage. J Appl Physiol. 2006;100:1267–1277. doi: 10.1152/japplphysiol.01059.2005. [DOI] [PubMed] [Google Scholar]
- 42.Dikalov S, Fink B. ESR techniques for the detection of nitric oxide in vivo and in tissues. Methods Enzymol. 2005;396:597–610. doi: 10.1016/S0076-6879(05)96052-7. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 44.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Searles CD. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am J Physiol Cell Physiol. 2006;291:C803–816. doi: 10.1152/ajpcell.00457.2005. [DOI] [PubMed] [Google Scholar]
- 46.Gao F, Gao E, Yue TL, et al. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- 47.Bevers LM, Braam B, Post JA, et al. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47:87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- 48.Haruna Y, Morita Y, Komai N, et al. Endothelial dysfunction in rat adjuvant-induced arthritis: vascular superoxide production by NAD(P)H oxidase and uncoupled endothelial nitric oxide synthase. Arthritis Rheum. 2006;54:1847–1855. doi: 10.1002/art.21891. [DOI] [PubMed] [Google Scholar]
- 49.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- 50.Mannick JB. Regulation of apoptosis by protein S-nitrosylation. Amino Acids. 2007;32:523–526. doi: 10.1007/s00726-006-0427-6. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto A, Comatas KE, Liu L, Stamler JS. Screening for nitric oxide-dependent protein-protein interactions. Science. 2003;301:657–661. doi: 10.1126/science.1079319. [DOI] [PubMed] [Google Scholar]
- 52.Whalen EJ, Foster MW, Matsumoto A, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 53.Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Up-regulation of nitric oxide synthase by estradiol in human aortic endothelial cells. FEBS Lett. 1995;360:291–293. doi: 10.1016/0014-5793(95)00124-r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.