Abstract
Purpose
To determine the possibility of synergistic anti-leukemic activity and the underlying molecular mechanisms associated with cytarabine combined with valproic acid (VPA) [a histone deacetylase inhibitor (HDACI) and an FDA-licensed drug for treating both children and adults with epilepsy] in pediatric acute myeloid leukemia (AML).
Experimental Design
The type and extent of anti-leukemic interactions between cytarabine and VPA in clinically relevant pediatric AML cell lines and diagnostic blasts from children with AML were determined by MTT assays and standard isobologram analyses. The effects of cytarabine and VPA on apoptosis and cell cycle distributions were determined by flow cytometry analysis and caspase enzymatic assays. The effects of the two agents on DNA damage and Bcl-2 family proteins were determined by Western blotting.
Results
We demonstrated synergistic antileukemic activities between cytarabine and VPA in 4 pediatric AML cell lines and 9 diagnostic AML blast samples. t(8;21) AML blasts were significantly more sensitive to VPA and showed far greater sensitivities to combined cytarabine and VPA than non-t(8;21) AML cases. Cytarabine and VPA cooperatively induced DNA double strand breaks, reflected in induction of γH2AX and apoptosis, accompanied by activation of caspases 9 and 3. Further, VPA induced Bim expression and shRNA knockdown of Bim resulted in significantly decreased apoptosis induced by cytarabine, and by cytarabine plus VPA.
Conclusions
Our results establish global synergistic antileukemic activity of combined VPA and cytarabine in pediatric AML and provide compelling evidence to support the use of VPA in the treatment of children with this deadly disease.
STATEMENT OF TRANSLATIONAL RELEVANCE.
In this study, we demonstrate highly synergistic antileukemic activities of combined cytarabine (ara-C) and VPA in a panel of pediatric AML cell lines and diagnostic blast samples derived from children with de novo AML. Thus, VPA could be an attractive agent for combination therapy for children with this deadly disease. Based on our results, VPA was recently incorporated into one of the treatment arms for high risk AML in the St. Jude Children’s Research Hospital AML08 clinical trial “A Randomized Trial of Clofarabine Plus Cytarabine Versus Conventional Induction Therapy and of Natural Killer Cell Transplantation Versus Conventional Consolidation Therapy in Patients with Newly Diagnosed Acute Myeloid Leukemia”. In this trial, children with AMkL without t(1;22) and other high risk patients without FLT3-ITD will receive a combination of VPA with low dose ara-C, daunorubicin and etoposide (LD-ADE) during the second induction course.
INTRODUCTION
Acute myeloid leukemia (AML) accounts for one-fourth of acute leukemias in children, but it is responsible for more than half of the leukemia deaths in this patient population.1 In contrast to the tremendous success in treating acute lymphoblastic leukemia over the last three decades, resulting in a >80% cure rate, improvements in AML therapy have been limited1. Resistance to cytarabine, the most active drug in the treatment of AML, is a major cause of treatment failure.2,3 Therefore, new therapies for children with AML are urgently needed.
Cytarabine is a prodrug that must be converted to a triphosphate derivative (ara-CTP) to exert its cytotoxic effects.4 Cytarabine cytotoxicity is believed to result from a combination of DNA polymerase inhibition and incorporation of ara-CTP into DNA, resulting in chain termination and a blockade of DNA synthesis.4 In addition, previous studies have documented the ability of cytarabine to trigger apoptosis in human leukemia cells.4
Histone deacetylase (HDAC) inhibitors (HDACIs) promote histone acetylation and subsequent chromatin relaxation and uncoiling, which facilitates transcription of different genes, especially those involved in cellular differentiation.5 HDACIs may also disrupt the function of HDACs in co-repressor complexes implicated in the differentiation blockade exhibited by certain forms of AML [e.g., t(8;21) AML and APL involving t(15;17)].6,7 HDACI cytotoxicity is regulated by diverse mechanisms including activation of stress-related pathways or inactivation of cytoprotective pathways, up-regulation of death receptors, induction of p21CIP1, ceramide production, disruption of heat shock proteins, and induction of oxidative damage.8 Further, emerging evidence suggests that HDACIs can directly induce DNA damage in leukemia cells.9 A number of HDACIs are currently being tested in clinical trials and encouraging results have been reported for their use in treating both hematological malignancies and solid tumors.10–16 However, no HDACIs have yet been approved by the US Food and Drug Administration (FDA) for treating children with cancer.
Recently, the anticonvulsant drug valproic acid (VPA) was reported to exhibit powerful HDACI activity,17,18 and to induce apoptosis in leukemia cells but not in normal cells at clinically achievable concentrations (100–150 μg/ml).18–20 VPA is usually well tolerated in children and the extensive clinical experience with this drug makes it a very attractive agent for treating pediatric AML. In fact, preclinical and clinical studies have demonstrated additive-to-synergistic antileukemic effects on AML when VPA is used in combination with other chemotherapy agents including idarubicin,21 5-aza-2’-deoxycytidine,22 gemtuzumab ozogamicin,23 and NPI-0052.24 Recently, VPA was reported to markedly increase cytarabine cytotoxicity in a single AML cell line.25 However, neither the mechanisms of interaction between VPA and cytarabine nor the extent to which these results can be generalized to different AML subtypes have been established.
In this study, we hypothesize that VPA synergizes with cytarabine, resulting in enhanced antileukemic activity in AML cells, by inducing apoptosis. To model this concept, we examined the impact of VPA on cytarabine cytotoxicities in 4 pediatric AML cell lines and 9 diagnostic blast samples from children with de novo AML. We demonstrate highly synergistic antileukemic activities of combined cytarabine and VPA in all of the cell lines and diagnostic blast samples, especially those with t(8;21). Our mechanistic studies reveal cooperative induction of DNA damage by cytarabine and VPA and induction of Bim by VPA that underlie the synergistic activity of this drug combination. Collectively, our results provide compelling evidence to support the use of VPA in combination with standard chemotherapy drugs in clinical trials for treating pediatric AML.
MATERIAL AND METHODS
Clinical Samples
Diagnostic bone marrow samples (n=9) from children with de novo AML were obtained from the Children’s Hospital of Michigan leukemia cell bank. Cell bank samples were selected from cases with sufficient cell numbers (minimum 5 × 106, blast percentage >75%, viability >85%). Patient characteristics are summarized in supplementary Table S1. Mononuclear cells were purified by standard Ficoll-Hypaque density centrifugation. Informed consent was provided according to the Declaration of Helsinki. Sample handling and data analysis protocols were approved by the Human Investigation Committee of the Wayne State University School of Medicine.
Drugs
Cytarabine and VPA were purchased from Sigma Chemical Company (St Louis, MO).
Cell Culture
The THP-1 [derived from a 1-year-old male with AML M5 and t(9;11)], Kasumi-1 [derived from a 7-year-old male with AML M2 and t(8;21)], and MV4-11 [derived from a 10-year-old male with AML M5 and t(4;11)] pediatric AML cell lines were purchased from American Type Culture Collection (Manassas, VA). The CMS (derived from a 2-year-old female with AML M7) pediatric AML cell line was a gift from Dr. A Fuse from the National Institute of Infectious Diseases, Tokyo, Japan. These cell lines were cultured in RPMI 1640 with 10–20% fetal bovine serum (Hyclone, Logan, UT) and 2 mM L-glutamine, plus 100 U/ml penicillin and 100 μg/ml streptomycin, in a 37 °C humidified atmosphere containing 5% CO2/95% air.
In Vitro Cytotoxicity Assays
In vitro cytarabine and VPA cytotoxicities of pediatric AML cell lines and diagnostic blasts were measured by using MTT (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyltetrazolium-bromide, Sigma) assays, as previously described.26 IC50 values were calculated as drug concentrations necessary to inhibit 50% proliferation compared to untreated control cells. The extent and direction of cytarabine and VPA cytotoxic interactions were evaluated as described previously.27,28 Briefly, synergism, additivity or antagonism was quantified by determining the combination index (CI), where CI<1, CI=1, and CI>1 indicate synergistic, additive, and antagonistic effects, respectively. Based on the classic isobologram, the CI was calculated using the following equation: CI=[(D)1/(Dx)1] + [(D)2/(Dx)2]. At the 50% inhibition level, (Dx)1 and (Dx)2 are concentrations of cytarabine and VPA, respectively, which induce a 50% inhibition in cell proliferation when administered individually. (D)1 and (D)2 are concentrations of cytarabine and VPA, respectively, which inhibit cell proliferation by 50% when combined.
Assessment of Baseline and Drug Induced Apoptosis
Diagnostic AML blasts from patient 7 [46, XY, t(8;21)], THP-1, and Kasumi-1 cells cultured in RPMI1640 plus 10–20% FBS were treated with VPA (0.5, 0.66, and 0.5 mM, respectively) or cytarabine (1000, 900, and 100 nM, respectively) alone or in combination for 24 h (for the patient sample) or 96 h (for the cell lines). The VPA and cytarabine doses for the cell lines were IC20s, while those for patient AML blasts were ~IC50s, determined by MTT assays. The same concentrations of VPA and cytarabine were used in the rest of the studies unless specified. The cells were harvested, vigorously pipetted and triplicate samples taken to determine baseline and drug-induced apoptosis using the Apoptosis Annexin-V fluorescein isothiocyanate (FITC)/propidium iodide (PI) Kit (Beckman Coulter; Brea, CA), as previously described.29 Apoptotic events were recorded as a combination of Annexin-V+/PI- (early apoptotic) and Annexin-V+/PI+ (late apoptotic/dead) events and results were expressed as percent of Annexin-V+ cells after subtracting results for untreated cells. Synergy was quantified using the cooperativity index (cooperativity index = sum of apoptosis of single agent treatment/apoptosis upon combined treatment). Cooperativity index <1, =1, or >1 is termed synergistic, additive, or antagonistic, respectively.23
Effects of VPA and Cytarabine on Cell Cycle Progression in AML Cells
THP-1 or Kasumi-1 cells were treated with VPA or cytarabine alone or in combination for 96 h. Cells were harvested and fixed with ice-cold 70% (v/v) ethanol for 24 h. After centrifugation at 200 x g for 5 min, the cell pellets were washed with PBS (pH 7.4) and resuspended in PBScontaining PI (50 μg/ml), Triton X-100 (0.1%,v/v), and DNase-free RNase (1 μg/ml). DNA contents were determinedby flow cytometry using a FACScan flow cytometer (BD Biosciences, San Jose, CA). Cell cycle analysis was performed with the Multicycle software (Phoenix Flow Systems Inc, San Diego, CA).
Western Blot Analysis
Extracted or immunoprecipitated proteins were subjected to SDS-polyacrylamide gel electrophoresis. Separated proteins were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Thermo Fisher Inc., Rockford, IL) and immunoblotted with anti-acetyl-histone 3 (ac-H3), -ac-H4, -H4 (Upstate Biotechnology, Lake Placid, NY), -Bak, -Bax, -Bid, -Bim, -Bad, -Puma, -p21, -Bcl-2, -Bcl-xL, -Mcl-1, -γH2AX (Cell Signaling Technology, Danvers, MA), or -β-actin (Sigma, St Louis, MO) antibody, as described previously.30 Immunoreactive proteins were visualized using the Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE), as described by the manufacturer.
Caspase-9 and Caspase-3 Assays
THP-1 and Kasumi-1 cells were treated with cytarabine or VPA alone or combined for up to 96 h. Caspase-3 and caspase-9 enzymatic activities were assayed using the Caspase-3 Fluorometric Kit and the Caspase-9 Colorimetric Kit, respectively, purchased from R&D Systems (Minneapolis, MN), based on the manufacturer’s instructions. THP-1 and Kasumi-1 cells treated with 500 and 1000 nM daunorubicin, respectively, for 16 h (results in >70% apoptosis) were used as positive controls.
shRNA Knockdown of Bim in THP-1 Cells
Bim shRNA lentivirus clones were purchased from the RNAi Consortium (Sigma-Aldrich). THP-1 cells were infected by shRNA lentivirus clones. After selection with puromycin, a pool of infected cells was expanded and tested for Bim expression by Western blotting (designated Bim-shRNA). A pool of cells from the negative control transduction was used as the negative control (designated NTC-shRNA).
Statistical Analysis
Differences in cytarabine IC50s between VPA treated and untreated AML cells and differences in cell apoptosis between cytarabine and VPA treated (individually or combined) and untreated cells were compared using the paired t-test. The relationship between the levels of γH2AX and caspase-3 activities was determined by the Pearson test. Statistical analyses were performed with GraphPad Prism 4.0.
RESULTS
Synergistic Antileukemic Interactions between Cytarabine and VPA in Pediatric AML Cell Lines and Diagnostic Blasts
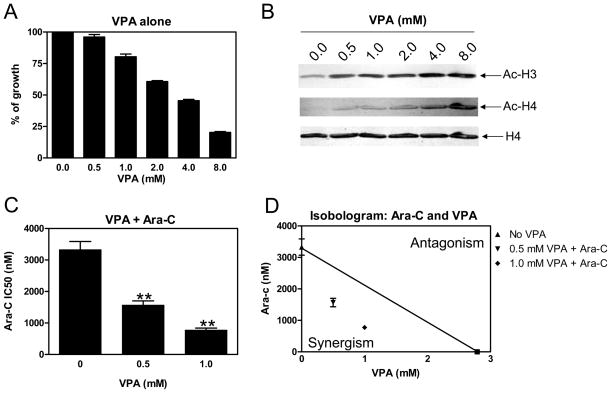
To explore the possibility of synergistic cytotoxicity when cytarabine was combined with HDACIs to treat pediatric AMLs, we tested VPA [a short-chain fatty acid HDACI which inhibits class I and class IIa HDACs5] with cytarabine toward THP-1 AML cells using MTT assays. In vitro incubation of THP-1 cells with VPA alone resulted in inhibition of cell proliferation with an IC50 of 2.97 mM (Figure 1A). This was accompanied by hyperacetylation of histones H3 and H4, but not total histone H4 (Figure 1B). This VPA concentration was in excess of the maximally achievable plasma concentration in children (1 mM), at which there was only modest inhibition of cell proliferation (Figure 1A). When simultaneously administered with cytarabine, VPA at 0.5 and 1 mM significantly enhanced cytarabine sensitivity [as reflected in decreased IC50s] by 2.1- and 4.3-fold, respectively (Figure 1C). The combined effects of cytarabine with VPA on cell proliferation were clearly synergistic, as determined by standard isobologram analysis (Figure 1D) and by calculating CI values.28 A CI<1, indicative of synergism, was calculated for each of the drug combinations (Table 1).
Figure 1. Synergistic cytotoxic interactions between VPA and cytarabine toward THP-1 cells.
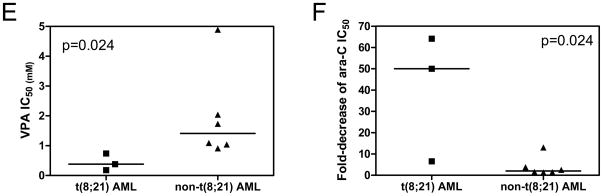
Panel A: THP-1 cells were cultured at 37 °C for 96 h in complete medium with dialyzed fetal bovine serum in 96-well plates at a density of 4 × 104 cells/ml, with a range of concentrations of VPA, and viable cell numbers were determined using the MTT reagent and a visible microplate reader. The IC50 values were calculated as the concentrations of drug necessary to inhibit 50% proliferation compared to control cells cultured in the absence of drug. The data are presented as mean values ± standard errors from at least 3 independent experiments. Panel B: THP-1 cells were harvested and lysed after incubation with a range of concentrations of VPA (0–8 mM) for 48 h. Soluble proteins were analyzed on Western blots probed by anti-ac-H3, -ac-H4, or -H4 antibody. Panels C: Cytarabine IC50s of THP-1 cells were determined in the absence or presence of VPA treated simultaneously. ** indicates statistically significant difference (p<0.005). Panel D: Standard isobologram analysis of THP-1 cell proliferation inhibition by VPA and cytarabine. The IC50 values of each drug are plotted on the axes; the solid line represents the additive effect, while the points represent the concentrations of each drug resulting in 50% inhibition of proliferation. Points falling below the line indicate synergism between drug combinations whereas those falling above the line indicate antagonism. Panel E: In vitro VPA sensitivities of the diagnostic AML blasts were measured by MTT assay, as described in the Materials and Methods. The horizontal lines indicate median VPA IC50s in each group of patient samples. The p value was determined by the nonparametric Mann-Whitney U test. Panel F: Fold decrease of cytarabine IC50s for the diagnostic AML blasts measured by MTT assays in the presence of 0.5 mM or lower VPA compared to that from cytarabine alone. The horizontal lines indicate the median fold change in each group of patient samples. The p value was determined by the nonparametric Mann-Whitney U test.
Table 1.
Effect of VPA on cytarabine sensitivity in AML cell lines and primary AML blasts
| Cell line/Patient | Cytogenetics | VPA IC50 (mM) | Cytarabine IC50 (nM) | p value | ||||
|---|---|---|---|---|---|---|---|---|
| 0.0 mM VPA | 0.15 mM VPA | 0.30 mM VPA | 0.50 mM VPA | 1.0 mM VPA | ||||
| Kasumi-1 | 45<2n>-X, t(8;21), complex karyotype | 0.79±0.03 | 436.3±41.9 | 144.7±35.3 (0.522) | 52.1±15.4 (0.499) | 26.2±1.6 (0.693) | ND | <0.004 |
| CMS | 46, complex karyotype | 2.70±0.16 | 253.5±7.7 | ND | ND | 132.6±2.7 (0.705) | 125.0±6.9 (0.863) | <0.004 |
| MV4-11 | 48 (46–48) <2n>XY, t(4;11), complex karyotype | 0.37±0.03 | 106.3±63 | 23.2±3.4 (0.759) | 3.1±0.9 (0.840) | ND | ND | <0.013 |
| THP-1 | 94 (88–96) <4n> XY/XXY, t(9;11), complex karyotype | 2.97±0.10 | 3328.5±258.4 | ND | ND | 1567.3±134.0 (0.641) | 775.3±62.1 (0.574) | <0.002 |
| Patient 1 | 46, XX | 1.09 | 14164.0 | ND | ND | 1086.0 (0.536) | 88.0 (0.923) | NA |
| Patient 2 | 46, XY, inv(16) | 4.89 | 6692.0 | ND | ND | 5072.0 (0.867) | 2645.0 (0.599) | NA |
| Patient 3 | 46, XY, inv(16) | 2.04 | 3848.0 | ND | ND | 2476.0 (0.888) | 1552.0 (0.893) | NA |
| Patient 4 | 46, XY | 1.73 | 2282.0 | ND | ND | 1578.0 (0.980) | 491.0 (0.793) | NA |
| Patient 5 | 46, XY, t(3;5) | 0.91 | 2191.0 | ND | ND | 578.0 (0.812) | ND | NA |
| Patient 6 | 46, XY, +9 | 1.04 | 440.3 | ND | ND | 175.2 (0.879) | ND | NA |
| Patient 7 | 46, XY, t(8;21) | 0.74 | 902.4 | ND | ND | 138.5 (0.825) | ND | NA |
| Patient 8 | 46, XX, t(8;21) | 0.72 | 2228.0 | 306.1 (0.346) | 89.51 (0.459) | 34.73 (0.714) | ND | NA |
| Patient 9 | 46, XX, t(8;21) | 0.18 | 495.9 | 9.177 (0.861) | ND | ND | ND | NA |
Note: Cytarabine IC50s are presented as mean plus standard errors from at least three independent experiments with the cell lines. NA, not applicable; ND, not determined. Numbers in the parentheses represent the combination index (CI) values.
To determine whether the synergistic antileukemic activity of VPA and cytarabine was unique to the THP-1 subline, analogous cytotoxicity experiments were performed with the Kasumi-1, MV4-11, and CMS sublines derived from children with different AML subtypes. VPA showed variable cytotoxicities in the 3 additional AML sublines, with IC50s ranging from 0.37 to 2.7 mM (Table 1). It is interesting that MV4-11 [harbors t(4;11)] and Kasumi-1 [harbors t(8;21)] cells were both substantially more sensitive to VPA than were the THP-1 and CMS sublines (Table 1). At 0.3 mM VPA, simultaneous treatment with cytarabine resulted in 8.4- and 34.3-fold decrease in cytarabine IC50s, respectively, in Kasumi-1 and MV4-11 cells, compared to that from cytarabine alone (Table 1). The results with the MV4-11 cells are particularly interesting since they harbor a FLT3 ITD in addition to t(4;11).31 For CMS cells, simultaneous administration of VPA and cytarabine also resulted in 2-fold decreased cytarabine IC50 at 1 mM VPA, compared to that from cytarabine alone (Table 1).
Analogous results were obtained when AML blasts collected at diagnosis from 9 children with de novo AML were evaluated following co-treatment with cytarabine and VPA (0.15 to 1 mM) (Table 1). As with Kasumi-1 cells, diagnostic blasts from t(8;21) AML cases (n=3, patients 7–9) were significantly more sensitive to VPA than non-t(8;21) AML blasts (n=6, patients 1–6) (median VPA IC50 0.38 mM vs 1.41 mM, p=0.024, Table 2 and Figure 1E) and showed 6.5- to 64.1-fold decreased cytarabine IC50s when combined with VPA at doses 0.5 mM or lower, compared to that from cytarabine alone. By contrast, non-t(8;21) AML blasts only showed 1.3-to 13-fold decrease in cytarabine IC50s when combined with 0.5 mM VPA (p=0.024, Table 2 and Figure 1F).
For both AML cell lines and diagnostic blast samples, cytarabine and VPA were again synergistic by isobologram analyses (not shown) and by CI values (Table 1). Collectively, our results demonstrate that synergistic antileukemic effects of combined cytarabine and VPA are broad-ranging and occur in multiple AML subtypes.
VPA and Cytarabine Synergistically Induce Apoptosis of Pediatric AML Cells
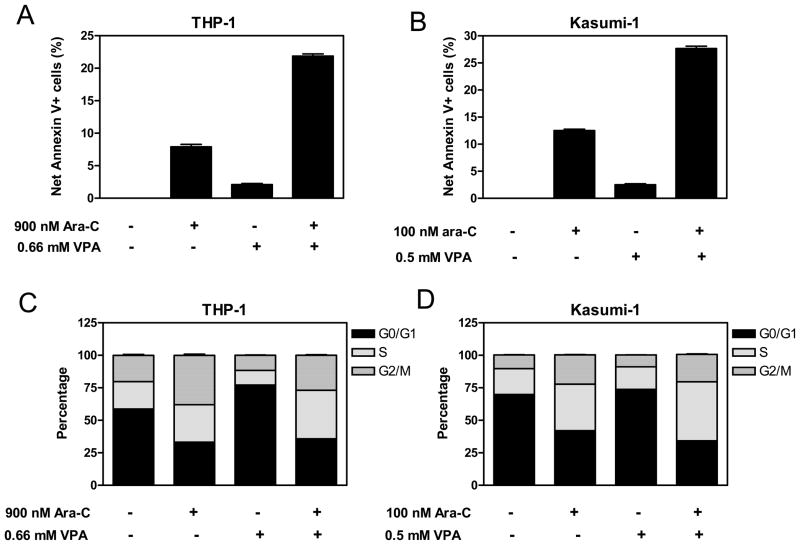
We hypothesized that VPA may lower the apoptotic threshold in pediatric AML cells, rendering them more susceptible to apoptosis induced by cytarabine. Another possibility could be that VPA combines with cytarabine to induce cell cycle arrest, resulting in synergistic antileukemic activity on this basis. To test these hypotheses, THP-1 and Kasumi-1 cells, treated with cytarabine and VPA individually or in combination for 96 h, were analyzed by flow cytometry to determine impacts on cell cycle distribution and apoptosis. Treatment with cytarabine alone substantially induced apoptosis in both THP-1 and Kasumi-1 cells, while treatment with VPA by itself resulted in only marginally increased apoptosis in both cell lines (Figures 2A and 2B). Combined VPA and cytarabine caused a substantial and synergistic induction of apoptosis compared to that resulting from the individual drug treatments (cooperativity index = 0.46 and 0.55, respectively, Figures 2A and 2B).
Figure 2. VPA augments apoptosis and S phase arrest induced by cytarabine in pediatric AML cells.
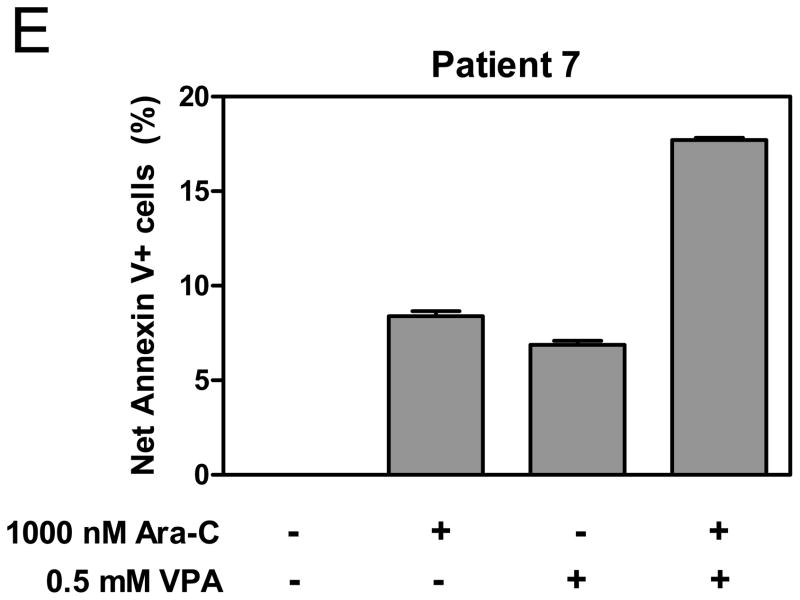
Panels A, B, and E: THP-1 (panel A), Kasumi-1 cells (panel B), and t(8;21) AML diagnostic blasts (panel E) were treated with cytarabine or VPA alone or in combination for 96 h, 96 h, and 24 h, respectively. Early and late apoptosis events in the cells were determined by annexin V/PI staining and flow cytometry analyses. Data are presented as net percent of annexin-V+ cells relative to that of untreated cells. Panels C&D: THP-1 (panel C) and Kasumi-1 cells (panel D) were treated with cytarabine or VPA alone or combined for 96 h. Cell cycle distribution was determined by PI staining and flow cytometry analysis.
As expected, treatment of THP-1 and Kasumi-1 cells with cytarabine alone resulted in S phase and G2/M phase blockade compared to untreated cells (Figures 2C and 2D). Treatment with VPA by itself caused arrest in G1/S progression in THP-1 cells (Figure 2C). However, VPA treatment of Kasumi-1 cells caused at most marginal effects on cell cycle progression (e.g. slight increase of G1 phase and slight decrease of S phase) (Figure 2D). In both cell lines, co-treatment with VPA and cytarabine resulted in additional S arrest compared to that from cytarabine alone; in THP-1 cells, combined treatment resulted in an abrogation of the G1 arrest by VPA alone (Figures 2C and 2D). These results demonstrate that VPA augments both apoptosis and S phase arrest induced by cytarabine in THP-1 and Kasumi-1 cells.
To extend these latter results to diagnostic AML patient samples, blasts from patient 7 (Table 1) for which there were sufficient cells were treated with cytarabine and VPA individually or in combination for 24 h and analyzed by flow cytometry for apoptosis and cell cycle distribution. Again, there was a synergistic induction of apoptosis by combined cytarabine and VPA (cooperativity index = 0.82, Figure 2E). Changes in cell cycle distribution in the blasts could not be determined due to lack of cell proliferation (not shown).
Cytarabine and VPA Synergistically Activate Caspases 9 and 3 Pediatric AML Cells
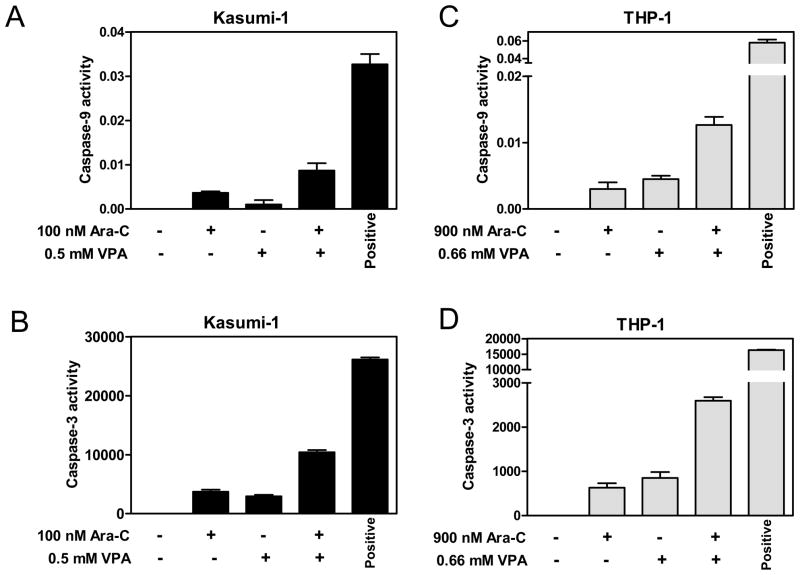
To determine if apoptosis induced by cytarabine and VPA was associated with caspase activation, THP-1 and Kasumi-1 cells treated with cytarabine and VPA alone or combined for 96 h were subjected to caspase-9 and caspase-3 enzymatic assays. In Figure 3, co-treatments with cytarabine and VPA resulted in synergistic activation of caspases 9 and 3 in both cell lines. These results demonstrate that cytarabine and VPA synergistically induce apoptosis of pediatric AML cells through the intrinsic apoptotic pathway.
Figure 3. Synergistic activation of caspase-9 and caspase-3 by cytarabine and VPA in THP-1 and Kasumi-1 cells.
Whole cell lysates from Kasumi-1 (panels A&B) and THP-1 (panels C&D) cells treated with cytarabine or VPA alone or in combination for 96 h were subjected to caspase-9 and caspase-3 enzymatic assays, respectively, as described in the Materials and Methods. THP-1 and Kasumi-1 cells treated with 500 and 1000 nM daunorubicin, respectively, for 16 h were used as the positive controls.
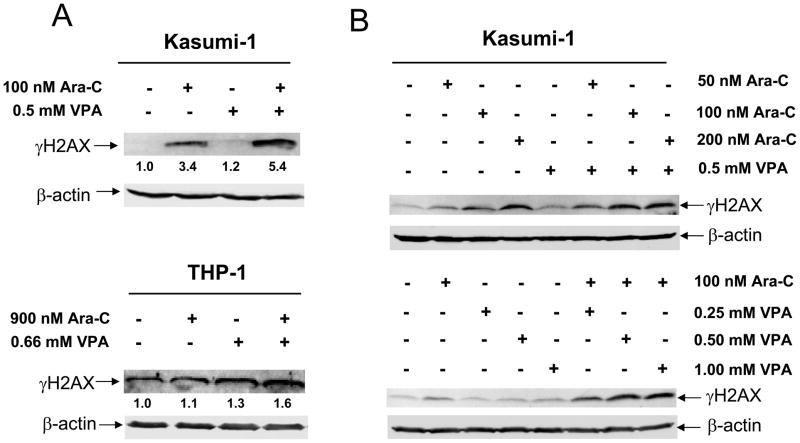
VPA and Cytarabine Cooperatively Induce DNA Damage in THP-1 and Kasumi-1 Cells
Efforts were then undertaken to determine the molecular mechanisms that underlie the synergistic induction of apoptosis by the two agents. Cytarabine is a DNA damaging agent which causes DNA double strand breaks. A previous study suggested that HDACIs can also cause DNA damage in leukemia cells.9 Thus, we hypothesized that cytarabine and VPA cooperate in causing DNA damage, which subsequently triggers apoptosis. To test this possibility, THP-1 and Kasumi-1 cells were treated with variable concentrations of cytarabine or VPA, alone or combined for 96 h, and protein lysates were subjected to Western blotting to detect γ H2AX, a biomarker of DNA double strand breaks.32 Interestingly, co-treatment with VPA and cytarabine resulted in distinctly cooperative induction of γ H2AX in both cell lines (Figure 4A). In Kasumi-1 cells, this cooperative induction of γ H2AX was both cytarabine and VPA concentration dependent (Figure 4B). These results establish that VPA augments cytarabine-induced DNA double strand breaks which may trigger apoptosis. It is important to note that there was no difference in the extent of synergy of VPA (0.5 mM) with 100 or 200 nM cytarabine in terms of triggering DNA damage. This suggests that combing the two agents would allow for a dose reduction in cytarabine.
Figure 4. Cooperative induction of DNA double strand breaks by VPA and cytarabine in THP-1 and Kasumi-1 cells.
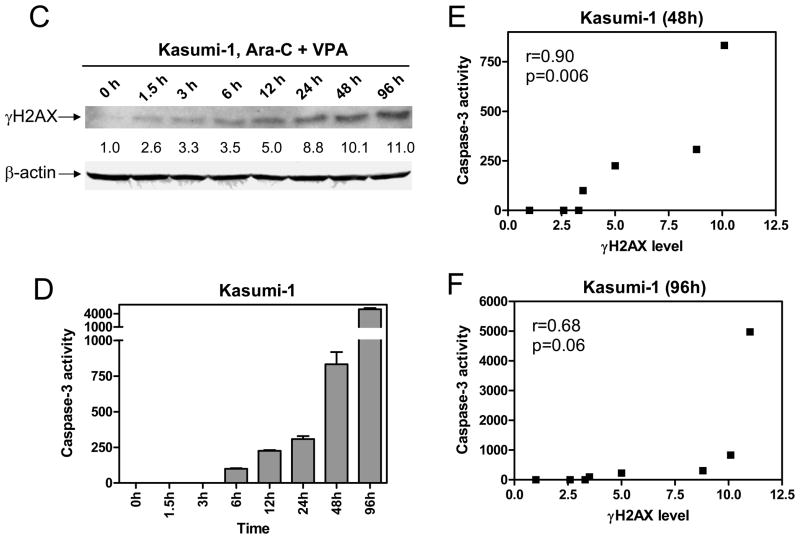
Panel A: Whole cell lysates were prepared from Kasumi-1 (upper panel) and THP-1 (lower panel) cells treated with VPA and cytarabine alone or in combination for 96 h and subjected to Western blotting probed by anti-γ H2AX or -actin antibody. Panel B: Kasumi-1 cells were treated with variable concentrations of cytarabine and fixed concentration of VPA or variable concentrations of VPA and fixed concentration of cytarabine alone or in combination for 96 h. Whole cell lysates were extracted and subjected to Western blotting probed by anti-γ H2AX or -actin antibody. Panels C&D: Kasumi-1 cells were treated with combined cytarabine and VPA for up to 96 h and cell lysates were extracted and subjected to Western blotting probed by anti-γ H2AX or – actin antibody (panel C) or to caspase-3 assays as described in the “Methods” (panel D). Panels E&F: The relationships between the levels forγ H2AX and the activities of caspase-3 in Kasumi-1 cells treated with combined cytarabine and VPA for up to 48 h (panel E) or 96 h (panel F) were determined by the Pearson tests.
Induction of γ H2AX by combined VPA/cytarabine was an early molecular event in Kasumi-1 cells, as revealed by a time course study (Figure 4C). Thus, substantial induction ofγ H2AX (2.6-fold increase relative to control) was detected by Western blotting as early as at 1.5 h (Figure 4C), accompanied by caspase-3 activation starting at 6 h (Figure 4D). Further, the levels of γ H2AX significantly correlated with caspase-3 activities over 48 h (r = 0.90, p = 006, Figure 4E). However, this association was abolished when the 96 h time data were included (r = 0.68, p = 0.06, Figure 4F). These results strongly suggest that DNA damage was associated with caspase-3 activation in Kasumi-1 cells treated with combined cytarabine and VPA during early times (within 48 h). There may be other factor(s) contributing to the late time (96 h) caspase-3 activation in this experiment.
Bim is a Critical Determinant of Apoptosis Induced by Cytarabine and Combined VPA and Cytarabine in Pediatric AML Cells
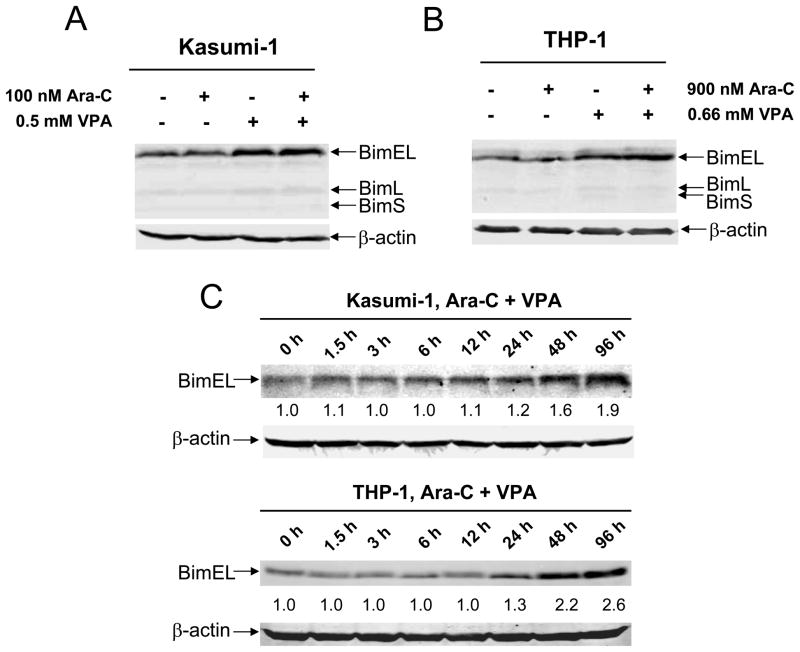
Previous studies showed that HDACIs can induce Bim to promote apoptosis in cancer cells.33,34 It is conceivable that VPA also induces Bim expression in pediatric AML cells, thus contributing to apoptosis induced by combined VPA and cytarabine. As shown in Figures 5A and 5B, modest induction of the BimEL isoform by VPA and VPA plus cytarabine was detected in both THP-1 and Kasumi-1 cells. In contrast, levels for other Bcl-2 family proteins were largely unchanged (Supplementary Figure S1). These results suggest that Bim could be another important determinant for the antileukemic activities of combined VPA/cytarabine in pediatric AML cells. In contrast to the DNA damage response, induction of Bim appeared to be a later molecular event in both sublines (post 48 h treatment, Figure 5C). This could explain the disproportionately increased caspase-3 activation seen at later times in Kasumi-1 cells (48 h and 96 h, Figure 4D).
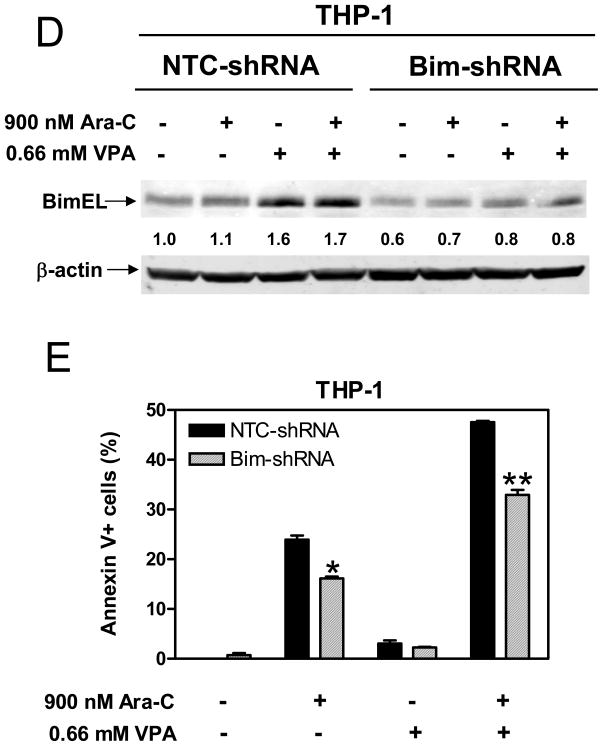
Figure 5. Bim plays a critical role in apoptosis induced by cytarabine and cytarabine plus VPA in pediatric AML cells.
Panels A&B: Kasmui-1 (panel A) and THP-1 (panel B) cells were treated with cytarabine or VPA alone or in combination for 96 h. Whole cell lysates were extracted and subjected to Western blotting probed by anti-Bim, or – actin antibody. Panel C: Kasumi-1 and THP-1 cells were treated with combined cytarabine and VPA for up to 96 h and cell lysates were extracted and subjected to Western blotting probed by anti-Bim or – actin antibody. Panels D&E: THP-1 cells were infected by Bim or negative (NTC) control shRNA lentivirus clones. After selection with puromycin, infected THP-1 cells were expanded and treated with cytarabine or VPA alone or combined for 96 h. The treated cells were then subjected to Western blotting for Bim expression (panel C) and to flow cytometry analysis for apoptosis (panel D).
To provide direct evidence that Bim is a critical effector of the antileukemic activities of cytarabine with and without VPA, lentivirus shRNA knockdown of Bim was performed in THP-1 cells. shRNA knockdown of Bim (~40%) substantially abolished its induction by VPA and combined VPA/cytarabine (Figure 5D). This was accompanied by significantly decreased apoptosis induced by cytarabine alone and combined cytarabine/VPA (Figure 5E).
Collectively, these results strongly support our hypothesis that cytarabine and VPA cause DNA double strand breaks in a cooperative fashion, which in turn triggers caspase activation and apoptosis. Further, VPA induces expression of Bim which promotes apoptosis induced by cytarabine.
DISCUSSION
HDAC inhibition represents one of the most promising epigenetic treatments for cancer because HDACIs have been established to reactivate silenced genes and exert pleiotropic antitumor effects selectively in cancer cells.5 The ability of HDACIs to induce cell differentiation, cell cycle arrest, and apoptosis in human leukemic cells but not in normal cells has stimulated significant interest in clinical applications as anti-leukemic agents.5,18–20 Currently, HDACIs including the anti-epileptic agent VPA are being evaluated in the treatment of acute leukemias.13–15 Despite their well-characterized molecular and cellular effects, single-agent activity of this class of drugs has been modest.5 Accordingly, there has been significant interest in developing rationally designed combination therapies using HDACIs.
In this study, we analyzed the cellular and molecular effects of combined cytarabine and VPA in a panel of clinically relevant pediatric AML cell lines and diagnostic blasts from children with de novo AML. Our rationale was based on the central role of cytarabine in AML chemotherapy1–3 and on the documented ability of VPA to induce apoptosis specifically in leukemia cells, without causing proliferation inhibition of normal hematopoietic progenitor cells35. Indeed, phase I/II studies using VPA as a single agent for adults with refractory AML or myelodysplastic syndrome have shown that VPA is well tolerated.15,36
The activity of VPA alone or in combination with cytarabine was initially evaluated against THP-1 AML cells, the most cytarabine resistant subline tested in our study. In vitro incubations of THP-1 cells with VPA resulted in inhibition of cell proliferation in a dose-dependent manner, accompanied by hyperacetylation of histones H3 and H4. Interestingly, when VPA was incubated simultaneously with cytarabine, there was a synergistic loss of cell proliferation. When this was expanded to include three additional cell lines derived from children with different AML subtypes, synergism was again demonstrated, suggesting that this mechanism may be broadly applicable to pediatric AMLs. Further, synergistic interactions between VPA and cytarabine were observed in 9 diagnostic blast samples from children with AML. Of particular interest, t(8;21) AML cells were significantly more sensitive to VPA and showed the greatest response to co-treatment with cytarabine and VPA. This was not unexpected, given that several fusion proteins (AML-1/ETO, PML-RARA, etc) recruit nuclear co-repressor complexes (which contain HDACs)7. Thus AML cases harboring these fusion genes might be preferentially susceptible to HDACIs. Previous pharmacokinetic studies have shown that clinically achievable trough levels of VPA used in the treatment of children with epilepsy37 approximate the in vitro concentrations of VPA that synergized with cytarabine in our study.
The synergistic cytotoxicity of combined cytarabine and VPA is clearly due to cell death since synergistic induction of apoptosis by the two agents in both pediatric AML cell lines and diagnostic blasts was detected. In THP-1 cells, VPA inhibited cell cycle progression at G1/S, which may block apoptosis mediated by the HDACI.38 Interestingly, combined cytarabine and VPA completely abolished VPA-induced G1 arrest and resulted in additional S phase arrest, which may favor apoptosis induced by co-treatment with these agents.
Our mechanistic studies in THP-1 and Kasumi-1 cells suggested that induction of apoptosis through caspase activation directly contributed to the potent synergism between cytarabine and VPA. Interestingly, this was accompanied by cooperative induction of DNA double strand breaks, as reflected by the induction of γ H2AX. Induction of γ H2AX was significantly associated with caspase-3 activation, suggesting that DNA double strand breaks were responsible for the apoptotic response upon treatment with the two agents. However, the molecular mechanism(s) underlying VPA-induced DNA damage in pediatric AML cells remains elusive. Additional studies are underway to further determine the effects of HDACIs in inducing DNA damage in this disease.
Besides induction of DNA damage, both VPA and combined VPA/cytarabine also induced expression of the BH3-only pro-apoptotic protein, Bim, in both Kasmui-1 and THP-1 cells. Bim has been classified as an “activator” in view of its purported ability to engage directly and activate Bax and Bak.39 It has been well documented that Bim is critical for HDACI-induced apoptosis of both solid tumor and leukemia cells.33,34 In this study, we demonstrated that Bim also plays critical roles in cytarabine and cytarabine plus VPA induced apoptosis in pediatric AML cells. However, Bim may not be responsible for the synergy between the two agents since only VPA, but not cytarabine, induced Bim expression in our experiments.
Together, our results document global synergistic antileukemic activities of combined VPA/cytarabine in pediatric AMLs and suggest that VPA could be an attractive agent for combination therapy of this deadly disease. Based on our results, VPA was recently incorporated into one of the treatment arms for high risk AML in the St. Jude Children’s Research Hospital AML08 clinical trial “A Randomized Trial of Clofarabine Plus Cytarabine Versus Conventional Induction Therapy and of Natural Killer Cell Transplantation Versus Conventional Consolidation Therapy in Patients with Newly Diagnosed Acute Myeloid Leukemia”. In this trial, children with AMkL without t(1;22) and other high risk patients without FLT3-ITD will receive a combination of VPA with low dose cytarabine, daunorubicin and etoposide (LD-ADE) during the second induction course. The incorporation of VPA as a new agent for treating high risk AML patients has potential advantages based on its well-characterized toxicity profile and safety in children. Based on our results, incorporation of VPA into cytarabine based clinical trials for treatment of different risk groups of pediatric AML should be strongly considered.
Supplementary Material
Acknowledgments
This work was supported by the Karmanos Cancer Institute Start-up Fund, the Children’s Research Center of Michigan, Leukemia Research Life, the Herrick Foundation, the Children’s Leukemia Foundation of Michigan, the National Cancer Institute (CA120772), the Leukemia and Lymphoma Society, the ELANA Fund, Justin’s Gift Charity, the Sehn Family Foundation, St. Baldrick’s Foundation, the Dale Meyer Memorial Endowment for Leukemia Research, the Ring Screw Textron Endowed Chair for Pediatric Cancer Research (J.W.T.), and the Natural Science Foundation of China (NSFC30873093).
References
- 1.Meshinchi S, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. Oncologist. 2007;12:341–355. doi: 10.1634/theoncologist.12-3-341. [DOI] [PubMed] [Google Scholar]
- 2.Kaspers GJ, Zwaan CM. Pediatric acute myeloid leukemia: towards high-quality cure of all patients. Haematologica. 2007;92:1519–1532. doi: 10.3324/haematol.11203. [DOI] [PubMed] [Google Scholar]
- 3.Zwaan CM, Kaspers GJ. Possibilities for tailored and targeted therapy in paediatric acute myeloid leukaemia. Br J Haematol. 2004;127:264–279. doi: 10.1111/j.1365-2141.2004.05167.x. [DOI] [PubMed] [Google Scholar]
- 4.Grant S. Ara-C: cellular and molecular pharmacology. Adv Cancer Res. 1998;72:197–233. doi: 10.1016/s0065-230x(08)60703-4. [DOI] [PubMed] [Google Scholar]
- 5.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 7.Berman JN, Look AT. Targeting transcription factors in acute leukemia in children. Curr Drug Targets. 2007;8:727–737. doi: 10.2174/138945007780830818. [DOI] [PubMed] [Google Scholar]
- 8.Gao N, Rahmani M, Shi X, Dent P, Grant S. Synergistic antileukemic interactions between 2-medroxyestradiol (2-ME) and histone deacetylase inhibitors involve Akt down-regulation and oxidative stress. Blood. 2006;107:241–249. doi: 10.1182/blood-2005-06-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaymes TJ, Padua RA, Pla M, et al. Histone deacetylase inhibitors (HDI) cause DNA damage in leukemia cells: a mechanism for leukemia-specific HDI-dependent apoptosis? Mol Cancer Res. 2006;4:563–573. doi: 10.1158/1541-7786.MCR-06-0111. [DOI] [PubMed] [Google Scholar]
- 10.Ellis L, Pan Y, Smyth GK, et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008;14:4500–4510. doi: 10.1158/1078-0432.CCR-07-4262. [DOI] [PubMed] [Google Scholar]
- 11.Kelly WK, O'Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuendgen A, Strupp C, Aivado M, et al. Treatment of myelodysplastic syndromes with valproic acid alone or in combination with all-trans retinoic acid. Blood. 2004;104:1266–1269. doi: 10.1182/blood-2003-12-4333. [DOI] [PubMed] [Google Scholar]
- 13.Cimino G, Lo-Coco F, Fenu S, et al. Sequential valproic acid/all-trans retinoic acid treatment reprograms differentiation in refractory and high-risk acute myeloid leukemia. Cancer Res. 2006;66:8903–8911. doi: 10.1158/0008-5472.CAN-05-2726. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 16.Munster P, Marchion D, Bicaku E, et al. Phase I trial of histone deacetylase inhibition by valproic acid followed by the topoisomerase II inhibitor epirubicin in advanced solid tumors: a clinical and translational study. J Clin Oncol. 2007;25:1979–1985. doi: 10.1200/JCO.2006.08.6165. [DOI] [PubMed] [Google Scholar]
- 17.Gottlicher M, Minucci S, Zhu P, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008;34:206–222. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Tang R, Faussat AM, Majdak P, et al. Valproic acid inhibits proliferation and induces apoptosis in acute myeloid leukemia cells expressing P-gp and MRP1. Leukemia. 2004;18:1246–1251. doi: 10.1038/sj.leu.2403390. [DOI] [PubMed] [Google Scholar]
- 20.Insinga A, Monestiroli S, Ronzoni S, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11:71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Gonzalez B, Yang H, Bueso-Ramos C, et al. Antileukemia activity of the combination of an anthracycline with a histone deacetylase inhibitor. Blood. 2006;108:1174–1182. doi: 10.1182/blood-2005-09-008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Hoshino K, Sanchez-Gonzalez B, Kantarjian H, Garcia-Manero G. Antileukemia activity of the combination of 5-aza-2'-deoxycytidine with valproic acid. Leuk Res. 2005;29:739–748. doi: 10.1016/j.leukres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 23.ten Cate B, Samplonius DF, Bijma T, de Leij LF, Helfrich W, Bremer E. The histone deacetylase inhibitor valproic acid potently augments gemtuzumab ozogamicin-induced apoptosis in acute myeloid leukemic cells. Leukemia. 2007;21:248–252. doi: 10.1038/sj.leu.2404477. [DOI] [PubMed] [Google Scholar]
- 24.Miller CP, Ban K, Dujka ME, et al. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110:267–277. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siitonen T, Koistinen P, Savolainen ER. Increase in Ara-C cytotoxicity in the presence of valproate, a histone deacetylase inhibitor, is associated with the concurrent expression of cyclin D1 and p27(Kip 1) in acute myeloblastic leukemia cells. Leuk Res. 2005;29:1335–1342. doi: 10.1016/j.leukres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Taub JW, Huang X, Matherly LH, et al. Expression of chromosome 21-localized genes in acute myeloid leukemia: differences between Down syndrome and non-Down syndrome blast cells and relationship to in vitro sensitivity to cytosine arabinoside and daunorubicin. Blood. 1999;94:1393–1400. [PubMed] [Google Scholar]
- 27.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- 28.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 29.Edwards H, Xie C, LaFiura KM, et al. RUNX1 regulates phosphoinositide 3-kinase/AKT pathway: role in chemotherapy sensitivity in acute megakaryocytic leukemia. Blood. 2009;114:2744–2752. doi: 10.1182/blood-2008-09-179812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge Y, Stout ML, Tatman DA, et al. GATA1, cytidine deaminase, and the high cure rate of Down syndrome children with acute megakaryocytic leukemia. J Natl Cancer Inst. 2005;97:226–231. doi: 10.1093/jnci/dji026. [DOI] [PubMed] [Google Scholar]
- 31.Quentmeier H, Reinhardt J, Zaborski M, Drexler HG. FLT3 mutations in acute myeloid leukemia cell lines. Leukemia. 2003;17:120–124. doi: 10.1038/sj.leu.2402740. [DOI] [PubMed] [Google Scholar]
- 32.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–6169. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci U S A. 2005;102:16090–16095. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawagoe R, Kawagoe H, Sano K. Valproic acid induces apoptosis in human leukemia cells by stimulating both caspase-dependent and -independent apoptotic signaling pathways. Leuk Res. 2002;26:495–502. doi: 10.1016/s0145-2126(01)00151-5. [DOI] [PubMed] [Google Scholar]
- 36.Kuendgen A, Schmid M, Schlenk R, et al. The histone deacetylase (HDAC) inhibitor valproic acid as monotherapy or in combination with all-trans retinoic acid in patients with acute myeloid leukemia. Cancer. 2006;106:112–119. doi: 10.1002/cncr.21552. [DOI] [PubMed] [Google Scholar]
- 37.Gerstner T, Bell N, Longin E, Konig SA. Oral rapid loading of valproic acid--an alternative to the usual saturation scheme? Seizure. 2006;15:630–632. doi: 10.1016/j.seizure.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Weiss RH. p21Waf1/Cip1 as a therapeutic target in breast and other cancers. Cancer Cell. 2003;4:425–429. doi: 10.1016/s1535-6108(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 39.Letai A. BCL-2: found bound and drugged! Trends Mol Med. 2005;11:442–444. doi: 10.1016/j.molmed.2005.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.