Abstract
Objective
To determine the time between first intercourse and first sexually transmitted infection (STI) with Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis and time between repeated infections.
Design
Observational study.
Setting
Three adolescent medicine clinics.
Participants
A cohort of 386 urban young women aged 14 to 17 years at enrollment.
Main Outcome Measures
Age at first intercourse; organism-specific interval between first intercourse and first STI diagnosis; interval between repeated infections; and age at first STI test prior to study participation.
Results
Participants had first intercourse at a young age (first, second, and third quartiles were 13, 14, and 15 years of age, respectively). By age 15 years, 25% of the women acquired their first STI, most often C trachomatis. Median interval between first intercourse and first STI diagnosis was 2 years. Within 1 year of first intercourse, 25% had their first C trachomatis infection. Repeated infections were common; within 3.6, 6, and 4.8 months, 25% of the women with prior C trachomatis, N gonorrhoeae, and T vaginalis infection were reinfected with the respective organisms. Considerable delay in STI testing was found for those who began sex at a younger age. The median interval between first sex and first test were 4.9, 3.5, 2.1, 1.8, and 1.2 years for those who had first sex at ages 10, 11, 12, 13, and 14 years, respectively.
Conclusions
Timely screening and treatment are important for prevention of STI sequelae. For urban adolescent women, STI screening (especially for C trachomatis) should begin within a year after first intercourse and infected individuals should be retested every 3 to 4 months.
Screening adolescent women for selected sexually transmitted infections (STIs)—by sexual history to identify risk and by laboratory testing to verify infection—is endorsed by clinical practice guidelines and implemented in at least some proportion of health visits for adolescents.1–3 Sexually transmitted infection screening is justified by disproportionate STI morbidity among young women, including pelvic inflammatory disease, ectopic pregnancy, tubal infertility, preterm birth, and increased susceptibility to human immunodeficiency virus infection.3–11
The 2006 Sexually Transmitted Diseases Treatment Guidelines recommend Chlamydia screening for women younger than 26 years but do not suggest a beginning age for such screening.12 Similarly, the US Preventive Services Task Force makes no recommendations about the beginning age of STI screening and periodicity of screening because of limitations of epidemiological data.13,14 These limitations include relatively short follow-up periods, infrequent testing schedules, and the inability to reliably ascertain infection outside the observational period and study venues. A clearly delineated timeline of major events related to STI acquisition in the life of young women would provide a basis for more effective STI screening strategies. Additionally, such data could shed new light on the natural history of STI during adolescence and young adulthood.
Herein, we used data from a longitudinal study in combination with participants’ medical records. The combined data offered a unique opportunity to reconstruct infection histories in a group of inner-city young women. We addressed 3 questions about the timing of STI-related events. First, what is the interval between a young woman’s first sexual intercourse and her first Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis infection? Answers to this question could better guide decisions about the appropriate timing of first STI screening. Second, what is the interval between first and subsequent infections by C trachomatis, N gonorrhoeae, or T vaginalis? Better understanding of this interval would provide information as to the most appropriate screening frequency as well as the duration of such screening in adolescence. Finally, what is the relationship between the age at first coitus and age at first STI test prior to enrollment in our study? Defining this relationship can help determine if risk ascertainment and provider testing behavior vary by age.
METHODS
STUDY DESIGN
This analysis is based on a convenience sample of young women enrolled in a longitudinal study of factors related to STI, referred to as the Young Women’s Project (YWP). Enrollment started in 1999, with maximal length of follow-up approaching 8.2 years (2995 days). The research was approved by the institutional review board of Indiana University–Purdue University at Indianapolis.
Enrollment
Young women between the ages of 14 and 17 years, able to understand English, without serious psychiatric diagnoses or substance use disorder and attending 1 of 3 adolescent medicine clinics were eligible for enrollment. The 3 adolescent medicine clinics primarily serve the inner-city population of Indianapolis. Young women were identified by clinic schedule and those who agreed to participate were enrolled at the current or subsequent clinical visit. Young women were enrolled without regard to sexual experience. Participants’ written informed consent and written parental permission were obtained at enrollment.
At enrollment, participants completed a self-administered questionnaire and a face-to-face interview with trained research associates to establish lifetime and recent (past 3 months) sexual behaviors, as well as lifetime STI history. Cervical and vaginal specimens were collected by a research nurse practitioner.
Follow-up
Participants returned for follow-up every 3 months. At each 3-month visit, participants completed face-to-face interviews to establish sexual behaviors in the previous 3 months. Participants had high completion rates for quarterly interviews, with only 5% of possible follow-up interviews missing. The self-administered questionnaire was repeated annually. Cervical and vaginal specimens were obtained for STI testing every 3 months.
In alternating quarters, study participants were instructed to complete daily behavioral diaries and submit weekly self-administered vaginal swabs for STI testing. Self-administered vaginal swabs have shown high acceptability and excellent sensitivity and specificity.15 Vaginal swab samples were retrieved by study personnel each week. Samples were stored at −20°C and processed in batches and test results were made available to study personnel just before each participant’s subsequent quarterly visit, at which time infected participants were treated.16 Study participants were compensated $2 for each completed diary and $5 for each cervicovaginal specimen.
ASCERTAINMENT OF DEMOGRAPHIC AND SEXUAL BEHAVIORAL INFORMATION
Demographic and sexual behavioral information was ascertained from the interviews and diaries. At each interview, participants were asked about lifetime and past 3 months’ experiences of vaginal, oral, and anal sex; lifetime and past 3 months’ sexual partners; and intercourse frequency and condom use in the past 3 months. Age at first sexual intercourse and lifetime history of STI were obtained at enrollment and annually.
The age at first sexual intercourse was primarily established by self-report provided during the face-to-face interview at enrollment. To establish the reliability of such self-reports, we compared age at first sex reported at enrollment with that reported 1 year later. This analysis showed 94.0% agreement within 1 year. For participants without lifetime intercourse experience at enrollment, age at first sex was determined from follow-up interviews and diaries.
STI TESTING AND DETERMINATION OF INFECTION EPISODES
Nucleic acid amplification tests (NAATs) were used to analyze all study specimens for C trachomatis and N gonorrhoeae (Amplicor CT/NG PCR; Roche Diagnostics, Indianapolis). Detection of T vaginalis DNA was performed using a modification of the Amplicor CT/NG PCR assay that included primers and probes specific for T vaginalis.17
The following decision rule was used to identify infections in this repetitively tested cohort. First, positive test results for C trachomatis, N gonorrhoeae, or T vaginalis obtained from participants’ medical records were considered as true infection episodes. Positive test results based on cervical swab samples conducted at quarterly clinical visits were also considered as true infection episodes, as consistent with current practice. In the absence of quarterly test results, we used weekly vaginal samples to determine the presence of C trachomatis or T vaginalis. For weekly samples, we required at least 3 positive NAAT results to avoid misinterpreting transient DNA deposited during recent coitus as new infection.18 We also required that these positive test results be at least 3 weeks after treatment of a previous infection to avoid misinterpreting transient DNA shedding following successful treatment as a new infection.16,18 Because of false-positive NAAT results for N gonorrhoeae,19 samples testing positive by the Amplicor CT/NG PCR were confirmed by Gen-Probe (San Diego, California) Aptima, which amplifies a different molecular target.16,18,20
DATA OBTAINED FROM PARTICIPANTS’ MEDICAL RECORDS
To reduce potential bias due to failure to identify sexual behavior and STI diagnosed before or outside of study participation, we examined the participants’ medical records for test results for C trachomatis, N gonorrhoeae, and T vaginalis. Medical records were searched with the aid of the Regenstrief Medical Record System (Regenstrief Institute, Inc, Indianapolis).The Regenstrief Medical Record System is a comprehensive electronic medical record system that links most major regional hospitals located in Indianapolis, including all primary care and acute care facilities affiliated with Wishard Health Services, to which the study sites belong. Additionally, we extracted and incorporated study participants’ test and treatment data from the county sexually transmitted disease clinic. Together, these clinics represent nearly all nonprivate STI testing facilities in the greater Indianapolis metropolitan area. Sexually transmitted infection testing methods were heterogeneous, including culture-based diagnostics (approximately 10% of diagnoses) and DNA amplification tests (about90% of diagnoses) for C trachomatis and N gonorrhoeae. For T vaginalis, diagnoses were made by wet preparation microscopy (17%) and NAAT (83%). The high rates of NAAT diagnosis, especially for T vaginalis, reflected the fact that all research test results from the study laboratory, which were based on amplification methods, were incorporated into the Regenstrief Medical Record System. The medical record data were extracted on April 27, 2008.
DOCUMENTATION OF TREATMENT
At enrollment and quarterly follow-up visits, STI test results were provided to research and clinic staff within 72 hours of sample collection. Treatment according to published guidelines was provided, typically within 1 week of testing. Infections identified from samples collected during the weekly vaginal sampling periods were treated at the end of each 3-month period. The antibiotic types and regimen details were recorded for each treatment. To identify potentially effective treatments occurring outside of study participation, all prescription antibiotic orders were extracted from participants’ medical records in the Regenstrief Medical Record System. Antibiotics were considered effective for a given STI if listed in the 2006 Sexually Transmitted Diseases Treatment Guidelines.12 Antibiotic regimens not listed in the treatment guidelines were considered effective on the basis of published data documenting activity or cure of at least 80% for the STI in question.21,22 More than 96% of relevant treatments offered and documented by our study were consistent with guidelines.
DATA ANALYSIS
Demographic, behavioral, and clinical characteristics of study participants were summarized and reported in tabular form. First intercourse was the event of interest. Age at first intercourse was ascertained from the enrollment interview if the participant had been sexually active prior to study enrollment or from subsequent quarterly interviews or diaries if she had become sexually active during the study. Age at first sex for participants who had reported no intercourse either before or during the study was censored at the last study visit. The distribution of age at first sex was depicted by a Kaplan-Meier curve.23 We also used survival analyses to examine the time between the first sexual intercourse and the first diagnosis with C trachomatis, N gonorrhoeae, or T vaginalis infection. In this analysis, the events of interest were the first documented infections with each of the 3 organisms. The event times were censored at the last visit time if no infection was documented during the observation period. Selected quartiles (25th and 50th percentiles) of the event times were obtained and results reported in tabular form. Using similar techniques, we reported the interval from first to next infection as well as intervals between subsequent infections. Similarly, we examined the age distributions of the first infection with each of the 3 organisms.24
Finally, we examined the timing of first STI test in relationship to the age at first intercourse. Because the test results obtained after study enrollment reflect study procedures instead of normal clinical STI testing behavior, we only used data from participants who had received STI tests as documented in their medical records prior to enrollment; participants who had received no STI tests before study participation were censored at enrollment. A scatterplot was used to present the relationship between the age at the first coitus and age at first STI test; different symbols were used to differentiate the observed non-study STI tests from the censored observations.
Descriptive and survival analyses were implemented using SAS25 and all figures were made using Splus (Insightful Corporation, Seattle, Washington).
RESULTS
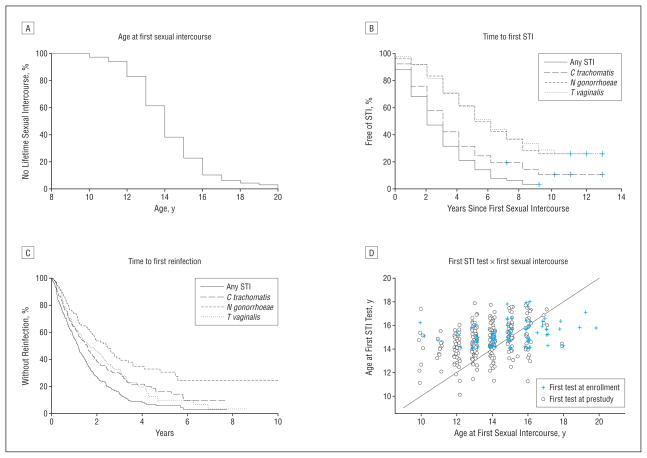
Baseline demographic, behavioral, and clinical characteristics of the 386 study participants measured at the time of enrollment are summarized in Table 1. Study participants reported an average of 3 partners in their lifetime (median, 2; range, 0–28) at enrollment. Three hundred sixty-four of the 386 participants (94%) reported first sexualinter course prior to completing the study. Among the 364 sexually active women, 285 (78.3%) reported sexual intercourse prior to study enrollment and 79 (21.7%) became sexually active during the follow-up period. Age distribution of first sexual intercourse is shown in the Figure, A.
Table 1.
Demographic and Behavioral Characteristics of the Young Women’s Project Participants
| Subject Characteristic | Mean (SD) |
|---|---|
| Age at enrollment, y (range, 14–17 y) | 15.3 (1.1) |
| African American ethnicity, No. (%) | 344 (89.1) |
| Length of follow-up, y (range, 0–8.2 y) | 3.2 (2.0) |
| No. of partners in the last 2 mo, reported at enrollment | 1 (1) |
| No. of partners during lifetime, reported at enrollment [median] | 3 (4) [2] |
| Age at first sexual intercourse, ya | 14.2 (2.0) |
| 25th, 50th, 75th Percentiles | 13, 14, 15 |
Age at first sex ascertained from interviews was accurate to the year.
Figure.
Distributions of age at first sexual intercourse (A), time from first sexual intercourse to first diagnosis of sexually transmitted infection (STI) with Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis (B), time from initial STI to first reinfection (C), and relationship between the age at first sexual intercourse and age at first STI test (D).
The average number of sexual partners in the 2 months prior to enrollment was 1 (median, 1; range, 0–10). Based on extracted medical record data, 147 (51.6%) of the 285 sexually experienced participants had 1 or more laboratory-documented C trachomatis (41.4%), N gonorrhoeae (14.0%), or T vaginalis (13.7%) infections prior to enrollment (Table 2). Self-reported STI history data were in agreement with the medical records. Of the 285 sexually experienced participants, 44% reported at least 1 STI prior to YWP enrollment; 37.2%, 15.8%, and 16.1% of these women reported prior C trachomatis, N gonorrhoeae, or T vaginalis infections.
Table 2.
Percentage of Subjects With STI History, Organism-Specific Baseline STI Prevalence, Age at First STI, and Time of First and Subsequent Sexually Transmitted Infections
| No. (%) |
25th, 50th Percentile, y |
|||||
|---|---|---|---|---|---|---|
| Subjects With Positive STI Test Result Prior to Enrollmenta | Subjects Who Tested Positive for STI at Enrollmentb | Time From First Sexual Intercourse to First STIc | Age at First Documented STIc | Time From Initial STI to First Reinfectiond | Time Between Infectionsd,e | |
| C trachomatis | 118 (41.4) | 42 (10.9) | 1, 3 | 15, 17 | 0.7, 1.6 | 0.3, 0.7 |
| N gonorrhoeae | 40 (14.0) | 17 (4.4) | 3, 5 | 17, 19 | 1.0, 2.4 | 0.5, 1.2 |
| T vaginalis | 39 (13.7) | 23 (6.0) | 3, 6 | 17, 19 | 0.7, 1.7 | 0.4, 0.7 |
| Any STI | 147 (51.6) | 67 (17.4) | 1, 2 | 15, 16 | 0.5, 1.2 | 0, 0.5 |
Abbreviations: C trachomatis, Chlamydia trachomatis; N gonorrhoeae, Neisseria gonorrhoeae; STI, sexually transmitted infection; T vaginalis, Trichomonas vaginalis; YWP, Young Women’s Project.
Based on examination of medical records of 285 young women who reported sexual intercourse before enrollment.
Based on 386 young women enrolled in the YWP study; the prevalence rates of C trachomatis, N gonorrhoeae, T vaginalis, and any of the 3 STIs for 364 sexually active women were 11.5%, 4.7%, 6.3%, and 18.4%, respectively.
Based on 364 young women who reported first sex either before or during the YWP study; age at first sex ascertained from enrollment interview was accurate to the year.
Based on 303 young women who had at least 1 reinfection.
Intervals between all infections considered.
The interval between first sexual intercourse and first STI diagnosis was examined by Kaplan-Meier estimates of the proportion of participants without C trachomatis, N gonorrhoeae, or T vaginalis infections (Figure, B). C trachomatis infections were identified significantly sooner than either N gonorrhoeae or T vaginalis infections. The median times between first sexual intercourse and first C trachomatis, N gonorrhoeae, or T vaginalis infections were 3, 5, and 6 years, respectively. A similar pattern was seen when time to first STI was examined as a function of chronologic age. The median ages of the first C trachomatis, N gonorrhoeae, or T vaginalis infection were 17 years, 19 years, and 19 years, respectively. However, the median age of the first STI of any type was 16 years. The median length of infection-free interval following first sexual intercourse was only 2 years.
The intervals between the initial STI and first reinfection are shown in the Figure, C. Median time to first reinfection was 1.2 years (426 days). Median time to the first C trachomatis reinfection was about 1.6 years (581 days), with about 36% of these infections occurring in the first year. The median time to the first N gonorrhoeae reinfection was about 2.4 years (878 days), with 26% occurring in the first year following initial diagnosis of gonorrhea. Median time to the first T vaginalis reinfection was about 1.7 years (616 days). Within 2 years, about 75% of participants with an initial STI were diagnosed with a second STI, not necessarily of the same type. Within 4 years of an initial STI, virtually all (92%) of the participants had a second STI. When all repeated STIs were taken into account, intervals between infections were even shorter (Table 2). The shorter intervals between later infections as opposed to first infections possibly reflect the increased frequency of testing during the study period compared with the pre-study period.
According to the extracted medical records, 283 of the 386 participants (73%) had received STI tests prior to YWP enrollment. The median age at first STI test received before YWP enrollment was 15 years (mean, 15.4 years), approximately 1 year after the median age of first sexual intercourse. Closer examination revealed that age at first STI test was not strongly correlated with the age at first intercourse (Figure, D) (correlation coefficient=0.4). For women who had first sexual intercourse at younger ages (eg, at 10, 11, 12, 13, and 14 years), the median intervals between first sexual intercourse and first STI test were 4.9, 3.5, 2.1, 1.8, and 1.2 years, respectively.
COMMENT
Most women become sexually active during adolescence, and earlier age at first sexual intercourse is associated with increased STI risk. The US Preventive Services Task Force recommends screening women younger than 25 years for C trachomatis and N gonorrhoeae,13,26 and the Centers for Disease Control and Prevention suggests annual screening for C trachomatis for sexually active women within 1 year of first coitus and screening for N gonorrhoeae for women at increased risk until age 26 years.12 However, neither group has made evidence-based recommendations on the most appropriate starting age and the most appropriate frequency of screening.14
To our knowledge, this research provides the first data on the timing of the initial and subsequent STIs following the onset of sexual activity in a high-risk sample of urban young women. Half of the study participants became infected within 2 years of first sexual intercourse, with C trachomatis infection detected earlier than N gonorrhoeae and T vaginalis infections(P<.001).Repeated STIs were common, and the time to reinfections usually was very short, especially for C trachomatis. This is consistent with the results of previous studies supporting relatively early rescreening following an initial STI, especially if the index infection is due to C trachomatis.27 However, continuing surveillance may be necessary because of the continuing high risk of infection even if the first rescreening test result is negative.
The findings highlight the importance of early STI screening in urban adolescent women, especially considering the minimal harm of screening.28 For example, our data show that 25% of young women will have their first STI within 1 year after first intercourse (Table 2). Therefore, if screening starts within 1 year of first intercourse, a great majority (75%) of young women will have the benefit of screening before acquiring their first STI. Alternatively, our data can be used to support the beginning ages of screening in the absence of information about prior sexual activity. For example, 25% of first infections with C trachomatis, N gonorrhoeae, and T vaginalis occur at ages 15, 17, and 17 years, respectively (Table 2). Therefore, beginning screening at age 15 years would bring benefit to more than 75% of the young women before acquiring the first infection. Similarly, our data can be used to guide screening frequency. If we wish to screen at a time when no more than 25% of reinfections have occurred with C trachomatis, N gonorrhoeae, and Tvaginalis, our screening intervals would be 3.6, 6, and 4.8 months, respectively (Table 2). If the 10th percentile is used, more aggressive screening for these organisms (in 2.4 months) would be needed. Similar rescreening intervals have been suggested for an urban adult population.29
Despite the current guidelines’ dependence on clinical determination of sexual activity, the interval between first sexual intercourse and first STI test was especially prolonged for those with earliest onset of first intercourse. For example, the median delay of the first STI test was almost 5 years after the first coitus for those young women who reported first intercourse at age 10 years. A wide range of factors may have contributed to delayed screening, including vague language of the guidelines, patients’ deferral of appointments, and misperception among some providers that STI risk begins at later ages. Statutory requirement to report underage sexual activities may also discourage risk assessment. Regardless, these data are consistent with findings that physicians fail to obtain sexual history from a large proportion of adolescent patients.30,31 An important implication of these findings is the need for renewed emphasis on training, skills, and incentives to conduct such screenings by those who care for adolescent patients. Quality assurance standards such as the Health Plan Employer Data and Information Set (http://www.ncqa.org/tabid/892/Default.aspx) can serve as important reminders for screening for sexual activity among young women, although the Health Plan Employer Data and Information Set does not define sexual activity. Within the context of standards for preventive health care for young women, perhaps a more specific and achievable national health objective would be obtaining initial STI screening tests within 12 months of young women’s first sexual intercourse, as our data suggest.
Intervals between first sexual intercourse and first STI appear to be different for the 3 organisms, which to our knowledge, has not been prospectively demonstrated among adolescent women. This differential time to infection could at least in part be explained by the organisms’ respective prevalence rates among the partner population. Previous studies show that young age is associated with increased risk of C trachomatis infection, while older age is associated with increased risk of T vaginalis infections.32,33 Cervicovaginal tissue immaturity, cervical ectopy, or immunologic naivete are proposed as explanations for age-related differences in C trachomatis infections.34 However, the same ordering of STI was seen for each sexual intercourse onset cohort (data not shown). While the issue is beyond the scope of the current article, there may be a need for a closer examination of the transmission risks of these organisms. Whatever the explanation, these data lend more credence to current emphasis on early C trachomatis screening among young women.
Our study focuses on a sample of urban adolescents at elevated STI risk, characterized by early age at first coitus, multiple sexual partners, and high STI rates. The results may not be readily generalizeable to suburban and rural youths or urban residents of higher socioeconomic status. However, considering that inner-city residents of lower socioeconomic status bear much of the disease burden of STI, such a focus is justified. The research points to the need for future studies to examine the STI time patterns in other groups. Second, the use of STI tests obtained from electronic medical records raises the possibility that some tests were obtained for diagnostic evaluations rather than for routine screening. To the extent that this is true, it would lead to an underestimate of the STI incidence before the study period. Therefore, the actual occurrence of the first STI events following the onset of sex could be sooner than what we reported, thus justifying an even earlier starting age of screening. In this sense, our data represent a potentially more conservative timeline of STI testing events in adolescent women.
Timely screening and treatment of STIs in women, particularly C trachomatis, decrease the risk of complications resultingfromuntreatedinfection.35 To achieve such a goal, STI screening should begin within a year after first intercourse. Furthermore, because of ongoing high risk for subsequent infection, our study suggests the need for follow-up screening as frequently as every 3 to 4 months. We recognize the many financial and practical barriers to such intensive programs. Nevertheless, screening and treatment efforts based on evidence-based estimates of onset and level of risk will be the mainstay of prevention of STI complications.
Acknowledgments
Funding/Support: This work was supported by grants R01 HD042404, HD044387, and U19 AI031494-14 from the National Institutes of Health.
Role of the Sponsor: The sponsors provided financial support for the study only and had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the study; or in the preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: Dr Van Der Pol has a consulting relationship with Roche Diagnostic Corporation.
Author Contributions: Drs Fortenberry and Tu had full access to the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Tu, Batteiger, Wiehe, Katz, Orr, and Fortenberry. Acquisition of data: Batteiger, Van Der Pol, Orr, and Fortenberry. Analysis and interpretation of data: Tu, Batteiger, Wiehe, Ofner, Katz, Orr, and Fortenberry. Drafting of the manuscript: Tu, Ofner, Katz, and Fortenberry. Critical revision of the manuscript for important intellectual content: Tu, Batteiger, Wiehe, Van Der Pol, Katz, Orr, and Fortenberry. Statistical analysis: Tu, Ofner, and Katz. Obtained funding: Tu, Batteiger, Orr, and Fortenberry. Administrative, technical, and material support: Van Der Pol, Orr, and Fortenberry. Study supervision: Fortenberry.
Additional Contributions: Stanley Taylor, MA, and Jane Wang, PhD, assisted with data management. We thank Stanley Spinola, MD, for comments on the manuscript.
References
- 1.Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Baltimore, MD: Williams & Wilkins; 1994. [Google Scholar]
- 2.Green M, Palfrey JS. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 2. Arlington, VA: National Center for Education in Maternal and Child Health; 2000. [Google Scholar]
- 3.Shafer MA, Tebb KP, Pantell RH, et al. Effect of a clinical practice improvement intervention on Chlamydial screening among adolescent girls. JAMA. 2002;288(22):2846–2852. doi: 10.1001/jama.288.22.2846. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Trends in Reportable Sexually Transmitted Diseases in the United States, 2006. Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 5.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 6.Cates W, Jr, Wasserheit JN. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol. 1991;164(6 pt 2):1771–1781. doi: 10.1016/0002-9378(91)90559-a. [DOI] [PubMed] [Google Scholar]
- 7.Hillis SD, Owens LM, Marchbanks PA, Amsterdam LF, Mac Kenzie WR. Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol. 1997;176(1 pt 1):103–107. doi: 10.1016/s0002-9378(97)80020-8. [DOI] [PubMed] [Google Scholar]
- 8.Paavonen J, Westrom L, Eschenbach D. Pelvic inflammatory disease. In: Holmes KKSP, Stamm WE, Piot P, et al., editors. Sexually Transmitted Diseases. 4. New York, NY: McGraw-Hill; 2008. pp. 1017–1050. [Google Scholar]
- 9.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195(5):698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 11.Van Der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197(4):548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep. 2006;55(RR-11):1–94. [PubMed] [Google Scholar]
- 13.US Preventive Services Task Force. Screening for chlamydial infection: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;147 (2):128–134. doi: 10.7326/0003-4819-147-2-200707170-00172. [DOI] [PubMed] [Google Scholar]
- 14.Meyers D, Wolff T, Gregory K, et al. USPSTF. USPSTF recommendations for STI screening. Am Fam Physician. 2008;77(6):819–824. [PubMed] [Google Scholar]
- 15.Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005;32(12):725–728. doi: 10.1097/01.olq.0000190092.59482.96. [DOI] [PubMed] [Google Scholar]
- 16.Van Der Pol B, Williams JA, Orr DP, Batteiger BE, Fortenberry JD. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis. 2005;192(12):2039–2044. doi: 10.1086/498217. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Pol B, Kraft CS, Williams JA. Use of an adaptation of a commercially available PCR assay aimed at diagnosis of chlamydia and gonorrhea to detect Trichomonas vaginalis in urogenital specimens. J Clin Microbiol. 2006;44(2):366–373. doi: 10.1128/JCM.44.2.366-373.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batteiger BE, Tu W, Katz BP, et al. Application of Chlamydia trachomatis diagnostic PCR to test multiple serial vaginal samples in a longitudinal study of adolescent women. In: Chernesky M, Caldwell H, Christiansen G, editors. Chlamydial Infections, Proceedings of the 11th International Symposium on Human Chlamydial Infections. San Francisco, CA: International Chlamydia Symposium; 2006. pp. 513–516. [Google Scholar]
- 19.Whiley DM, Tapsall JW, Sloots TP. Nucleic acid amplification testing for Neisseria gonorrhoeae: an ongoing challenge. J Mol Diagn. 2006;8(1):3–15. doi: 10.2353/jmoldx.2006.050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shew ML, Fortenberry JD, Tu W, et al. Association of condom use, sexual behaviors, and sexually transmitted infections with the duration of genital human papillomavirus infection among adolescent women. Arch Pediatr Adolesc Med. 2006;160(2):151–156. doi: 10.1001/archpedi.160.2.151. [DOI] [PubMed] [Google Scholar]
- 21.Stamm WE, Guinan ME, Johnson C, Starcher T, Holmes KK, McCormack WM. Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. N Engl J Med. 1984;310(9):545–549. doi: 10.1056/NEJM198403013100901. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2006 Supplement: Gonococcal Isolate Surveillance Project (GISP) Annual Report 2006. Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 23.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. New York, NY: Springer; 2003. [Google Scholar]
- 24.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 25.SAS. SAS/STAT 9.1 User’s Guide. Carey, NC: SAS Institute, Inc; 2004. [Google Scholar]
- 26.US Preventive Services Task Force. Screening for gonorrhea: recommendation statement. Ann Fam Med. 2005;3(3):263–267. doi: 10.1370/afm.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortenberry JD, Brizendine EJ, Katz BP, Wools KK, Blythe MJ, Orr DP. Subsequent sexually transmitted infections among adolescent women with genital infection due to Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis. Sex Transm Dis. 1999;26(1):26–32. doi: 10.1097/00007435-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Nelson HD, Helfand M. Screening for chlamydial infection. Am J Prev Med. 2001;20(3 suppl):95–107. doi: 10.1016/s0749-3797(01)00253-7. [DOI] [PubMed] [Google Scholar]
- 29.Peterman TA, Tian LH, Metcalf CA, et al. RESPECT-2 Study Group. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med. 2006;145(8):564–572. doi: 10.7326/0003-4819-145-8-200610170-00005. [DOI] [PubMed] [Google Scholar]
- 30.Blum RW, Beuhring T, Wunderlich M, Resnick MD. Don’t ask, they won’t tell: the quality of adolescent health screening in five practice settings. Am J Public Health. 1996;86(12):1767–1772. doi: 10.2105/ajph.86.12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiscus LC, Ford CA, Miller WC. Infrequency of sexually transmitted disease screening among sexually experienced U.S. female adolescents. Perspect Sex Reprod Health. 2004;36(6):233–238. doi: 10.1363/psrh.36.233.04. [DOI] [PubMed] [Google Scholar]
- 32.Burstein GR, Gaydos CA, Diener-West M, Howell MR, Zenilman JM, Quinn TC. Incident Chlamydia trachomatis infections among inner-city adolescent females. JAMA. 1998;280(6):521–526. doi: 10.1001/jama.280.6.521. [DOI] [PubMed] [Google Scholar]
- 33.Miller WC, Swygard H, Hobbs MM, et al. The prevalence of trichomoniasis in young adults in the United States. Sex Transm Dis. 2005;32(10):593–598. doi: 10.1097/01.olq.0000179874.76360.ad. [DOI] [PubMed] [Google Scholar]
- 34.Ethier KA, Orr DP. Behavioral interventions for prevention and control of STDs among adolescents. In: Aral SO, Douglas JM, Lipshutz JA, editors. Behavioral Interventions for Prevention and Control of Sexually Transmitted Diseases. New York, NY: Springer US; 2007. [Google Scholar]
- 35.Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med. 1996;334(21):1362–1366. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]