Abstract
AMPK is a cellular energy sensor that negatively regulates the mTOR signaling pathway. As mTOR plays critical roles in cell growth and tumorigenesis of renal cell carcinoma (RCC), we examined whether exogenous induction of AMPK activity exhibits inhibitory effects on growth and survival of renal cell carcinoma cells. Activation of AMPK by AICAR resulted in potent suppressive effects on RCC growth, while combinations of AICAR with statins were potent inducers of apoptosis in such cells. The effects of AICAR resulted from inhibition of mTOR and its effectors, resulting from induction of AMPK activity. Similar results on RCC cell growth were obtained when combinations of metformin with statins were examined. Importantly, studies to examine the effects of AICAR or metformin, alone or in combinations with statins, on anchorageindependent growth demonstrated potent suppressive effects on RCC tumorigenicity in vitro. Altogether, our studies demonstrate that AMPK plays critical regulatory roles in the regulation of growth of RCC cells and raise the prospect of future use of AMPK activators in the treatment of renal cell carcinoma in humans.
Key words: AMPK, mammalian target of rapamycin (mTOR), renal cell carcinoma
Background
The serine-threonine kinase AMP-activated kinase (AMPK) is a cellular metabolic protein that is activated by elevated AMP: ATP levels in the cell under stressful cellular conditions. 1 Structurally, AMPK exists as a trimeric protein with a catalytic alpha subunit and the two regulatory subunits, beta and gamma.1,2 Following elevation of cellular AMP levels relative to ATP levels, the AMP binds to the gamma domain of AMPK, facilitating a conformational change in the alpha subunit, resulting in phosphorylation/activation of AMPK at threonine 172.2 This traditional notion on the mechanism of AMPK activation has been challenged by recent evidence indicating that binding of AMP to AMPK promotes LKB1-dependent phosphorylation of residue Thr-172 through inhibition of dephosphorylation, making the AMPK complex a less efficient substrate for protein phosphatases.3 Phosphorylation of AMPK at this active site results in subsequent induction of its kinase activity.1,2 In turn, such activation directly results in a variety of cellular metabolic responses aimed at replenishing the cellular energy supply, including enhanced fatty acid oxidation and glycolysis and decreased synthesis of glycogen, fatty acids and proteins.2
In recent years, research efforts have begun to highlight the intrinsic connection between cancer and metabolism. Malignant cells acquire the characteristic of enhanced anabolism, resulting in energy-consuming processes such as increased protein translation and DNA synthesis that can be targeted in cancerous cells due to the detectable changes in ATP levels as compared to AMP levels.4–6 Previous work has demonstrated that 5-aminoimidazole-4-carboxamide riboside (AICAR), the pharmacological activator of AMPK,7 suppresses tumor growth of established human colon,8 acute lymphoblastic leukemia,9 prostate10 and breast cancer6 cell lines in vitro. It is also known that AMPK is critically linked to the phosphatidyl-inositol-3 kinase/AKT/mTOR signaling pathway, a vital cellular signaling cascade that is essential for cell growth in response to mitogenic stimuli or pathways activated by growth factor receptors.11,12 AMPK activation directly inhibits phosphorylation and subsequent activation of the mTORC1 complex and is controlled in part by the upstream kinase AKT, whose activation decreases the AMP:ATP ratio.13–15 AKT also directly inhibits activation of AMPK by phosphorylation of AMPK at Ser 485/491.16,17 Other studies have shown that AICAR exerts its effects on cancer cells by inducing S-phase arrest and inhibition of the PI3-K/AKT pathway.18
Renal cell carcinoma (RCC) is a highly aggressive genitourinary cancer for which the treatment options are limited.19 This malignancy is characterized by over-activation of this AKT/mTOR signaling pathway.20 Extensive work over the last few years has demonstrated the effectiveness of targeting the mTOR pathway for the treatment of RCC.21 Temsirolimus, a known mTOR pathway inhibitor, has significant clinical activity in the treatment of RCC and it is now an FDA-approved agent in the treatment of patients with RCC.22 There is also evidence that 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) inhibit RCC cell growth and induce RCC cell death by de-phosphorylation/de-activation of AKT and its downstream signaling components.23 In the present study we examined the effects of AICAR, a pharmacological activator of AMPK, on RCC cells. Our data demonstrate that AICAR exhibits growth inhibitory and pro-apoptotic effects on RCC cells and suppresses the mTOR pathway, while the combination of AICAR with new generation statins results in enhancement of RCC cell death and suppression of anchorage-independent growth, suggesting that combinations of AICAR with statins may provide a novel approach to generate antitumor responses.
Results
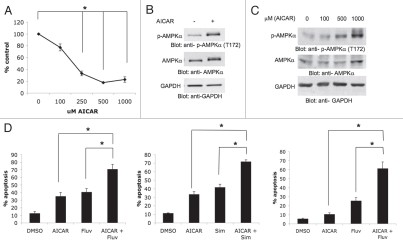
We first examined the effects of the AMPK activator AICAR on human renal cell carcinoma cell growth and viability. 786-0 cells were treated with increasing doses of AICAR and cell proliferation was assessed by MTT assays. AICAR clearly inhibited the growth of 786-0 cells in a dose-dependent manner (Fig. 1A). Also, as expected, AICAR induced phosphorylation/activation of the AMP-activated kinase (AMPK) in these cells (Fig. 1B), suggesting that its growth suppressive effects reflect engagement of AMPK and downstream suppression of mTOR. Such AICARinducible phosphorylation in renal cell carcinoma cells was also dose-dependent (Fig. 1C).
Figure 1.
AICAR-dependent growth suppression and apoptosis in renal cell carcinoma cells. (A) 786-0 cells were treated for 4 days with solvent control or with the indicated concentrations of AICAR. Cell proliferation was assessed by MTT. Data are expressed as % control-treated cells and represent means ± SE of three independent experiments. Paired t-test analysis for the growth of 786-0 cells treated with 250 µmol/L AICAR, 500 µmol/L AICAR and 1,000 µmol/L AICAR versus control treated cells showed a 2-tailed p values of 0.003, 0.0001 and 0.004, respectively. (B) Top, 786-0 cells were treated with solvent control or AICAR (2 mmol/L) for 48 hours, as indicated. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated/activated form of AMPKα at Thr 172. Middle, equal protein aliquots from the same experiment were resolved on a separate gel and probed with an anti-AMPKα antibody. Bottom, the top blot was subsequently reprobed with an antibody to detect anti-glyceradledhyde-3-phosphate dehydrogenase (anti-GAPDH). (C) Top, 786-0 cells were treated with solvent control or the indicated concentrations of AICAR for 48 hours. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated/activated form of AMPKα at Thr 172. Middle, bottom, the blot was subsequently stripped and reprobed with an antibody to detect anti-AMPKα⊠, middle, and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH), bottom. (D) Left, 786-0 cells were treated with solvent control (DMSO), AICAR (500 µmol/L), fluvastatin (3 µmol/L), or the indicated combinations for 96 hours. Data are expressed as % apoptotic cells and represent means ± SE of four independent experiments. Paired t-test analysis for the induction of apoptosis of 786-0 cells treated with AICAR versus fluvastatin and AICAR showed a 2-tailed p value = 0.013. Paired t-test analysis for the induction of apoptosis of 786-0 cells treated with fluvastatin versus fluvastatin and AICAR treated cells showed a 2-tailed p value = 0.0005. Middle, 786-0 cells were treated with solvent control (DMSO), AICAR (500 µmol/L), simvastatin (3 µmol/L), or the indicated combinations for 96 hours. Data are expressed as % apoptotic cells and represent means ± SE of 3 independent experiments. Paired t-test analysis for the induction of apoptosis of 786-0 cells treated with AICAR versus simvastatin and AICAR showed a 2-tailed p value = 0.003. Paired t-test analysis for the induction of apoptosis of 786-0 cells treated with simvastatin versus simvastatin and AICAR showed a 2-tailed p value = 0.003. Right, ACHN cells were treated with solvent control (DMSO), AICAR (500 µmol/L), fluvastatin (3 µmol/L), or the indicated combinations for ninety-six hours. Data are expressed as % apoptotic cells and represent means ± SE of four independent experiments. Paired t-test analysis for the induction of apoptosis of ACHN cells treated with AICAR versus fluvastatin and AICAR showed a 2-tailed p value = 0.008. Paired t-test analysis for the induction of apoptosis of ACHN cells treated with fluvastatin versus fluvastatin and AICAR treated cells showed a 2-tailed p value = 0.003.
In subsequent studies, we examined whether AMPK induction ultimately induces apoptosis of 786-0 cells. Treatment with AICAR resulted in apoptotic death of approximately 30–35% of cells at 96 hours (Fig. 1D). As previous work from our group has established that statins induce apoptosis of RCC cells by targeting the mTOR pathway,23 we also determined whether addition of fluvastatin or simvastatin to the cultures enhances AICARdependent apoptosis. Combining AICAR with either fluvastatin (Fig. 1D, left) or simvastatin (Fig. 1D, middle) resulted in much higher apoptosis than each agent alone, suggesting that statins enhance the pro-apoptotic effects of AICAR on RCC cells. When the effects of AICAR or fluvastatin on the induction of apoptosis were examined in the ACHN renal carcinoma cell line, we noticed minimal induction of apoptosis in these cells, but there was dramatic enhancement of apoptosis when the two agents were added together to the cultures (Fig. 1D, right), further establishing that AICAR promotes statin-induced cell death.
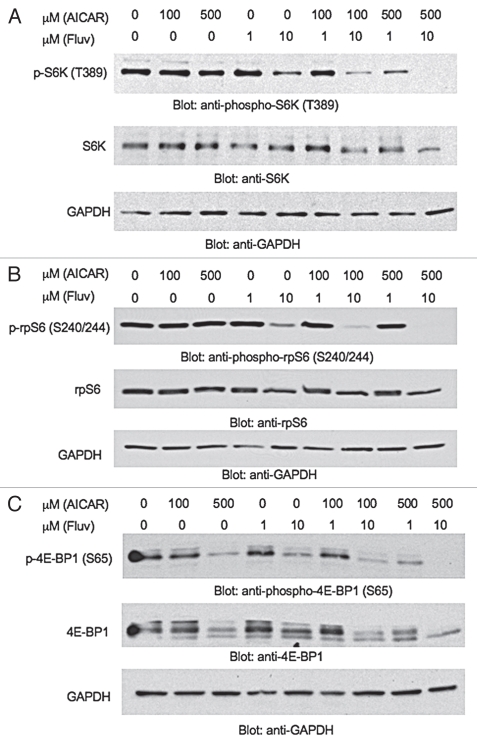
Extensive work in recent years has highlighted the therapeutic potential of targeting the mTOR pathway in renal cell carcinoma.21,22,24 In fact, temsirolimus was recently approved by the FDA for the treatment of such patients.22 Also, based on our previous work, statins exhibit suppressive effects on the phosphorylation/activation of AKT and downstream mTOR engagement in renal cell carcinoma cells.23 We examined the effects of AICAR, or combinations of AICAR with statins on the activation of downstream signaling effectors of AKT/mTOR in the 786-0 RCC cell line. AICAR had minimal effects on the phosphorylation of the S6 kinase (S6K) or S6 ribosomal protein (rpS6) (Fig. 2A and B), but partially suppressed phosphorylation of 4E-BP1 on serine 65 (Fig. 2C). However, when increasing concentrations of both AICAR (500 µmol/L) and fluvastatin (10 µmol/L) were added together to the cultures, we observed potent dephosphorylation of S6K on its active site, threonine 389 (Fig. 2A). Similarly, the phosphorylation of rpS6 and 4E-BP1 were dramatically decreased (Fig. 2B and C).
Figure 2.
Suppressive effects of combinations of AICAR and fluvastatin on the activation of the mTOR pathway in renal cell carcinoma cells. (A–C) 786-0 cells were treated with solvent control (DMSO), AICAR, fluvastatin or the indicated combinations of different concentrations of AICAR and fluvastatin for 48 hours, as indicated. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies against the phosphorylated/activated form of (A) Top, S6K on Thr 389 (B) Top, rpS6 on Ser 240/244 and (C) Top, 4E-BP1 on Ser 65. Equal protein aliquots from the same experiments in (A) top, (B) top or (C) top were resolved on separate gels and probed with (A) Middle, anti-S6K, (B) Middle, anti-rpS6 or (C) Middle, anti-4E-BP1 antibodies, respectively. The (A and B) top or (C) middle blots were subsequently reprobed with an (A–C) Bottom, anti-GAPDH antibody.
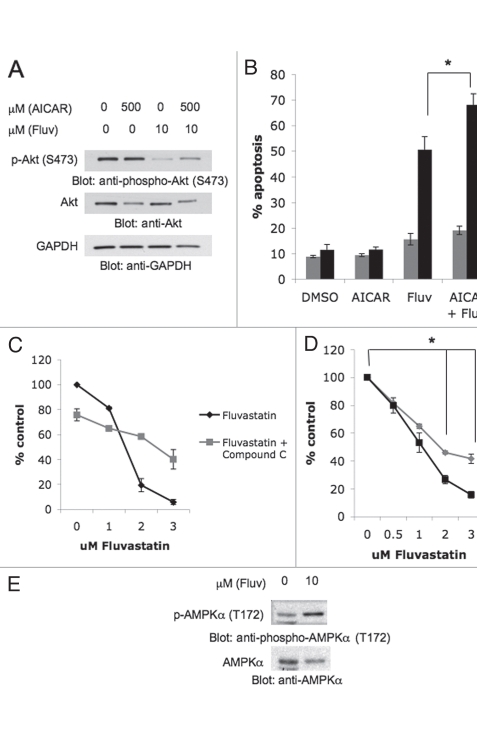
Previous work has established that AKT and AMPK converge on the tuberous sclerosis complex (TSC) 1-TSC2 complex, which lies upstream of mTORC1.12 Both AKT and AMPK phosphorylate TSC2, albeit at different sites that have opposing effects on eventual mTORC1 activation.12 AKT phosphorylation of TSC2 inhibits its inherent GAP activity, while AMPK phosphorylation enhances such GAP function of TSC2.12 Interestingly, despite their antagonistic regulation of TSC2 phosphorylation, AMPK activation is also directly controlled by AKT, suggesting that AKT lies upstream of AMPK in the convergence of these two distinct cellular signaling pathways.15–17 Treatment of renal cell carcinoma cells with AICAR did not induce de-phosphorylation of AKT (Fig. 3A). As expected,23 fluvastatin inhibited such phosphorylation, while such inhibition was also noticeable in cells treated with the AICAR + fluvastatin combination (Fig. 3A). To better understand the mechanisms of the enhanced antitumor effects seen in response to combinations of AICAR and statins, we tested whether overexpression of a constitutively active AKT mutant rescues the pro-apoptotic effects of the AICAR and fluvastatin combination. As shown in Figure 3B, Rat1a cells expressing a constitutively active form of AKT were nearly resistant to the apoptotic effects of AICAR and fluvastatin (Fig. 3B). Although, one can not exclude cell-dependent variations on the role of AKT, these findings strongly suggest that inhibition of AKT activity is critical for the generation of such pro-apoptotic effects by the combination. We also determined the effects of compound C, an agent known to inhibit AMPK activation.25 In contrast to what we observed in the case of AICAR, concomitant treatment of 786-0 cells with fluvastatin and compound C resulted in partial reversal of the suppressive effects of fluvastatin on 786-0 cell proliferation (Fig. 3C). As compound C is a non-specific inhibitor of AMPK and to exclude the possibility that such reversal reflects non-specific effects of compound C unrelated to AMPK inhibition, additional studies using cells with targeted disruption of both AMPK1 and AMPK2 were performed. In experiments with AMPK 1-/-2-/- mouse embryonic fibroblasts (MEFs) we found that these cells are more resistant to the inhibitory effects of statins on cell growth than wild type MEFs, indicating that fluvastatin's effects are mediated in part through AMPK (Fig. 3D). Consistent with this, immunoblotting experiments demonstrated that fluvastatin induces AMPK activation in RCC cells (Fig. 3E), further suggesting that engagement of AMPK accounts in part for the antitumor effects of statins.
Figure 3.
Role of AKT regulation in the induction of apoptosis by AICAR and fluvastatin. (A) Top, 786-0 cells were treated with solvent control (DMSO) or AICAR and/or fluvastatin at the indicated concentrations for 48 hours. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated/activated form of AKT on Ser 473. (A) Middle, equal protein aliquots from the same experiment were resolved on a separate gel and probed with an anti-AKT antibody. (A) Bottom, the top blot was subsequently reprobed with an anti-GAPDH antibody. (B) Control Rat1a cells or Rat1a cells stably transfected with myristoylated AKT (Myr-AKT) were treated with solvent control (DMSO), AICAR (500 µmol/L), fluvastatin (10 µmol/L) or AICAR and fluvastatin for 72 hours. The induction of apoptosis was evaluated by flow cytometry using Annexin V/propidium iodide staining. Data are expressed as % apoptotic cells. Means ± SE of five independent experiments are shown. Paired t-test analysis for the induction of apoptosis of control Rat1a cells treated with fluvastatin versus fluvastatin and AICAR showed a 2-tailed p value = 0.0008. (C) 786-0 cells were treated for 4 days with solvent control (DMSO) or with the indicated concentrations of fluvastatin alone or fluvastatin in combination with 10 µmol/L compound C and cell proliferation was assessed by MTT assays. Data are expressed as % control-treated cells and represent means ± SE of three independent experiments. (D) Wild-type (WT) AMPK mouse embryonic fibroblasts (MEFs) or AMPK 1-/-2-/- MEFs were treated with solvent control (DMSO) or the indicated concentrations of fluvastatin. Cell proliferation was assessed by MTT. Data are expressed as % control-treated cells and represent means ± SE of three independent experiments. Paired t-test analysis comparing values between AMPK 1-/-2-/- cells and AMPK WT cells treated with 2 µmol/L fluvastatin or 3 µmol/L fluvastatin showed a 2-tailed p values of 0.014 and 0.002, respectively. (E) Top, 786-0 cells were treated with solvent control (DMSO) or fluvastatin (10 µmol/L) for 48 hours, as indicated. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated/activated form of p-AMPKα (Thr 172). (E) Bottom, the top blot was subsequently stripped and reprobed with an anti-AMPKα antibody.
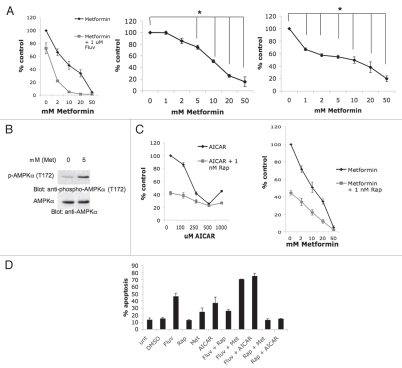
Much like AICAR, metformin is also known to act as a pharmacological activator of AMPK.25 When the effects of this agent on 786-0 cell proliferation were examined, potent dosedependent suppressive effects on cell growth were noticeable (Fig. 4A). Furthermore, combinations of metformin and fluvastatin significantly inhibited cell growth as compared to metformin or fluvastatin alone (Fig. 4A). Metformin also inhibited the growth of the ACHN and CAKI-2 renal cell carcinoma cell lines in a dose-dependent manner (Fig. 4A). As expected, treatment of RCC cells with metformin resulted in AMPK activation in RCC cells (Fig. 4B). In subsequent studies, we sought to better understand the mechanisms by which statins enhance the antitumor effects of AMPK activators via mTOR inhibition. Specifically, we sought to obtain information on the contributions of mTORC1 and mTORC2 targeting in the process. It should be noted that our previous work has strongly suggested that statin-treatment targets both mTORC1 and mTORC2 complexes in renal cell carcinoma cells, as evidenced by the inhibition of AKT phosphorylation on Ser473.23 To determine the contribution of mTORC1 in the process, we examined the effects of rapamycin that selectively targets mTORC1.12 Upon analyzing the combined effects of either AICAR or metformin and rapamycin on 786-0 cell growth, we noticed that rapamycin enhanced the growth suppressive effects of both AICAR (Fig. 4C, left) and metformin (Fig. 4C, right), although such an enhancement was not noticeable at the high doses of AICAR (Fig. 4C). We also examined in parallel the effects of dual targeting of mTORC1 or mTORC1/mTORC2 and AMPK activation on the generation of apoptosis in renal cell carcinoma cells. As expected,23 fluvastatin induced apoptosis of 786-0 cells, while rapamycin did not (Fig. 4D). Metformin and AICAR also induced various degrees of apoptosis (Fig. 4D) and such apoptosis was strongly potentiated by concomitant treatment of the cells with fluvastatin, but not rapamycin (Fig. 4D). In fact, there was some reversal of the pro-apoptotic effects of AICAR or metformin by concomitant use of rapamycin (Fig. 4D), suggesting that negative feedback of mTORC2 activation in response to rapamycin partially reverses the pro-apoptotic effects of these agents. Thus, combined inhibition of mTORC1 and/or mTORC2 enhances generation of growth inhibitory responses by AMPK activators in RCC cells, but only mTORC2 targeting enhances pro-apoptotic responses.
Figure 4.
Effects of combined AMPK activation and mTOR inhibition on the growth of renal cell carcinoma cells. (A) Left, 786-0 cells were treated for 4 days with solvent control or with the indicated concentrations of metformin alone or metformin in combination with 1 µmol/L fluvastatin. Cell proliferation was assessed by MTT assays. Data are expressed as % control-treated cells and represent means ± SE of three independent experiments. Middle, CAKI-2 cells were treated for 4 days with solvent control or with the indicated concentrations of metformin. Cell proliferation was assessed by MTT. Data are expressed as % control-treated cells and represent means ± SE of three independent experiments. Paired t-test analysis for the growth of CAKI-2 cells treated with 5, 10, 20 and 50 mmol/L metformin versus control-treated cells showed a 2-tailed p values of 0.017, 0.003, 0.001 and 0.01 respectively. Right, ACHN cells were treated for 4 days with solvent control or with the indicated concentrations of metformin. Cell proliferation was assessed by MTT. Data are expressed as % control-treated cells and represent means ± SE of four independent experiments. Paired t-test analysis for the growth of ACHN cells treated with 1, 2, 5, 10, 20 and 50 mmol/L metformin versus control treated cells showed a 2-tailed p values of 0.0007, 0.0003, 0.0004, 0.002, 0.006 and 0.0004 respectively. (B) Top, 786-0 cells were treated with solvent control or 5 mmol/L metformin for 48 hours, as indicated. Equal amounts of total cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated/activated form of AMPKα at Thr 172. (B) Bottom, the top blot was subsequently stripped and reprobed with an antibody to detect anti-AMPKα. (C) Left, 786-0 cells were treated for 4 days with solvent control or with the indicated concentrations of AICAR alone or AICAR in combination with 1 nmol/L rapamycin. Cell proliferation was assessed by MTT assays. Data are expressed as % control-treated cells and represent means ± SE of three independent experiments. Right, 786-0 cells were treated for 4 days with solvent control or with the indicated concentrations of metformin alone or metformin in combination with 1 nmol/L rapamycin. Cell proliferation was assessed by MTT assays. Data are expressed as % control-treated cells and represent means ± SE of three independent experiments. (D) 786-0 cells were treated with solvent control (DMSO), rapamycin (10 nmol/L), fluvastatin (3 µmol/L), metformin (10 mmol/L), AICAR (500 µmol/L), or the indicated combinations for 96 hours. The induction of apoptosis was evaluated by flow cytometry using Annexin V/propidium iodide staining. Data are expressed as % apoptotic cells. Mean ± SE of three independent experiments are shown.
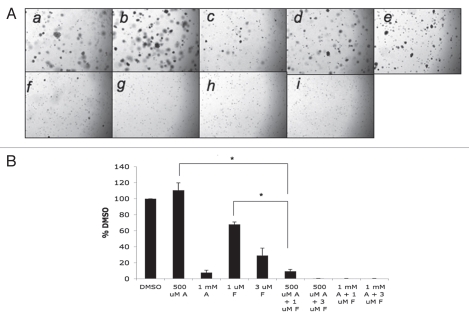
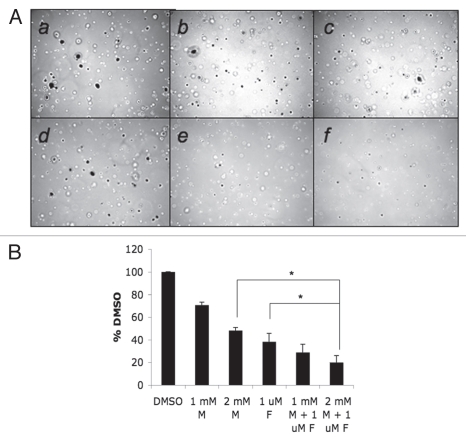
Finally, we examined the effects of AICAR and/or fluvastatin on anchorage-independent growth of RCC cells, by examining colony formation from malignant cells in soft agar assays.26,27 As shown in Figure 5, anchorage-independent growth of RCC cells was inhibited at high concentrations of AICAR or fluvastatin (Fig. 5). However, when both AICAR and fluvastatin were added to the cultures, there was dramatic enhancement of such inhibition, with almost complete suppression of anchorage-independent growth seen at combinations of AICAR at a concentration of 500 µmol/L, with either lower or higher concentrations of fluvastatin (Fig. 5A and B). Similarly, 786-0 colony formation was suppressed by metformin, while combinations of metformin with fluvastatin resulted in much more potent suppression than either treatment alone (Fig. 6A and B).
Figure 5.
Effects of AICAR and/or fluvastatin on renal cell carcinoma cell anchorage-independent growth. (A) Equal numbers of 786-0 cells were plated in a soft-agar assay system and treated with either AICAR (500 µmol/L or 1,000 µmol/L), fluvastatin (1 or 3 µmol/L), or the indicated combinations of AICAR and fluvastatin. Colony formation was analyzed after 14 days of culture. A representative area (x20 magnification) of each treatment point is shown (a–i). Cells were treated with solvent control (DMSO) (a) 500 µmol/L AICAR, (b) 1,000 µmol/L AICAR, (c) 1 µmol/L fluvastatin, (d) 3 µmol/L fluvastatin (e) the combination of 500 µmol/L AICAR and 1 µmol/L fluvastatin, (f) 500 µmol/L AICAR and 3 µmol/L fluvastatin, (g) 1,000 µmol/L AICAR and (h) 1 µmol/L fluvastatin or (i) 1,000 µmol/L AICAR and 3 µmol/L fluvastatin. Colonies were stained with crystal violet. (B) Colonies were counted and results were expressed as % control of DMSO-treated colonies. Data shown represent means ± SE of three independent experiments. Paired t-test analysis for the inhibition of colony formation of 786-0 cells treated with 500 µmol/L AICAR versus 1 µmol/L fluvastatin and 500 µmol/L AICAR showed a 2-tailed p value = 0.007. Paired t-test analysis for the inhibition of colony formation of 786-0 cells treated with 1 µmol/L fluvastatin versus 1 µmol/L fluvastatin and 500 µmol/L AICAR showed a 2-tailed p value = 0.009.
Figure 6.
Effects of metformin and/or fluvastatin on renal cell carcinoma cell anchorage-independent growth. (A) Equal numbers of 786-0 cells were plated in a soft-agar assay system and treated with either metformin (1 mmol/L or 2 mmol/L), fluvastatin (1 µmol/L), or the indicated combinations of metformin and fluvastatin. Colony formation was analyzed after 14 days of culture. A representative area (x20 magnification) of each treatment point is shown (a–f). Cells were treated with solvent control (DMSO), (a) 1 mmol/L metformin, (b) 2 mmol/L metformin, (c) 1 µmol/L fluvastatin, (d) the combination of 1 mmol/L metformin and 1 µmol/L fluvastatin, (e) 2 mmol/L metformin and (f) 1 µmol/L fluvastatin. Colonies were stained with crystal violet. (B) Colonies were counted and results were expressed as % control of DMSO-treated colonies. Data shown represent means ± SE of four independent experiments. Paired t-test analysis for the inhibition of colony formation of 786-0 cells treated with 2 mmol/L metformin versus 1 µmol/L fluvastatin and 2 mmol/L metformin showed a 2-tailed p value = 0.014. Paired t-test analysis for the inhibition of colony formation of 786-0 cells treated with 1 µmol/L fluvastatin versus 1 µmol/L fluvastatin and 2 mmol/L metformin showed a 2-tailed p value = 0.048.
Discussion
There has been accumulating evidence over the last several years for an integral link between cancer and metabolism. It is now well established that highly anabolic cancer cells need to constantly generate new proteins to support their ongoing growth and for their survival,4–6 ultimately resulting in changes to cellular energy levels-detected by AMPK.1,2 Pharmacological targeting of AMPK may provide a novel approach for the treatment of malignancies, alone or in combination with other therapeutic approaches.4–6 One such AMPK-targeting drug, 5-aminoimidazole-4-carboxamide riboside (AICAR), activates AMPK by acting as a cellular AMP mimic, binding AMPK and facilitating a conformational change that reveals its active site.7 Another AMPK activator is the antidiabetic drug metformin, which has been previously shown to exhibit antitumor effects in vitro and in animal models in vivo, and for which there is emerging interest for its potential use in clinical oncology.28
Recent work has shown that AMPK activators sensitize colon cancer cell lines to TNFα8 and suppress the growth of prostate and/or breast carcinoma lines.6,10 In addition, AICAR slows the growth of MDA-MB-231 breast cancer tumor xenografts in nude mice.6 Despite the emerging evidence suggesting antitumor effects for AMPK activators, very little is known on the potential clinical-translational relevance of targeting this pathway in renal cell carcinoma, a malignancy in which the mTOR cascade plays a prominent role in growth and survival because of its regulatory effects on the hypoxia inducible factor (HIF) 1α and 2α proteins.29,30 The importance of targeting this signaling cascade in the treatment of renal cancer has been firmly established by studies demonstrating that beyond temsirolimus, other mTOR inhibitors such everolimus and deferolimus have also significant clinical activity in the treatment of this malignancy.30,31 In fact everolimus was the second mTOR inhibitor after temsirolimus recently approved by the FDA for the treatment of RCC patients.31 Studies to identify other agents that may synergistically enhance the activity of mTOR inhibitors and/or overcome resistance to such inhibitors may prove to be important in further expanding the treatment options of patients with renal cancer. Recently, we demonstrated that statins (atorvastatin, fluvastatin) inhibit mTOR activation in renal carcinoma cells and generate growth inhibitory and pro-apoptotic responses,23 suggesting that combinations of these agents with other drugs with activity in RCC may provide a novel approach to enhance antitumor responses.
In the present study we provide the first evidence that activating AMPK using AICAR or metformin results in suppression of RCC cell growth and induction of apoptosis of RCC cells. Additionally, our data suggest a possible enhancement of antitumor responses when AICAR is combined with fluvastatin or simvastatin, likely reflecting enhanced dephosphorylation of downstream elements of the AKT signaling cascade. It is of particular interest that statins work by inhibiting the rate-limiting enzyme of the mevalonate pathway, HMG Coenzyme-A reductase,32—a pathway that is ultimately linked to a number of cellular signaling cascades. Interestingly, AMPK, the protein-target of phosphorylation and activation by AICAR, has also been shown to inhibit this same enzyme.33 Notably, we detected statin-dependent phosphorylation of AMPK in RCC cells and found that the effects of fluvastatin on cell growth are partially reversible by concomitant treatment of the cells with the AMPK inhibitor compound C. Moroever, statin-dependent growth inhibition is decreased in MEFs with targeted disruption of the Ampk1/Ampk2 genes, suggesting that the antiproliferative effects of statins are also mediated in part via modulation of AMPK-regulated signals and responses. Consistent with this, previous studies have demonstrated atorvastatin-34 and simvastatin35-dependent phosphorylation and activation of AMPK in epithelial cells. The findings of these studies, taken together with our data, are consistent with a functional role of AMPK in the induction of statin-responses in both normal and malignant cells. In addition, our data have shown that overexpression of a constitutively activated AKT mutant rescues the apoptotic effects of the AICAR/fluvastatin combination, suggesting that AKT may be the converging point of both AMPK-dependent and independent signals that generate such responses. Such a role for AKT is further supported by our previous work demonstrating statin-dependent suppression of serine 473 AKT phosphorylation,23 as well as by the fact that AKT is known to directly control activation of AMPK activity.15–17
The antiproliferative and pro-apoptotic effects we detected using AICAR in RCC cells, including enhanced effects when combined with statins, were also seen in experiments using another AMPK activator, metformin. Moreover, in experiments to determine anchorage-independent growth of renal cell carcinoma cells, we established that combinations of either AICAR or metformin dramatically enhanced suppression of clonogenic tumor cell growth. Previous studies had demonstrated statin-dependent suppression of anchorage-independent growth of breast carcinoma cells36 and metformin-induced suppression of anchorage-independent growth of prostate cancer cells.37 Our data provide the first evidence that AICAR and metformin induce suppression of anchorage-independent growth of RCC cells and that such properties of these AMPK activators are dramatically enhanced when combined with fluvastatin.
Altogether our data provide direct evidence that activators of AMPK exhibit potent inhibitory effects on RCC cells and provide the rationale for future clinical-translational efforts involving AICAR or other AMPK activators for the treatment of renal cell carcinoma. It is particularly encouraging that pharmacological analysis from a clinical trial involving intravenous administration of AICAR in diabetic patients38 demonstrated that achievable serum concentrations of AICAR in such patients are similar to concentrations used in the present study. Based on that, it is likely that such concentrations may be also achievable in clinical trials in patients with renal cell carcinoma or other malignancies, and this should be directly addressed in future studies.
Methods
Cell lines and reagents.
The 786-0, CAKI-2 and ACHN renal carcinoma cell lines were obtained from ATCC (catalog numbers CRL-1932, HTB-47, CRL-1611) in 2006 and authenticated in February 2010, via the Johns Hopkins Genetic Resources Core Facility. The 786-0 cell line was grown in RPMI 1640 supplemented with 10% fetal bovine serum, antibiotics, 10 mmol/L HEPES buffer and 1 mmol/L sodium pyruvate. The CAKI-2 cell line was grown in McCoy's Media supplemented with 10% fetal bovine serum and antibiotics. The ACHN cell line was grown in Minimal Essential Medium supplemented with 10% fetal bovine serum and antibiotics. Rat1a cells and Rat1a-Myr-AKT cells39 were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics. Immortalized Ampkα wild type (WT) and Ampkα1-/-2-/- mouse embryonic fibroblasts (MEFs)40 were grown in DMEM supplemented with 10% fetal bovine serum and antibiotics. 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) was purchased from Sigma-Aldrich. Metformin was purchased from Sigma-Aldrich. Fluvastatin was kindly provided from Novartis or purchased from Calbiochem. Simvastatin and compound C were purchased from Calbiochem. Antibodies against the phosphorylated forms of AKT (Ser 473), S6K (Thr 389), S6 ribosomal protein (rpS6; Ser 240/Ser 244), eukaryotic initiation factor 4E (eIF4E)-binding protein (4E-BP1; Ser 65) and AMP-activated kinase alpha (AMPKα; Thr 172) were obtained from Cell Signaling Technology. Antibodies to detect AKT, S6K, rpS6, 4E-BP1 and AMPKα were also purchased from Cell Signaling Technology. An anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody was obtained from Millipore.
Cell proliferation assays.
Cells were seeded in 96-well plates and incubated in the presence of control solvent or the indicated concentrations of either AICAR, metformin, fluvastatin, compound C and/or rapamycin at 37°C for 4 days. Cell proliferation was assessed using a methyl-thiazolyl-tetrazolium assay system as described previously.41
Flow cytometric analysis.
Flow cytometric studies to detect apoptosis by Annexin V/propidium iodide staining were done as in our previous studies.42,43
Cell lysis and immunoblotting.
Cells were treated with DMSO (control), AICAR, metformin and/or fluvastatin for the indicated times and were subsequently lysed in phosphorylation lysis buffer as described previously.44,45 Immunoblotting using an enhanced chemiluminescence method was done as in previous studies.42,44,45
Soft-agar assays.
Anchorage-independent growth was assessed in a soft-agar assay system using established methodology26 and set up as in our previous studies.27
Acknowledgements
This work was supported by National Institutes of Health grants CA121192, CA77816, CA100579, and by a grant from the Department of Veterans Affairs (to L.C.P.). J.W. was supported by a NIH training grant T32CA009560.
Abbreviations
- AMPK
AMP-activated kinase
- AICAR
5-aminoimidazole-4-carboxamide riboside
- PI3-K
phosphatidyl-inositol-3 kinase
- mTOR
mammalian target of rapamycin
- rps6
S6 ribosomal protein
- S6K
S6 kinase
- RCC
renal cell carcinoma
- TSC
tuberous sclerosis complex
References
- 1.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Science. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 3.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandolfi PP. Aberrant mRNA translation in cancer pathogenesis: an old concept revisited comes finally of age. Oncogene. 2004;23:3134–3137. doi: 10.1038/sj.onc.1207618. [DOI] [PubMed] [Google Scholar]
- 5.Avdulov S, Li S, Michalek V, Burrichter D, Peterson M, Perlman DM, et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell. 2004;5:553–563. doi: 10.1016/j.ccr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Swinnen JV, Beckers A, Brusselmans K, Organe S, Segers J, Timmermans L, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–2448. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 7.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 8.Su RY, Chao Y, Chen TY, Huang DY, Lin WW. 5-Aminoimidazole-4-carboxamide riboside sensitizes TRAIL- and TNF{alpha}-induced cytotoxicity in colon cancer cells through AMP-activated protein kinase signaling. Mol Cancer Ther. 2007;6:1562–1571. doi: 10.1158/1535-7163.MCT-06-0800. [DOI] [PubMed] [Google Scholar]
- 9.Sengupta TK, Leclerc GM, Hsieh-Kinser TT, Leclerc GJ, Singh I, Barredo JC. Cytotoxic effect of 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) on childhood acute lymphoblastic leukemia (ALL) cells: implication for targeted therapy. Mol Cancer. 2007;6:46. doi: 10.1186/1476-4598-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 11.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Hardie DG. AMPK and Raptor: matching cell growth to energy supply. Mol Cell. 2008;30:263–265. doi: 10.1016/j.molcel.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 16.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 17.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 18.Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280:39582–39593. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 19.Longo R, D'Andrea MR, Sarmiento R, Salerno F, Gasparini G. Integrated therapy of kidney cancer. Ann Oncol. 2007;18:141–148. doi: 10.1093/annonc/mdm244. [DOI] [PubMed] [Google Scholar]
- 20.Hager M, Haufe H, Kemmerling R, Hitzl W, Mikuz G, Moser PL, et al. Increased activated Akt expression in renal cell carcinomas and prognosis. J Cell Mol Med. 2009;13:2181–2188. doi: 10.1111/j.1582-4934.2008.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho D, Signoretti S, Regan M, Mier JW, Atkins MB. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13:758–763. doi: 10.1158/1078-0432.CCR-06-1986. [DOI] [PubMed] [Google Scholar]
- 22.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferonalpha or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 23.Woodard J, Sassano A, Hay N, Platanias LC. Statin-dependent suppression of the Akt/mammalian target of rapamycin signaling cascade and programmed cell death 4 upregulation in renal cell carcinoma. Clin Cancer Res. 2008;14:4640–4649. doi: 10.1158/1078-0432.CCR-07-5232. [DOI] [PubMed] [Google Scholar]
- 24.Clark PE. Recent advances in targeted therapy for renal cell carcinoma. Curr Opin Urol. 2007;17:331–336. doi: 10.1097/MOU.0b013e3282c508e0. [DOI] [PubMed] [Google Scholar]
- 25.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 27.Katsoulidis E, Carayol N, Woodard J, Konieczna I, Majchrzak-Kita B, Jordan A, et al. Role of Schlafen 2 (SLFN2) in the generation of IFNα-induced growth inhibitory responses. J Biol Chem. 2009;284:25051–25054. doi: 10.1074/jbc.M109.030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:62–67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor A, Figlin RA. Targeted inhibition of mammalian target of rapamycin for the treatment of advanced renal cell carcinoma. Cancer. 2009;115:3618–3630. doi: 10.1002/cncr.24409. [DOI] [PubMed] [Google Scholar]
- 31.Atkins MB, Yasothan U, Kirkpatrick P. Everolimus. Nat Rev Drug Discov. 2009;8:535–536. doi: 10.1038/nrd2924. [DOI] [PubMed] [Google Scholar]
- 32.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 33.Ching YP, Davies SP, Hardie DG. Analysis of the specificity of the AMP-activated protein kinase by site-directed mutagenesis of bacterially expressed 3-hydroxy 3-methylglutaryl-CoA reductase, using a single primer variant of the unique-site-elimination method. Eur J Biochem. 1996;237:800–808. doi: 10.1111/j.1432-1033.1996.0800p.x. [DOI] [PubMed] [Google Scholar]
- 34.Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;14:2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 35.Kou R, Sartoretto J, Michel T. Regulation of Rac1 by simvastatin in endothelial cells: differential roles of AMP-activated protein kinase and calmodulindependent kinase kinase-beta. J Biol Chem. 2009;284:14734–14743. doi: 10.1074/jbc.M808664200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotamraju S, Williams CL, Kalyanaraman B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67:7386–7394. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- 37.Sahra Ben I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 38.Boon H, Bosselaar M, Praet SF, Blaak EE, Saris WH, Wagenmakers AJ, et al. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia. 2008;51:1893–1900. doi: 10.1007/s00125-008-1108-7. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy SG, Wagner AJ, Conzen SD, Jordán J, Bellacosa A, Tsichlis PN, et al. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 40.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colamonici OR, Domanski P, Platanias LC, Diaz MO. Correlation between interferon (IFN)a resistance and deletion of the IFNα/β genes in acute leukemia cell lines suggests selection against the IFN system. Blood. 1992;80:744–749. [PubMed] [Google Scholar]
- 42.Sassano A, Katsoulidis E, Antico G, Altman JK, Redig AJ, Minucci S, et al. Suppressive effects of statins on acute promyelocytic leukemia cells. Cancer Res. 2007;67:4524–4532. doi: 10.1158/0008-5472.CAN-06-3686. [DOI] [PubMed] [Google Scholar]
- 43.Giafis N, Katsoulidis E, Sassano A, Tallman MS, Higgins LS, Nebreda AR, et al. Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res. 2006;66:6763–6771. doi: 10.1158/0008-5472.CAN-05-3699. [DOI] [PubMed] [Google Scholar]
- 44.Yetter A, Uddin S, Krolewski JJ, Jiao H, Yi T, Platanias LC. Association of the interferon-dependent tyrosine kinase Tyk-2 with the hematopoietic cell phosphatase. J Biol Chem. 1995;270:18179–18182. doi: 10.1074/jbc.270.31.18179. [DOI] [PubMed] [Google Scholar]
- 45.Uddin S, Sassano A, Deb DK, Verma A, Majchrzak B, Rahman A, et al. Protein kinase C-delta (PKC-delta) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J Biol Chem. 2002;277:14408–14416. doi: 10.1074/jbc.M109671200. [DOI] [PubMed] [Google Scholar]