Abstract
Rationale
Vascular endothelial (VE)-cadherin localized at adherens junctions (AJs) regulates endothelial barrier function. As WNT (wingless) signaling-induced activation of the transcription factor Krüppel-like factor-4 (KLF4) may have an important role in mediating the expression of VE-cadherin and AJ integrity, we studied the function of KLF4 in regulating VE-cadherin expression and the control of endothelial barrier function.
Objective
The goal of this study was to determine the transcriptional role of KLF4 in regulating VE-cadherin expression and endothelial barrier function.
Methods and Results
Expression analysis, microscopy, chromatin immunoprecipitation (ChIP), electrophoretic mobility shift assays (EMSA), and VE-cadherin-luciferase reporter experiments demonstrated that KLF4 interacted with specific domains of VE-cadherin promoter and regulated the expression of VE-cadherin at AJs. KLF4 knockdown disrupted the endothelial barrier, indicating that KLF4 is required for normal barrier function. In vivo studies in mice showed augmented lipopolysaccharide-induced lung injury and pulmonary edema following Klf4 depletion.
Conclusion
Our data show the key role of KLF4 in the regulation of VE-cadherin expression at the level of the AJs and in the acquisition of VE-cadherin-mediated endothelial barrier function. Thus, KLF4 maintains the integrity of AJs and prevents vascular leakage in response to inflammatory stimuli.
Keywords: Barrier function, Endothelial cells, KLF4, VE-cadherin, WNT
Introduction
The vascular endothelium controls the exchange of solutes, hormones, and leukocytes between the blood and tissues. The regulation of vascular endothelial permeability participates critically in an array of physiological and pathological processes including developmental, tumor angiogenesis as well as immunity and inflammation.1-4 Vascular endothelial (VE)-cadherin is a single-pass homophilic cell adhesion protein localized at adherens junctions (AJs) that regulates endothelial barrier function, leukocyte trafficking, and angiogenesis.1-4 However, the underlying transcriptional mechanisms regulating VE-cadherin expression in endothelial cells (ECs) remain to be fully understood.
The Krüppel-like factors (KLFs) comprise a family of transcription factors containing the conserved C2H2 zinc finger DNA binding domain.5 KLF4, a homolog of KLF1, is a downstream target of WNT (wingless).6-10 KLF4 has an acidic transcriptional activation domain at the N-terminus, and C-terminus contains 81 conserved amino acid residues that form three C2H2 zinc fingers that serve as the DNA-binding domain.5,11,12 The presence of proline (P), glutamic acid (E), serine (S), and threonine (T) (PEST)-like sequence located between the transcriptional activation and inhibitory domains11,12 suggests that KLF4 may also be a target of ubiquitin-proteosomal proteolysis. The highly homologous zinc finger regions of KLF1 and KLF4 interact with a “CACCC” DNA sequence element of promoters/enhancers of target genes.11,12 Human and mouse KLF4 share 90% amino acid identity and 103 amino acid residues of the C-terminus are 100% conserved.11,12 The ability of KLF4 to regulate the terminal differentiation of goblet cells13 and to suppress expression of cyclin D1 and ornithine decarboxylase (ODC)14,15 suggests its critical role in cell cycle arrest. In vascular smooth muscle cells, KLF4 induces the expression of p21, p27, p53, and retinoblastoma, thereby inhibiting synthetic phenotypes of these cells.16,17 Although KLFs are expressed in ECs18-22 and they may have a role in inflammation,23,24 the specific role of KLF4 in regulating VE-cadherin expression and thereby endothelial barrier function remains unclear.
Klf4-deficient mice displayed a defect in the acquisition of skin barrier function and rapid loss of body fluid as neonates.25 The ectopic expression of Klf4 enhanced barrier function in the epidermis.26,27 Recent studies have shown expression of KLF4 in ECs under physiologic conditions, and elevated expression of KLF4 in cultured ECs induced the expression of several anti-inflammatory and anti-thrombotic factors, notably endothelial nitric oxide synthase (eNOS) and thrombomodulin (TM). In contrast, depletion of KLF4 enhanced the expression of tumor necrosis factor alpha (TNFα)-induced vascular cell adhesion molecule-1 (VCAM-1) and tissue factor (TF).22-24 Thus, KLF4 appears to play a potentially role in inflammation and monocyte differentiation.28,29 Conditional deletion of Klf4 in the surface ectoderm-derived tissues of the eye also resulted in corneal epithelial fragility,30,31 whereas elevated expression of Klf4 displayed an athero-protective phenotype in ECs.32-36
Deletion of the VE-cadherin gene in mice results in mid-gestational embryonic lethality due to severe vascular development defects.37 VE-cadherin gene expression is regulated by several transcription factors including Ets-binding sites (EBS) and hypoxia response element (HRE) as well as non-specific promoter elements.38,39 Our goal here was to address the role of KLF4 in mediating the expression of VE-cadherin and thereby determine whether KLF4 regulates endothelial barrier function.
Materials and Methods
Antibodies and Reagents
Mouse anti-human KLF4 mAb (H00009314-M01) was purchased from AbNOVA (Walnut, CA). Goat anti-VE-cadherin (sc-6458), rabbit anti-VE-cadherin (sc-28644), and mouse anti-GAPDH (sc-51906) antibodies, control non-silencing siRNA, Klf4-siRNA for mouse, VE-cadherin-siRNA, and KLF4-siRNA for human were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). VE-cadherin cDNA was purchased from Origene Technologies, Inc., (Rockville, MD). Anti-β-catenin (clone 14) mAb was purchased from BD Biosciences (San Jose, CA). Human, native, citrate-free thrombin was obtained from EMD Biosciences (La Jolla, CA). Rabbit anti-mouse Klf2 was bought from Genway Biotech (San Diego, CA). Anti-Klf6 was bought from Biolegend (San Diego, CA).
Methods are provided as online supplement materials.
Results
Expression of KLF4 in endothelial cells and response to WNT3A stimulation
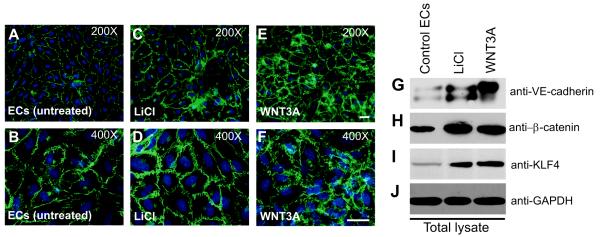
We observed Klf4 expression in all tissues tested (Online Figure I). In addition to the 55kDa polypeptide, we observed fast mobility anti-KLF4 immunoreactive species in few tissues (Online Figure I). Next we analyzed expression of KLF4 and its function in early passage primary HUVECs to determine its role in EC junction homeostasis. HUVECs displaying the cobblestone morphology of confluent monolayers expressed abundant VE-cadherin protein and formed adherens junctions (AJs). To test the hypothesis that WNT3A regulate expression of VE-cadherin, we treated HUVECs with either lithium chloride (LiCl) or recombinant WNT3A. We used LiCl as a positive control because it has been shown to induce Wnt signaling by binding to and inactivating GSK-3β, thereby stabilizing β-catenin. We observed uniform VE-cadherin zipper-like staining throughout the HUVEC monolayer (Figure 1A-F). Interestingly, we also observed increased VE-cadherin staining in HUVECs treated with LiCl and WNT3A (Figure 1C-F). For additional images, see online Figure II. Next cell extracts prepared from these cells were analyzed by antibodies against VE-cadherin, β-catenin, and KLF4. Control HUVECs showed typical basal expression of VE-cadherin, β-catenin, and KLF4 proteins, whereas addition of LiCl and WNT3A increased the expression of these proteins without changing GAPDH expression (Figure 1G-J). We expected LiCl (20 ng/ml) to induce a greater phenotype HUVECs than the canonical Wnt ligand WNT3A. However, HUVECs expressed higher levels of VE-cadherin, KLF4 and β-catenin in response to WNT3A addition. We observed at least two anti-VE-cadherin immunoreactive polypeptides in these cells (Figure 1G). WNT3A stimulation decreased the level of faster mobility species and conversely increased the level of the slower mobility species (Figure 1G, last lane). Using RT-PCR, we also detected increased expression of β-catenin and KLF4 transcripts in HUVECs stimulated with LiCl or WNT3A (Online Figure IIIA-D). Quantitative RT-PCR showed the ability of LiCl or WNT3A to induce expression of β-catenin (1.3-fold) and KLF4 (<1-fold) transcripts in cultured HUVECs (Online Figure IIID). In contrast, SOX2 expression was unchanged (Online Figure IIID). Because both KLF4 and VE-cadherin are implicated in the acquisition of barrier function, we investigated the importance of this novel relationship between KLF4 and VE-cadherin.
Figure 1. WNT3A induces the expression of VE-cadherin.
A-F, Untreated or ECs treated with LiCl (20 ng/mL) or with recombinant WNT3A (50 ng/mL) for 3 d and stained for VE-cadherin. Representative images of control and treated ECs at 200× (A, C, E) and 400× (B, D, F) magnification. For additional images, see Figure S2. G-J) Cell extracts were analyzed by Western blot (WB) for VE-cadherin, β-catenin, KLF4, and GAPDH. Results are representative of at least three separate experiments.
KLF4 depletion disrupts AJs in microvascular endothelial cells and increases transendothelial permeability
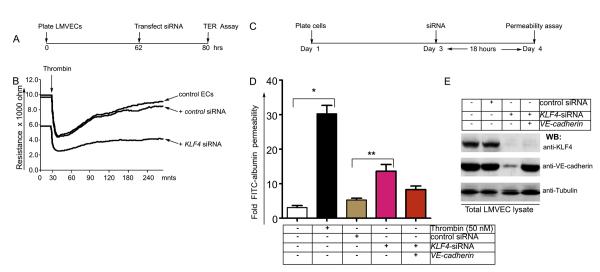
EC monolayer junction barrier integrity was monitored in real-time by transendothelial electrical resistance (TER) measurements. As thrombin induces EC contraction and endothelial barrier disruption, it was used as a positive control for endothelial barrier disruption in the TER assay. To test the hypothesis that KLF4 depletion impairs endothelial barrier function, TER was monitored in human pulmonary microvessel endothelial cells (HLMVECs) treated with control siRNA or KLF4-siRNA (time-line of experiments is shown in Figure 2A). We observed decreased TER in control HLMVECs and HLMVECs treated with control siRNA following thrombin challenge (arrow), indicating thrombin-induced opening of AJs (Figure 2B). TER returned to baseline within 3 h of thrombin (50 nM) challenge (Figure 2B). KLF4-depleted HLMVECs however showed lower baseline TER (Figure 2B) indicating higher basal AJ permeability compared with control HLMVECs or control siRNA-treated HLMVECs (p<0.05, n=7). Addition of thrombin to KLF4-depleted HLMVECs showed a further drop in TER (Figure 2B), indicating greater loss of AJ barrier integrity. KLF4-depleted monolayers also did not recover to baseline values following thrombin in contrast to control cells similarly challenged with thrombin (Figure 2B). Thus, KLF4 depletion in ECs showed loss of AJ integrity and enhanced endothelial barrier dysfunction in response to thrombin.
Figure 2. KLF4 depletion increases endothelial barrier permeability.
A, Timeline of TER assay. B, HLMVECs plated on gold microelectrodes were left untreated (control) or transfected with a non-silencing siRNA or KLF4-silencing siRNA, followed by TER assay. Note that KLF4 silencing inhibited the thrombin response relative to untreated group (no transfection) or negative control group (non-silencing siRNA; n = 5 per group). Mean value (± s.e.m.) of maximal TER responses to thrombin (50 nM) stimulation (n = 7). Thrombin-induced decrease in TER was significantly attenuated in HLMVECs transfected by KLF4-depletion compared with untreated control or negative control group transfected with a non-silencing siRNA. C-E, KLF4 knockdown increases transendothelial permeability of fluorescein isothiocyanate (FITC)-conjugated albumin by decreasing VE-cadherin expression and AJ integrity. C, Timeline of experiments. D, Confluent HLMVEC monolayers were grown on microporous filters for 36 h, either left alone (control) or treated with control siRNA or with KLF4-siRNA for 12 hours. At 18 hr post transfection, transendothelial FITC-albumin permeability was measured. Control HLMVECs showed basal transendothelial FITC-albumin permeability values, while KLF4 knockdown increased transendothelial FITC-albumin permeability. Re-expression of VE-cadherin into KLF4-depleted ECs partially restored the effect of loss of KLF4. Values are mean ± s.e.m. (n= 10). *p < 0.05 vs other control (untreated) group. **p <0.01 KLF4 siRNA vs control siRNA. E, The efficiency of KLF4-knockdown and VE-cadherin re-expression in HLMVECs was determined by Western blotting.
Next to address the role of KLF4 in transendothelial permeability to albumin, we used Transwell membrane filters to grow confluent endothelial monolayers (Figure 2C-E). The time-line of these experiments is shown in Figure 2C. Control HLMVECs and control siRNA showed basal permeability while FITC-albumin transendothelial permeability increased after thrombin addition (50 nM) or following KLF4 depletion (Figure 2D). FITC-albumin permeability was significantly higher in thrombin treated HLMVEC compared with KLF4 knockdown. Re-expression of VE-cadherin cDNA in KLF4-depleted HLMVECs restored albumin permeability (Figure 2E); however, the restoration was incomplete suggesting that VE-cadherin may not be the only target of KLF4. The efficiency of KLF4-knockdown and VE-cadherin re-expression in HLMVECs was determined by Western blotting (Figure 2E).
KLF4 binds to and activates the VE-cadherin promoter
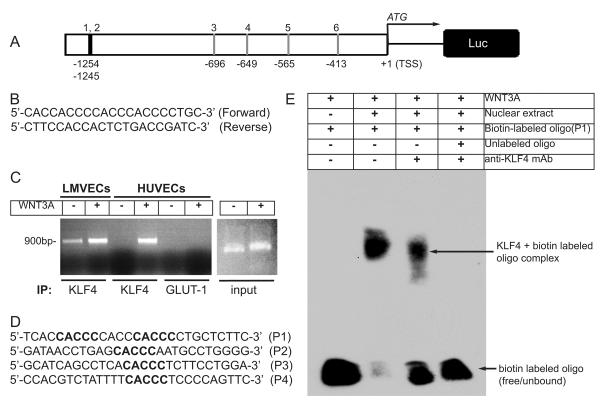
Since ECs constitutively express KLF4 and KLF4 expression increases in response to WNT3A stimulation and as shown above KLF4 regulates endothelial junctional permeability, we determined the role of KLF4 in activating the VE-cadherin promoter. Analysis of human VE-cadherin promoter −1260 to +1 relative to transcription start site (TSS) revealed 6 putative KLF4 binding sites (CACCC) in the human VE-cadherin promoter (VE-cadherin promoter is shown in Figure 3A). The first two binding sites (CACCC) are located close to each other at −1254 to −1245 positions, whereas four binding sites are located at −696, −649, −565, and −413 upstream of TSS.
Figure 3. KLF4 binds to VE-cadherin promoter.
A, Schematic of human VE-cadherin promoter −1.3 kb upstream of transcription start site (TSS). Potential KLF4 binding (CACCC) sites are indicated. B, Sequence of human VE-cadherin primer pair used for ChIP experiments. C, HLMVECs and HUVECs were grown in complete media, and left untreated (-) or treated for 3 days with WNT3A. ChIP assay was performed with indicated antibodies. PCR product of VE-cadherin promoter using input chromatin. D, Biotin-labeled oligonucleotide probes (P1-P4) containing CACCC sites used for EMSA. E, Representative image of EMSA blot. Probe-1 (P1) of KLF4 site from VE-cadherin-promoter was incubated with nuclear extracts prepared from ECs treated with WNT3A or by pre-incubation of nuclear extract with anti-KLF4 antibody in the presence or absence of cold-unlabeled oligonucleotide. Results are representative of at least three separate experiments.
To test the hypothesis that KLF4 binds to the VE-cadherin promoter, primers were designed to bind sequences flanking the 6 putative KLF4-binding sites and amplify a 900 base-pair product from the Chromatin in the ChIP assay (Figure 3B). ECs were either left untreated or treated with WNT3A for 3days. Chromatin was obtained, and immunoprecipitations were performed using antibodies specific to KLF4 or GLUT-1 (control). We observed minimal basal binding of KLF4 to VE-cadherin promoter in control, untreated HLMVECs but binding increased in response to WNT3A stimulation (Figure 3C). There was no basal binding in HUVECs, but as with HLMVECs increased KLF4 binding to the VE-cadherin promoter sequence in response to WNT3A stimulation was seen in these cells. The immunoprecipitation was specific for KLF4 since there was no detection of the VE-cadherin promoter when anti-GLUT-1 antibody was used for immunoprecipitation.
Next we performed electrophoretic mobility shift assays (EMSA) to determine interactions between KLF4 and the VE-cadherin-promoter. We designed and synthesized 4 biotin-labeled oligonucleotide probes flanking both sites at −1254 and −1245 (probe-1, P1), −696 (probe-2, P2), −649 (probe-3, P3), and −565 (probe-4, P4) nucleotides (Figure 3D). Nuclear extracts were prepared from ECs stimulated with WNT3A. As shown in representative EMSA assay, we observed an interaction of KLF4 with the VE-cadherin DNA sequence element flanking nucleotide sites at −1254 and −1245 (Figure 3E, third lane). In contrast, incubation with unlabeled oligos displaced the specific binding of KLF4 to the biotin-labeled VE-cadherin promoter DNA sequence (Figure 3E, far right). We also observed interaction of KLF4 with P2, P3, and P4 biotinylated oligonucleotides (data not shown). Thus, WNT3A induced the expression of KLF4 and KLF4 binds to the VE-cadherin promoter DNA sequence at one or more sites upstream of TSS.
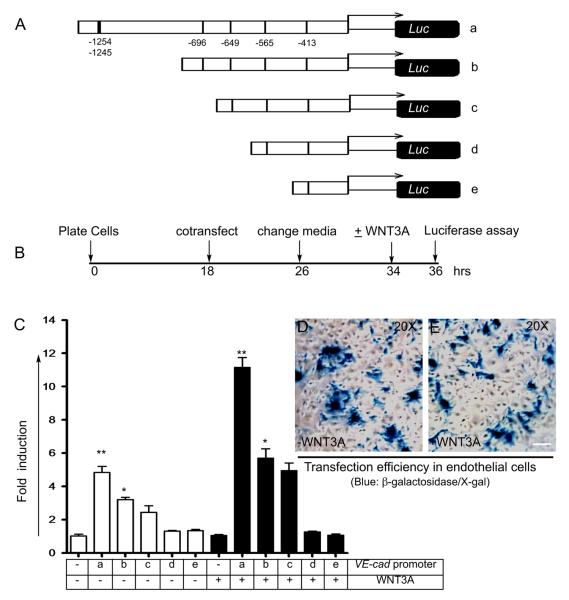
To determine whether WNT3A-induced activation of KLF4 is required for the VE-cadherin promoter activity, we also used the VE-cadherin promoter luciferase reporter constructs and performed transfection experiments (Figure 4). Wild-type (construct-a) and deletion constructs (b-e) are shown in Figure 4A. The potential KLF4 binding sites are shown in Figure 4A, construct-a. The wild-type VE-cadherin reporter construct-a contains 6 putative KLF4 binding sites, and deletion constructs (b-e) contains 4, 3, 2, and 1 site, respectively. ECs were co-transfected with a tracer amount of β-galactosidase together with wild-type construct-a or with deletion constructs (b-e). The timeline of experiments is shown in Figure 4B. We observed basal luciferase activity in HLMVECs transfected with β-galactosidase alone (Figure 4C). However, HLMVECs co-transfected with wild-type construct-a and deletion constructs-b and -c showed 2-, 1.5-, and 1.0-fold increase in luciferase activity (Figure 4C). Importantly, compared to untreated cells, WNT3A induced greater increase in luciferase activity in HLMVECs co-transfected with construct-a (5-fold), construct-b (2.5-fold), and construct-c (2.0-fold). In contrast, there was no induction in HLMVECs transfected with deletion mutant constructs-d and -e (Figure 4C). Regardless of WNT3A treatment, there was no detectable luciferase activity in KLF4-knockdown HLMVECs co-transfected with wild-type construct-a (Online Figure IV). The efficiency of transfecting HLMVECs with or without WNT3A treatment subsequently was assessed by X-gal staining (Figure 4D and E).
Figure 4. Analysis of VE-cadherin luciferase promoter reporter constructs.
A, Human wild-type VE-cadherin promoter and truncated promoter constructs driving luciferase reporter gene. B, Time-line of transfection and luciferase assay. C, Indicated constructs were transiently transfected into ECs together with a tracer amount of β-galactosidase. Fold-luciferase activity is shown as mean ± s.e.m. *p < 0.05 vs control (untreated) group; **p <0.01 vs control (without reporter construct) calculated from three independent experiments, each carried out in triplicate. D and E, Transfection efficiency with or without addition of WNT3A was determined by staining with X-gal.
Klf4 depletion augments LPS-induced lung polymorphonuclear neutrophil sequestration and vascular permeability
To determine if depletion of mouse Klf4 decreases endothelial barrier function in vivo, we used LPS (7.5 mg/kg BW) to increase lung vascular permeability (Figure 5). We monitored polymorphonuclear neutrophil (PMN) sequestration and extravascular water content in lungs as increased lung vascular permeability is coupled to increases in these parameter. MPO activity (used to monitor PMN sequestration) increased steadily at 1, 3, and 6 h after LPS (Figure 5B). At 1, 3 and 6 h, LPS increased MPO activities to 4-, 6-, and 6-fold, respectively, in control and control siRNA treated groups. Klf4-siRNA + LPS resulted in significantly greater lung tissue MPO activities (5-, 7- and 8-fold/mg lung tissue). The extent of Klf4-knockdown and down-regulation of VE-cadherin expression is shown in Figure 5C. There was no change in Cyclin-D1 or GAPDH protein levels while siRNA-mediated knockdown of Klf4 decreased VE-cadherin expression (Figure 5C). Control untreated mice exhibited normal lung architecture (Online Figure VA and B) in contrast to LPS-challenged mice (Online Figure VC-H). Importantly, Klf4-depleted group showed severe alveolar wall thickening, alveolar hemorrhage, and marked PMN sequestration (Online Figure VH). Lung tissue was also collected from these mice to measure extravascular water content (Online Figure V,I). Untreated control and mice receiving control siRNA showed no edema; wet/dry weight (w/d) ratios of 4.6 ± 1.6 and 4.3 ± 1.8, respectively (n=24, * p < 0.01). In contrast, control mice (treated with control siRNA construct or untreated) challenged with LPS showed increases in mean w/d ratio of 6.0 ± 1.4 and 7.2 ± 1.4, respectively (n=24, **p < 0.05). The mean lung w/d weight ratio was however markedly increased in Klf4-depleted mice challenged with LPS (9.1 ± 1.6). Thus, Klf4 depletion significantly augmented both lung PMN sequestration and water content in response to LPS, which was coupled to reduction in VE-cadherin expression and endothelial barrier integrity.
Figure 5. Klf4 depletion worsens bacterial endotoxin LPS-induced lung inflammation and vascular leakage.
A, Time-line of siRNA administration, LPS challenge, myeloperoxidase (MPO) as a measure of lung neutrophil sequestration, and lung extravascular water content assays. B, Lung MPO activities were assayed in mice receiving either control siRNA or Klf4-specific siRNA with or without LPS challenge at 20 min, 1 h, 3 h, and 6 h. C, Lung tissue extracts were prepared 18 h after siRNA administration (but prior to receiving LPS) and efficacy of Klf4-knockdown in lung tissues was evaluated by immunoblotting with the indicated antibodies. Values are mean ± s.e.m. (n=12 per group). *p < 0.01 vs control (untreated) group. **p <0.05 Klf4 siRNA vs control siRNA.
Although, Klf2 and Klf6 are also expressed in mouse lung microvascular endothelial cells (mLECs) (Online Figure VI), we observed that Klf2 and Klf6 did not compensate for the reduction in Klf4 expression (Online Figure VI). Furthermore, comparison of accumulation of 131I-albumin in lung parenchyma (used as a measure of vascular permeability) following Klf4- or VE-cadherin knockdown showed that reduction in expression of either Klf4 or VE-cadherin induced vascular leakage (Online Figure VII).
Discussion
ECs respond to Wnt stimulation based on their ability to express and release multiple Wnt ligands, cell surface expression of Wnt receptors, and secretion of modulators of Wnt signaling pathway.9 Activation of the canonical Wnt signaling pathway promotes the stabilization of a fraction of β-catenin.6-10 Stabilized β-catenin thereby functions to transduce Wnt signals and acts as a co-activator for the transcription factor T cell factor/lymphocyte enhancer binding factor (TCF/LEF-1).6-10 Transcription factor KLF4 has been identified as a key target of the Wnt signaling pathway.6-8 Here we determined the possible role of the KLF4 in regulating VE-cadherin expression, and thus KLF4’s role as a crucial determinant of endothelial barrier function.
KLF4 is known to promote differentiation of vascular cells and induce cell cycle arrest of synthetic smooth muscle cells.29,32,33,35,36 However, its role in regulating VE-cadherin expression and endothelial barrier function has not been addressed. Previous studies have described the obligatory function of VE-cadherin in the formation of AJs and regulation of endothelial permeability.2-4,42 Since Klf4−/− mice display impaired barrier function during development,25 we posited that a functionally important link exists between KLF4 and VE-cadherin expression that thereby regulates endothelial barrier function.
We demonstrated first Klf4 expression in all tissues tested, including ECs in their basal state;18,20,24 moreover, KLF4 was markedly upregulated in WNT3A-treated ECs. We also observed the fast mobility anti-KLF4 immunoreactive species. Because KLF4 has two PEST-like sequences, the fast mobility immunoreactive anti-KLF4 could represent the proteasome-mediated proteolytic product. However, the fast mobility protein may also be due to non-specific immunoreactivity or presence of a minor antibody contaminant. We confirmed the expression of Klf4 by RT-PCR in lungs and heart (data not shown) and ECs. Although KLF4 expression is typically seen in pluripotent and embryonic stem cells, our data as well as previous finding 18,20,24 clearly identified its presence in adult tissue and differentiated cells. Thus, it appears that the transcriptional activity of KLF4 is involved in homeostasis of fully differentiated cells.
The observation that Klf4 was constitutively expressed in ECs and its expression could be induced by WNT prompted us to address the relationship between KLF4 and VE-cadherin expression. Hence we determined KLF4-mediated transcriptional regulation of VE-cadherin. Both human and mouse VE-cadherin promoters contain binding sites for transcription factors ETS1, HRE, and zinc finger domain-containing factors such as KLF4.38,39 Whereas WNT is known to induce β-catenin and KLF4 expression in tumor cells,6-8 here we observed the upregulation of β-catenin and KLF4 in normal ECs. We also demonstrated by immunostaining and Western blotting increased expression of VE-cadherin in ECs stimulated with LiCl, a direct activator of Wnt signaling. To establish further the relationship between KLF4 and VE-cadherin promoter, we focused on the −1.3-kb promoter elements containing at least 6 CACCC sites, which are the putative KLF4 binding elements.11,12 Results from ChIP and EMSA experiments showed that KLF4 binds at least 4 of the CACCC sites in the VE-cadherin promoter. Results using the luciferase assay also demonstrated the essential role of KLF4 in activating the VE-cadherin promoter and the requirement of Wnt signaling in this response. It is important to note however that the VE-cadherin promoter is responsive to multiple stimuli including hypoxia and bacterial toxins,38,39 thus it is likely that VE-cadherin promoter is not only under the sole transcriptional control of KLF4. Our experiments do not rule out the possibility that transcription factors (such as SP1, ETS, or HRE) can also cooperate with KLF4 to regulate expression of VE-cadherin.
Studies have shown that increased VE-cadherin expression typically stabilizes AJs and promotes endothelial barrier function.1,2 Hence we used the TER assay to measure changes in AJ integrity. In confluent endothelial monolayers, KLF4 knockdown decreased basal TER, indicating a key role of KLF4 in controlling endothelial barrier function. Also depleting KLF4 resulted in AJ instability evidenced by a further decrease in TER in response to thrombin challenge of these cells and failure of the barrier function to fully recover. KLF4 knockdown also increased transendothelial albumin permeability of FITC-albumin consistent with decreased TER values and opening of AJs secondary to the lowered VE-cadherin expression seen in these cells. This was also seen in lung vascular endothelium of mice in which Klf4 depletion disrupted the vascular barrier as evidenced by greater pulmonary edema formation in response to response to LPS challenge as well as greater lung PMN sequestration. Therefore, our data demonstrate that KLF4 depletion results in decreased TER and increased transendothelial albumin permeability by preventing the expression of VE-cadherin. As KLFs regulate the expression of inflammatory mediators ,28 we can not rule out the possibility that the observed effects of KLF4 depletion in increasing endothelial permeability and PMN sequestration were secondary to increased production of such permeability-increasing mediators. An important caveat in these studies is that KLF4 does not solely regulate VE-cadherin expression and integrity of endothelial junctions. Other factor such as tyrosine phosphorylation of VE-cadherin and VE-cadherin cleavage by A disintegrin and metallopeptidase 10 (ADAM10) are also known to influence junctional integrity by interfering with VE-cadherin function.40,41
In summary, we have shown for the first time that KLF4 binds to the VE-cadherin promoter in mature ECs and induces VE-cadherin transcription, and is required for the maintenance of normal endothelial barrier function. It is possible in this context that KLF4 makes AJ barrier more resistant to inflammatory stimuli and serves to prevent vascular leakage. This function of KLF4 may be important in development of blood vessels with normal AJs during angiogenesis, and it is possible therefore that agent capable of activating KLF4 function could promote of normalization of blood vessels in ischemic cardiac diseases.
Novelty and Significance
What is known?
KLF4 is one of the four transcription factors used to produce induced pluripotent stem (iPS) cells.
Klf4-deficient mice display a defect in the acquisition of skin barrier function, while elevated KLF4 expression has atheroprotective effects.
Conventional VE-cadherin gene knockout in mice results in severe vascular defects including disruption of endothelial barrier function.
What new information does this article contribute?
For the first time, a new mechanistic link is established between transcription factor KLF4 and VE-cadherin protein as they relate to endothelial barrier function and vascular leakage.
We show that KLF4 binds to and activates the VE-cadherin promoter. Accordingly, KLF4-depletion results in the loss of VE-cadherin from the adherens junctions which disrupt the vascular endothelial barrier function.
Thus, KLF4 activating agents could potentially promote normalization of leaky vessels in ischemic vascular diseases.
Endothelial cells (ECs) that line the walls of all blood vessels, are participate in many physiological processes, such as hemostasis, leukocyte trafficking and angiogenesis. They are also involved, in pathological changes such as inflammation and ischemic vascular disease. In the current study, we show that the transcription factor KLF4 is constitutively expressed in endothelial cells and that it is upregulated upon WNT3A stimulation. We investigated the role of KLF4 in regulating VE-cadherin expression and endothelial barrier function. We also mapped KLF4 binding sites on the VE-cadherin promoter. KLF4 depletion disrupted VE-cadherin-mediated function of adherens junctions in endothelial monolayers and increased transendothelial permeability in vitro., In mice, Klf4 knockdown augmented LPS-induced lung injury and pulmonary edema. These data show that KLF4 plays a major role in the maintenance of normal endothelial barrier function and makes the barrier more resistant to inflammatory stimuli and vascular leakage. Agents capable of specifically activating KLF4 function may help normalize changes in endothelial barrier function associated with ischemic vascular diseases.
Supplementary Material
Acknowledgments
We thank Sakina Petiwala for excellent technical assistance.
Sources of Funding This work was supported by American Heart Association (10GRNT4520014) and National Institutes of Health (R01HL079356; HL079356-03S1) and by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) Award Number UL1RR029879 from the National Center for Research Resources to K.K.W. C.E.C. was supported by T32HL072742, E.E.K. by T32GM070388 and T32HL072742, and T.A.D. by T32HL007829 NIH training grants.
Non-standard Abbreviations and Acronyms
- ALI
Acute Lung Injury
- BW
Body weight
- ChIP
Chromatin immunoprecipitation
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HMVECs
human lung microvascular endothelial cells
- KLF
Krüppel-Like Factor
- LPS
Lipopolysaccharide
- MPO
Myeloperoxidase
- siRNA
Small interfering RNA
- shRNA
Short hairpin RNA
- TER
Transendothelial Electrical Resistance
- VE-cadherin
Vascular Endothelial cadherin
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 2.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 3.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J. Biol. Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Chen X, Kato Y, Evans PM, Yuan S, Yang J, Rychahou PG, Yang VW, He X, Evers BM, Liu C. Novel cross talk of Kruppel-like factor 4 and beta-catenin regulates normal intestinal homeostasis and tumor repression. Mol Cell Biol. 2006;26:2055–2064. doi: 10.1128/MCB.26.6.2055-2064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghaleb AM, McConnell BB, Nandan MO, Katz JP, Kaestner KH, Yang VW. Haploinsufficiency of Krüppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis. Cancer Res. 2007;67:7147–7154. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans PM, Chen X, Zhang W, Liu C. KLF4 interacts with beta-CATENIN/TCF4 and blocks P300/CBP recruitment by beta-catenin. Mol Cell Biol. 2010;30:372–381. doi: 10.1128/MCB.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin AM, Sullivan KM, D’Amore PA. Cultured Endothelial Cells Display Endogenous Activation of the Canonical Wnt Signaling Pathway and Express Multiple Ligands, Receptors, and Secreted Modulators of Wnt Signaling. Developmental Dynamics. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 10.Turner J, Crossley M. Mammalian Krüppel-like transcription factors: more than just a pretty finger. Trends Biochem. Sci. 1999;24:236–240. doi: 10.1016/s0968-0004(99)01406-1. [DOI] [PubMed] [Google Scholar]
- 11.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel like family of transcription factors. Int. J. Biochem. Cell Biol. 2000;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bieker JJ. Kruppel-like factors: three fingers in many pies. J. Biol. Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 13.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Whitney EM, Gao SY, Yang VW. Transcriptional profiling of Krüppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326:665–677. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon HS, Yang VW. Requirement of Krüppel-like factor 4 in preventing entry into mitosis following DNA damage. J Biol Chem. 2004;279:5035–5041. doi: 10.1074/jbc.M307631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor β control element required for expression of the smooth muscle cell differentiation marker SM22α in vivo. J. Biol Chem. 2000;275:37798–37806. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ. Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yet SF, McA’Nulty MM, Folta SC, Yen HW, Yoshizumi M, Hsieh CM, Layne MD, Chin MT, Wang H, Perrella MA, Jain MK, Lee ME. A Krüppel-like zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J. Biol. Chem. 1998;273:1026–1031. doi: 10.1074/jbc.273.2.1026. [DOI] [PubMed] [Google Scholar]
- 19.Mazzanti CM, Tandle A, Lorang D, Costouros N, Roberts D, Bevilacqua G, Libutti SK. Early genetic mechanisms underlying the inhibitory effects of endostatin and fumagillin on human endothelial cells. Genome Res. 2004;14:1585–1593. doi: 10.1101/gr.2552804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerszten RE, Edelman ER, Jain MK. Kruppel-like Factor 4 Regulates Endothelial Inflammation. Journal of Biological Chemistry. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 21.Methe H, Balcells M, Mdel C Alegret, Santacana M, Molins B, Hamik A, Jain MK, Edelman ER. Vascular bed origin dictates flow pattern regulation of endothelial adhesion molecule expression. Am J Physiol Heart Circ Physiol. 2007;292:H2167–75. doi: 10.1152/ajpheart.00403.2006. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Carlson EC, Chen ZY, Hamik A, Jain MK, Dunwoodie SL, Yang YC. Conditional deletion of Cited2 results in defective corneal epithelial morphogenesis and maintenance. Dev Biol. 2009;334:243–252. doi: 10.1016/j.ydbio.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowland BD, Peeper DS. KLF-4, p21 and context-dependent opposing forces in cancer. Nature Reviews. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 24.Jain MK, Atkins GB. Role of Kruppel-like Transcription Factors in Endothelial Biology. Circ. Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 25.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–260. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 26.Patel S, Xi ZF, Seo EY, McGaughey D, Segre JA. Klf4 and corticosteroids activate an overlapping set of transcriptional targets to accelerate in utero epidermal barrier acquisition. Proc Natl Acad Sci U S A. 2006;103:18668–18673. doi: 10.1073/pnas.0608658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- 29.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007;27:182–194. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swamynathan SK, Davis J, Piatigorsky J. Identification of candidate Klf4 target genes reveals the molecular basis of the diverse regulatory roles of Klf4 in the mouse cornea. Invest Ophthalmol Vis Sci. 2008;49:3360–3370. doi: 10.1167/iovs.08-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida T, Gan Q, Owens GK. Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Physiol Cell Physiol. 2008;295:C1175–82. doi: 10.1152/ajpcell.00288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous Deficiency of Kruppel-Like Factor 2 Augments Experimental Atherosclerosis. Circ. Res. 2008;103:690–699. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherepanova OA, Pidkovka NA, Sarmento OF, Yoshida T, Gan Q, Adiguzel E, Bendeck MP, Berliner J, Leitinger N, Owens GK. Oxidized phospholipids induce type VIII collagen expression and vascular smooth muscle cell migration. Circ Res. 2009;104:609–618. doi: 10.1161/CIRCRESAHA.108.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deaton RA, Gan Q, Owen GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotype modulation of smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1027–1037. doi: 10.1152/ajpheart.01230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 38.Lelievre E, Mattot V, Huber P, Vandenbunder B, Soncin F. ETS1 lowers capillary endothelial cell density at confluence and induces the expression of VE–cadherin. Oncogene. 2000;19:2438–2446. doi: 10.1038/sj.onc.1203563. [DOI] [PubMed] [Google Scholar]
- 39.Le Bras A, Lionneton F, Mattot V, Lelièvre E, Caetano B, Spruyt N, Soncin F. HIF-2alpha specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene. 2007;26:7480–7489. doi: 10.1038/sj.onc.1210566. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, Reiss K. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008;102:1192–1201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.