Abstract
HGP is a 24-amino acid peptide derived from HIV gp41 that increases vesicular escape when incorporated into gene delivery vehicles. The typical yield of HGP from solid phase peptide synthesis is low due to its length and hydrophobicity. The goal of this work was to investigate truncated sequences that maintained activity in order to improve the ease and yield of synthesis. A shortened, 15-amino acid sequence retained comparable lytic activity and the ability to interact with lipids when compared to the full length peptide. A scrambled peptide showed poor lytic activity, confirming that the activity of these endosomal escape peptides is sequence specific. Peptides were covalently attached to polyethylenimine (PEI) and used to condense plasmid DNA to form nanoparticulate carriers. When delivery efficiencies of PEI-peptide conjugates were compared in vitro, PEI modified with the truncated HGP sequence increased transgene expression over unmodified PEI and full length HGP.
Keywords: gene delivery, peptide, polyethylenimine
Introduction
Efficient release of delivery vehicles from endocytic vesicles remains a major barrier for efficient nonviral gene therapy. The majority of nonviral gene delivery vehicles are entrapped within endocytic vesicles after internalization1, limiting their ability to deliver their nuclear- or cytosolic-active cargo. Widely used methods for mediating delivery to the cytosol include the use of buffering polymers such as polyethylenimine (PEI), which is one of the most efficient cationic polymer-based vehicles2. However, it has been shown that an excess of polymer is necessary for the efficiency of PEI3, resulting in poor delivery efficiencies in vivo since polymer can be separated from DNA. It is also advantageous to limit the amount of PEI administered, as increased amounts can cause cellular toxicity4. Peptides are promising candidates for potent mediators of vesicular escape and are also relatively easy to synthesize and incorporate into nanoparticulate delivery vehicles. Examples of peptides that are membrane-active which have been employed for polymeric gene delivery include GALA5, Tat6, melittin7, and hemagglutinin8.
We have previously reported the modification of a polymer-based gene delivery vehicle with a membrane-lytic 24-amino acid peptide taken from the carboxyl terminus of HIV gp419. The peptide-polymer conjugate mediated increased transfection efficiency despite unchanged levels of internalization compared to unmodified polymer. Microscopy was used to elucidate the mechanism of HGP; diffuse cytoplasmic localization of HGP-modified delivery vehicles after internalization suggested HGP mediated release of particles from endocytic vesicles9. However, the synthesis of HGP results in low yield after purification due its hydrophobicity and length, limiting its application. It was therefore desirable to survey peptides of shorter length that were able to maintain the same properties as full-length HGP.
It is known that alpha helices are one of two main secondary structures that membrane-proteins adopt10. The carboxyl terminus of gp41 is comprised of several peptides that have been shown to form amphipathic alpha-helical structures that interact with membranes11, 12. The goal of this work was to identify a truncated peptide derived from HGP that could similarly facilitate endosomal release when incorporated into drug carriers. The candidate for a shortened peptide was selected to have of 15 amino acid residues based on calculations that a peptide of this length possessing 80% alpha-helical character would have a predicted length of ~3 nm, the thickness of a membrane. An additional consideration is that peptides of this length can be synthesized by solid phase peptide synthesis without significant interchain aggregation13. For these reasons, a helical wheel applet14 was used to scan the sequence of HGP in 15 amino acid segments. The shortened peptide, referred to as sHGP, was chosen where alignment of hydrophilic and hydrophobic residues was optimally separated. A scrambled peptide sequence was chosen by randomly permuting the sequencing of sHGP and using the helical wheel applet to ensure there was no alignment of hydrophilic and hydrophobic residues. The lytic activities of sHGP and scrHGP were tested using a liposome leakage assay and peptides were conjugated to PEI to determine transfection abilities.
In this work, we evaluated a shortened sequence of HGP, referred to as sHGP, that was able to retain its lytic activity and transfection properties. We also evaluated a scrambled sequence, referred to as scrHGP, to demonstrate the sequence specificity of sHGP activity. A shortened peptide sequence would have the advantage of improved synthetic yield broadening its applicability to delivery vehicles.
Materials and Methods
Peptide synthesis
HGP, sHGP, and scrHGP (Table 1) were synthesized with a C-terminal cysteine by standard solid phase peptide synthesis techniques using Fmoc chemistry and were HPLC purified to >95% purity or purchased from GenScript Corporation (Piscataway, NJ).
Table 1.
Amino acid sequences of HGP, sHGP, and scrHGP
Peptide sequences
| Peptide | Abbreviation | Sequence |
|---|---|---|
| HGP | HGP | LLGRRGWEVLKYWWNLLQYWSQEL |
| short HGP | sHGP | RGWEVLKYWWNLLQY |
| scrambled HGP | scrHGP | KYWQLWNELRVGLYW |
Liposome preparation
Liposomes composed of a 5:1 ratio of L-α-phosphatidylcholine and cholesterol (Avanti Polar Lipids, Alabaster, AL) were synthesized in 10 mM Tris pH 7.4, 20 mM NaCl, 0.1 mM EDTA (Buffer A) with and without 50 mM sulforhodamine B (Invitrogen, Carlsbad, CA) by extrusion through a 100 nm polycarbonate membrane (Whatman, Florham Park, NJ) using the Avanti Mini-Extruder. For liposomes prepared with sulforhodamine B, unencapsulated dye was removed by dialysis into Buffer A.
Dye Release Assay
The extent of liposome lysis was monitored by measuring the fluorescence intensity of dequenched sulforhodamine B released from liposomes using a microplate reader as described previously9. Complete (100%) dye release was established as the fluorescence intensity after treatment with a 0.05% solution of Triton X-100. The data are reported as the average relative fluorescence units (RFU) of three replicates ± SD.
Tryptophan fluorescence
Peptide stock solutions were prepared in DMSO and concentrations were determined by measuring the absorbance at 280 nm. Peptides were diluted to the same concentration in DMSO and were added at a 2 μM final concentration to either Buffer A or a 0.1 mM solution of liposome diluted in Buffer A. The fluorescence intensity of tryptophan was measured by exciting at 280 nm and reading emission between 300 to 400 nm using a Tecan Safire2 microplate reader (Tecan Systems, Inc., San Jose, CA). Fluorescence intensities were measured at 1 nm increments using a 10 nm bandwidth. A corrected spectrum was obtained after averaging three sample measurements and subtracting either a Buffer A or liposome blank. The center of weight was calculated as an intensity weighted average using the equation: Center of weight = (Σ Ii λi)/ Σ Ii.
PEI-peptide conjugate preparation
Branched PEI with molecular weight 25,000 (Sigma, St. Louis, MO) was modified with 10 mole equivalents of N-Succinimidyl 3-(2-pyridyldithio)-propionate (SPDP) purchased from Pierce (Rockford, IL) according to the manufacturer’s protocols. Unreacted crosslinker was removed by dialysis and the product was concentrated using an Amicon Ultra Centrifugal Filter Unit (Millipore, Billerica, MA). Concentrated PEI solution was added to DMF at a 1:9 ratio and 5 equivalents of peptide or cysteamine were added. Peptide or cysteamine was allowed to react for 48 hours at 60 °C and any unreacted pyridyldithiol groups were quenched with cysteamine for 1 hour. PEI-peptide conjugate was acidified to pH 3 and dialyzed against water for 3 days using a 10,000 MWCO membrane (Pierce). The resulting solution was filtered using a 0.2 μm syringe filter. PEI concentration was determined at each step by measuring the absorbance at 630 nm after addition of copper (II) acetate, as described previously15. Efficiency of peptide conjugation was determined by quantifying the absorbance at 280 nm after subtracting control PEI. PEI modified with cysteamine, HGP, sHGP, or scrHGP will be referred to as PEI, PEI-HGP, PEI-sHGP, and PEI-scrHGP, respectively.
Polyplex formulation and characterization
Polycation-complexed nucleic acid, termed polyplexes, were formulated by adding equal volumes of polymer to nucleic acid at the desired polymer nitrogen to nucleic acid phosphate (N/P) ratio. Polyplexes were allowed to incubate at room temperature for 10 minutes to allow for complete complexation.
The association of nucleic acid with polymer was monitored by agarose gel electrophoresis. Polyplexes prepared at various N/P ratios were loaded into a 1% agarose gel and stained with ethidium bromide. The hydrodynamic diameter of complexes were measured in triplicate using a ZetaPALS zeta potential and particle size analyzer (Brookhaven Instruments Corp., Holtsville, NY) as described previously16.
Cell culture
HeLa cells were purchased from ATCC (Manassas, VA) and cultured in complete growth medium (minimum essential medium supplemented with 10% fetal bovine serum and antimycotic/antibiotic solution). Cells were passaged when flask reached ~80% confluency.
Evaluation of Transfection Efficiencies
Transfection experiments were performed in six replicates. Cells were seeded at 30,000 cells/well in 24-well plates the day before transfection. Polyplexes were formulated using 1 μg of gWiz-Luciferase plasmid DNA (Aldevron, Fargo, ND) and diluted in prewarmed OptiMEM medium (Invitrogen). Cells were rinsed with phosphate buffered saline, pH 7.4 (PBS), and 200 μL of polyplex solution was added to each well and incubated at 37 °C in at 5% CO2 atmosphere. After 4 hours, polyplex solution was removed and replaced with complete growth medium. Cells were allowed to incubate for a further 44 hours and luciferase expression was quantified using a luciferase assay kit (Promega Corp., Madison, WI) as described previously 9. The data is reported as the average relative luminescence units (RLU) of six replicates ± SD.
Results
Liposome leakage assay
The lytic activity of peptides was evaluated by measuring the fluorescence dequenching of encapsulated dye upon release from liposomes exposed to different peptide concentrations (Figure 1). At lower peptide concentrations, HGP has a higher lytic activity which is superseded by sHGP at higher peptide concentrations. The increase in lytic activity of sHGP over HGP at peptide concentrations of 250 nM and 500 nM is statistically significant with p-values <0.05.
Figure 1.
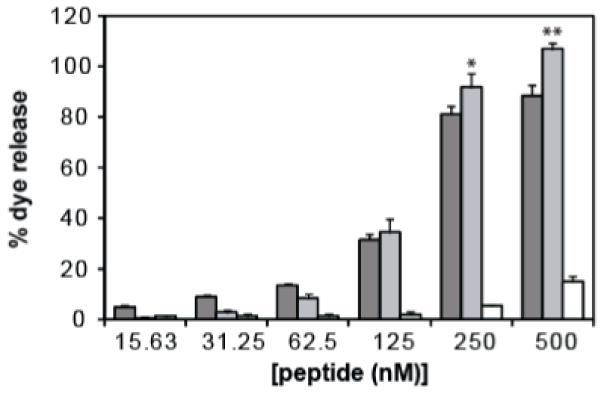
Lytic activity of HGP (dark grey bars), sHGP (light grey bars), and scrHGP (white bars) were measured by the dequenching of a fluorescent dye upon release from liposomes. 0% dye release corresponds to the fluorescence level of untreated liposome solution and 100% dye release corresponds to the fluorescence level after treatment with Triton X-100. Results are reported as mean percent dye release ± SD of triplicate samples. (Student’s t-test, *p<0.05, **p<0.001)
Tryptophan fluorescence
Tryptophan residues can be used as an intrinsic probe for the solvent exposure of a peptide17, 18. In decreasingly polar environments, such as when partitioned into a lipid bilayer, tryptophan fluorescence will have increased quantum yield and become shifted towards shorter wavelengths (blue-shift). The tryptophan fluorescence of each peptide was measured in Buffer A or in a solution of liposomes and the appropriate blank was subtracted (Figure 2A). In order to clearly delineate the wavelength shift of each spectra, each fluorescence profile was normalized by the highest RFU value (Figure 2B). The center of weight calculated for each peptide in Buffer A was 353.3 nm, 358.4 nm, and 359.4 nm for HGP, sHGP, and scrHGP, respectively. The center of weight calculated for each peptide in liposome solution was 351.7 nm, 353.0 nm, and 359.4 nm for HGP, sHGP, and scrHGP, respectively.
Figure 2.
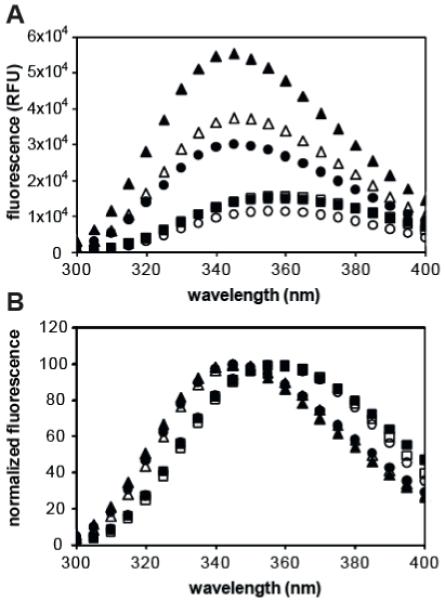
(A) Fluorescence spectra of tryptophan in HGP (circles), sHGP (triangles), and scrHGP (squares) were measured by excitation at 280 nm and reading emission between 300 and 400 nm in 1 nm increments. Fluorescence spectra were taken with (filled) and without (empty) liposome solution. (B) Each fluorescence spectra was normalized by its highest intensity value in order to delineate emission spectra shifts.
PEI-peptide conjugate preparation
PEI-peptide conjugates were prepared using the bifunctional crosslinker SPDP to react the primary amines of PEI with cysteine-terminated HGP, sHGP, or scrHGP. PEI modified with cysteamine was synthesized following the same protocol as a control. The amount of peptide was determined by measuring the absorbance at 280 nm using UV/vis spectrometry. Each conjugate had an average loading between 4-5 peptides per PEI molecule.
Polyplex characterization
Polyplexes were formed by the addition of equal volumes of peptide conjugates to plasmid DNA followed by rapid mixing and incubation. The ability of the peptide conjugates to complex nucleic acid was evaluated using gel electrophoresis. DNA associated with polymer are retained within the loading well of an agarose gel whereas free DNA migrates into the gel. All conjugates were able to complex DNA between N/P ratios 2 and 2.5 (data not shown). The hydrodynamic diameters of triplicate prepared polyplexes were measured using dynamic light scattering techniques (Figure 3). Sizes were greater than 200 nm for polyplexes formulated at N/P ratio 2 and between 90 nm and 130 nm for polyplexes formulated at N/P ratios 3 and 4 for all conjugates. The sizes reported are the average diameter measurement of three polyplex preparations ± SD.
Figure 3.
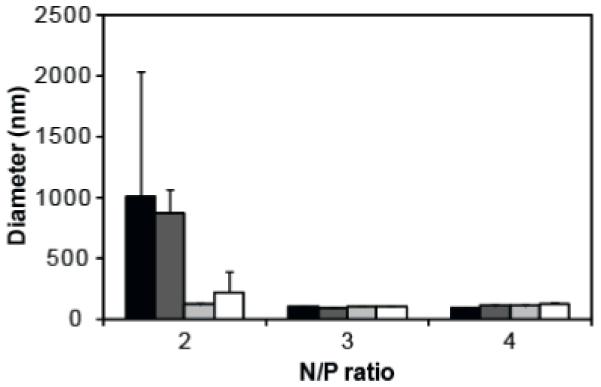
Hydrodynamic diameters of PEI (black bars), PEI-HGP (dark grey bars), PEI-sHGP (light grey bars), and PEI-scrHGP (white bars) polyplexes were measured using dynamic light scattering at N/P ratios 2, 3, and 4. Measurements are the average of triplicate samples ± SD.
Plasmid delivery of PEI-peptide conjugates
The efficiency of plasmid delivery mediated by the PEI-peptide conjugates was evaluated using the luciferase reporter gene system in HeLa cells in vitro (Figure 4A). Polyplexes were formulated at N/P ratios 2, 3, and 4 and cell lysate was collected 48 hours after transfection. At N/P ratios 3 and 4, PEI-HGP and PEI-sHGP had statistically significant increases in transfection efficiency over PEI while PEI-scrHGP had statistically significant decreases in transfection efficiency at these N/P ratios (Figure 4B). There were no statistically significant differences between any formulations at N/P 2.
Figure 4.
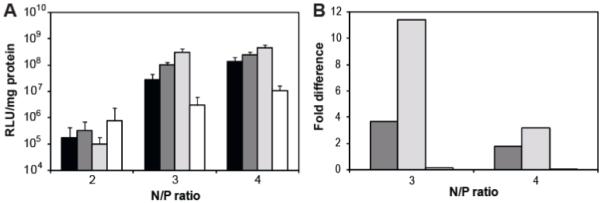
(A) Luciferase activity of PEI (black bars), PEI-HGP (dark grey bars), PEI-sHGP (light grey bars), and PEI-scrHGP (white bars) were evaluated in HeLa cells at N/P ratios 2, 3, and 4. Results are reported as the average of six replicates ± SD. (B) Fold difference in expression of PEI-HGP (dark grey bars), PEI-sHGP (light grey bars), and PEI-scrHGP (white bars) compared to PEI at the corresponding N/P ratio. Differences in expression were statistically significant for all represented data points (p<0.05).
Discussion
Efficient vesicular escape is necessary for nucleic acid delivery vehicles that are internalized through endocytic mechanisms. In previous work, we incorporated the HIV gp41 derived peptide HGP into a polymer-based gene delivery vehicle to increase nucleic acid delivery efficiency through enhanced endosomal escape9. However, due to its length and hydrophobicity, HGP is challenging to synthesize and incorporate into delivery vehicles. The goal of this work was to (1) investigate a shortened sequence of HGP that retains the same lytic activity and transfection properties as full length peptide and (2) evaluate a scrambled sequence to demonstrate the sequence specificity of the shortened peptide HGP.
First, the properties of free HGP, sHGP, and scrHGP peptide were investigated. The liposome leakage assay evaluates the ability of peptides to cause disruption of lipid bilayers by the extent of fluorescent dye release from the interior liposomes (Figure 1). It was found that sHGP had an effect as potent as HGP at concentrations higher than 125 nM. In addition, scrHGP had a significantly decreased activity with less than 20% dye release at 500 nM. To investigate the partitioning of peptides into lipids, tryptophan fluorescence was measured with and without the presence of liposomes (Figure 2). It was found that when liposome solution was added, the tryptophan fluorescence intensities of both HGP and sHGP increased whereas the tryptophan fluorescence intensity of scrHGP was unchanged (Figure 2A). Tryptophan fluorescence spectra were normalized by the maximum intensity of each spectrum in order to clearly delineate the blue-shift of tryptophan fluorescence (Figure 2B). The center of weight for each spectrum was calculated by an intensity weighted average. Upon the addition of liposome solution, the center of weight of HGP was blue-shifted from 353.3 nm to 351.7 nm, sHGP was blue-shifted from 358.4 nm to 353.0 nm, and scrHGP remained at 359.4 nm. The 4.6 nm blue-shift in center of weight observed in sHGP after addition of liposome solution indicates lipid partitioning whereas the stationary center of weight observed in scrHGP would suggest it does not interact with liposomes. The center of weight for HGP was shifted to wavelengths comparable to a sHGP /liposome solution prior to addition of liposomes. The longer structure of HGP may allow for intra-peptide shielding of tryptophan, as observed in folded proteins19. Circular dichorism (CD) spectroscopy was performed on peptides to determine differences between secondary structure between HGP, sHGP, and scrHGP (data not shown) for the purpose of understanding structure-function relationships. However, peptides were poorly soluble in solvents suitable for the collection of biologically relevant CD spectra and the high tryptophan content of peptides made interpretation of spectra convoluted.
Peptides were covalently attached to PEI in order to incorporate them into a model delivery vehicle. PEI-peptide conjugates were prepared with similar peptide substitutions per polymer to separate peptide activity from differences in loading. In order to ensure that differences observed were due to peptide and not the molecular weight distribution or primary amine content of PEI, control polymer was prepared exactly as peptide-modified polymer with the exception that cysteamine was used in substitution for peptide. Additionally, PEI concentrations were carefully measured by monitoring cuprammonium complex formation after addition of copper (II) acetate to ensure that polymer concentrations were matched. Complexation of plasmid DNA was monitored by gel retardation, which showed that complete DNA condensation was achieved at similar N/P ratios for each conjugate (data not shown). The hydrodynamic sizes of polyplexes formed at N/P ratios 3 and 4, polymer concentrations at which complexes were fully formed, were comparable between each PEI conjugate, with sizes ranging from 95 nm to 130 nm (Figure 3). Because sizes were similar, differences in transfection efficiency between the conjugates were likely due to peptide modification and not size. The ability of conjugates to deliver plasmid DNA was evaluated using the luciferase reporter gene system (Figure 4). Both PEI-HGP and PEI-sHGP improved the transfection efficiency over unmodified PEI at N/P ratios 3 and 4, with the extent of improvement decreased at N/P 4 since increased amounts of free PEI polymer concentration also assists in endosomal escape3, 20. PEI-sHGP demonstrated increases in transfection efficiencies of 1.8-fold increase at N/P 4 and a 3.1-fold increase at N/P 3 over PEI-HGP. The fold increase of PEI-HGP over PEI observed is decreased when compared to previously unpublished and published data9. This difference may be due to the addition of a step in these studies to first dialyze the PEI polymers in order to remove low molecular PEI. Higher molecular weight PEI has been reported to have higher transfection efficiencies4. Although it is unknown why sHGP shows improvement over the full length peptide, data are consistent with observations from the liposome leakage assay (Figure 1). At N/P ratios 3 and 4, the transfection efficiency of PEI-scrHGP was significantly depressed compared to PEI-HGP and PEI-sHGP, consistent with its lytic activity. The transfection efficiency of PEI-scrHGP was depressed compared to unmodified PEI. Complexation and unpackaging assays were performed in order to determine whether the decrease in transfection efficiency was due to polyplex formation and stability, but no differences were observed between the conjugates (data not shown). It remains unknown why scrHGP modification causes decreases in transfection efficiency. No differences in transfection efficiency were observed between any conjugates at N/P ratio 2, likely due to aggregation of complexes at this low charge ratio, which is supported by hydrodynamic size data (Figure 3).
We have demonstrated that the lytic activity and transfection properties of HGP could be preserved in a truncated sequence, sHGP. The evaluation of a scrambled peptide established that the activity of HGP peptides are sequence specific. A shortened sequence has the advantage of improved synthetic yield and ease of purification. Unoptimized crude preparations of HGP and sHGP resulted in purities of 5% and 20%, respectively, as determined by HPLC. Since initial attempts, optimization of sHGP synthesis conditions has resulted in the preparation of peptide that is 70% pure. This shortened membrane-active peptide can be combined with other bioactive peptides, such as targeting peptides and nuclear localization peptides, for the preparation of multi-functional delivery vehicles21.
Acknowledgements
The authors would like to acknowledge Dr. Niels H. Andersen, Irene Shu, and D. Victoria Williams for their help with circular dichroism. This work was supported by grants from the National Science Foundation (CBET 0448547) and the National Institute of Health (R01NS064404) awarded to S.H.P. E.J.K was supported by the National Institute of Health (5F31NS064805).
Abbreviations
- CD
circular dichroism
- DMSO
dimethylsulfoxide
- PBS
phosphate buffered saline
- PEI
Polyethylenimine
- SD
standard deviation
- SPDP
N-Succinimidyl 3-(2-pyridyldithio)-propionate.
References
- 1.Akita H, Ito R, Khalil IA, Futaki S, Harashima H. Quantitative three-dimensional analysis of the intracellular trafficking of plasmid DNA transfected by a nonviral gene delivery system using confocal laser scanning microscopy. Mol Ther. 2004;9(3):443–51. doi: 10.1016/j.ymthe.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med. 2004;6(10):1102–11. doi: 10.1002/jgm.598. [DOI] [PubMed] [Google Scholar]
- 4.Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16(8):1273–9. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 5.Haensler J, Szoka FC., Jr. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem. 1993;4(5):372–9. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph C, Plank C, Lausier J, Schillinger U, Muller RH, Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J Biol Chem. 2003;278(13):11411–8. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 7.Ogris M, Carlisle RC, Bettinger T, Seymour LW. Melittin enables efficient vesicular escape and enhanced nuclear access of nonviral gene delivery vectors. J Biol Chem. 2001;276(50):47550–5. doi: 10.1074/jbc.M108331200. [DOI] [PubMed] [Google Scholar]
- 8.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci U S A. 1992;89(17):7934–8. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon EJ, Bergen JM, Pun SH. Application of an HIV gp41-derived peptide for enhanced intracellular trafficking of synthetic gene and siRNA delivery vehicles. Bioconjug Chem. 2008;19(4):920–7. doi: 10.1021/bc700448h. [DOI] [PubMed] [Google Scholar]
- 10.Deisenhofer J, Epp O, Miki K, Huber R, Michel H. Structure of the Protein Subunits in the Photosynthetic Reaction Center of Rhodopseudomonas-Viridis at 3a Resolution. Nature. 1985;318(6047):618–624. doi: 10.1038/318618a0. [DOI] [PubMed] [Google Scholar]
- 11.Costin JM, Rausch JM, Garry RF, Wimley WC. Viroporin potential of the lentivirus lytic peptide (LLP) domains of the HIV-1 gp41 protein. Virol J. 2007;4(1):123. doi: 10.1186/1743-422X-4-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivas SK, Srinivas RV, Anantharamaiah GM, Segrest JP, Compans RW. Membrane interactions of synthetic peptides corresponding to amphipathic helical segments of the human immunodeficiency virus type-1 envelope glycoprotein. J Biol Chem. 1992;267(10):7121–7. [PubMed] [Google Scholar]
- 13.Chan WC, White PD. Fmoc solid phase peptide synthesis: a practical approach. Oxford University Press; Oxford; New York: 2000. p. xxiv.p. 346. [Google Scholar]
- 14.2010 < http://cti.itc.virginia.edu/~cmg/Demo/wheel/wheelApp.html>.
- 15.von Harpe A, Petersen H, Li Y, Kissel T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J Control Release. 2000;69(2):309–22. doi: 10.1016/s0168-3659(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 16.Park IK, Lasiene J, Chou SH, Horner PJ, Pun SH. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly(ethylenimine) J Gene Med. 2007;9(8):691–702. doi: 10.1002/jgm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beechem JM, Brand L. Time-resolved fluorescence of proteins. Annu Rev Biochem. 1985;54:43–71. doi: 10.1146/annurev.bi.54.070185.000355. [DOI] [PubMed] [Google Scholar]
- 18.Subbarao NK, Parente RA, Szoka FC, Jr., Nadasdi L, Pongracz K. pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry. 1987;26(11):2964–72. doi: 10.1021/bi00385a002. [DOI] [PubMed] [Google Scholar]
- 19.Brand L, Everse J, Kaplan NO. Structural characteristics of dehydrogenases. Biochemistry. 1962;1:423–34. doi: 10.1021/bi00909a009. [DOI] [PubMed] [Google Scholar]
- 20.Sonawane ND, Szoka FC, Jr., Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278(45):44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 21.Kwon EJ, Bergen JM, Park IK, Pun SH. Peptide-modified vectors for nucleic acid delivery to neurons. J Control Release. 2008;132(3):230–5. doi: 10.1016/j.jconrel.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


