Abstract
Transplantation of glial restricted precursor (GRP) cells has been shown to reduce glial scarring after spinal cord injury (SCI) and, in combination with neuronal restricted precursor (NRP) cells or enhanced expression of neurotrophins, to improve recovery of function after SCI. We hypothesized that combining GRP transplants with rolipram and cAMP would improve functional recovery, similar to that seen after combining Schwann cell transplants with increasing cAMP. A short term study, 1)uninjured control, 2)SCI+vehicle, and 3)SCI+cAMP, showed that spinal cord [cAMP] were increased 14 days after SCI. We used 51 male rats subjected to a thoracic SCI for a 12-week survival study: 1)SCI+vehicle, 2)SCI+GRP, 3)SCI+cAMP, 4)SCI+GRP+cAMP, and 5)uninjured endpoint age-matched control (AM). Rolipram was administered for 2 weeks after SCI. At 9 days after SCI, GRP transplantation and injection of dibutyryl-cAMP into the spinal cord were performed. GRP cells survived, differentiated, and formed extensive transplants that were well integrated with host tissue. Presence of GRP cells increased the amount of tissue in the lesion; however, cAMP reduced the graft size. White matter sparing at the lesion epicenter was not affected. Serotonergic input to the lumbosacral spinal cord was not affected by treatment, but the amount of serotonin immediately caudal to the lesion was reduced in the cAMP groups. Using telemetric monitoring of corpus spongiosum penis pressure we show that the cAMP groups regained the same number of micturitions per 24 hrs when compared to the AM group, however, the frequency of peak pressures was increased in these groups compared to the AM group. In contrast, the GRP groups had similar frequency of peak pressures compared to baseline and the AM group. Animals that received GRP cells regained the same number of erectile events per 24 hrs compared to baseline and the AM group. Since cAMP reduced the GRP transplant graft, and some modest positive effects were seen that could be attributable to both GRP or cAMP, future research is required to determine how cAMP affects survival, proliferation, and / or function of progenitor cells and how this is related to function. cAMP may not always be a desirable addition to a progenitor cell transplantation strategy after SCI.
Keywords: rolipram, micturition, sexual function, chronic, telemetry, hPLAP transgenic rat, serotonin, therapeutic strategy, astrocytes, oligodendrocytes
1. Introduction
Endogenous repair has been shown to occur following contusion spinal cord injury (SCI; (Beattie, et al., 1997, Mothe and Tator, 2005, Namiki and Tator, 1999, Vaquero, et al., 1981, Wallace, et al., 1987), however, the response of the endogenous progenitor cell population is insufficient and does not lead to adequate recovery (Beattie, et al., 1997, Horner, et al., 2000, Mothe and Tator, 2005, Namiki and Tator, 1999). This insufficient endogenous response may be due to insufficient numbers of progenitor cells, the hostile milieu of the lesion environment, lack of neurotrophic stimulation, absence of permissive substrates, and presence of inhibitory factors (Cao, et al., 2002, Horner and Gage, 2000, Popovich, et al., 1999, Schmidt and Leach, 2003). Our laboratory has previously shown that when glial restricted precursor (GRP) cells, derived from transgenic embryonic rats harboring the heat-stable human placental alkaline phosphatase (hPLAP) gene (Rao, et al., 1998), are transplanted into acute thoracic contusion SCI, the genetically-identified donor cells survived 6 weeks and differentiated into cells with glial markers, including CC-1 and glial fibrillary acidic protein (GFAP). In addition, these cells appeared to integrate into the white matter, produced some apparent remyelination, and modulated the inflammatory response and the formation of a glial scar (Hill, et al., 2004). Furthermore, GRP cell transplants in combination with neuronal restricted precursor (NRP) cells from the same embryonic stage, and genetically modified multineurotrophin expressing GRP cells, have been shown to improve functional recovery after spinal cord lesions (Cao, et al., 2005, Mitsui, et al., 2005). Transplantation of astrocytes derived from bone morphogenetic protein-4 (BMP-4) treated GRP cells have also been shown to result in significant histopathological and functional improvement (Davies, et al., 2006, Davies, et al., 2008). Also, combining transplantation of neural precursor cells with growth factors and anti-inflammatory and immunosuppressive treatment, enhanced survival of donor cells and improved functional recovery (Karimi-Abdolrezaee, et al., 2006). More recently, a group has shown positive effects of GRP/NRP and bone marrow stromal cell transplantations on bladder and sexual function after SCI (Temeltas, et al., 2009, Temeltas, et al., 2009).
cAMP has been shown to play an important role in axon regeneration in the adult central nervous system, largely in part through downstream effects of cAMP-dependent protein kinase (PKA), that ultimately help overcome the inhibitory cues of the injured spinal cord milieu (Bregman, et al., 1998, Cai, et al., 1999, Gao, et al., 2004, Hannila and Filbin, 2008, Spencer and Filbin, 2004). The phosphodiesterase (PDE) -IV inhibitor rolipram increases intracellular cAMP and cGMP concentrations in various tissues, and mainly in monocytes, macrophages, granulocytes, T-lymphocytes, and dopaminergic and adrenergic nerve cells. Furthermore, rolipram has the ability to suppress pro-inflammatory cytokines (predominantly TNF-α) and chemokines (Zhu, et al., 2001). Recent evidence suggests that rolipram not only enhances axonal regeneration, but also protects against apoptotic insults, for example by reducing caspase-3 activity (Chen, et al., 2007). In addition rolipram reduces oligodendrocyte death at 24 - 72 hours after SCI (Whitaker, et al., 2008).
Pearse et al. (2004) demonstrated in vivo that SCI results in a reduction of spinal cord cAMP concentrations, and that this could be prevented by exogenous administration of rolipram and dibutyryl (db) -cAMP. Additionally, the increase of TNF-α that occurred within the first 6 hrs following SCI was retarded in animals that received rolipram. Moreover, he showed that the combination of Schwann cell transplants and elevation of cAMP after SCI resulted in significant improvement of locomotor function and promoted supraspinal and propriospinal axon sparing and myelination, as well as serotonergic fiber growth into and beyond the graft (Pearse, et al., 2004). Another study showed similar beneficial effects after transplanting embryonic spinal tissue and administering rolipram in a hemisection model of SCI (Nikulina, et al., 2004). These findings suggest a beneficial role for elevating cAMP in the injured spinal cord.
In the present work, we sought to use the Pearse et al (2004) strategy aimed at elevating cAMP concentrations to determine whether we could enhance the therapeutic potential of GRP cells transplanted into subacute lesions, 9 days after mid-thoracic SCI. In order to monitor a wider variety of functional outcomes, we included in-cage monitoring of bladder and sexual function to the functional testing database. Autonomically mediated functions, such as urogenital tract function, following SCI and its subsequent complications are highly prevalent and clinically very important (Anderson, 2004, Hicken, et al., 2001, Noreau, et al., 2000). Recently, we developed a method to assess recovery of micturition and erectile function in conscious freely moving rats by monitoring corpus spongiosum penis (CSP) pressure using telemetry (Nout, et al., 2007, Nout, et al., 2005). In the following study we determine recovery of both micturition and erectile events following delayed transplantation of GRP cells and administration of local db-cAMP and systemic rolipram after SCI. Furthermore, detailed histopathology allows assessment of graft characteristics and fate of transplanted cells.
2. Materials and Methods
2.1 Study design
2.1.1. Long-term survival study
Forty-five male rats, age 71 ± 2 days (mean ± SE), were divided into 4 groups: 1) Operated control group (OP control; n=11): SCI, vehicle (0.45% NaCl in dimethyl sulfoxide, subcutaneously (sc) by osmotic pump for 14 days), control transplant (3x3.3μl phosphate buffered saline injected into 3 sites of the lesion region), and control injections (2×0.25μl 0.9% NaCl at 0.5cm cranial and 0.5cm caudal to the lesion center); 2) GRP control group; n=11: SCI, vehicle sc, GRP cell transplant (2-3x106 GRP cells in 10μl PBS divided into 3 sites in the lesion region), and control injections; 3) cAMP control group; n=12; SCI, rolipram sc (0.5mg/kg/day), control transplant, and cAMP injections (2×0.25μl 50mM db-cAMP); 4) GRP cAMP group; n=11: SCI, rolipram sc, GRP cell transplant, and cAMP injections. In addition, 6 animals, age 155 days, served as an endpoint age-matched uninjured control (AM control) group for collection of telemetric and histopathological data.
2.1.2. Short-term survival study
Twelve male rats, age 71 ± 1 days, were used to determine cAMP concentrations in spinal cord, serum, cerebrospinal fluid (CSF) using a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA) at day 14 after SCI. Rats were randomly divided into an uninjured control group (UI control; n=4), OP control group (n=4), and cAMP control group (n=4).
Rats were housed individually in plastic cages, maintained on a 12-h light/dark cycle, and had free access to food and water. All animal experiments were conducted after approval by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University and were performed in compliance with NIH guidelines and recommendations.
2.2. Surgical Procedures and Post-Operative Care
2.2.1. Transducer implantation
For the long-term survival study, telemetric pressure transducers (TA11PA-C40, Data Sciences International, St. Paul, MN, USA) were implanted in the bulb of the CSP as described previously (Nout, et al., 2007, Nout, et al., 2005, Schmidt, et al., 1995) in 7 out of 11 OP control rats, 7 out of 11 GRP control rats, 7 out of 12 cAMP control rats, 9 out of 11 GRP cAMP rats, and 5 out of 6 AM control rats. Telemetric studies started 4 – 5 days post operatively. Animals had access to maple syrup water (60ml/L, 20ml/day).
2.2.2. Spinal cord injury and osmotic pump placement
Animals were anesthetized with pentobarbital intraperitoneal (IP) (Abbott Laboratories, Chicago, IL, USA; 50 mg/kg). Six days after transducer implantation, a 25 g-cm SCI was delivered with a MASCIS/NYU device as previously described (Gruner, 1992). Afterwards an Alzet® osmotic pump (model 2ml2; Durect Corporation, Cupertino, CA, USA) was placed sc to deliver either rolipram (Sigma, St. Louis, MO, USA) in DMSO (Sigma, St. Louis, MO, USA) or 0.45% NaCl in DMSO for 14 days.
2.2.3. Transplantation
On days 9 or 10 after SCI, rats were administered cyclosporin (Bedford Laboratories, Bedford, OH, USA; 5mg/kg/day IP) as described by Ibarra et al. (1996 a, b). Animals were anesthetized with inhaled isoflurane (2-3%). A pre-operative dose of buprenorphine hydrochloride (Buprenex, Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA; 0.05mg/kg sc) was administered. The dorsal midline incision was re-opened, the injured spinal cord was re-exposed, and the cranial half lamina of T11 was removed. The transplant procedure was carried out using a 10μl bevel-tip FlexiFil microsyringe (World Precision Instruments Inc., Sarasota, FL, USA; diameter = 180 μm) assembled in a micromanipulator. The first 3.3μl of either a GRP cell suspension (2-3×106 cells) or PBS was injected over 3 min in the lesion center. The second and third 3.3μl aliquots were injected into the lesion region at 3.5 ± 0.1 mm cranial and caudal to the first injection site. Depth of injections was approximately 1 mm. Using a similar 10μl FlexiFil syringe assembled in the micromanipulator, 0.25μl db-cAMP (Sigma, St. Louis, MO, USA) or 0.9% NaCl was administered on either side (0.7mm) of midline approximately 1mm cranial and caudal to the cranial and caudal injections sites, respectively. Depth of injections was 1 mm. On day 11 after SCI, the maple syrup water was replaced by 20ml/day of 1.2% liquid vitamin C (Vedco, Inc., St. Joseph, MO, USA) prepared in maple syrup water (60ml/L) and cyclosporin was added to the drinking water (10mg/kg/day by mouth assuming rats drank 120-144ml/day; (Ibarra, et al., 1996, Ibarra, et al., 1996) throughout the remainder of the study. On day 14 after SCI the osmotic pump was removed under inhaled isoflurane (1-2%) anesthesia.
2.2.4. Sacrifice
At 12 weeks after SCI, the 45 long-term survival animals were anesthetized with xylazine (TranquiVed™, Vedco Inc., St. Joseph, MO, USA; 10 mg/kg IP) and ketamine (ketamine HCl, Abbott Laboratories, N.Chicago, IL, USA; 80 mg/kg IP) and transcardially perfused with 0.9% NaCl and 4% paraformaldehyde in PBS. The lesion region and the L4-S2 segment of the spinal cord were isolated and kept in 4% paraformaldehyde for 24 hours and cryoprotected in 30% sucrose in PBS for 24-48 hours. The tissue was frozen at −80°C until further analysis. The 12 short-term survival animals were anesthetized with xylazine and ketamine at 14 days after SCI. The dorsal midline incision was re-opened and the spinal cord was exposed. CSF (250μl) was collected from the atlantoccipital site, whole blood was collected from the heart, and 3 thoracic spinal cord segments were removed. The lesion center (5mm), a cranial adjacent section (5mm), and a caudal adjacent section (5mm) were all flash frozen and stored at −80°C for further analysis. Blood was allowed to clot, and serum and CSF were frozen at – 80°C until further analysis.
2.3. Glial Restricted Precursor Cells
Transgenic E13.5 rat embryos were used that harbor the heat-stable hPLAP gene. GRP cells were isolated as described previously (Hill, et al., 2004, Rao, et al., 1998). When cultures reached 50-70% confluence, cells were harvested at University of Rochester and transported to The Ohio State University overnight for transplantation the following day. Cells were grafted the day of arrival. On arrival, prior to transplantation, viability and number of GRP cells were assessed. The suspension (15 ml total volume) was centrifuged (Centra CL3R, International Equipment Company, Needham Heights, MA, USA) at 300g (1000rpm) for 5 min at room temperature. The pellet containing the GRP cells was then re-suspended in 10μl PBS and used for transplantation as described above.
2.4. Behavioral Tests
2.4.1. Locomotor
Open-field walking was evaluated before SCI (Baseline; BL) and at 24 hours, 48 hours, and weekly thereafter using the 21-point BBB Locomotor Rating Scale (Basso, et al., 1995). In addition, activity in the cage was determined by telemetry. Movement of the transducer over different detection fields of the receiver was recorded and a moving average (counts per minute) was acquired using Dataquest ART software (Data Sciences International, St. Paul, MN, USA). Data were recorded on days 1, 5-8, 10-15, 21, 28, 35, 42, 49, 56, 63, 70, 77, and 84 after SCI.
2.4.2. Micturition and erectile function
Micturition and erectile characteristics derived from telemetric obtained CSP pressures were evaluated using Spike2 data acquisition and analysis software (version 3.1, Cambridge Electronic Design Limited, Science Park, Milton Road, Cambridge CB40FE, U.K.) as previously described (Nout, et al., 2007, Nout, et al., 2005). CSP pressure was analyzed for days 2, 5, 14, and 84. CSP pressure data were analyzed for the total number of micturitions, full erectile events (tumescence pressure of at least 30 mmHg above baseline on top of which at least 1 suprasystolic pressure peak of 100 mmHg above the tumescence pressure occurs), and partial erectile events (similar to full erectile events with the exception that peaks never reach 100 mmHg over the tumescence pressure) in a 24 hour period. Furthermore, a detailed waveform analysis was performed for 4 days (BL, day 5, 15, 84). For micturitions and partial erectile events this was performed on the complete 24 hour period and for full erectile events this was performed on an 8 hour window (9pm – 5am) within the 24 hour recording period. Data analyzed were duration of the events, area under the curve (AUC), total number of pressure peaks, peak frequency and mean and maximum pressures. Furthermore, a peak analysis was performed. For micturition events this included the number of afterpeaks, and afterpeak duration, AUC, and mean and maximum pressures. For erectile events this included the duration, AUC, and mean and maximum pressures of the peaks that occurred during those events.
2.4.3. Reflex erection tests
Ex copulatory reflex erection tests were performed as previously described (Hart, 1968, Nout, et al., 2007, Nout, et al., 2005, Schmidt, et al., 1995). Reflex testing was performed twice prior to SCI and on days 2, 7, 16, 22, 30, 44, 58, 72, and 86 following SCI. The 6 animals in the AM control group were tested twice, once prior to and once after transducer implantation.
2.5. Histopathology and Analysis – Lesion
Lesion regions (10mm) were sectioned at 20 μm on a cryostat transversally (5 OP control animals, 5 GRP control animals, 6 cAMP control animals, and 5 GRP cAMP animals) or horizontally (5 OP control animals, 5 GRP control animals, 5 cAMP control animals, and 4 GRP cAMP animals).
2.5.1 Immunohistochemistry and staining
Transverse sections were stained for hPLAP and GFAP for astrocytes, hPLAP and CC-1 for oligodendrocytes, hPLAP and nestin for undifferentiated cells, and hPLAP and NG2 as well as hPLAP and platelet derived growth factor receptor (PDGFR) α for oligodendrocyte precursor cells. Horizontal sections was stained for hPLAP and serotonin (Table 1). Transverse sections were also stained with Luxol Fast Blue for myelin to determine white matter sparing.
Table 1.
Antibodies used for immunohistochemistry. Source, concentration, and incubation time are shown. The Nestin Rat-401 hybridoma was developed by Susan Hockfield and obtained from the Developmental Systems Hybridoma Bank (DSHB) developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, USA. The PDGFR〈 was generously supplied by Dr. Stallcup, Burnham Institute for Medical Research, La Jolla, CA.
| Primary antibody | Secondary antibody | Buffer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | [ ] | Inc. time |
Isotype | Source | Name | [ ] | Inc. time |
Source | |
| hPLAP | 1:200 | O/N | Mouse monoclonal IgG2a |
Sigma, St. Louis, MO |
AF-594 GAM IgG2a |
1:200 | 2 hours |
Invitrogen, Carlsbad, CA |
PBS |
| AF-488 GAM IgG |
1:200 | 2 hours |
Invitrogen | PBS | |||||
| CC-1 | 1:100 | O/N | Mouse monoclonal IgG2b |
Abcam Cambridge, MA |
AF-488 GAM IgG2b |
1:200 | 2 hours |
Invitrogen | PBS |
| GFAP | 1:1000 | O/N | Rabbit polyclonal IgG |
Sigma | AF-488 GAR IgG |
1:200 | 2 hours |
Invitrogen | PBS |
| Nestin | 1:10 | O/N | Mouse hybridoma supernatant IgG1 |
DSHB, Iowa City, IA US |
AF-488 GAM IgG1 |
1:200 | 2 hours |
Invitrogen | PBS |
| NG2 | 1:200 | O/N | Mouse monoclonal IgG1 |
Biological, Swampscott, MA |
AF-488 GAM IgG1 |
1:200 | 2 hours |
Invitrogen | PBS |
| PDGFRα | 1:50 | O/N | Rabbit polyclonal IgG |
Dr. Stallcup, La Jolla, CA |
AF-488 GAR IgG |
1:200 | 2 hours |
Invitrogen | PBS |
| 5-Ht | 1:500 | O/N | Rabbit polyclonal IgG |
Immunostar, Hudson, WI |
AF-488 GAR IgG |
1:200 | 2 hours |
Invitrogen | PBS |
| Syn. | 1:200 | O/N | Mouse monoclonal IgG1 |
Chemicon, Temecula, CA |
AF-546 GAM IgG |
1:200 | 2 hours |
Invitrogen | PBS |
[ ] = concentration, Inc. time = incubation time, hPLAP = human placental alkaline phosphatase, O/N = overnight, CC-1 = APC antibody CC-1, GFAP = glial fibrillary acidic protein, PDGFR〈 = platelet derived growth factor receptor alpha, 5-Ht = serotonin, Syn. = synaptophysin, GAM = goat-anti-mouse, GAR = goat-antirabbit, AF = Alexa-Fluor, PBS = phosphate buffered saline (ph=7.4).
2.5.2. Analysis
The amount of tissue in the lesion region was determined from transverse sections as a percentage of the expected volume of tissue based on lesion region length and average area of a cranial and caudal section. For this, every other section on slides stained for hPLAP and GFAP were imaged and analyzed using the tracing tool in MetaMorph software.
The volume of GRP cells that occupied the lesion region was determined from transverse sections (10 animals) by imaging every other section on slides stained for hPLAP and GFAP and analyzing images using MetaMorph software. The hPLAP positive area in 35 sections was determined by using one threshold parameter that would pick up the hPLAP positive immunofluorescence within the MetaMorph software. Tissue and spared white matter at the lesion center was determined from transverse sections stained for hPLAP and GFAP (21 animals). Slides were examined and images were taken under standard fluorescence at 10x (Zeiss Axioplan2 microscope, Carl Zeiss, Hallergmoos, Germany). We determined the GFAP and hPLAP positive tissue area in one section at the lesion center using Adobe Photoshop vs.5 (Adobe Systems Incorporated, San Jose, CA, USA). This section contained the smallest amount of tissue, and this area was expressed as a percentage relative to a more caudal section to correct for tissue loss due to cord collapse. In addition the spared white matter at lesion center was determined from the Luxol Fast Blue–stained sections and expressed as percentage relative to a caudal section. GRP cell fate was determined by examining sections for the presence of double-labeled cells expressing hPLAP and nestin, hPLAP and GFAP, hPLAP and CC-1, or hPLAP and NG2/PDGFRα. Slides from 10 animals were examined and images were taken on a Zeiss 510 META confocal laser scanning microscope (Hallergmoos, Germany) at 63x. Stacks of images were collected with a distance of 0.37 μm between consecutiveslices. These stacks were processed using AutoDeblur & AutoVisualize vs.9.3 software (AutoQuant Imaging Inc., Watervliet, NY, USA) and analyzed using MetaMorph vs.6.3 software (Molecular Devices Corporation, Downingtown, PA, USA). Relative to the lesion center, a cranial, center, and caudal section were used for this analysis. Images were taken in the area where the transplant integrated with the host tissue. Three images were made from 3 different randomly selected parts of the transplant in that section and while choosing the area to be imaged, the slide was examined for hPLAP only. This ensured our sampling technique was not based on double-labeling. Within the stacked image, the slice showing most co-localization was determined using MetaMorph software, and cells in this slice were manually counted. This analysis was done along the margin of the transplant, since we expected differentiated cells to be present in that area. To quantity serotonin cranial and caudal to the lesion center, images were taken from the horizontal sections stained for hPLAP and serotonin under standard fluorescence at 2.5x. Three sections per animal were examined. Using one threshold parameter within MetaMorph software the quantity of serotonin was determined in an area that spanned the entire width of the cord over a length of 0.8mm immediately cranial and immediately caudal to the lesion center.
2.6. Histopathology and Analysis – Lumbosacral Spinal Cord
Lumbosacral spinal cord blocks for all available animals (n = 44) were sectioned transversally at 20 μm on a cryostat. Immunohistochemistry for synaptophysin and serotonin was performed on every other section (Table 1). Images were taken on a Zeiss 510 META confocal laser scanning microscope (Hallergmoos, Germany) at 63x with 2x zoom. For each animal 12 motor neurons located in the DL nuclei (6 left side, 6 right side) and 8 motor neurons located in the DM nuclei (4 left side, 4 right side) were imaged. Images were made based on synaptophysin and TOTO-3 staining. Serotonin staining was not examined prior to collecting the images. Stacks of images were collected with a distance of 0.37 μm between consecutive slices. These stacks were processed as described above using AutoDeblur & AutoVisualize vs.9.3 software and analyzed using MetaMorph vs.6.3 software. Serotonin in close proximity to motor neurons in the DM and DL nuclei was quantified by determining the amount of serotonin within the stack.
2.7. Statistics
Data are presented as means ± standard error of the mean (SE). A factorial repeated measures analysis of variance (ANOVA) and a factorial repeated measures analysis of covariance (ANCOVA) with the covariate factor being baseline data was used to analyze all behavioral data. The null hypothesis was rejected at α = 0.05. A one-way ANOVA was used to compare BL and endpoint data of the OP control, GRP control, cAMP control, and GRP cAMP groups to that of the AM control group. A one-way repeated measures ANOVA was used to determine significant differences between BL and endpoint data. Significant differences identified by the ANOVA were isolated using the Holm-Sidak procedure for pair wise multiple comparison post-hoc test.
A t-test was used to analyze the area of the lesion occupied by GRP cells and the differentiation of the GRP cells. Other histopathological data and ELISA results were analyzed using a one-way ANOVA. Significant differences identified by the ANOVA were isolated using the Holm-Sidak procedure for pair wise multiple comparison post-hoc test. The statistical computations were performed with software packages (Sigmastat 3.0, SPSS, Chicago, IL and SPSS 12.0, SPSS, Chicago, IL).
3. Results
3.1 Histopathology
In the majority of rats (28 of 40) the lesion consisted of a single large cavity that gradually enlarged to only be surrounded by a thin rim of spared white matter at the level of the lesion center. At this level, a large number of DAPI positive cell bodies were present, suggestive of scar tissue that appeared to have entered the lesion cavity from the surface of the spinal cord. Concomitant with this infiltration of cells was the development of multiple cavities separated by trabeculae, spared white matter, and tissue containing graft cells. Moving caudad the lesion was gradually filled in until it was no longer visible. In the other animals, the lesion consisted of more than 1 cavity that gradually was filled in until no longer visible (Figure 1A top panel).
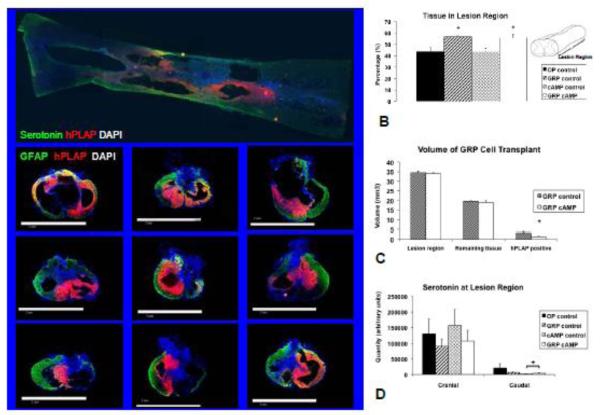
Figure 1.
Lesion region analysis. In A the top panel shows a horizontal section through the lesion in which the extent of the hPLAP positive transplant can be seen; cranial to the left. The bottom panel shows representative images of 9 of the 10 animals that received GRP cell transplants are shown. Sections are 20 μm thick and stained for serotonin (top panel; green), astrocytes (bottom panel; GFAP = green), GRP cells (hPLAP = red), and cell nuclei (DAPI = blue). In B the area of GFAP positive tissue in the lesion region shown as a percentage relative to the cranial and caudal cord and in C the lesion region and transplant volumes are shown determined from transverse sections. The bars to the left show the expected volumes should there be no tissue loss, the bars in the middle show the volumes of tissue remaining, and the bars to the right show the volumes of the GRP transplants for both groups. D shows quantification of serotonin cranial and caudal to the lesion center.
✦: significantly different from OP control and cAMP control (B); significantly different from hPLAP positive volume in GRP control (C); percentage reduction significantly different from OP control (D).
In all animals that received GRP cell transplants, extensive areas consisting of hPLAP positive cells were identified in the injured spinal cord at 84 days after SCI (Figures 1 and 2). In the bottom panel of Figure 1A, representative images are shown of the transplants of 9 of the 10 animals from which the spinal cord was sectioned transversally. Images are shown from an area in the lesion region, adjacent but not at lesion center. Grafts were present throughout the lesion region and were well integrated with the spared tissue of the host. Graft cells bordered the lesion cavity and were present in the spared tissue and trabeculae within the lesion cavity. The density of hPLAP positive cells was high in the center of the graft and gradually reduced as the graft integrated with the host tissue. The three injection sites and their respective cell deposits were not individually recognizable, as the hPLAP positive cells formed a continuous graft in 9 out of 11 animals (sectioned transversally). Needle tracts from the injection were present in 5 out of 11 animals and were limited to either the cranial (3) and/or caudal (4) injection sites.
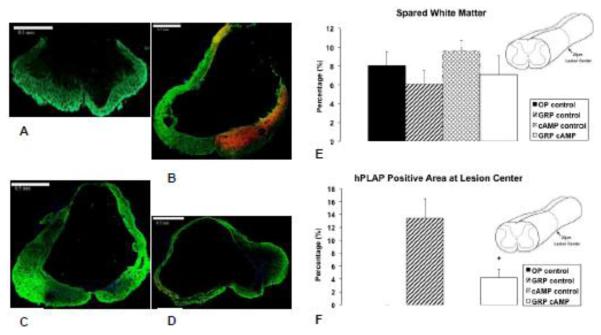
Figure 2.
Lesion center analysis. Representative lesion centers show in A = OP control, B = GRP control, C = cAMP control, and D = GRP cAMP. Sections are 20 μm thick and stained for GFAP, hPLAP, and DAPI. Green represents astrocytes (GFAP), red / yellow represents GRP cells (hPLAP), and blue represents nuclei (DAPI). In E the spared white matter is shown as a percentage of the area of a caudal section of thoracic spinal cord. In F the area of hPLAP positive tissue at lesion center is shown as a percentage of GFAP positive tissue in a more caudal section of thoracic spinal cord. ✦: significantly different from GRP control.
The area occupied by tissue (GFAP positive) and GRP cells (hPLAP positive) was determined from the transversally cut spinal cords from 10 animals, 35 sections were imaged per animal. The distance between consecutively imaged sections was approximately 200μm. Figure 1B shows that there was significantly more tissue present throughout the lesion region in animals that received a GRP cell transplant (p=0.003; F=6.9). In Figure 1C the volumes of the lesion region and transplants are shown. The expected cord volume not accounted for tissue loss of the lesion region is shown as well as the actual volume of the remaining tissue and the volume of the GRP transplant. Figure 1C demonstrates that the volume occupied by GRP cells in the lesion region in the GRP control group was significantly (p=0.047; F=5.5) larger than that in the GRP cAMP group. At the lesion center, there was no residual gray matter, but a thin rim of white matter remained (Figures 2A – D). The percentage white matter sparing was not different between groups and was 8.1 ± 1.5 % for OP control, 6.1 ± 1.4% for GRP control, 9.6 ± 1.1% for cAMP control, and 7.1 ± 2.0% for GRP camp (Figure 2E). The hPLAP positive area at the lesion center was significantly larger in the GRP control group compared to the GRP cAMP group (p<0.001; F=15.6; Figure 2F).
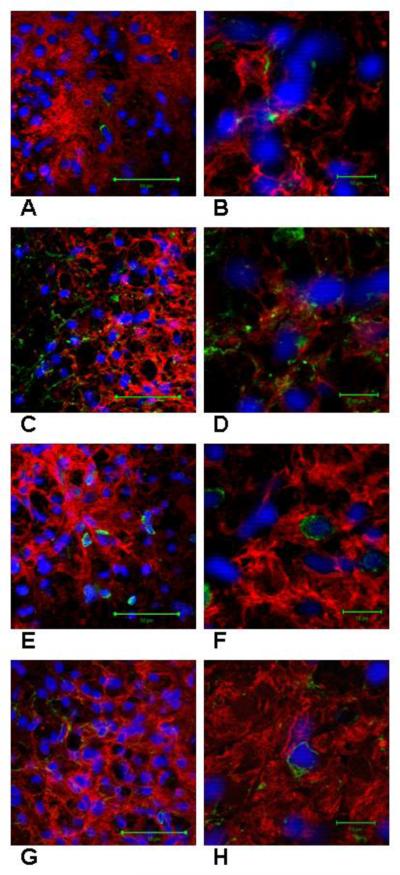
At the time of sacrifice, 75 days after transplantation GRP cells expressed nestin, GFAP, CC-1, and NG2 (Figure 3). Our quantification method allowed determination of cell differentiation of a total of 22,684 hPLAP positive cells (12,291 GRP control and 10,393 GRP cAMP); 5,356 were examined for nestin expression, 5,018 for GFAP expression, 5,672 for CC-1 expression, and 6,638 for NG2 expression. The proportion of hPLAP positive cells that expressed nestin was 12 ± 4% for GRP control and 10 ± 2% for GRP cAMP. The proportion of hPLAP positive cells that expressed GFAP was 60 ± 7% for GRP control and 42 ± 6% for GRP cAMP (p=0.07; F=4.4). The proportion of hPLAP positive cells that expressed CC-1 was 33 ± 5% for GRP control and 41 ± 5% for GRP cAMP. The proportion of hPLAP positive cells that expressed NG2 was 8.8 ± 1.8% for GRP control and 11.2 ± 1.4% for GRP cAMP. Examination of sections immunostained for hPLAP and PDGFRα demonstrated few cells expressing both markers, consistent with the results of immunostaining for hPLAP and NG2.
Figure 3.
Differentiation of hPLAP positive GRP cells. Each confocal image (A-H) represents one optical slice from a 20 μm section. In A and B GRP cells are shown that express both hPLAP (red) and nestin (green). In C and D GRP cells are shown that express both hPLAP (red) and GFAP (green). In E and F GRP cells are shown that express both hPLAP (red) and CC-1 (green). In G and H GRP cells are shown that express both hPLAP (red) and NG2 (green). Cell nuclei are stained with DAPI (blue). Scale bar in A, C, E, and G = 50μm and B, D, F, and H = 10μm.
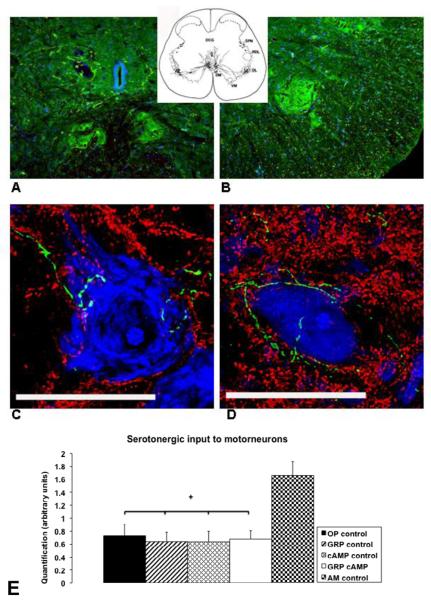
In all groups a significant reduction in serotonin caudal to the lesion was seen when compared to the amount cranial to the lesion (Figure 1D). The percentage of serotonin found caudal to the lesion compared to cranial to the lesion was 33 ± 6% for OP control, 18 ± 7% for GRP control, 3 ± 1% for cAMP control, and 9 ± 4% for GRP cAMP. In both groups that received cAMP, this reduction was significantly more (p=0.004; F=6.8) than the reduction seen in the OP control group. For 44 animals 20 cells from the DM and DL nuclei in the lumbosacral spinal cord were imaged. The location of the DM and DL nuclei is shown in Figure 4A and B, respectively. Quantification of the amount of serotonin in close proximity to these motor neurons (Figure 4 C and D) was significantly less following SCI when compared to the AM control group (p<0.001; F=5.4), however, no differences between groups were found (Figure 4 E).
Figure 4.
Location of dorsomedial (A) and dorsolateral nuclei (B) in the lumbosacral spinal cord, examples of motor neurons located in the dorsomedial or dorsolateral nuclei (C and D), and quantification of serotonin in close proximity to these motor neurons (E). Sections in A and B are stained for serotonin (green) and cell nuclei (blue; TOTO-3 cyanine dimer nucleic acid stain, Invitrogen Corporation, Carlsbad, CA, USA; 2mM) by immunohistochemistry. In A the 2 dorsomedial nuclei are shown left and right from midline just ventral to the central canal. B shows the right dorsolateral nucleus in the ventral horn of the lumbosacral spinal cord. Sections in C and D are 20 μm thick and stained for serotonin (green), synaptophysin (red), and DNA/RNA (TOTO-3) by immunohistochemistry. The extensive dendritic field with synapses in these nuclei and the serotonergic input (green) to the motor neurons is shown here. Scale bar (C and D) = 50 μm. In E the quantification of serotonin in close proximity to motor neurons is shown in arbitrary units for all groups of rats. ✦: significantly different from AM control.
3.2. Functional outcomes
In the short-term study, rats were approximately 80 days and weighed 325 ± 3 g at the time of SCI. At the time of transplant, animals in the OP control group weighed significantly more than animals in the cAMP control group (304 ± 6 g and 285 ± 2 g, respectively). At the time of sacrifice (14 days post SCI) the UI control animals weighed 363 ± 4 g, which was significantly more (p<0.001) than the OP control (291 ± 6 g) and cAMP control (279 ± 1 g) animals. In the long-term study, rats were 71 ± 2 days and weighed 293 ± 1 g for transducer implantation. Rats were 77 ± 2 days and weighed 318 ± 2 g at the time of SCI. At the time of transplant and sacrifice, rats weighed 289 ± 3 g and 385 ± 6, respectively. Average weights of animals at the time of sacrifice were similar in all groups (OP control: 392 ± 11 g; GRP control: 383 ± 14 g; cAMP control: 380 ± 13 g; GRP cAMP: 391 ± 12 g); however, when grouping animals together the animals in the combined OP and GRP control groups weighed significantly more than animals in the combined GRP cAMP and cAMP control groups (295 ± 4 and 284 ± 3; p=0.05). Five animals died unexpectedly during the course of this study; 1 animal from the OP control group died on day 9 following SCI, 1 animal in the cAMP control group died on day 11 following SCI, 1 animal in the GRP control group died on day 22 following SCI, and 2 animals in the GRP cAMP group died on days 53 and 79 following SCI. Three animals (1 GRP control, 2 GRP cAMP) had episodes of autophagia that resolved spontaneously.
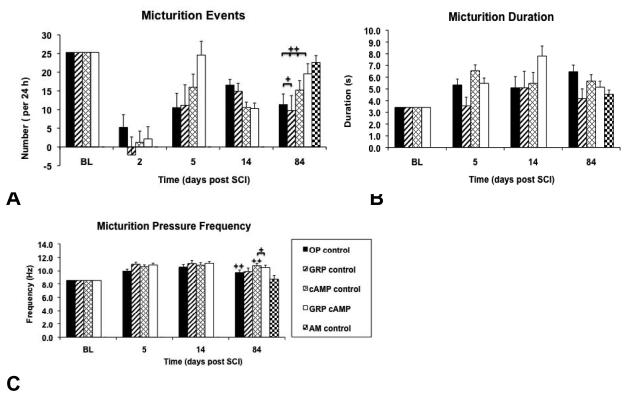
The BL average number of micturition events per 24 hours per rat was 22 ± 2 (OP control), 20 ± 2 (GRP control), 29 ± 2 (cAMP control), and 27 ± 1 (GRP cAMP). First spontaneous micturition events were detected by telemetry 2 – 4 days following SCI. Although in most animals recovery of micturition was sufficiently effective that manual bladder expression was no longer required, the number of micturitions per 24 hours at the endpoint of the study remained significantly lower than that at BL for all groups (OP control: p=0.03; F=12; GRP control: p=0.05; F=11; cAMP control: p=0.02; F=11; GRP cAMP: p=0.008; F=18). However, the number of micturitions at the endpoint of the study in the cAMP control (15 ± 3) and GRP cAMP (19 ± 1) groups was not lower than that recorded from the AM control group (23 ± 2; Figure 5A). In contrast, the OP control (12 ± 3) and GRP control (10 ± 2) groups did have significantly fewer micturitions per 24 hours at endpoint compared to the AM control group (p=0.008; F=4.6; Figure 5A). Detailed pressure waveform analysis revealed increased duration of micturition events and increased frequency of micturition pressure peaks over time (Figures 5B and C). Micturition peak frequency is a robust parameter as shown by small standard errors and little variability between groups. Also, the micturition peak frequency recorded in the AM control group was not significantly different from BL data of the other 4 groups. Comparing day 84 to BL revealed a significant increase of micturition peak frequency for the OP control and cAMP control groups (p=0.03; F=11.6 and p=0.01; F=12.7, respectively), whereas in the animals that received GRP cells there was no significant difference in this parameter. At day 84 the micturition peak frequency of the cAMP control and the GRP cAMP groups were significantly larger when compared to the AM control group (p=0.02; F=3.6).
Figure 5.
Recovery of micturition function shown for all groups. A shows the estimated marginal means of the number of micturitions per 24 hours determined by ANCOVA set with the coveriate baseline (BL) at 25.3 micturitions/24h for BL and days 2, 5, 14, and 84 after SCI. In B the estimated marginal means of the micturition duration are shown determined by ANCOVA set at BL=3.4s (covariate) and in C the estimated marginal means of the micturition peak frequency are shown determined by ANCOVA set at BL=8.6Hz (covariate) for BL and days 5, 14, and 84 after SCI.
✦✦: significantly different from BL; ✦: significantly different from AM control
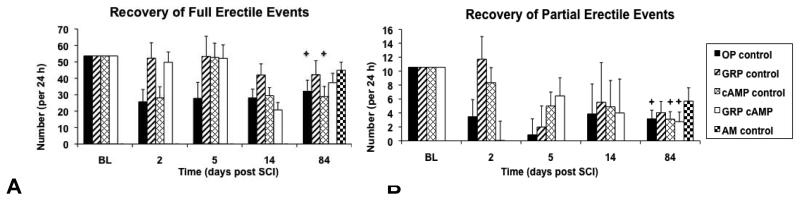
Number of full erectile events per 24 hours per rat at BL was 52 ± 3 (OP control), 63 ± 4 (GRP control), 47 ± 6 (cAMP control), and 63 ± 9 (GRP cAMP; Figure 6A). At 84 days after SCI the total number of full erectile events was significantly reduced from BL in the OP control (32 ± 2; p=0.006; F=28) and cAMP control groups (30 ± 4; p=0.03; F=8.7). In contrast the reduction of events in the GRP control and GRP cAMP groups was not significant (40 ± 10 and 37 ± 7, respectively). No significant differences were present between groups or between the injured groups and the AM control group (45 ± 5 erectile events per 24h). The number of partial erectile events per 24 hours per rat at BL was 8 ± 2 (OP control), 8 ± 2 (GRP control), 7 ± 2 (cAMP control), and 13 ± 3 (GRP cAMP; Figure 6B). The total number of partial erectile events on day 84 following SCI was reduced in all groups, but only in the GRP control group (3 ± 2) was this reduction not significant from BL. In the other groups there were significantly fewer partial erectile events (OP control: 3 ± 1, p=0.002, F=54; cAMP control: 3 ± 1, p=0.005, F=19; GRP cAMP: 3 ± 1, p=0.007, F=19). In all groups the number of partial erectile events per 24 hours at 84 days after SCI was not significantly lower than in the AM group (6 ± 3).
Figure 6.
Recovery of erectile events shown for all groups. The estimated marginal means determined by ANCOVA with baseline being the covariate is shown for baseline (BL) and days 2, 5, 14, and 84 after SCI. In A full erectile events are shown set at BL=54 erectile events per 24h and in B partial erectile events are shown set at BL=10.6 partial erectile events per 24h.
✦: significantly different from BL.
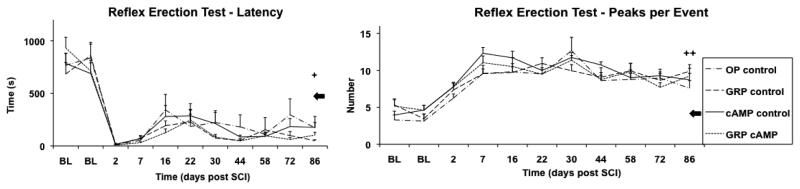
Following SCI, latency (time of first erectile event following sheath retraction) decreased at all time points (Figure 7A). Although partial recovery occurred, latency remained significantly lower than BL for all groups (OP control: p<0.001; F=28; GRP control: p<0.001; F=29; cAMP control: p=0.002; F=16; GRP cAMP: p<0.001; F=61). Furthermore, latency recorded in the AM group was significantly lower than latency measured at BL in the injured groups (p<0.001; F=14). At day 86 after SCI, latency in the GRP control and GRP cAMP groups remained significantly lower than that of the AM group (p<0.001; F=14). The number of suprasystolic pressure peaks that occurred during erectile events increased from BL at all time points following SCI (Figure 7B). At day 86 after SCI the number of pressure peaks per event was significantly higher than at BL for the GRP control (p=0.002; F=28) and cAMP control (p=0.016; F=92) groups. Furthermore, the number of pressure peaks at day 86 following SCI remained significantly greater than that recorded in the AM control group for all groups (p<0.001; F=7.6).
Figure 7.
Ex copulatory reflex erection test results. For all groups of rats the latency (time to first erection after sheath retraction) (A) and the number of peaks per event (B) are shown for baseline (BL) and for the duration of the study.
✦: all groups significantly different from BL; GRP control and GRP cAMP groups significantly different from AM control.
✦✦: GRP control and cAMP control significantly different from BL; all groups significantly different from AM control.
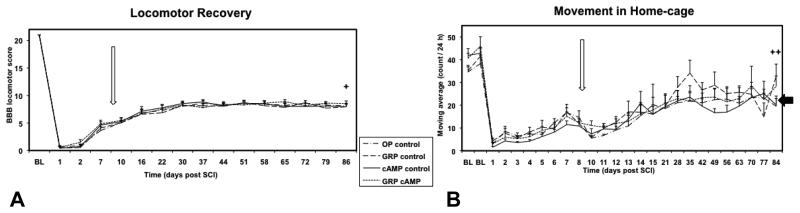
The 25 g-cm SCI resulted in an initial profound paraplegia in all rats characterized by BBB locomotor scores of 0.6 ± 0.3 (OP control) 0.5 ± 0.3 (GRP control) 0.4 ± 0.3 (cAMP control), and 0.6 ± 0.1 (GRP cAMP) at 24 hours after SCI. Gradually, rats showed some recovery of hind limb locomotor function (Figure 8A), however, no significant differences were found between groups. BBB locomotor scores were 8.2 ± 0.4 (OP control) 8.0 ± 0.6 (GRP control) 8.0 ± 0.5 (cAMP control), and 8.6 ± 0.4 (GRP cAMP) at the time of sacrifice. This remained significantly different from BL (p<0.001; F=404). In the short-term survival study, BBB locomotor scores at 14 days after SCI for the OP control (6.6 ± 1.0) and cAMP control (7.1 ± 1.1) animals were not significantly different from those in the long-term study at 16 days after SCI. The level of activity of the rats when housed in their cages, determined by telemetry, was reduced immediately after SCI (Figure 8B). This was followed by a steady recovery; however, the transplantation surgery at day 9 resulted in a second drop in level of activity. The level of activity reached a plateau between 3 – 5 weeks following SCI, and at the endpoint of this study was similar to that of the AM control group, which was significantly less than activity recorded for rats at BL for the OP control (p=0.03; F=9), cAMP control (p<0.001; F=36), and GRP cAMP (p=0.009; F=14) groups.
Figure 8.
Recovery of locomotion function. For the 4 groups of rats the BBB locomotor score (A) and level of activity in home cage (B; AVG ± SE) are shown for baseline (BL) and for the duration of the study. The open arrow indicates time of transplantation surgery (day 9). The level of activity of the AM control group in B is indicated with the black arrow.
✦: all groups significantly different from BL; ✦✦: OP control, cAMP control, GRP cAMP, and AM control significantly different from BL.
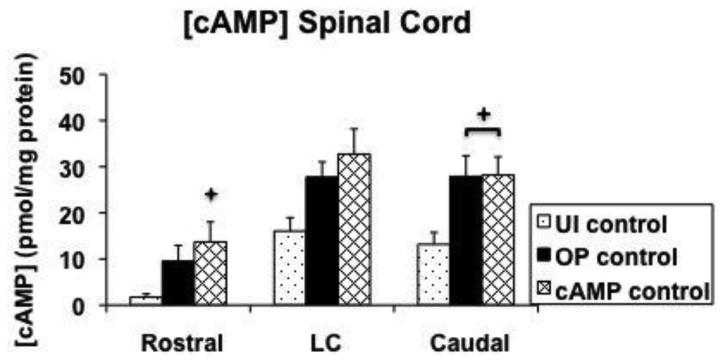
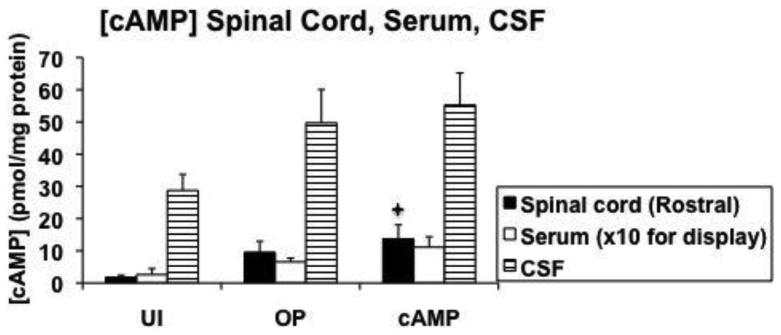
3.3 cAMP concentrations
Concentrations of cAMP were determined in cranial spinal cord, lesion center, caudal spinal cord, serum, and CSF 14 days after SCI (Figure 9). Lesion center [cAMP] were not significantly different between the groups, however, in the cranial sections significantly more cAMP was found in the cAMP control group vs. the UI control group (p=0.035). In addition, in the caudal sections significantly more cAMP was found in both the OP control and cAMP control groups vs. the UI control group (p=0.04 and p=0.02, respectively; Figure 9A). Concentrations of cAMP measured in serum and CSF were not different between groups (Figure 9B).
Figure 9.
Quantification of cAMP concentrations at 14 days after injury. A shows cAMP concentrations determined in a cranial section, lesion center, and caudal section of spinal cord. B shows cAMP concentrations determined in cranial spinal cord, serum, and cerebrospinal fluid. The value for serum is graphed at 10x the actual value for display purposes. LC = lesion center; UI = uninjured control; OP = operated control; cAMP = cAMP control; CSF = cerebrospinal fluid.
✦: significantly different from uninjured control.
4. Discussion
Here we show that hPLAP positive GRP cells transplanted at 9 days after contusion SCI exhibited robust survival and integration into host tissue for up to 85 days, and expressed markers for both oligodendrocytes (CC1) and astrocytes (GFAP). As GRP cells are immunoreactive negative for CC1 and GFAP prior to transplantation, the expression of these makers suggest in vivo differentiation of the GRP cells along the astrocytic and oligodendrocytic pathways. Extensive analysis of histopathology and neurological outcome showed some desirable effects attributable to the GRP transplant such as the increased tissue volume throughout the injured cord suggesting, again, that these transplants are neuroprotective. In addition, animals with GRP cell transplants did not have significantly increased micturition peak frequencies and they did not have a significant reduction of erectile events per 24 hours compared to baseline data at the end point of the study. On the down side, animals with GRP cells had significantly shorter latency times in reflex erection tests. The only positive effect we could attribute to cAMP treatment was that by the end of the study these animals had a similar number of micturitions per 24 hours as the age-matched control animals. Adverse effects associated with administration of rolipram and db-cAMP were plentiful and included increased body-weight loss in the acute and chronic phase after SCI and increased micturition peak frequencies compared to age-matched control animals. Perhaps surprisingly, analysis of the 12-week survival transplants also showed that in rats that were administered db-cAMP and rolipram for two weeks after SCI, a smaller area of the injured cord was occupied by hPLAP-positive cells from the donor, suggesting that this treatment reduced survival or proliferation of GRP cells. In addition, in animals that received db-cAMP and rolipram, the amount of serotonin immediately caudal to the lesion was reduced and there was a trend towards less differentiation of precursor cells into astrocytes. Although rolipram + cAMP and GRP cells affected some of the functional outcome parameters, there was no consistent beneficial effect across outcomes from either treatment or from the combination of both.
The present results are consistent with recent reports suggesting that undifferentiated GRP cells may not be therapeutically effective without further in vitro or in vivo treatments to promote astrocyte differentiation (Davies, et al., 2006, Davies, et al., 2008). Further, while treatments with db-cAMP and rolipram appear to be anti-inflammatory and may enhance neurite outgrowth and/or attenuate oligodendrocyte death in a number of in vitro and in vivo assays (Bregman, et al., 1998, Cai, et al., 1999, Pearse, et al., 2004, Spencer and Filbin, 2004, Whitaker, et al., 2008), the protocol applied here in combination with GRP cells was not therapeutically effective. In addition, it may have reduced the size of the 12-week survival transplant and reduced serotonergic fibers immediately caudal to the lesion. A recent study reported beneficial effects of a one-time administration of rolipram on histopathological and functional outcome measurements after SCI only when it was administered in combination with thalidomide (Koopmans, et al., 2009).
Our moderate contusion injury produced a very consistent lesion with the area of spared white matter at the lesion center in the OP control group (8.1 ± 1.5%) in the same range as described previously for this level of injury (9.9 ± 4.8%; Basso et al., 1996). There were no group differences with respect to spared white matter or functional outcomes, consistent with the idea that these two parameters are highly correlated (Basso, et al., 1996). Furthermore, in this study, cAMP concentrations in the spinal cord appeared to be increased 14 days after SCI, contrary to what others have shown previously (Pearse, et al., 2004).
Pearse et al. (2004) demonstrated in vivo that spinal cord [cAMP] are reduced after SCI (up till 14 days after SCI), and showed that administration of rolipram and db-cAMP, using the same methodology as we did in our study, prevented this SCI-induced reduction of spinal cord [cAMP]. They demonstrated that the combination of Schwann cell transplants and elevation of [cAMP] following a 12.5g-cm SCI resulted in significant improvement of the BBB score at 8 weeks after transplant (injured-only group: 10.4; treated group: 15.0). At 1 week after transplant the injured-only group had a BBB score of 7 vs 10.5 in the treated group but no BBB scores were reported prior to 1 week after transplant. This treatment strategy promoted supraspinal and propriospinal axon sparing and myelination, as well as serotonergic fiber growth into and beyond the graft. The extent of the transplant grafts within the injury lesion region and whether or not this was affected by cAMP were not quantified. Noting that Schwann cells are very different from the progenitor cells we used here, the results still suggest that the effects of cAMP and rolipram on progenitor cell proliferation and survival warrant further careful study if this approach is to be used in other combination strategies for repairing SCI. Another study by this group showed that treatment with rolipram improved functional recovery, promoted axonal growth, and attenuated astrogliosis (Nikulina, et al., 2004). This is particularly interesting in light of our findings of slightly reduced differentiation of GRP cells into astrocytes. Perhaps reduced activation of endogenous astrocytes and reduced glial scarring is produced through similar mechanisms of cAMP that alter lineage differentiation of GRP cells. In our study less hPLAP-GFAP positive cells were seen in the animals that received db-cAMP and rolipram, and although more hPLAP-CC-1 positive cells were seen in the GRP cAMP group, this was not statistically significant and there was no beneficial effect on functional outcome. With increased availability of oligodendrocytes, one would expect to see enhanced re-myelination and potentially improved functional outcome. However, it is possible that even if the proportion of hPLAP-CC-1 positive cells was larger, the total number of hPLAP-positive oligodendrocytes would not have been increased in our GRP cAMP group since cAMP appeared to have a negative effect on GRP cell transplant size. In addition, we identified very few hPLAP-NG2-positive and hPLAP-PDGFRα-positive cells, suggesting that few donor cells were differentiating into oligodendrocyte precursor cells.
As far as the authors are aware, our study is the only one that has quantified extent of transplant within the injured spinal cord. Certainly there appear not to be any other reports on the effect of cAMP on survival of transplanted cells within the injured spinal cord. Our study suggests that administering db-cAMP and rolipram is not necessarily beneficial to survival of GRP cells. This is unlikely to be due to the altered immune response that is caused by cAMP, since it has been well established that cAMP has predominantly anti-inflammatory effects. Macrophages failed to differentiate into activated macrophages when exogenous cAMP was added in an in vitro model (Peters, et al., 1990) and phagocyte function of macrophages was inhibited by cAMP (Aronoff, et al., 2005, Peters, et al., 1990). However, numerous reports have shown that cAMP is involved in regulation of cell survival, in particular of neoplastic and progenitor cells.
In B-cell chronic lymphocytic leukemia (B-CLL) PDE-IV is the predominant PDE expressed in the neoplastic cells. Rolipram induces apoptosis in B-CLL cells through a cAMP dependent and caspase dependent manner. Rolipram increases the efficacy of glucocorticoid-mediated apoptosis in B-CLL cells by modulating glucocorticoid receptor signal transduction, a process that requires PKA (Tiwari, et al., 2005). Interestingly, this up-regulation of glucocorticoid receptor transcript levels does not take place in normal circulating hematopoietic cells, or in cells of T-cell chronic lymphocytic leukemia (T-CLL) (Meyers, et al., 2009, Meyers, et al., 2007). Also, rolipram was found to suppress the anti-apoptotic members of the Bcl-2 family and induce the pro-apoptotic protein Bax. Combining these mechanisms results in a shift of the balance between pro- and anti-apoptotic members of the Bcl-2 family towards a pro-apoptotic direction (Siegmund, et al., 2001). Both acute and chronic lymphoblastic leukemia cells undergo apoptosis following administration of rolipram. Contributing mechanisms include G1 and G2/M cell cycle arrest (Ogawa, et al., 2002), mitochondrial depolarization, release of cytochrome c into the cytosol, and caspase-9 and -3 activation (Moon and Lerner, 2003). Elevation of cAMP concentration has also been shown to inhibit cell growth and induce apoptosis in other cancer cell lines such as retinoblastoma cells (Fassina, et al., 1997), papilloma cells (Marko, et al., 1998), glioma cells (Chen, et al., 2002), neuroblastoma cells (Kumar, et al., 2004), and esophageal cancer cells (Wang, et al., 2005). In cancer cell lines this effect of PDE-IV inhibition is an obvious area of interest since halting growth of these cells is a much-desired therapeutic goal. Furthermore, in the treatment of bronchial asthma, PDE inhibition is thought to be an important mechanism of the anti-inflammatory actions of theophylline. Theophylline and rolipram have been shown to increase eosinophil intracellular cAMP concentrations and inhibit eosinophil survival (Momose, et al., 1998, Takeuchi, et al., 2002, Wang, et al., 2005). Recently it has been demonstrated that cAMP elevation resulted in significant inhibition of colony growth and induced apoptosis of progenitor cells in asthmatics, but not in normal subjects. These effects were not limited to the eosinophil lineage alone (Wang, et al., 2003). A number of possible mechanisms for this are internucleosomal DNA cleavage, G1 cell arrest, and effects through CREB gene regulation. Another potential mechanism for the pro-apoptotic effect of cAMP is given in a recent study of pulmonary hypertension. Pulmonary arterial hypertension is a proliferative vascular disease characterized by aberrant regulation of smooth muscle cell proliferation and apoptosis in distal pulmonary arteries. Elevating cAMP concentrations through administration of PDE inhibitors suppressed proliferation and matrix metalloproteinase activity and promoted apoptosis in these cells. One of the important mechanisms appeared to be attenuation of DNA synthesis (Growcott, et al., 2006). These studies, and the results of our study, suggest that (progenitor) cell survival should be carefully examined in treatment strategies that include the use of elevating cAMP.
In the present study, GRP cells alone did not improve locomotor and only modestly improved some autonomic behavioral outcomes. Similarly, Davies et al (2006) showed no beneficial effects from transplantation of undifferentiated GRP cells, however transplantation of GRP-derived astrocytes (GDAs) resulted in improvement of histopathological and locomotor outcomes. Other studies examining the effects of different glial progenitor cells such as the human embryonic oligodendrocyte precursor cells (OPCs) (Keirstead, et al., 2005) and oligodendrocyte precursors (Bambakidis and Miller, 2004) have demonstrated positive effects on locomotor outcome, but these studies were conducted in less severe SCI models. One elegantly performed study in male rats of similar weight as ours, demonstrated a positive effect of transplanting oligodendrocyte-type 2 astrocyte progenitors (O-2A) into the lesion center on locomotor recovery after a 25 g-cm SCI (Lee, et al., 2005). Although a significant difference was found in BBB score at 6 weeks after transplantation (9.5 in operated control animals vs. 12.3 in treated animals), few significant effects of transplantation were seen during electrophysiological assessment of these animals. The O-2A cells used in that study differentiated into oligodendrocytes that had the ability to promote remyelination and regeneration of axons. Differences between GRP cells and O-2A cells include different developmental age of isolation, different chemokine response patterns, different responses to inducers of differentiation, and different behaviors of the GRP cells versus O-2As following transplantation (Rao, et al., 1998). The latter is most striking and perhaps reflects the embryonic nature of the GRP cells that have the potential to differentiate into two distinct types of astrocytes and O-2A cells (Gregori, et al., 2002). In contrast, the postnatally generated O-2A cells predominantly differentiated into oligodendrocytes while their ability to generate astrocytes is still controversial (Noble, et al., 2004). Differences in characteristics of these progenitor cells undoubtedly affect eventual functional outcome and need to be studied in detail in future experiments. In addition to finding the best progenitor cell characteristics to use in the development of transplantation strategies, investigators also need to consider the occurrence of harmful side effects. Davies et al (2008) saw evidence of allodynia with GRP cells and GDAs and, although we did not formally test for this as we have done in other studies (Lindsey, et al., 2000), we did not see obvious features of allodynia or hyperreflexia related to GRP cell transplants in this study. We note, however, that the effects reported in Davies et al (2008) are quite modest, and may not have been severe enough to affect sensation to the degree that we would notice it in the normal handling of the animals. The reports from Davies et al (2006, 2008) strongly suggest that future studies should employ these tests.
Recovery of autonomic function after transplantation strategies has been studied by Mitsui et al. using fibroblasts engineered to secrete brain-derived neurotrophic factor (BDNF) and neurotrophin (NT)-3 (Mitsui, et al., 2005) and a combined application of NRP and GRP cells (Mitsui, et al., 2005). In these studies, rats were observed in metabolic cages and recovery of micturition from the period of bladder areflexia appeared accelerated in the treatment groups; however treatment differences were no longer apparent by 4 weeks after transplantation. Both of these treatments appeared to reduce signs of detrusor sphincter dyssynergia and detrusor hyperreflexia as determined by cystometry and determination of bladder weights. In the combined NRP GRP transplant study, rats underwent a protocol similar to ours in which SCI was followed by transplantation of precursor cells in 3 sites of the lesion at 9 days after injury. Transplant injections in the dorsal midline of the spinal cord were performed using a Hamilton syringe with a tip of 310μm which is significantly larger than what we used (180μm) and transplanting a combination of NRP and GRP cells resulted in improvement of the BBB locomotor score from 7.1 (operated control group) to 9.4 (NRP GRP group). The comparatively low BBB score seen in the operated control group was attributed to the modified-moderate contusion injury that is used, in which the impactor rod is left on the spinal cord for an additional 5 sec following a 25 g-cm contusion (Mitsui, et al., 2005). Interestingly, in the previous study that demonstrated a positive effect of transplanting fibroblasts expressing BDNF and NT-3 on recovery of locomotor function (BBB from 3.5 to 8.2), (Mitsui, et al., 2005), this modified-moderate contusion model resulted in BBB scores of 3.5 in the operated control group at 8 weeks after transplant. The fibroblast study was performed using a similar experimental paradigm as the NRP GRP study. Considering the difference in BBB locomotor scores between the 2 groups (3.5 vs 8.2) after transplant of genetically modified fibroblasts, it is perhaps not surprising other behavioral outcomes improved as well. Development of thermal hypersensitivity was not affected by this treatment. In addition, both of these studies reported increased serotonergic input to the DL nucleus in the lumbosacral spinal cord; however from the data shown it is unclear what nucleus was examined for serotonin (Mitsui, et al., 2005). Although in our study the number of micturitions per 24 hours, micturition duration, and erectile event duration appeared to have recovered more in the GRP cAMP treatment groups, none of these outcomes were unequivocally positively affected by our intervention. We did not find significant beneficial effects of our transplantation strategy on micturition or sexual outcome measurements. Another recent study, demonstrated only very slight improvement of some urodynamic bladder function outcomes following transplantation of either a combination of NRP and GRP cells or bone marrow stromal cells (Temeltas, et al., 2009). Locomotor outcomes and histopathological quantification of transplant size and white matter sparing were not provided. The same group also showed improvement in erectile function outcomes using the same treatment (Temeltas, et al., 2009). GRP cells have been shown to have beneficial effects on locomotor recovery when they are genetically modified to express multineurotrophins (Cao, et al., 2005). The experimental paradigm in this study was similar to our study and the studies conducted by Mitsui et al. (2000a and 2000b), in that transplantation was performed 9 days after SCI. However, the level of injury (150 kdyn; Infinite Horizon device) was much less severe and resulted in BBB scores of 10 – 12 prior to transplant. This strategy resulted in BBB scores of 12.2 – 15.6 (operated control and multineurotrophin-expressing GRP group, respectively) at 6 weeks after SCI.
In this study we used telemetry to monitor micturition and erectile events in rats as a measurement of autonomic function. We believe this technique is very useful because anesthesia, animal handling, or animal restraint does not affect the results. Although we found some significant differences in some of the measurements, there was no unequivocal positive outcome of the applied treatment, similar to the results we showed for the locomotor function outcome. Telemetry also provided us with a measurement of activity in the home-cage, which appears a useful indicator of gross voluntary movement. This measurement was the only one that showed clear deterioration of the recovery curve at the time of the transplantation surgery. Telemetry is a valuable system to obtain data on recovery of spontaneous functions, in particular autonomically mediated outcomes.
In conclusion, our study showed modest beneficial effects on some functional outcome measurements of transplanting GRP cells and administering cAMP after SCI. Although elevation of cAMP has been reported to have beneficial effects in neural regeneration and may have beneficial effects on lineage differentiation of GRP cells, from our study it appears that cAMP is not always reduced after SCI and that administering rolipram and db-cAMP may reduce survival or proliferation of GRP cells following transplantation into the injured spinal cord. Continued research should be undertaken to specifically study the effects of cAMP on endogenous and exogenously administered progenitor cells and future studies should examine effects of combined treatment strategies, such as this one, for SCI. Transplant strategies appear promising; however it seems appropriate to use combinatorial strategies in which cell transplants are combined with other therapies such as treatments that induce differentiation of progenitor cells into populations with desired functional characteristics, treatments that affect regeneration, and/or treatments that alter the spinal cord milieu. It is important that investigators critically examine effects and interactions of all these treatments, not only on functional outcome measurements but also on histopathological features. Furthermore, effects of the complimentary treatments such as administered neurotrophins or cAMP administration may have different effects in normal adult cells vs. in progenitor cells such as used in current transplantation strategies.
Research Highlights.
GRP cells survived, differentiated, and formed extensive transplants.
GRP cells and cAMP had modest positive effects on micturitions and erections.
cAMP reduced the graft size throughout the lesion region and at the lesion center.
Serotonin immediately caudal to the lesion was reduced in the cAMP groups.
5. Acknowledgements
We would like to thank Rochelle Deibert, Cristal Forrider, and John Komon for their contributions to this study.
Funding: NIH NS-31193; New York State Center for Research Excellence: contract number CO19772
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 2.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174:595–599. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 3.Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4:16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 6.Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young W. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148:453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- 7.Bregman BS, Broude E, McAtee M, Kelley MS. Transplants and neurotrophic factors prevent atrophy of mature CNS neurons after spinal cord injury. Exp Neurol. 1998;149:13–27. doi: 10.1006/exnr.1997.6669. [DOI] [PubMed] [Google Scholar]
- 8.Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 9.Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao QL, Howard RM, Dennison JB, Whittemore SR. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177:349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- 11.Chen RW, Williams AJ, Liao Z, Yao C, Tortella FC, Dave JR. Broad spectrum neuroprotection profile of phosphodiesterase inhibitors as related to modulation of cell-cycle elements and caspase-3 activation. Neurosci Lett. 2007;418:165–169. doi: 10.1016/j.neulet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Chen TC, Wadsten P, Su S, Rawlinson N, Hofman FM, Hill CK, Schonthal AH. The type IV phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitors p21(Cip1) and p27(Kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant A-172 glioma cells. Cancer Biol Ther. 2002;1:268–276. doi: 10.4161/cbt.80. [DOI] [PubMed] [Google Scholar]
- 13.Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJ. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fassina G, Aluigi MG, Gentleman S, Wong P, Cai T, Albini A, Noonan DM. The cAMP analog 8-Cl-cAMP inhibits growth and induces differentiation and apoptosis in retinoblastoma cells. Int J Cancer. 1997;72:1088–1094. doi: 10.1002/(sici)1097-0215(19970917)72:6<1088::aid-ijc25>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Gregori N, Proschel C, Noble M, Mayer-Proschel M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J Neurosci. 2002;22:248–256. doi: 10.1523/JNEUROSCI.22-01-00248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Growcott EJ, Spink KG, Ren X, Afzal S, Banner KH, Wharton J. Phosphodiesterase type 4 expression and anti-proliferative effects in human pulmonary artery smooth muscle cells. Respir Res. 2006;7:9. doi: 10.1186/1465-9921-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126-128. [DOI] [PubMed] [Google Scholar]
- 20.Hannila SS, Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2008;209:321–332. doi: 10.1016/j.expneurol.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart BL. Sexual reflexes and mating behavior in the male rat. J Comp Physiol Psychol. 1968;65:453–460. doi: 10.1037/h0025842. [DOI] [PubMed] [Google Scholar]
- 22.Hicken BL, Putzke JD, Richards JS. Bladder management and quality of life after spinal cord injury. Am J Phys Med Rehabil. 2001;80:916–922. doi: 10.1097/00002060-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Hill CE, Proschel C, Noble M, Mayer-Proschel M, Gensel JC, Beattie MS, Bresnahan JC. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 24.Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 25.Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibarra A, Guizar-Sahagun G, Correa D, Kretschmer R, Grijalva I, Flores-Murrieta FJ, Castaneda-Hernandez G, Odor A, Lopez RM, Franco-Bourland R, Espitia AL, Salgado-Ceballos H, Madrazo I. Alteration of cyclosporin-A pharmacokinetics after experimental spinal cord injury. J Neurotrauma. 1996;13:267–272. doi: 10.1089/neu.1996.13.267. [DOI] [PubMed] [Google Scholar]
- 27.Ibarra A, Reyes J, Martinez S, Correa D, Guizar-Sahagun G, Grijalva I, Castaneda-Hernandez G, Flores-Murrieta FJ, Franco-Bourland R, Madrazo I. Use of cyclosporin-A in experimental spinal cord injury: design of a dosing strategy to maintain therapeutic levels. J Neurotrauma. 1996;13:569–572. doi: 10.1089/neu.1996.13.569. [DOI] [PubMed] [Google Scholar]
- 28.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koopmans GC, Deumens R, Buss A, Geoghegan L, Myint AM, Honig WH, Kern N, Joosten EA, Noth J, Brook GA. Acute rolipram/thalidomide treatment improves tissue sparing and locomotion after experimental spinal cord injury. Exp Neurol. 2009;216:490–498. doi: 10.1016/j.expneurol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Kumar B, Hanson AJ, Prasad KN. Sensitivity of proteasome to its inhibitors increases during cAMP-induced differentiation of neuroblastoma cells in culture and causes decreased viability. Cancer Lett. 2004;204:53–59. doi: 10.1016/j.canlet.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Lee KH, Yoon do H, Park YG, Lee BH. Effects of glial transplantation on functional recovery following acute spinal cord injury. J Neurotrauma. 2005;22:575–589. doi: 10.1089/neu.2005.22.575. [DOI] [PubMed] [Google Scholar]
- 33.Lindsey AE, LoVerso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil Neural Repair. 2000;14:287–300. doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- 34.Marko D, Romanakis K, Zankl H, Furstenberger G, Steinbauer B, Eisenbrand G. Induction of apoptosis by an inhibitor of cAMP-specific PDE in malignant murine carcinoma cells overexpressing PDE activity in comparison to their nonmalignant counterparts. Cell Biochem Biophys. 1998;28:75–101. doi: 10.1007/BF02737806. [DOI] [PubMed] [Google Scholar]
- 35.Meyers JA, Su DW, Lerner A. Chronic lymphocytic leukemia and B and T cells differ in their response to cyclic nucleotide phosphodiesterase inhibitors. J Immunol. 2009;182:5400–5411. doi: 10.4049/jimmunol.0804255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers JA, Taverna J, Chaves J, Makkinje A, Lerner A. Phosphodiesterase 4 inhibitors augment levels of glucocorticoid receptor in B cell chronic lymphocytic leukemia but not in normal circulating hematopoietic cells. Clin Cancer Res. 2007;13:4920–4927. doi: 10.1158/1078-0432.CCR-07-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsui T, Fischer I, Shumsky JS, Murray M. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp Neurol. 2005;194:410–431. doi: 10.1016/j.expneurol.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Mitsui T, Shumsky JS, Lepore AC, Murray M, Fischer I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005;25:9624–9636. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Momose T, Okubo Y, Horie S, Suzuki J, Isobe M, Sekiguchi M. Effects of intracellular cyclic AMP modulators on human eosinophil survival, degranulation and CD11b expression. Int Arch Allergy Immunol. 1998;117:138–145. doi: 10.1159/000024001. [DOI] [PubMed] [Google Scholar]
- 40.Moon EY, Lerner A. PDE4 inhibitors activate a mitochondrial apoptotic pathway in chronic lymphocytic leukemia cells that is regulated by protein phosphatase 2A. Blood. 2003;101:4122–4130. doi: 10.1182/blood-2002-10-3208. [DOI] [PubMed] [Google Scholar]
- 41.Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Namiki J, Tator CH. Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J Neuropathol Exp Neurol. 1999;58:489–498. doi: 10.1097/00005072-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noble M, Proschel C, Mayer-Proschel M. Getting a GR(i)P on oligodendrocyte development. Dev Biol. 2004;265:33–52. doi: 10.1016/j.ydbio.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Noreau L, Proulx P, Gagnon L, Drolet M, Laramee MT. Secondary impairments after spinal cord injury: a population-based study. Am J Phys Med Rehabil. 2000;79:526–535. doi: 10.1097/00002060-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Nout YS, Bresnahan JC, Culp E, Tovar CA, Beattie MS, Schmidt MH. Novel technique for monitoring micturition and sexual function in male rats using telemetry. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1359–1367. doi: 10.1152/ajpregu.00532.2006. [DOI] [PubMed] [Google Scholar]
- 47.Nout YS, Schmidt MH, Tovar CA, Culp E, Beattie MS, Bresnahan JC. Telemetric monitoring of corpus spongiosum penis pressure in conscious rats for assessment of micturition and sexual function following spinal cord contusion injury. J Neurotrauma. 2005;22:429–441. doi: 10.1089/neu.2005.22.429. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa R, Streiff MB, Bugayenko A, Kato GJ. Inhibition of PDE4 phosphodiesterase activity induces growth suppression, apoptosis, glucocorticoid sensitivity, p53, and p21(WAF1/CIP1) proteins in human acute lymphoblastic leukemia cells. Blood. 2002;99:3390–3397. doi: 10.1182/blood.v99.9.3390. [DOI] [PubMed] [Google Scholar]
- 49.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 50.Peters JH, Borner T, Ruppert J. Accessory phenotype and function of macrophages induced by cyclic adenosine monophosphate. Int Immunol. 1990;2:1195–1202. doi: 10.1093/intimm/2.12.1195. [DOI] [PubMed] [Google Scholar]
- 51.Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 52.Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt MH, Valatx JL, Sakai K, Debilly G, Jouvet M. Corpus spongiosum penis pressure and perineal muscle activity during reflexive erections in the rat. Am J Physiol. 1995;269:R904–913. doi: 10.1152/ajpregu.1995.269.4.R904. [DOI] [PubMed] [Google Scholar]
- 55.Siegmund B, Welsch J, Loher F, Meinhardt G, Emmerich B, Endres S, Eigler A. Phosphodiesterase type 4 inhibitor suppresses expression of anti-apoptotic members of the Bcl-2 family in B-CLL cells and induces caspase-dependent apoptosis. Leukemia. 2001;15:1564–1571. doi: 10.1038/sj.leu.2402232. [DOI] [PubMed] [Google Scholar]
- 56.Spencer T, Filbin MT. A role for cAMP in regeneration of the adult mammalian CNS. J Anat. 2004;204:49–55. doi: 10.1111/j.1469-7580.2004.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeuchi M, Tatsumi Y, Kitaichi K, Baba K, Suzuki R, Shibata E, Takagi K, Miyamoto K, Hasegawa T. Selective phosphodiesterase type 4 inhibitors reduce the prolonged survival of eosinophils stimulated by granulocyte-macrophage colony-stimulating factor. Biol Pharm Bull. 2002;25:184–187. doi: 10.1248/bpb.25.184. [DOI] [PubMed] [Google Scholar]
- 58.Temeltas G, Dagci T, Evren V, Lekili M. Effects of Neuronal and Glial Restricted Precursor Cells Transplantation on Erectile Function after Experimentally Induced Spinal Cord Injury. J Sex Med. 2009 doi: 10.1111/j.1743-6109.2009.01376.x. [DOI] [PubMed] [Google Scholar]
- 59.Temeltas G, Dagci T, Kurt F, Evren V, Tuglu I. Bladder function recovery in rats with traumatic spinal cord injury after transplantation of neuronal-glial restricted precursors or bone marrow stromal cells. J Urol. 2009;181:2774–2779. doi: 10.1016/j.juro.2009.01.093. [DOI] [PubMed] [Google Scholar]
- 60.Tiwari S, Dong H, Kim EJ, Weintraub L, Epstein PM, Lerner A. Type 4 cAMP phosphodiesterase (PDE4) inhibitors augment glucocorticoid-mediated apoptosis in B cell chronic lymphocytic leukemia (B-CLL) in the absence of exogenous adenylyl cyclase stimulation. Biochem Pharmacol. 2005;69:473–483. doi: 10.1016/j.bcp.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Vaquero J, Ramiro MJ, Oya S, Cabezudo JM. Ependymal reaction after experimental spinal cord injury. Acta Neurochir (Wien) 1981;55:295–302. doi: 10.1007/BF01808445. [DOI] [PubMed] [Google Scholar]
- 62.Wallace MC, Tator CH, Lewis AJ. Chronic regenerative changes in the spinal cord after cord compression injury in rats. Surg Neurol. 1987;27:209–219. doi: 10.1016/0090-3019(87)90031-0. [DOI] [PubMed] [Google Scholar]
- 63.Wang CH, Lin HC, Lin CH, Yu CT, Liu SL, Huang KH, Chung KF, Kuo HP. Effect of theophylline and specific phosphodiesterase IV inhibition on proliferation and apoptosis of progenitor cells in bronchial asthma. Br J Pharmacol. 2003;138:1147–1155. doi: 10.1038/sj.bjp.0705131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang HM, Zheng NG, Wu JL, Gong CC, Wang YL. Dual effects of 8-Br-cAMP on differentiation and apoptosis of human esophageal cancer cell line Eca-109. World J Gastroenterol. 2005;11:6538–6542. doi: 10.3748/wjg.v11.i41.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W, Masu K, Tamura G, Suzuki K, Ohwada K, Okuyama K, Shirato K, Takayanagi M, Ohno I. Inhibition of eosinophil survival by a selective inhibitor of phosphodiesterase 4 via the induction of apoptosis. Biol Pharm Bull. 2005;28:515–519. doi: 10.1248/bpb.28.515. [DOI] [PubMed] [Google Scholar]
- 66.Whitaker CM, Beaumont E, Wells MJ, Magnuson DS, Hetman M, Onifer SM. Rolipram attenuates acute oligodendrocyte death in the adult rat ventrolateral funiculus following contusive cervical spinal cord injury. Neurosci Lett. 2008;438:200–204. doi: 10.1016/j.neulet.2008.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 2001;7:387–398. doi: 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]