Abstract
Importance of the field
Mesenchymal adult stem cells have properties that make them attractive for use in tissue engineering and regenerative medicine. They are inherently plastic, enabling them to differentiate along different lineages, and promote wound healing and regeneration of surrounding tissues by modulating immune and inflammatory responses, promoting angiogenesis and secreting other trophic factors. Unlike embryonic stem cells, clinical uses of mesenchymal stem cells are not encumbered by ethical considerations or legal restrictions.
Areas covered in this review
We discuss skeletal muscle as a source of mesenchymal stem and progenitor cells by reviewing their biology and current applications in tissue engineering and regenerative medicine. This paper covers literature from the last 5 – 10 years.
What the reader will gain
Skeletal muscle is a plentiful source of mesenchymal stem and progenitor cells. This tissue may be obtained via routine biopsy or collection after surgical debridement. We describe the biology of these cells and provide an overview of therapeutic applications currently being developed to take advantage of their regenerative properties.
Take home message
There is potential for stem and progenitor cells derived from skeletal muscle to be incorporated in clinical interventions, either as a cellular therapy to modify the natural history of disease or as a component of engineered tissue constructs that can replace diseased or damaged tissues.
Keywords: MPCs, MSCs, regenerative medicine, skeletal muscle stem cells, tissue engineering
1. The promise of mesenchymal stem and progenitor cells
Mesenchymal stem cells (MSCs) are a population of adult stem cells with many properties that make them attractive for use in the fields of tissue engineering and regenerative medicine [1–4]. These cells are inherently plastic, enabling them to differentiate along different lineages under the appropriate induction conditions. They also appear to exhibit a number of trophic properties that promote regeneration in the surrounding tissue [5]. MSCs can be harvested from a variety of adult tissues, and unlike embryonic stem cells, their use is not encumbered by ethical considerations or legal restrictions. As a result, there is considerable hope that MSCs will be incorporated in a variety of clinical interventions, either as a cellular therapy to modify the natural history of diseases or as a component of engineered tissue constructs that can replace diseased or damaged tissues. This paper reviews skeletal muscle as a potential source of MSCs, highlights a variety of current applications for muscle-derived MSCs, and suggests some potential advantages of harvesting MSCs from skeletal muscle.
MSCs were originally discovered as adherent, fibroblastic cells in bone marrow aspirates that were capable of multilineage differentiation [6,7]. Following this discovery, the differentiation capacity of MSCs has been verified in vivo by inducing the cells to form ectopic bone [7]. Given their ability to differentiate, an obvious application for the cells was to incorporate them into a suitable biomaterial scaffold that would encourage them to form tissues de novo, either in vivo or in vitro [8,9]. In the last decade, there have been substantial developments in the field of tissue engineering [10,11]. Although it is not yet possible to grow replacement tissues and organs in the laboratory for widespread clinical use, MSCs continue to be considered as an important component in a variety of tissue engineering applications.
More recently, it has been noted that the regenerative benefit of MSCs does not appear to correlate solely or directly with their ability to differentiate into the diseased tissue type [12]. In a number of studies, MSCs have been injected into diseased tissue, such as the heart [13,14] and brain [15,16]. Although there was functional improvement to these tissues following MSC injection, there was little evidence of the MSCs differentiating into the surrounding cell types, as was expected. Instead, these studies have led to the discovery that MSCs promote wound healing and regeneration in the surrounding tissues by modulating the local inflammatory responses [17,18] and by limiting fibrosis of the functional tissues [19]. It has since been shown that MSCs also promote angiogenesis and secrete trophic (i.e., pro-growth and pro-survival) factors that augment the endogenous regeneration process [20,21]. Although substantial investigation is still needed to elucidate fully the mechanisms of their trophic behavior, MSCs are an important cell type used in many strategies for cell-based therapy.
Despite the potentially far-reaching promise of MSCs in many aspects of regenerative medicine and tissue engineering, any approaches using these cells are limited by the availability of a suitable MSC population in a clinical setting. The most common source of MSCs for clinical use is the bone marrow. However, the low concentration of MSCs in the bone marrow necessitates the application of specialized equipment to concentrate the MSCs prior to use [22], and may still yield too few cells for many tissue engineering applications [23]. Furthermore, in many countries, including the USA, there is a substantial regulatory burden associated with ex vivo expansion and maintenance of these cells for clinical use [24]. Similar limitations also exist for MSCs derived from adipose tissue, which is another commonly used source of MSCs [25]. As a result, there is considerable research into alternative sources of MSCs that may be able to overcome these clinical limitations. This review will focus on skeletal muscle as an alternative source of MSCs and on the potential clinical advantages of using the cells derived from this source.
2. Skeletal muscle-derived mesenchymal stem and progenitor cells
MSCs were first identified in the adherent fraction of cells in a bone marrow aspirate when it was observed that they can be induced to differentiate into osteoblasts and adipocytes in vitro [26]. Although these are important characteristics of MSCs, there is no definitive, agreed-upon marker to positively identify a population that is capable of these functions [27]. Instead, there is a set of three minimal criteria that must be met by an MSC population, which are designed to encourage consistency between investigators [28]: i) the population must be adherent to tissue culture plastic (TCP) and capable of in vitro expansion on TCP; ii) the cell surface epitope profile of the population must meet specific requirements (Table 1) to ensure a uniform cell type and minimal contamination by leukocytes and hematopoietic progenitor cells; and iii) the cells must be capable of differentiating into osteoblasts, adipocytes and chondrocytes in vitro. These criteria were developed for cell populations harvested from the bone marrow, and although applicable to MSCs harvested from other tissues, additional tissue-specific criteria may also apply to these other MSC populations.
Table 1.
Required cell surface epitope profile for a population of MSCs [28].
| Surface marker | Protein name | Indicator for HSC* |
|---|---|---|
| ≥95% of the cell population must be positive for | ||
| CD73 | ecto-5′-nucleotidase (SH3 and SH4) | Non-specific |
| CD90 | thy-1 | Non-specific |
| CD105 | endoglein (SH2) | Non-specific |
| ≤98% of the cell population must be negative for | ||
| CD34 | Unknown antigen | Primitive hematopoietic progenitors and endothelial cells |
| CD45 | Protein tyrosine phosphatase receptor type C | Pan-leukocyte marker |
| CD14 or CD11b‡ | Unknown antigen or integrin α M | Monocytes and macrophages |
| CD79α or CD19‡ | Immunoglobulin-associated α or unknown antigen | B-cells |
| HLA-DR§ | MHC class II (heterodimer) | Immunologically simulated cells |
HSC: Hematopoietic stem cell.
Either marker is sufficient for the given requirement.
Cells positive for HLA-DR may still be considered MSCs, but this marker indicates that they have been stimulated (e.g., by IFN-γ), which should be indicated when describing the cell type (i.e., as ‘stimulated’ MSCs).
The proteins associated with CD34, CD19 and CD19 are currently unknown.
In addition to bone marrow aspirate, MSCs have been isolated from a variety of other adult tissues. In particular, the discovery of MSCs in adipose tissue aspirates was exciting from a clinical standpoint because it provided an additional source of tissue that is clinically available and can be processed to yield MSCs [25,29]. Adipose-tissue-derived MSCs could also yield a viable allogeneic source by harvesting cells from tissue collected during liposuction procedures [30]. MSCs have been isolated from other connective tissues, such as the marrow space of long bones [31,32], trabecular bone chips [33–35], periosteum [36,37], synovial fluid [38–40], periodontal ligament [41,42], palatine tonsil [43], parathyroid gland [44] and fallopian tube [45]. Cells derived from these tissues are of scientific interest; however, due to the limited clinical availability of these tissues, applications for their use as a source of cells for biological therapy are not obvious. Finally, MSCs have been harvested from tissues that are lost as a result of development, such as umbilical cord [46,47], umbilical cord blood/Wharton’s Jelly [48,49] and primary tooth dental pulp [50,51], and these have been identified as potential sources of MSCs for cell banking [18].
Recently, there have been several reports of harvesting human MSC-like cells from adult skeletal muscle. The muscle tissue used to harvest the cells was obtained from healthy muscle tissue biopsies [52], surgical waste tissue from orthopaedic reconstructions ([53] and unpublished observations: Nesti et al.), or surgically debrided muscle tissue following orthopaedic trauma [53]. Given that these cells can be obtained from surgical waste tissue or with minimally invasive biopsy procedures, there is growing evidence that skeletal muscle may be an important clinical source of MSCs for use in therapeutic applications. The purpose of this review is to highlight the work that has been done to characterize the muscle-derived MSCs and the use of these cells in tissue engineering and regenerative medicine.
3. Origins of stem and progenitor cells in muscle tissue
Several cell populations with the properties of mesenchymal stem cells have been previously isolated from the skeletal muscle. One of the best characterized populations are the muscle derived stem cells (MDSCs) harvested from murine skeletal muscle [54,55]. MDSCs are isolated from the muscle homogenate using a pre-plating technique, which enriches the population of MDSCs by eliminating the contaminating populations of more adherent cell types [56]. The more slowly adherent MDSCs have demonstrated enhanced differentiation potential, and can be readily induced to become osteoblasts, adipocytes and chondrocytes in vitro. An additional feature of this cell type is their ability to differentiate into myoblasts in vitro and to promote muscle regeneration in vivo [57].
Interestingly, this population of MDSCs has not been harvested from human muscle tissue solely on the basis of their adhesion characteristics. Instead, a population of cells with similar in vitro characteristics has been identified in human skeletal muscle using FACS to isolate the cells that are positive for CD34, CD56 and CD144 [52,58]. These cells, called myoendothelial cells, express surface markers of both endothelial (CD34 and CD144) and satellite cells (CD56), which are skeletal muscle stem cells. Typically, satellite cells are quiescent myoblast precursors that lie adjacent to the myofibers beneath the basal lamina, and divide asymmetrically in response to muscle injury [59]. Although most satellite cells are committed to the myogenic lineage, the small subset of satellite cells that co-express the endothelial makers (less than 0.5% of all satellite cells) are associated with the vasculature in vivo and capable of osteogenic, adipogenic and osteogenic differentiation [52]. Myoendothelial cells appear to have enhanced myogenic potential in vivo compared with satellite cells. There is also evidence that these cells can be induced to differentiate into endothelial cells under the appropriate conditions. However, these are not the only multipotent stem cell type in skeletal muscle with an anatomical affiliation to the vasculature.
Pericytes are cells intimately associated with capillaries and microvessels. They play several roles in the maintenance of the vasculature [60], and recent experiments suggest that these cells may have other important functions for tissue regeneration. Isolated pericytes are capable of regenerating muscle tissue in vivo [61], and they can differentiate into myocytes, osteoblasts, adipocytes and chondrocytes in vitro [62,63]. Given that pericytes are present in almost every tissue in the body, it has been suggested that MSCs harvested from various tissues were in fact pericytes, or a similar cell type, which originated in the vasculature of those tissues [64]. Recently, there has been compelling evidence to support this hypothesis by the demonstration that a sub-population of pericytes express the markers used to identify MSCs and exhibit the in vitro differentiation characteristics of MSC populations [65]. Based on these observations, it has been proposed that pericytes serve as a reservoir of multipotent cells that can be recruited from the vasculature as needed to repair the tissue in response to injury.
Another distinct population of multipotent cells has been harvested from skeletal muscle following traumatic injury [53]. One important distinction that has been made about the traumatized-muscle-derived progenitor cells is that they are rapidly adherent during the harvesting procedure, as opposed to the MDSCs, which are selected on the basis of their slow adherence. The MPCs are also present in substantial numbers at the time of harvest. Multipotent cells in uninjured muscle are rare, whereas approximately one million cells per gram of tissue can be isolated from injured muscle [66]. The debrided muscle tissue from which MPCs are harvested was in the process of wound healing and tissue remodeling in response to a traumatic injury [67]. These observations support the hypothesis that multipotent cells are recruited from their niche following injury, and that they proliferate in the tissue to fulfill their regenerative function [64,68]. As a result, the cells harvested from traumatized muscle are referred to as mesenchymal progenitor cells (MPCs) to indicate that these cells may not have been in a quiescent, stem cell state when they were harvested (Figure 1). However, except that they are available in greater numbers in the tissue, there do not appear to be any major differences between the traumatized-muscle-derived MPCs and a typical MSC population [53,66].
Figure 1. Origin and characteristics of muscle-derived MSCs.
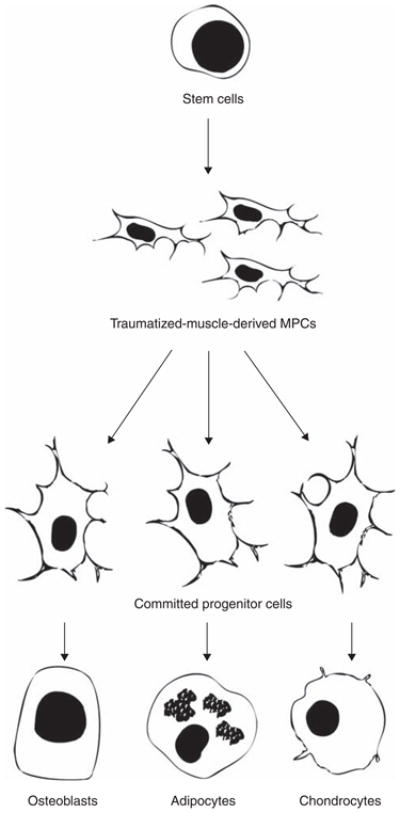
Traumatized-muscle-derived MPCs are undifferentiated progenitor cells that have been activated in response to the injury and are proliferating in the tissue. These cells have not yet terminally differentiated and maintain the ability to differentiate into osteoblasts, adipocytes or chondrocytes.
Traumatized muscle-derived MPCs also exhibit several of the trophic properties that are associated with MSCs. In vitro studies have shown that MPCs can modulate local inflammatory responses [69], promote angiogenesis by increasing the rate of endothelial cell proliferation and inhibit apoptosis of nearby cells [70,71]. The MPCs also appear to be intrinsic to severely injured muscle that has compromised tissue architecture [67]. These observations suggest that MPCs play a role in promoting the functional regeneration of skeletal muscle following traumatic injury. By providing a biochemical environment that favors tissue growth, the MPCs could augment the ability of committed myogenic progenitor cells to remodel the tissue and generate new skeletal muscle.
The origin of these proliferating cells is not currently known, although it is possible that the population of pericytes serves as a cellular reservoir for MPCs as well as the myoendothelial and satellite cell types. A model for pericyte recruitment can be constructed in which the local tissue architecture determines the pericyte fate (Figure 2). In response to minor, routine muscle damage, pericytes may be recruited from their vascular niche and travel along the architecture of the skeletal muscle to the site of muscle damage where they can augment satellite cell regeneration of the myofibers and may differentiate into satellite cells after the myofiber has been repaired [52]. Alternatively, following severe injury, the architecture of the skeletal muscle may be disrupted such that the recruited pericytes have no orientation in the tissue. In this case, they are free to proliferate in the cellular milieu, which includes inflammatory leukocytes and other wound healing cell types (e.g., fibroblasts and myofibroblasts), and assume the MPC phenotype. According to this model, skeletal muscle contains several stem cell types, which can respond to the severity of injury and repair minor muscle damage or remain as a more plastic stem cell type to promote regeneration following major tissue damage. The distinctions made between the MDSCs, myoendothelial cells, pericytes and traumatized-muscled-derived MPCs are based on their in vitro characteristics and species of origin, which are summarized in Table 2.
Figure 2. Functional characteristics of pericytes.
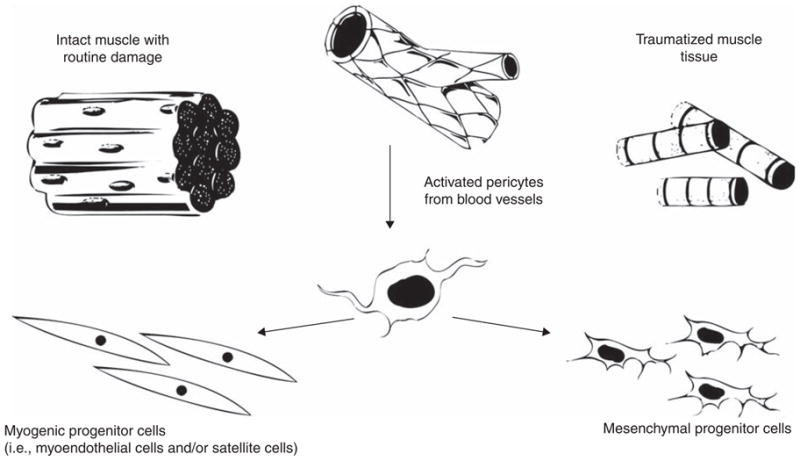
Pericytes may respond to injury by leaving their vascular niche and migrating into the damaged muscle tissue. In an environment where there is sufficient muscle fiber architecture, they will retain a muscle stem cell phenotype. However, if the tissue damage is extensive, the pericytes may not have sufficient environmental signals and will instead display a functional phenotype that resembles that of MSCs or traumatized-muscle-derived MPCs.
Table 2.
Mesenchymal stem and progenitor cells identified in skeletal muscle tissue.
| Cell type | Species |
In vitro differentiation* |
Trophic? | |||||
|---|---|---|---|---|---|---|---|---|
| OB | A | C | EC | MB | N | |||
| Muscle derived stem cells (MDSCs) | Mouse | + | + | + | + | + | + | Yes |
| Myoendothelial cells | Human | + | + | + | + | + | Yes | |
| Pericytes | Various (including mouse and human) | + | + | + | + | Yes | ||
| Mesenchymal progenitor cells (MPCs) | Human (traumatized muscle) | + | + | + | + | Yes | ||
A: Adipocytes; C: Chondrocytes; EC: Endothelial cells; MB: Myoblasts; N: Cell types with neurogenic characteristics; OB: Osteoblasts.
4. Muscle-derived MSCs and MPCs for tissue engineering
Muscle-derived stem cells have been used in various tissue engineering strategies to generate replacement structures for tissue that has damaged or diseased. The general approach to tissue engineering is to design a scaffold to support a stem cell population, and frequently at least one biochemical factor is used to promote and guide differentiation of the MSCs into the desired cell type. The engineered device can be implanted to replace the damaged tissue of the host, and over time, it will be remodeled and incorporated into the adjacent tissue. There has been exhaustive work to develop such tissue engineered devices using MSCs derived from bone marrow (reviewed in [1,32]), adipose tissue (reviewed in [72]) and other tissues, but recent work suggests that muscle stem cells could be an alternative cell source for several of these engineered tissues.
4.1 Bone
Following a complex fracture or traumatic injury, a gap or segmental defect may occur in the bone. A segmental defect can also be generated during the repair of a non-union fracture or when a tumor must be removed from the bone. Once formed, surgeons have limited options to repair the bone to its original length. A tissue engineered bone graft would provide an optimal solution for repairing the segmental defect.
Several populations of muscle-derived stem cells have osteogenic potential, including MDSCs [55], MPCs [53], pericytes [65] and myoendothelial cells [52]. Considerable work has been done to determine the optimal conditions for the MDSC cell type to generate bone tissue in vivo [73,74]. By pre-transfecting MDSCs with the gene for bone morphogenetic protein-4 (BMP-4), the MDSCs produced this osteoinductive factor in vivo upon implantation and then differentiated into osteoblasts [75]. When these cells were loaded onto an implantable scaffold, the MDSCs were able to fill a critical sized femoral defect with bone in a rat model. MDSCs expressing BMP-4 have also been seeded in both collagen and gelatin sponges and were sufficient to repair a critical sized calvarial defect in mice [73,76]. These cells are being evaluated for use with fibrin and collagen gels to fill bone defects in the craniofacial skeleton [77].
4.2 Articular cartilage
Osteoarthritis is a disease characterized by degeneration of the joint articular cartilage. Once a defect is initiated in the joint surface, for example as a result of trauma or degenerative joint disease such as osteoarthritis, the damaged articular cartilage cannot be regenerated by endogenous repair mechanisms [78]. The damaged region can be surgically removed to alleviate pain associated with the disease, but this procedure results in a reduction in the articular surface. Substantial effort has been made to tissue engineer a cartilage plug, which could replace the damaged tissue and restore full function to the joint [79].
MDSCs, pericytes and traumatized-muscle-derived MPCs have chondrogenic potential and might be applicable for the engineering of a cartilage tissue construct. The ability of these cell types to differentiate into chondrocytes has been evaluated based on their ability to express collagen type II and an extracellular matrix rich in sulfated proteoglycans [62,66,80]. Furthermore, MDSCs transfected with the gene for BMP-4 adopt a chondrogenic phenotype in vitro. Implantation of these cells into a full thickness osteochondral defect in the articular cartilage of rat knees using fibrin glue resulted in persistent repair at the defect site after 24 weeks [81]. In a similar experiment, MDSCs were seeded in bovine collagen type I gels [82], and after culturing for 3 weeks in vitro, the constructs were implanted into full thickness osteochondral defects in the knees of New Zealand white rabbits. After 24 weeks, the implanted MDSCs provided improved regeneration of the articular surface, based on expression of collagen type II and construct integration, compared with control constructs containing chondrocytes. These experiments demonstrate the potential of muscle-derived stem and progenitor cells for cartilage tissue engineering.
5. Muscle-derived MSCs and MPCs as trophic mediators of tissue regeneration
An alternative therapeutic approach using muscle-derived MSCs takes advantage of their trophic properties to enhance the endogenous mechanism of regeneration [83]. For this strategy, it is not necessary for the muscle-derived cells to differentiate in order for tissue repair to occur. Instead, muscle-derived cells will act to promote the surrounding cells to regenerate the desired tissue, and thus modify the natural history of some disease mechanisms. Although similar studies have been performed in MSCs harvested from other tissues (reviewed in [84–87]), there are several applications for tissue regeneration using muscle-derived MSCs and MPCs.
5.1 Bone and cartilage
An alternative approach to regenerating bone tissue with mesenchymal stem and progenitor cells may not require the differentiation of these cells into osteoblasts. In a recent study, co-transfection of MDSCs with the gene coding for BMP-4 and the gene for VEGF led to enhanced endochondral ossification to form bone tissue compared to MDSCs that were transfected with only BMP-4 [74]. VEGF is an important mediator of fracture healing [88], and the synergistic interaction of VEGF with BMP-4 appeared to enable greater recruitment of local MSCs from the bone marrow, promoted angiogenesis of the regenerating tissue and accelerated the process of cartilage tissue resorption [89]. A similar synergistic relationship has been observed in terms of VEGF and BMP-2 expression. MPCs derived from traumatized muscle tissue express high levels of VEGF [70], suggesting that they could provide a similar regenerative benefit to regenerating bone tissue without the need for gene transfection prior to implantation. These cells could be implanted on a scaffold to promote conduction of endogenous MSCs into a segmental defect, or the cells could be injected directly into a fracture callus to improve the rate of healing and reduce the likelihood of a non-union [90]. A similar approach has been used to promote chondrogenesis in a murine model of osteoarthritis by intra-articular injection of MDSCs expressing BMP-4 and VEGF [91].
5.2 Nerve
The trophic properties of muscle-derived stem and progenitor cells may also be useful to promote peripheral nerve regeneration. Peripheral nerve trauma frequently accompanies orthopaedic trauma, and may result in a segmental defect of the nerve that must be bridged to restore function to the distal extremities. The process of nerve regeneration is typically mediated by neurotrophic factors (NTFs) that are expressed by Schwann cells, which are the support cells in peripheral nerves [92]. MPCs derived from traumatized muscle also express many of the NTFs that are important to nerve regeneration, such as brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), ciliary neurotrophic factor (CNTF) and neurotrophin-3 (NT-3), and the factors secreted by the MPCs are sufficient to enhance the rate of axon growth in vitro [93]. The ability of these cells to promote nerve regeneration in vitro is currently being evaluated using a rat model of sciatic nerve injury. Similarly, MDSCs seeded in a silicone nerve guide have been shown to augment sciatic nerve regeneration similar to a population of neural progenitor cells in nude mice [94].
5.3 Skeletal/cardiac muscle
An obvious therapeutic application for muscle-derived stem and progenitor cells is to promote the regeneration of skeletal muscle. Skeletal muscle has substantial capacity to regenerate itself by activating the reservoir of muscle-specific stem cells and other mechanisms discussed earlier in this review. However, following severe injury in the muscle tissue, the regenerative activities of the satellite cells are in competition with the pro-fibrotic activities of fibroblasts that also proliferate in the region of injury [95,96]. One factor that determines which cell type can dominate the wound healing process is the ratio of TGF-β1 to TGF-β3 [97]. When this ratio is high, the excess amount of TGF-β1 stimulates the fibroblasts to secrete disorganized extracellular matrix proteins and leads to fibrosis in the tissue [98,99]. The fibrotic tissue physically impedes the ability of the satellite cells to repair the muscle tissue.
There have been several studies supporting the possibility that muscle-derived stem cells can augment wound healing following injury. MPCs derived from traumatized muscle express high levels of TGF-β3 (unpublished observations: Jackson et al.), which may help maintain a balance of cytokines to promote muscle regeneration. Furthermore, using models of skeletal muscle injury in mice, treatment with MDSCs results in improved muscle regeneration, which is attributed to ability of the MDSCs to recruit capillaries and nerves into the region of muscle regeneration [100]. The MDSCs can also differentiate directly into endothelial cells and cell types with neuronal characteristics [55]. As a result, treatment with MDSCs results in superior muscle regeneration compared with treatment with satellite cells. MDSCs may also provide some benefit to patients suffering from Duchenne Muscular Dystrophy, as mdx mice, which are a model of dystrophic muscle, exhibit an improved muscle structure following treatment with MDSC [101].
Stem and progenitor cells from skeletal muscle may also promote the regeneration of cardiac muscle tissue [102,103]. Ischemic or infarcted tissue that results from a coronary episode can result in a reduction in cardiac function. Injection of MDSCs into the infarcted region of cardiac tissue resulted in improved function compared with injection with myoblasts [104]. The improvement in function was attributed to enhanced engraftment of the injected MDSCs and fusion of these cells with the host cardiomyocytes [105]. Furthermore, there was evidence that MDSCs mediated a secondary mechanism of regeneration by promoting angiogenesis in the tissue [106]. Similar results have been observed after injection of myoendothelial cells into infarcted myocardium [107]. As a result, MDSCs and myoendothelial cells are a promising candidate cell type for cell transplantation therapies to improve cardiac function following infarction.
5.4 Other tissues
There are two other notable examples of therapeutic applications that are based on the trophic properties of muscle-derived stem and progenitor cells. First, muscle-derived stem and progenitor cells are currently being used in vascular regeneration. MDSCs seeded on a compound polyester urethane urea scaffold have been used to promote the proliferation and migration of smooth muscle and vascular endothelial cells into the luminal space of the construct in vivo [108]. As a result, regenerating blood vessels that were exposed to MDSC trophic factors exhibited greater patency, less fibrosis and greater mechanical strength than the acellular controls. MPCs derived from traumatized muscle also express many of the growth factors associated with MSC-mediated maintenance of vascular endothelial cells [70]. MPCs can also promote the proliferation of endothelial cells in vitro, and these cells are currently being vetted for use in a vascular graft device.
A variety of MSC populations have also been used to correct urological disorders (reviewed in [109]), including those derived from skeletal muscle tissue. MDSCs have been used as a cellular therapy to overcome stress urinary incontinence. In this disorder, a weakening of the urethral sphincter results in involuntary urine release as a result of increased intra-abdominal pressure. Using an animal model of stress urinary incontinence, periurethral injection of MDSC resulted in improved urological function [110]. In addition to differentiation into smooth muscle to enhance contractility in the sphincter muscle, these cells also appeared to promote innervation of the myofibers, which provided further enhancement to the urethral sphincter function [111]. MDSCs seeded on intestinal submucosa and implanted into a rat model to have also been shown to promote the repair of vaginal tissue in vivo [112].
6. Expert opinion
In recent years, skeletal muscle has emerged as a promising tissue source for mesenchymal stem and progenitor cells that can be used in a variety of therapeutic applications. Skeletal muscle is one of the most plentiful tissues in the body, accounting for approximately one third of body weight in a healthy individual [23]. The high capacity of muscle to repair itself after injury suggests that it serves as a reservoir for cells that participate in tissue regeneration processes [113]. Several research groups have characterized different muscle-derived stem cell populations that exhibit the ability to differentiate into multiple cell types, including osteoblasts, adipocytes, chondrocytes, myoblasts and endothelial cells. In addition, there is evidence that these cells exhibit the same trophic properties (i.e., pro-growth, anti-inflammation and anti-apoptotic) that are attributed to the pro-regeneration effects of bone marrow-derived MSCs. These cells, which can be obtained via minimally invasive muscle biopsy, may provide surgeons with clinically versatile populations of stem cells.
In addition to the stem cell populations that can be harvested from healthy muscle, it has also been reported that MPCs can be harvested from muscle tissue following traumatic injury. Harvesting traumatized-muscle-derived MPCs offers two substantial advantages over untraumatized tissue as a cell source. First, the MPCs can be harvested from tissues at the wound margins of an open fracture, which are surgically debrided as a part of the standard method of care for these injuries. Therefore, there is no need to perform additional procedures to acquire tissue for cell harvesting. Second, the MPCs are present in substantially greater numbers in the traumatized muscle compared with uninjured muscle tissue. It is speculated that the MPCs begin to proliferate (or migrate to the injury site) in response to the injury, and are thus harvested while they are still at the uncommitted progenitor cell state. As a result, the surgeon may have access to greater numbers of mesenchymal stem and progenitor cells for therapeutic applications without requiring a large mass of muscle tissue. A patient who has been exposed to substantial soft tissue damage and/or open fractures would be an ideal candidate for autologous traumatized-muscle-derived MPC therapy, as these cells could be used to regenerate the tissues in these wounds (e.g., bone, muscle, nerve and cartilage). Current research is directed towards identifying and characterizing the cell proliferation and stimulation factors that are associated with traumatic injury, with the goal of applying them to other populations of MSCs derived from uninjured muscle tissue.
One limitation to the use of muscle-derived stem and progenitor cells in therapeutic application is the lack of standards in the cell types that are currently being investigated. There are at least three distinct populations of cells harvested from human skeletal muscle (i.e., pericytes, myoendothelial cells and traumatized-muscle-derived MPCs) and one murine cell population (MDSC) that have the characteristics of an MSC population. Additionally, there are several other stem cell populations (i.e., mesoangioblasts [114], side population cells [115], and CD133+ cells [116]) that do not meet the requirements for MSCs, and were therefore not included in this review. Finally, there are muscle-specific stem cells (i.e., satellite cells and myoblasts) that are capable of only myogenic differentiation. Each of these cell types is characterized primarily on the basis of their in vitro characteristics after they have been harvested from the body. However, it is evident that the phenotype of these cells may shift in vivo as they migrate through different tissues and are exposed to different extracellular and environmental signals. While rudimentary models may be developed to describe the in vivo relationship among these stem cell populations (see Figure 2), substantial additional studies are needed to refine and verify these relationships. A better understanding of how the muscle-derived mesenchymal stem and progenitor cells are related will allow us to better predict the regenerative capabilities of cell populations that are harvested by differing methods.
There are two broad strategies for therapeutic applications using muscle-derived stem and progenitor cells (Figure 3). The first is a tissue engineering strategy, whereby the cells are loaded onto a construct (i.e., a scaffold) and induced to differentiate (using biochemical factors) into the appropriate cell type for the tissue that is being engineered. The second strategy takes advantage of the inherently trophic nature of the stem and progenitor cells to promote the endogenous mechanisms of tissue regeneration in vivo. While the promise of the former strategy to generate replacement organs and tissues has generated a great deal of investigation, the latter strategy may present more opportunities for clinical success in the immediate future.
Figure 3. Schematics of two general strategies in the application of muscle-derived mesenchymal stem and progenitor cells in regenerative medicine.
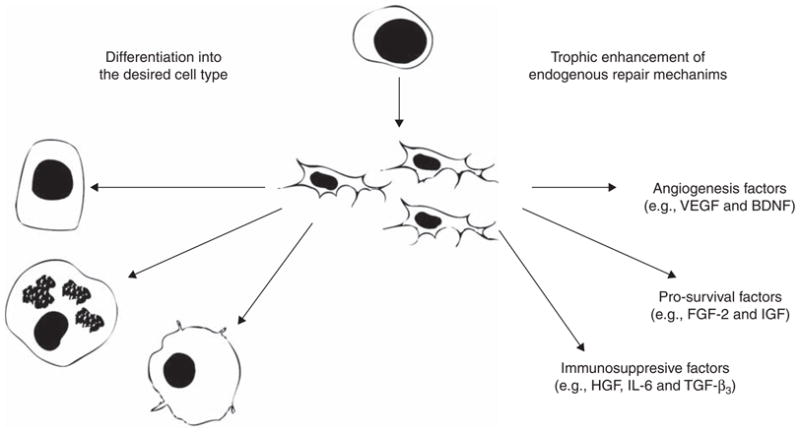
(1) The cells can be differentiated into a particular cell type that is needed to replace damaged tissues, or (2) the cells can be used as trophic mediators that will enhance endogenous tissue repair mechanisms.
There are several factors that limit the clinical success to the current approach of tissue engineering with muscle-derived stem cells. First, the majority of tissue engineering studies have been performed using the murine MDSC population, and the human cell type that is most similar to the MDSCs are the myoendothelial cells, which are rare in skeletal muscle. Substantial ex vivo expansion will be required to generate sufficient numbers of cells for tissue engineering applications. Second, in many of the tissue engineering studies using MDSC, the cells were transfected with genes that would promote differentiation prior to implantation. While this is an excellent system to study the cellular and molecular mechanisms involved in tissue engineering, whether the MDSCs are merely acting as gene delivery vehicles rather than as a stem cell population in the process should be taken into consideration [82]. Furthermore, the safety concerns about gene therapy approaches may limit direct application of these strategies to move from animal models towards clinical translation [117]. It is critical that the efficacy of these tissue engineering studies be tested using stem cell populations that can be harvested from human patients to facilitate the translation of these therapeutic applications into clinical use.
In comparison, the use of stem and progenitor cells as trophic mediators may present a more promising immediate future. In principle, this strategy requires substantially less development, as it chiefly takes advantage of the intrinsic trophic or pro-regenerative properties of the MSC populations. The regenerative benefits of several stem cell populations have already been demonstrated. In this review, we have described several applications using stem cells derived from human skeletal muscle to regenerate a variety of tissue types, many of which are nearing clinical translation. Taken together, these findings strongly illustrate the great potential of this approach for the development of therapeutic applications in the near-term, and underscore the critical importance of research efforts directed at elucidating and harnessing the regenerative properties of skeletal-muscle-derived stem and progenitor cells.
Article highlights.
Skeletal muscle is a reservoir for cells with properties that are similar to those of mesenchymal stem cells.
Several therapeutic cell types have been isolated from muscle tissue and are distinguished on the basis of their in vitro behavior. The relationship between these cells in vivo is unclear, but they are probably related to pericytes.
Mesenchymal stem and progenitor cells may be harvested from muscle tissue and used in a variety of therapeutic and regenerative medicine applications.
These cell types can be differentiated and delivered via seeding onto biomaterial scaffolds for tissue engineering applications designed to replace diseased or damaged tissues.
Muscle-derived mesenchymal stem and progenitor cells also secrete trophic factors that can promote and regulate endogenous mechanisms of tissue regeneration.
This box summarizes key points contained in the article.
Acknowledgments
Figures were produced using Servier Medical Art.
Footnotes
Declaration of interest
LJ Nesti has received funding from the Military Amputee Research Program #PO5-A011 and Comprehensive Neurosciences Program #CNP-2008-CR01. RS Tuan received NIH intramural Support (Z01 AR41131).
Bibliography
Papers of special note have been highlighted as either of interest
(•) or of considerable interest
(••) to readers.
- 1•.Chen F, Tuan R. Adult stem cells for cartilage tissue engineering and regeneration. Curr Rhematol Rev. 2008;4:149–54. This article provides a concise description of the general strategy for tissue engineering using MSCs through a focus on cartilage tissue engineering. [Google Scholar]
- 2.Patterson TE, Kumagai K, Griffith L, Muschler GF. Cellular strategies for enhancement of fracture repair. J Bone Joint Surg Am. 2008;90(Suppl 1):111–9. doi: 10.2106/JBJS.G.01572. [DOI] [PubMed] [Google Scholar]
- 3••.Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2007;15(2):109–16. doi: 10.1038/sj.gt.3303067. This review provides a broad overview of MSC biology and describes how these cells are currently being applied to clinical therapies. [DOI] [PubMed] [Google Scholar]
- 4.Nesselmann C, Ma N, Bieback K, et al. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008;12(5B):1795–810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7. doi: 10.1002/jcp.21200. This paper was the first to comprehesively review the trophic abilites of MSCs and to attribute the primary regenerative benefit of MSCs on these trophic properties. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein A, Piatetzky-Shapiro I, Petrakova K. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90. [PubMed] [Google Scholar]
- 7.Friedenstein A, Chailakhyan R, Gerasimov U. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–72. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–64. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 9.Tuan R, Boland G, Tuli R. Adult mesenchymal stem cells and cell based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Khademhosseini A, Vacanti JP, Langer R. How to grow new organs. Sci Am Mag. 2009;300(5):64–71. doi: 10.1038/scientificamerican0509-64. This article was written for the popular press by some of the pioneers in the field of tissue engineering. It provides a broad overview of the technological advances in the last decade and predicts how tissue engineering will make an impact on medicine in the future. [DOI] [PubMed] [Google Scholar]
- 11•.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8(6):457–70. doi: 10.1038/nmat2441. A synthesis of lessons learned from tissue engineering and a review of the ‘next generation’ tissue engineering devices featuring cell instructive materials. [DOI] [PubMed] [Google Scholar]
- 12.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 13.Shake J, Gruber P, Baumgartner W, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–26. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 14.Min JY, Sullivan MF, Yang Y, et al. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg. 2002;74(5):1568–75. doi: 10.1016/s0003-4975(02)03952-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–86. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–17. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 18•.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11(4):377–91. doi: 10.1080/14653240903080367. A comprehensive review of the immonosuppresive properties of MSCs and the clinical applications that have been designed to take advantage of their ability to modulate immune responses. [DOI] [PubMed] [Google Scholar]
- 19.Mias C, Lairez O, Trouche E, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27(11):2734–43. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 20.Tang YL, Zhao Q, Zhang YC, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117(1):3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Lozito TP, Kuo CK, Taboas JM, Tuan RS. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J Cell Biochem. 2009;107(4):714–22. doi: 10.1002/jcb.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muschler GF, Matsukura Y, Nitto H, et al. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res. 2005;(432):242–51. doi: 10.1097/01.blo.0000149812.32857.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Gates CB, Karthikeyan T, Fu F, Huard J. Regenerative medicine for the musculoskeletal system based on muscle-derived stem cells. J Am Acad Orthop Surg. 2008;16(2):68–76. doi: 10.5435/00124635-200802000-00004. This paper is a comprehesive review of the MDSC cell type and their use in gene therapy applications. [DOI] [PubMed] [Google Scholar]
- 24.Giordano A, Galderisi U, Marino I. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 25.Meliga E, Strem BM, Duckers HJ, Serruys PW. Adipose-derived cells. Cell Transplant. 2007;16(9):963–70. doi: 10.3727/096368907783338190. [DOI] [PubMed] [Google Scholar]
- 26.Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83–92. [PubMed] [Google Scholar]
- 27.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. This is a position paper that provides a rigorous definition of the generally accepted criteria for the identification MSC populations. [DOI] [PubMed] [Google Scholar]
- 29.Gimble JM, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362–9. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno H, Hyakusoku H. Mesengenic potential and future clinical perspective of human processed lipoaspirate cells. J Nippon Med Sch. 2003;70(4):300–6. doi: 10.1272/jnms.70.300. [DOI] [PubMed] [Google Scholar]
- 31.Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther. 2003;5(5):235–8. doi: 10.1186/ar991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo CK, Tuan RS. Tissue engineering with mesenchymal stem cells. IEEE Eng Med Biol Mag. 2003;22(5):51–6. doi: 10.1109/memb.2003.1256272. [DOI] [PubMed] [Google Scholar]
- 33.Song L, Young NJ, Webb NE, Tuan RS. Origin and characterization of multipotential mesenchymal stem cells derived from adult human trabecular bone. Stem Cells Dev. 2005;14(6):712–21. doi: 10.1089/scd.2005.14.712. [DOI] [PubMed] [Google Scholar]
- 34.Tuli R, Tuli S, Nandi S, et al. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21(6):681–93. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- 35.Noth U, Osyczka A, Tuli R, et al. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–9. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 36.Nakahara H, Dennis JE, Bruder SP, et al. In vitro differentiation of bone and hypertrophic cartilage from periosteal-derived cells. Exp Cell Res. 1991;195(2):492–503. doi: 10.1016/0014-4827(91)90401-f. [DOI] [PubMed] [Google Scholar]
- 37.Choi Y-S, Noh S-E, Lim S-M, et al. Multipotency and growth characteristic of periosteum-derived progenitor cells for chondrogenic, osteogenic, and adipogenic differentiation. Biotechnol Lett. 2008;30(4):593–601. doi: 10.1007/s10529-007-9584-2. [DOI] [PubMed] [Google Scholar]
- 38.Jones EA, English A, Henshaw K, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50(3):817–27. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 39.Vandenabeele F, De Bari C, Moreels M, et al. Morphological and immunocytochemical characterization of cultured fibroblast-like cells derived from adult human synovial membrane. Arch Histol Cytol. 2003;66(2):145–53. doi: 10.1679/aohc.66.145. [DOI] [PubMed] [Google Scholar]
- 40.Fan J, Varshney RR, Ren L, et al. Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng B. 2009;15(1):75–86. doi: 10.1089/ten.teb.2008.0586. [DOI] [PubMed] [Google Scholar]
- 41.Jo YY, Lee HJ, Kook SY, et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13(4):767–73. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 42.Gay IC, Chen S, Macdougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10(3):149–60. doi: 10.1111/j.1601-6343.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 43.Janjanin S, Djouad F, Shanti RM, et al. Human palatine tonsil: a new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis Res Ther. 2008;10(4):R83. doi: 10.1186/ar2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih YR, Kuo TK, Yang AH, et al. Isolation and characterization of stem cells from the human parathyroid gland. Cell Prolif. 2009;42(4):461–70. doi: 10.1111/j.1365-2184.2009.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jazedje T, Perin PM, Czeresnia CE, et al. Human fallopian tube: a new source of multipotent adult mesenchymal stem cells discarded in surgical procedures. J Transl Med. 2009;7:46. doi: 10.1186/1479-5876-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–92. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 47.Lee OK, Kuo TK, Chen W-M, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–75. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 48.Cetrulo CL., Jr Cord-blood mesenchymal stem cells and tissue engineering. Stem Cell Rev. 2006;2(2):163–8. doi: 10.1007/s12015-006-0023-x. [DOI] [PubMed] [Google Scholar]
- 49.Troyer DL, Weiss ML. Concise review: Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591–9. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang G, Sonoyama W, Liu Y, et al. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34(6):645–51. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Zheng B, Cao B, Crisan M, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25(9):1025–34. doi: 10.1038/nbt1334. This paper describes the identification and initial charaterization of myoendothelial cells. [DOI] [PubMed] [Google Scholar]
- 53•.Nesti LJ, Jackson WM, Shanti RM, et al. Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue. J Bone Joint Surg Am. 2008;90(11):2390–8. doi: 10.2106/JBJS.H.00049. This paper describes the identification and initial charaterization of traumatized-muscle-derived MPCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deasy BM, Huard J. Gene therapy and tissue engineering based on muscle-derived stem cells. Curr Opin Mol Ther. 2002;4(4):382–9. [PubMed] [Google Scholar]
- 55•.Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851–64. doi: 10.1083/jcb.200108150. This paper describes the identification and initial charaterization of MDSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gharaibeh B, Lu A, Tebbets J, et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protocol. 2008;3(9):1501–9. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 57.Cao B, Zheng B, Jankowski RJ, et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5(7):640–6. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 58.Crisan M, Deasy B, Gavina M, et al. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol. 2008;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. [DOI] [PubMed] [Google Scholar]
- 59.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441(7097):1080–6. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 60.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005;(94):115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 61.Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 62.Farrington-Rock C, Crofts NJ, Doherty MJ, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110(15):2226–32. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 63.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96(9):930–8. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 64.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3(3):229–30. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 65••.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. This paper describes the localization of MSC markers to the perivascular region and the identification of pericytes as the cells that exhibit the MSC phenotype in vitro. [DOI] [PubMed] [Google Scholar]
- 66.Jackson WM, Aragon AB, Djouad F, et al. Mesenchymal progenitor cells derived from traumatized human muscle. J Tissue Eng Regen Med. 2009;3(2):129–38. doi: 10.1002/term.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jackson WM, Aragon AB, Nesti LJ, Tuan RS. Putative heterotopic ossification progenitor cells derived from traumatized muscle. J Orthop Res. 2009;27(12):1645–51. doi: 10.1002/jor.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Djouad F, Jackson WM, Bobbick BE, et al. Activin A expression regulates multipotency of mesenchymal progenitor cells. Stem Cells Res Ther. doi: 10.1186/scrt11. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lozito T, Jackson WM, Tuan RS, Nesti LJ. Mesenchymal progenitor cells derived from traumatized muscle secrete factors that regulate angiogenesis. Trans Ortho Res Soc. 2010;56:739. [Google Scholar]
- 71.Jackson WM, Lozito TP, Djouad F, et al. Pro-regenerative functions of mesenchymal progenitor cells derived from traumatically injured muscle. Trans Ortho Res Soc. 2009;56:733. [Google Scholar]
- 72.Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76(2):56–66. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- 73.Lee JY, Qu-Petersen Z, Cao B, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150(5):1085–100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng H, Wright V, Usas A, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110(6):751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright V, Peng H, Usas A, et al. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6(2):169–78. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 76.Musgrave DS, Pruchnic R, Bosch P, et al. Human skeletal muscle cells in ex vivo gene therapy to deliver bone morphogenetic protein-2. J Bone Joint Surg Br. 2002;84(1):120–7. doi: 10.1302/0301-620x.84b1.11708. [DOI] [PubMed] [Google Scholar]
- 77.Usas A, Ho AM, Cooper GM, et al. Bone regeneration mediated by BMP4-expressing muscle-derived stem cells is affected by delivery system. Tissue Eng A. 2009;15(2):285–93. doi: 10.1089/ten.tea.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noth U, Steinert A, Tuan R. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371–80. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- 79.Chen F, Rousche K, Tuan R. Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–82. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto T, Kubo S, Meszaros LB, et al. The influence of sex on the chondrogenic potential of muscle-derived stem cells: implications for cartilage regeneration and repair. Arthritis Rheum. 2008;58(12):3809–19. doi: 10.1002/art.24125. [DOI] [PubMed] [Google Scholar]
- 81.Kuroda R, Usas A, Kubo S, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433–42. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 82.Adachi N, Sato K, Usas A, et al. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29:1920–30. [PubMed] [Google Scholar]
- 83.Caplan AI. New era of cell-based orthopaedic therapies. Tissue Eng B Rev. 2009;15(2):195–200. doi: 10.1089/ten.teb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84•.Patel S, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp. 2008;56(1):1–8. doi: 10.1007/s00005-008-0001-x. This paper reviews the immunological functions of MSCs and describes the role of these cells in tissue regeneration. [DOI] [PubMed] [Google Scholar]
- 85.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia-Castro J, Trigueros C, Madrenas J, et al. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J Cell Mol Med. 2008;12(6B):2552–65. doi: 10.1111/j.1582-4934.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Newman RE, Yoo D, Leroux MA, et al. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8(2):110–23. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 88.Wilkey JF, Buchberger G, Saucier K, et al. Cyclin D1 overexpression increases susceptibility to 4-nitroquinoline-1-oxide-induced dysplasia and neoplasia in murine squamous oral epithelium. Mol Carcinog. 2009;48(9):853–61. doi: 10.1002/mc.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng H, Usas A, Olshanski A, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20(11):2017–27. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 90.Tseng SS, Lee MA, Reddi AH. Nonunions and the potential of stem cells in fracture-healing. J Bone Joint Surg Am. 2008;90(Suppl 1):92–8. doi: 10.2106/JBJS.G.01192. [DOI] [PubMed] [Google Scholar]
- 91.Matsumoto T, Cooper GM, Gharaibeh B, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390–405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8(4):243–52. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Bulken-Hoover JD, Jackson WM, Ji Y, et al. Mechanisms to enhance the neurotrophic function of mesenchymal progenitor cells. Trans Ortho Res Soc. 2010;56:1627. [Google Scholar]
- 94.Koh JS, Lee JY, Lee JY. The effects of human muscle derived stem cells on the induction of peripheral nerve regeneration. Korean J Urol. 2008;49(4):350–9. [Google Scholar]
- 95.Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161(3):895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quintero AJ, Wright VJ, Fu FH, Huard J. Stem cells for the treatment of skeletal muscle injury. Clin Sports Med. 2009;28(1):1–11. doi: 10.1016/j.csm.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol. 2001;11(5):424–31. [PubMed] [Google Scholar]
- 98.Li Y, Foster W, Deasy BM, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164(3):1007–19. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu L, Saulis AS, Liu WR, et al. The temporal effects of anti-TGF-beta1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am Coll Surg. 2005;201(3):391–7. doi: 10.1016/j.jamcollsurg.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 100.Bedair HS, Karthikeyan T, Quintero A, et al. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008;36(8):1548–54. doi: 10.1177/0363546508315470. [DOI] [PubMed] [Google Scholar]
- 101.Deasy BM, Gharaibeh BM, Pollett JB, et al. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16(7):3323–33. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Itescu S, Schuster MD, Kocher AA. New directions in strategies using cell therapy for heart disease. J Mol Med. 2003;81(5):288–96. doi: 10.1007/s00109-003-0432-0. [DOI] [PubMed] [Google Scholar]
- 103.Clause KC, Tinney JP, Liu LJ, et al. A three-dimensional gel bioreactor for assessment of cardiomyocyte induction in skeletal muscle derived stem cells. Tissue Eng C Methods. 2009 doi: 10.1089/ten. TEC.2009.0098. published online 14 July 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haynesworth S, Goshima J, Goldberg V, Caplan A. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–8. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 105.Payne TR, Oshima H, Sakai T, et al. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12(16):1264–74. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- 106.Payne TR, Oshima H, Okada M, et al. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50(17):1677–84. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 107.Okada M, Payne TR, Zheng B, et al. Myogenic endothelial cells purified from human skeletal muscle improve cardiac function after transplantation into infarcted myocardium. J Am Coll Cardiol. 2008;52(23):1869–80. doi: 10.1016/j.jacc.2008.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nieponice A, Soletti L, Guan J, et al. In-vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng A. 2009 doi: 10.1089/ten.TEA.2009.0427. published online 6 November 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109•.Montzka K, Heidenreich A. Application of mesenchymal stromal cells in urological diseases. BJU Int. 2009 doi: 10.1111/j.1464-410X.2009.09077.x. published online 13 November 2009. A desciption of the wide array of strategies in which MPCs have been employed to promote regeneration and restore urological function. [DOI] [PubMed] [Google Scholar]
- 110.Huard J, Yokoyama T, Pruchnic R, et al. Muscle-derived cell-mediated ex vivo gene therapy for urological dysfunction. Gene Ther. 2002;9(23):1617–26. doi: 10.1038/sj.gt.3301816. [DOI] [PubMed] [Google Scholar]
- 111.Smaldone MC, Chen ML, Chancellor MB. Stem cell therapy for urethral sphincter regeneration. Minerva Urol Nefrol. 2009;61(1):27–40. [PubMed] [Google Scholar]
- 112.Ho MH, Heydarkhan S, Vernet D, et al. Stimulating vaginal repair in rats through skeletal muscle-derived stem cells seeded on small intestinal submucosal scaffolds. Obstet Gynecol. 2009;114(2 Pt 1):300–9. doi: 10.1097/AOG.0b013e3181af6abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Usas A, Huard J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials. 2007;28(36):5401–6. doi: 10.1016/j.biomaterials.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minasi MG, Riminucci M, De Angelis L, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129(11):2773–83. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 115.Uezumi A, Ojima K, Fukada S, et al. Functional heterogeneity of side population cells in skeletal muscle. Biochem Biophys Res Comm. 2006;341(3):864–73. doi: 10.1016/j.bbrc.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 116.Peault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15(5):867–77. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 117.Laurencot CM, Ruppel S. Regulatory aspects for translating gene therapy research into the clinic. Methods Mol Biol. 2009;542:397–421. [PubMed] [Google Scholar]


