Abstract
Objective
Knee surgery may alter the neuromuscular response to unexpected perturbations during functional, dynamic tasks. Long latency reflexes (LLR) follow a transcortical pathway and appear to be modifiable by task demands, potentially giving them a role in neuromuscular performance. We examined LLRs of the quadriceps and hamstrings in response to unexpected perturbations in individuals with a repaired anterior cruciate ligament (ACLR) during a weight-bearing task. We also investigated the anticipatory and volitional muscle activity that preceded and followed the LLR to quantify possible reflex adaptations associated with surgical repair.
Methods
Twelve females with ACLR and twelve healthy female controls performed a single leg squat maneuver, tracking a sinusoidal target. Random perturbations at the start of the flexion phase yielded tracking errors (“overshoot errors”) and triggered compensatory reflex activity.
Results
ACLR subjects demonstrated greater overshoot error and knee velocity during unexpected perturbations, increased LLR responses, and reduced absolute anticipatory, short-latency reflex, and voluntary quadriceps activity.
Conclusions
ACLR subjects showed impaired response to perturbation and a distinct EMG profile during a dynamic single leg weight-bearing task. Future research will determine the cause of neural adaptations in those with ACLR.
Significance
Neuromuscular adaptations may be a viable target for post-ACL injury rehabilitation interventions.
Keywords: neuromuscular control, single leg squat, weight bearing, reflexes
Introduction
An estimated 80,000 – 150,000 individuals rupture their anterior cruciate ligaments (ACL) annually in the United States (Griffin et al, 2000), with the incidence being 4 to 6 times greater in females (Hewett et al, 2006) due to a probable combination of neural and anatomical factors. Nearly 80% do not involve contact, instead occurring when an unexpected event (perturbation) occurs during voluntary contraction (Hewett et al, 2006). Approximately 60% of people who sustain ACL rupture in the United States undergo surgical reconstruction to repair the ACL (Owings and Kozak, 1998). The influence of knee surgery (ACL reconstruction) on the capability of the nervous system to respond to unexpected perturbations during dynamic conditions is unknown.
Neuromuscular control of the post-surgical knee may be undermined by altered lower extremity joint kinematics and kinetics (Bulgheroni et al, 1997, Ferber et al, 2003, Ristanis et al, 2005) tibiofemoral laxity and gait abnormalities (Brandsson et al, 2000, Knoll et al, 2004, Papannagari et al, 2006, Scarvell et al, 2006), and impaired joint position sense (Fremerey et al, 2000). Persistent quadriceps strength insufficiency occurs in some individuals (Elmqvist et al, 1989, Lorentzon et al, 1989, Snyder-Mackler et al, 1995) and may be linked to deficits in central activation (Urbach et al, 2001), perhaps via impaired gamma drive (Konishi et al, 2002). No consistent results have been reported regarding the effects of ACL reconstruction (ACLR) on muscle reflex responses. The hamstrings muscles are selectively activated in ACL deficient legs in response to support surface perturbations in an apparently reflex-mediated manner(Di Fabio et al, 1992). Delayed onset latencies of the quadriceps,(Wojtys and Huston, 2000) hamstrings(Bonfim et al, 2003, Wojtys and Huston, 2000)and gastrocnemius(Oeffinger et al, 2001) in response to a perturbation have been reported in ACLR limbs. These impairments offer a useful window into the physiologic basis of neuromuscular control of the lower limb.
In humans, long latency muscle stretch reflex responses, which occur in the window of 50–200 ms following a perturbation, are partially influenced by the motor cortex and may serve a significant purpose in preventing joint instability during perturbations (Marsden et al, 1983, Petersen et al, 1998, van Doornik et al, 2004). Recent studies have also provided direct evidence for the contribution of supraspinal centers to the long latency stretch reflexes of the quadriceps muscle (Mrachacz-Kersting et al, 2006). Reflexes adapt from operant forms of conditioning and learning (Horak et al, 1989, Wolf and Segal, 1996, Wolpaw and Carp, 1993) and may therefore be one way the neuromuscular system adapts to specific task demands or to an injury. Classic reflex studies perturb a limb relative to a fixed proximal segment in order to highlight the peripheral and or central contributions without vestibular or visual contributions (Carp and Wolpaw, 1994, Lewis et al, 2004, Mrachacz-Kersting et al, 2006). However, the behavior of reflexes in this artificial situation may be dissimilar to reflex activity during functional activities that commonly precipitate injury. Accordingly, we examined the effects of a significant environmental experience (injury, surgical repair, subsequent rehabilitation) on long latency responses during closed kinetic chain conditions, which more closely resemble tasks associated with injury and recovery.
The purpose of this study was to examine the long latency responses (LLR) of the quadriceps and hamstrings to unexpected perturbations in individuals with a repaired anterior cruciate ligament during a closed kinetic chain weight-bearing task. We also investigated the anticipatory and volitional muscle activity that preceded and followed the long latency responses to better understand contextual neural factors that may affect the LLR behavior. We hypothesize that individuals with ACLR knees, by virtue of their extensive surgical and post surgical experiences, show evidence for neural reorganization as compared to controls. Specifically, anticipatory, long latency reflexes, and volitional responses to unexpected perturbations in ACLR knees will be increased when compared to age matched controls.
Methods
Subjects
The study sample consisted of 24 female subjects (12 controls and 12 ACL reconstructed) between the ages of 18 – 30 years. Inclusion criteria were complete reconstruction of the ACL with either patellar tendon or hamstrings autograft, ability to climb stairs without difficulty, full joint ROM, absence of knee joint effusion, and regular physical activity. Individuals who had undergone meniscal repair along with ACL reconstruction were also included. Exclusion criteria included concomitant ligament injury, any other previous knee surgery, injury/surgery to the contralateral knee and pain during activity or rest. The mean time from the index surgery for the ACL reconstructed (ACLR) group was 3.7 yrs (SD=1.8) and anterior knee laxity measured by KT-2000 arthrometer was a side-to-side difference of 3.04 mm (SD=1). The control group had no previous history of knee pathology. Exclusion criteria for both groups included body mass index greater than 29, history of neurological deficits, musculoskeletal disorders, degenerative joint diseases and cardiovascular diseases. Demographic data are shown in Table 1.
Table 1.
Characteristics of Control and ACLR groups.
| Controls | ACLR | |
|---|---|---|
| Age (yrs) | 24.1 (3.2) | 22.4 (2.4) |
| Weight (lb) | 136.5 (20.3) | 144.1 (19) |
| Height (cm) | 163.8 (7.3) | 164.5 (5.28) |
| Yrs after surgery | - | 3.7 (1.8) |
| IKDC score * | 98.2 (4.8) | 85.2 (12.6) |
| Marx Activity Scale | 9.3 (4.6) | 10 (3.95) |
| Tegner Activity Scale (current) | 6.9 (2.1) | 7.1(2.4) |
| Knee laxity (max manual) (side to side diff in mm) * | 0.1 (0.91) | −3.13 (2.12) |
| SF 36 | ||
| Physical Function* | 100 (0) | 94.1 (8.7) |
| Role Physical | 97.9 (7.2) | 94.2 (20.8) |
| Body Pain* | 96.4 (2.4) | 84.5 (5.6) |
| General Health | 85.9(15.6) | 83.5(10.3) |
| Vitality* | 74.3(14.9) | 60(20.6) |
| Social Function | 100(0) | 93.7(12.5) |
| Role Emotional | 100(0) | 88.8(26) |
| Mental Health | 84(10.7) | 73.2(15.2) |
| KOOS | ||
| Symptoms* | 93.7(3.2) | 84.5(4.2) |
| Pain* | 98(3.4) | 91.9(9.35) |
| Activities of Daily Living* | 99.8(0.4) | 98(3.5) |
| Sports* | 99.6(1.24) | 82.8(25.7) |
| Quality of Life* | 97.1(7.4) | 74.5(18.2) |
Values are Mean (SD).
denotes significant difference between groups (p < 0.05).
The test side for the ACLR subjects was the reconstructed leg. The test side for the controls was pseudorandomly selected to counterbalance the ACLR limbs. Because neuromuscular adaptations likely include central components, we compared the ACLR limbs to age matched controls rather than to the opposite limb. Prior to participation subjects were given a brief description of the protocol and possible risks of participation, and were required to sign an informed consent statement approved by the University of Iowa’s Human Subjects Review board.
Screening examination
All subjects completed the following questionnaires: a general medical history form, the Short Form Medical Outcome Survey (SF 36) which assesses perception of quality of life(Ware, 1993), the IKDC Subjective Knee Evaluation form (Irrgang et al, 1998), the KOOS Knee Survey (Roos et al, 1998), the Tegner Activity Rating Scale (Tegner and Lysholm, 1985), and the Marx Activity Scale (Marx et al, 2001). We used a KT-2000 arthrometer to obtain tibiofemoral laxity measurements bilaterally in all subjects. We measured standing balance of all subjects with the commonly used single leg standing balance test on a strain gauge force plate (61 cm X 38 cm) (Balogun et al, 1997).
Experimental Task and Instrumentation
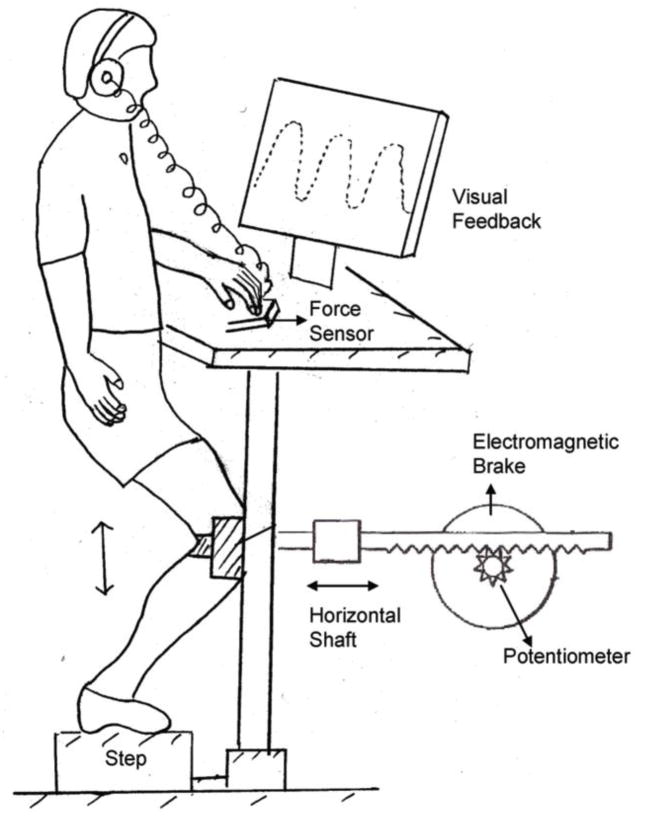
We used the single leg squat exercise (SLS), a commonly prescribed exercise for lower extremity rehabilitation, which simulates a common athletic position and requires fine control of the body over the planted leg (Livengood et al, 2004, Zeller et al, 2003). Subjects performed the resisted and controlled SLS exercise in a lower extremity perturbation device that has been described previously (Figure 1)(Madhavan and Shields, 2007, Shields and Madhavan, 2005). Briefly, the device consisted of a rack and pinion gear system that was attached to the anterior surface of the knee joint. The linear displacement of the rack during the SLS was measured by a potentiometer calibrated to convert angular displacement into linear displacement (cm). Pilot studies showed that this horizontal forward and backward translation of the knee has a strong correlation to knee angular position as measured by a video motion analysis system (R2 = 0.97). Subjects went through a range of 15 cm while performing the single leg squat, which corresponded to approximately 30 degrees of knee flexion.
Figure 1.
Schematic of the single leg squat device.
An electromagnetic brake, under computer software control, controlled the resistance of the pinion gear. The resistance of the brake was normalized to the body weight of each subject and was set at 17% body weight throughout knee flexion and extension. We chose 17% of body weight because pilot data supported that subjects could be given a significant perturbation without risk of injury. In addition, the brake also allowed near instantaneous drop in resistance to any level (perturbation), and the new level of resistance could be maintained for any desired duration. Linearity, repeatability, and hysteresis of the brake and potentiometer system were within 0.5% of full scale. Subjects were instructed to follow a sinusoidal tracking pattern that appeared on a computer monitor at a frequency of 0.4 Hz. Thus, one complete flexion and extension cycle took about 2500 milliseconds for the subjects to perform.
Subjects were permitted to place two fingers on a load sensor (Wafer Load cell, Model 872, Loadstar Sensor Inc) mounted on the left side of the device. They were instructed to put very little load through their finger, using it for light touch contact and not biomechanical support. The output from the load cell was used to provide an auditory warning if the force exceeded 5 N. Analysis of this touch force showed that subjects did not exceed 3 N of force throughout testing. Any attempt to lean on the hand would also jeopardize the ability of the subjects to complete the task.
We collected surface electromyographic (EMG) recordings from five muscles: the vastus medialis obliquus (VM), rectus femoris (RF), vastus lateralis (VL), lateral hamstrings (LH), and medial hamstrings (MH), of the exercised limb. Before fixing the electrodes, the skin was cleaned with alcohol to ensure adequate contact. Silver-silver chloride electrodes (eight millimeters in diameter) with on-site pre-amplification (gain * 35), further amplified at the main frame by 10K, were placed according to the landmarks described by Cram et al.(Cram et al, 1998). The amplifier uses a high-impedance circuit with a common mode rejection ratio of 87 dB at 60 Hz and a bandwidth of 15 to 4000 Hz (Model 544, Therapeutics Unlimited, Iowa City, IA).
Data Collection
Each subject attended one preliminary training session followed by a testing session after 24 hours. We positioned the subjects in the experimental apparatus with the test knee strapped to the movable segment of the device. The opposite leg was kept off the ground by flexing slightly at the knee and the left two fingers were allowed to make contact with the force sensor. We instructed the subjects to avoid leaning or rotating during the task and provided verbal corrections for deviations in the technique or form of exercise during the learning sessions. We marked the foot position so that any change in the position could be easily detected and corrected and the same foot placement could be maintained across days.
During the training session subjects were given sufficient practice to ensure that they were familiar in matching the sinusoidal target with knee displacement. We provided knowledge of results (error scores) during these practice trials. On the day of testing, we obtained EMG recordings during flexion and extension maximum voluntary isometric contractions (MVICs). Maximum contractions were performed with subjects seated on the chair of a Kin-Com isokinetic dynamometer (Kin-Com 125E+; Chattex Corp.; Chattanooga, TN) with the knee joint positioned in 40° of flexion per goniometric measurement. Subjects performed 3 maximum isometric contractions in extension followed by 3 maximum contractions in flexion. The trial with the highest recorded peak EMG was used to normalize the activity of each muscle during the resisted SLS task. We then positioned the subjects in the experimental device. Subjects performed 4 sets of 10 repetitions (unperturbed trials) of the SLS task to ensure that they retained the task from the previous day. The perturbation trials followed the unperturbed trials and consisted of an additional 4 sets of 10 repetitions of the SLS exercise. Representative EMG, displacement and velocity appear in Figure 2. Subjects were not exposed to the perturbation condition during the preliminary session. A perturbation consisted of release of the brake from 17% of BW to 0% BW. During each set of 10 repetitions, two unexpected perturbations (brake release) were delivered using a random number generator under software control. Each release lasted for 500 ms and was always given at 10 degrees of knee flexion, when an eccentric quadriceps contraction would consistently be underway. No verbal feedback or knowledge of results was given. The subjects were instructed to continue following the target pattern as accurately as possible even when they felt a sudden change in resistance level. A one-minute rest interval separated each set of 10 repetitions. Subjects were asked to rate their perceived exertion on a Modified Borg Scale with a range from 1, indicating no sensation of fatigue in the exercised leg, to 10 indicating a high level of fatigue in the exercised leg. No subject reported a sensation of fatigue greater than 1.5 supporting that the task did not induce localized muscle fatigue.
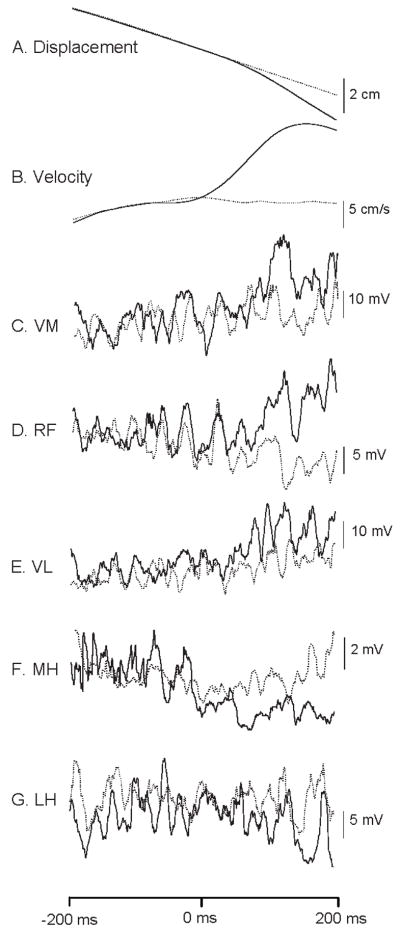
Figure 2.
Representative example of a. linear displacement, b. linear velocity, EMG traces of c. vastus medialis, d. rectus femoris, e. vastus lateralis, g. medial hamstrings and h. lateral hamstrings of a single subject (average of 8 trials). Dotted lines represent the unperturbed trials and solid lines are perturbed trials. X axis represents time (ms); the release of the brake occurred at 0 ms. EMG traces are root mean square averaged.
Data Reduction
We collected all experimental data online using Datapac 2K2 software (version 3.14; Run Technologies Inc., Vallejo, CA). We sampled electromyographic activity of the quadriceps and hamstring muscles at a rate of 2000 Hz. All other signals (linear potentiometer, target waveform, Schmitt trigger, brake and touch force) were digitized at 1000 Hz. We calculated linear velocity by differentiating the displacement signal (Tc = 10 ms) and low pass filtering at 6 Hz using a 5th order zero phase lag Butterworth filter. For the single leg balance assessment, we sampled movements of the center of pressure (COP) in the frontal and sagittal plane at a frequency of 500 Hz. The EMG signal was RMS (root mean square) processed with a time constant of 10 ms. We analyzed MVICs by finding the peak RMS EMG during each of the three contractions and calculating the mean RMS EMG for 200 ms on either side of the peak EMG. We expressed all EMG derivates as percentage of MVIC. During both perturbed and unperturbed repetitions of the task, a trigger pulse was recorded from a Schmitt trigger as the potentiometer attached to the rack and pinion gear of the exercise device passed the threshold voltage. For perturbed trials, the trigger pulse corresponded to the onset of the perturbation. For unperturbed trials, the trigger pulse served as a marker to indicate the point in the range of motion where the perturbation would have occurred if the software had allowed for it.
The first dependent variable of interest was overshoot or endpoint error, obtained by subtracting the end-point of the sine wave template target from the endpoint of subject’s flexion during the perturbation trials. The second dependent variable was peak velocity, obtained by differentiating the displacement signal. The peak of this velocity signal was measured in the 200 ms prior to perturbation, 50–200 ms after the perturbation, and 200 ms post perturbation to examine anticipatory, reflex, and voluntary phases, respectively. The third dependent variable was normalized LLR activity, obtained between 50 – 200 ms after the onset of perturbation. Normalized LLR was computed for each subject as the difference between the mean EMG of perturbation trials and the mean EMG of unperturbed trials, divided by the mean EMG of the unperturbed trials. The fourth dependent variable was the latency of peak LLR, calculated as the time to peak EMG activity between 50–200 ms following the perturbation. Finally, the fifth dependent variable was mean muscle EMG activity (expressed as % MVIC) in the 200 ms prior to perturbation, 50–200 ms after the perturbation, and 200 to 400 ms post perturbation. These time windows were analyzed to examine anticipatory, reflex, and voluntary activity, respectively. In the non-perturbation trials, the same time windows were analyzed with respect to the time when the perturbation could have occurred.
Statistical Analysis
We performed a two way repeated measures ANOVA with group (control vs. ACLR) as the between subject factor and perturbation (unperturbed vs. perturbed) as the repeated factor to test for significant differences in overshoot error, peak velocity, and mean EMG activity. We used a one factor ANOVA to compare time of peak LLR and normalized LLR of the quadriceps and hamstrings between the two groups. We performed a separate analysis for each of the five muscles sampled in the study. Before analysis we established the level of significance for all tests at p < 0.05.
A post study analysis supported that we had greater than 80% power to detect change in LLR. We also had less than 2% variation in the LLR between repeated perturbations within the same bout. We performed statistical analysis using SAS software (version 9.0).
Results
Descriptive Data
Subject descriptive data and questionnaire scores are shown in Table 1. IKDC scores, certain domains of the SF 36 (Physical Function, Bodily Pain, Vitality and Mental Health) and all domains of the KOOS survey differed significantly between the cohorts. The ACLR group scored lower on these questionnaires, reporting poorer function than the controls (p < 0.05). However, the groups did not differ significantly in their activity levels as assessed with the Marx and the Tegner activity scales. Individuals with ACLR had greater knee laxity in the knee that received the surgery (p < 0.05). Antero-posterior or medial-lateral movement amplitude of the center of pressure (COP) during single leg stance did not differ between ACLR group and controls (p > 0.05).
Overshoot Error
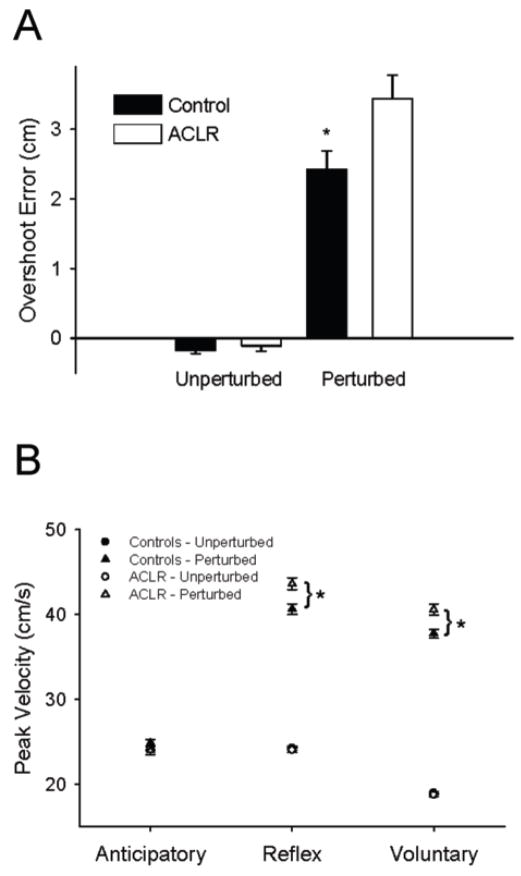
Random perturbations delivered during flexion of the SLS task caused the subjects to overshoot the target and elicited long latency muscle responses in the quadriceps and hamstrings (refer to Figure 2 for representative example). During the unperturbed trials, overshoot error did not differ between the ACLR group and controls (p > 0.05, mean error = 0.12 cm). However, the perturbation caused a 42% greater overshoot in the ACLR group as compared to the controls (p < 0.05). Mean overshoot error after a perturbation was 3.44 cm (SD = 1.72) in the ACLR group and 2.42 cm (SD = 1.37) in the control group (Figure 3A).
Figure 3.
A) Average overshoot error for the Controls (filled bars) and ACLRs (open bars) during unperturbed and perturbed trials. Values are means ± SE. * represents significant difference from ACLR (p < 0.05). B) Average peak velocity for the Controls (filled symbols) and ACLRs (open symbols) during unperturbed (circles) and perturbed (triangles) trials. Data are represented in 200 ms bins –anticipatory, reflex and voluntary- from the time perturbation occurred (perturbation trials) or would have occurred (unperturbed trials). Values are means ± SE. * represents significant difference between Controls and ACLR (p < 0.05).
Peak Velocity
Peak velocity did not differ between ACLR and control groups in the anticipatory time window for either the perturbed or the unpertubed trials (Figure 3B). Peak velocity was higher in perturbed trials than the unperturbed trials in the reflex and volitional time windows (p < 0.05). In the reflex and volitional time windows of the perturbed trials, peak velocity was higher for the ACLR group than the control group (p < 0.05).
Normalized Long Latency Responses
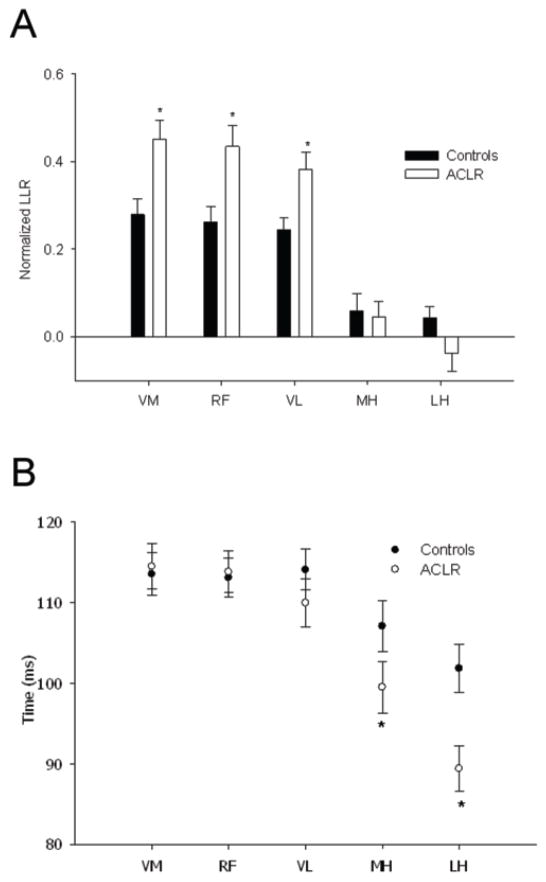
After a perturbation, normalized LLR was higher in the ACLR group than controls for VM, RF and VL muscles (all p < 0.05) (Figure 4A). Although controls showed increased LLR activity (compared to background EMG) for these muscles (+24%, +22% and +23%, respectively), ACLR EMG increases were nearly twice as high (+44%, +43% and +38%, respectively). No between-group differences appeared for MH and LH.
Figure 4.
A) LLRs normalized to background EMG activity (EMG of unperturbed trials) for the vastus medialis (VM), rectus femoris (RF), vastus lateralis (VL), medial hamstrings (MH) and lateral hamstrings (LH) during the 50 – 150 ms time bin following the perturbation, for the Controls (dark bars) and ACLRs (open bars). Values are means ± SE. * represents significant difference between Controls and ACLR (p < 0.05). B) Time at which the long latency response peaked for each of the muscles tested during the unexpected perturbations of the single leg squat task for the Controls (filled circles) and ACLRs (open circles). Values are means ± SE. * represents significant difference between Controls and ACLR (p < 0.05).
Time of Peak LLR
During the perturbation trials, latency of the peak LLR of the quadriceps muscles (VM, RF, VL) did not differ between ACLR and controls (Figure 4B). However, peak LLR latency was shorter for the ACLR group than controls for the hamstrings muscles (MH and LH, p < 0.05). For these muscles, the ACLR group triggered their peak LLR approximately 10 ms earlier than the controls.
EMG Response of Quadriceps Muscles
Anticipatory VM activity was not different for perturbed and unperturbed trials (p > 0.05), indicating that subjects did not predict when perturbations would be given. VM activity was ~44% lower for ACLR than controls in the anticipatory time window (p < 0.05) (Figure 5). Both control and ACLR subjects showed increased EMG (reflex time window) after perturbation (p < 0.05). In the reflex and volitional windows, EMG activity was lower for the ACLR group in perturbed and unperturbed trials (p < 0.05).
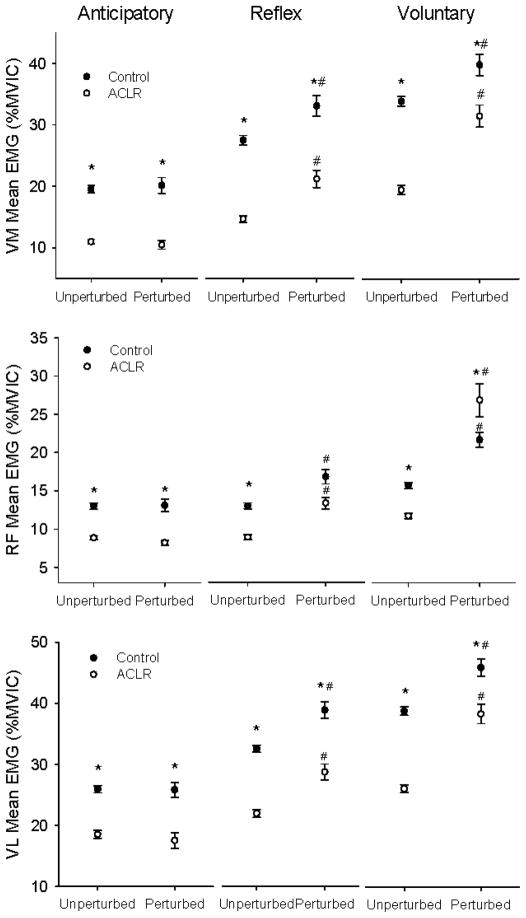
Figure 5.
Average EMG activity of the vastus medialis (VM), rectus femoris (RF) and vastus lateralis (VL) for the Controls (filled symbols) and ACLRs (open symbols) during unperturbed and perturbed trials. Data are represented in 200 ms bins -anticipatory, reflex and voluntary- from the time perturbation occurred (perturbation trials) or would have occurred (unperturbed trials). Values are means ± SE. * represents significant difference between Controls and ACLR (p < 0.05). # represents significant difference between unperturbed and perturbed trials (p < 0.05).
Anticipatory RF activity was not different for perturbed and unperturbed trials (p > 0.05). RF activity in the anticipatory bin was 30% lower for ACLR than controls (p < 0.05) (Figure 5). Both control and ACLR subjects showed increased EMG (reflex time window) after perturbation (p < 0.05). In the reflex time window, EMG activity was lower for the ACLR group than the control group only in the unperturbed trials (p < 0.05). In the volitional time window, EMG activity was lower for the ACLR group for the unperturbed trials (p < 0.05) but higher for the ACLR group in perturbed trails (p < 0.05).
Anticipatory VL activity was not different for perturbed and unperturbed trials (p > 0.05). Both groups of subjects demonstrated increased EMG activity after perturbations (p < 0.05). VL activity in the anticipatory, reflex and volitional time windows was lower for ACLR than controls (p < 0.05) (Figure 5). For both groups, VL activity in the reflex bin was higher for perturbed than for unperturbed trials (p < 0.05).
In summary, for the VM, RF, and VL muscles, perturbation yielded greater EMG activity in the reflex and volitional time windows for both subject cohorts. For VM and VL, control EMG for these muscles was consistently higher than ACLR EMG. On the other hand, the RF EMG response to perturbation was particularly strong for the ACLR group: ACLR EMG activity either met (reflex window) or exceeded (volitional window) the control group values.
EMG Response of Hamstrings Muscles
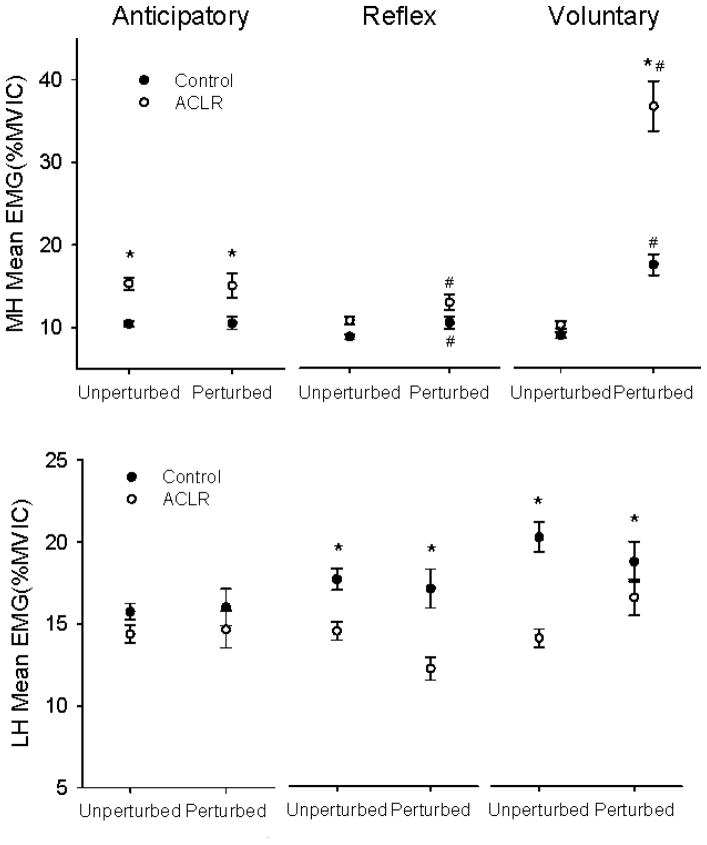
In contrast to the quadriceps, MH EMG activity for the ACLR group was higher than control values (+38%) in the anticipatory time window (p < 0.05) (Figure 6). EMG increased for both groups after perturbation (reflex bin, p < 0.05) but the between-group difference did not persist (p > 0.05). EMG in the volitional time window likewise increased for both groups after perturbation, but the increase for the ACLR group was particularly noteworthy. The volitional EMG increase for the ACLR group was nearly two-fold greater than for the control group (p < 0.05).
Figure 6.
Average EMG activity of the medial (MH) and lateral (LH) hamstrings for the Controls (filled symbols) and ACLRs (open symbols) during unperturbed and perturbed trials. Data are represented in 200 ms bins -anticipatory, reflex and voluntary- from the time perturbation occurred (perturbation trials) or would have occurred (unperturbed trials). Values are means ± SE. * represents significant difference between Controls and ACLR (p < 0.05. # represents significant difference between unperturbed and perturbed trials (p < 0.05).
LH activity was on average 15–20% in the anticipatory, reflex and voluntary bins for the two groups (Figure 6). Control group LH EMG was significantly higher than ACLR for the reflex and voluntary bins (p < 0.05).
Discussion
The purpose of this study was to examine the LLR responses of the quadriceps and hamstrings to unexpected perturbations in individuals with a repaired anterior cruciate ligament during a novel weight-bearing task. We also investigated the anticipatory and volitional muscle activity that preceded and followed the LLR responses in order to understand factors that may modulate the LLR responses. By virtue of their transcortical pathway (Mrachacz-Kersting et al, 2006), their adaptability to training (Horak et al, 1989) and their likely role in maintaining joint stability (Marsden et al, 1983, Petersen et al, 1998, van Doornik et al, 2004), LLR responses may adapt after significant events like ACL knee reconstruction surgery. We found that the quadriceps muscles of ACLR subjects exhibited significantly higher normalized LLR responses than controls after a perturbation (Figure 4A).
Three new findings emerged from this study. First, overall quadriceps EMG background activity was reduced in individuals with ACLR knees when compared to control subjects (anticipatory time window, unperturbed trials, Figure 5). Second, overshoot error and knee velocity during unexpected perturbations was greater in the ACLR group (Figure 3). Third and most importantly, although mean quadriceps reflex activity (50 – 200 ms following the perturbation) was lower in the ACLR limbs (Figure 5, reflex time window), the magnitude of the LLR when normalized to background EMG (mean EMG of unperturbed trials) was nearly two times higher in this group (Figure 4A). This is the first study to report up-regulated LLR responses in patients with ACLR knees, a finding that may not be evident in isolated limb perturbation studies.
The rationale for this study was to examine LLR responses during a task that has visual and vestibular activity, as is present during tasks when injury initially occurs. In addition, by developing a task that required significant (but safe) background muscle activity prior to the perturbation, we were able to discriminate responses between those with and without a major injury, surgery, and rehabilitation program. The basis for the enhanced LLR responses may be a protective response that develops because of the extensive rehabilitation, the surgery, or the severity of the injury that led to the surgery. Because most individuals with severe ACL injury have reconstructive surgery, it is difficult to find a large cohort of active females who did not have surgery, but engaged in a comparable level of intense rehabilitation. Future studies may strive to determine the event that specifically leads to the neuromuscular adaptations reported in this study.
The underlying the origin and the pathways responsible for LLR responses is highly controversial. In the upper limb (forearm, hand and finger muscles), evidence supports that the LLR pathway incorporates a supraspinal loop which travels to the motor cortex (Lewis et al, 2004, Marsden et al, 1983, Matthews, 1991). It has been suggested that the long latency response of the biceps brachii is mediated by velocity sensitive receptors (Ia receptors) (Lewis et al, 2005). In the lower limb, some studies have provided evidence for supraspinal contributions to long latency stretch responses of the tibialis anterior and rectus femoris (Mrachacz-Kersting et al, 2006). The increased normalized quadriceps LLR response to an unexpected perturbation in the ACLR group could be attributable to numerous causes. We found that the ACLR group did not increase their LLRs proportionally to background activity; rather, the magnitude of increase was almost two times higher than controls. This finding of greater normalized LLR responses suggests that the elicited quadriceps long latency response was not just an automatic compensation of reflex gain based on pre-existing (background) muscle activity, contrary to the classical view of “automatic gain compensation” (Matthews, 1986). Upper limb studies have shown that the need for LLR responses to accomplish a particular goal is lower when preparatory actions are observed (Johansson and Westling, 1988). In this study, since the individuals with ACLR knees had low “preparatory actions” (lesser anticipatory quadriceps activity), it is possible that they required larger long latency responses to compensate. Individuals with ACLR knees also showed higher peak velocities of knee flexion in response to the perturbation, accounting for a greater change in quadriceps muscle length and possibly a larger sensory input from the muscle spindles (group Ia, II afferents which are sensitive to changes in length and velocity). A greater input from the large diameter afferents (Ia and II’s) may be another possible reason for the larger normalized LLRs seen in ACLR individuals. Individuals with ACLR knees have been reported to show abnormal gamma loop sensitivity after surgery (Konishi et al, 2002). Alterations in muscle spindle gain could therefore also be another possible explanation for the altered normalized long latency responses in the ACLR individuals.
Changes in cortical excitability in individuals with ACL injuries have been previously reported (Heroux and Trenblay, 2006). Cortical mediation allows for better adjustment of the reflex to prevailing conditions. The LLR response in the ACLR group may be modulated in a manner appropriate to meet the motor demands (protection against perturbation) in these subjects. One way to achieve such modulation would be to vary the corticospinal drive to the lower limb motor neurons according to the task performed. Another point to be noted is that this greater magnitude of normalized LLRs was observed not just in the RF but also in the VM and VL. The RF has been suggested to have a different neural control than the other components of the quadriceps muscle (Mrachacz-Kersting et al, 2006). This study suggests that transcortical pathways also influence the LLR responses of the VM and VL. Depending on the type of task that is performed and the task demands, it is probable that LLR responses of different muscles come under different levels of cortical input.
Our findings of greater overshoot error, knee velocity and altered muscle activity in individuals with ACLR suggest that adaptations in neuromuscular control of the knee persist in patients even 4 years post surgery. Individuals with ACLR showed reduced activity of the quadriceps during the anticipatory, reflex and volitional phases of the SLS task. Considering the fact that unopposed quadriceps activity could translate the tibia forward and strain the ACL, this reduction in quadriceps activity could be a protective mechanism adapted by the CNS to protect the reconstructed ligament. We also observed that the ACLR group showed greater activity of the medial hamstrings compared to the controls during the anticipatory and volitional phases of knee flexion (Figure 6). In addition, the latency of the peak hamstrings LLR was shorter in the ACLR group (Figure 4B). Higher co-activation of the hamstrings has been reported previously during various activities in ACL injured individuals, complementing the action of the ACL (Grabiner et al, 1989, Solomonow and Krogsgaard, 2001). This increased activity of the hamstrings could partly explain the greater overshoot error seen in ACL reconstructed individuals. Congruent with this view, we previously observed that elderly individuals whose hamstrings activity was low (matching a younger cohort) demonstrated low overshoot error during the SLS task (Madhavan and Shields, 2009). Although medial hamstrings activity was greater in the anticipatory phase, activity of the lateral hamstrings did not differ from the Control cohort, suggesting that uncovering a protective role for the hamstrings may not be straightforward.
The present study reveals that increased overshoot error, enhanced LLRs, and overall reduced quadriceps activity all coexist in subjects with ACL reconstruction. Collectively, individuals with ACLR undergo a neural reorganization that is distinct from the neural control strategies portrayed by similar individuals without injury. Currently, we do not know if the neural reorganization demonstrated in this study was attributable to the injury, the surgery, the extensive rehabilitation or collectively the combination of all environmental factors that influence the CNS when developing movement control strategies. Future studies are necessary to determine if up regulated LLRs are protective against certain unexpected events, or, at times, contributory to injury. It is conceivable that the CNS can compensate for certain unexpected events in order to support a lower level of background quadriceps activity. Alternatively, up regulated LLRs may be a strategy that is effective when mild unexpected perturbations occur, but contribute to excessive anterior shear of the tibia during more violent unexpected perturbations. Future studies will also add to our understanding of possible transcortical elements of the LLR and its potential adaptability to rehabilitation strategies, including neuromuscular and cortical electrical stimulation.
Conclusions
Individuals with ACL reconstruction showed distinct neuromuscular control of the knee during a dynamic single leg weight-bearing task. ACLR subjects exhibited decreased quadriceps activity and enhanced long latency responses to unexpected perturbations. Additional research is needed to determine the role that up regulated LLRs play in response to various types of unexpected events in individuals with ACLR.
Acknowledgments
This work was supported in part by NIH R01-NR-010285-05 (RKS).
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balogun JA, Ajayi LO, Alawale F. Determinants of single limb stance balance performance. African Journal of Medicine & Medical Sciences. 1997;26:153–7. [PubMed] [Google Scholar]
- Bonfim TR, Jansen Paccola CA, Barela JA. Proprioceptive and behavior impairments in individuals with anterior cruciate ligament reconstructed knees. Arch Phys Med Rehabil. 2003;84:1217–23. doi: 10.1016/s0003-9993(03)00147-3. [DOI] [PubMed] [Google Scholar]
- Brandsson S, Kartus J, Larsson J, Eriksson BI, Karlsson J. A comparison of results in middle-aged and young patients after anterior cruciate ligament reconstruction. Arthroscopy. 2000;16:178–82. doi: 10.1016/s0749-8063(00)90033-1. [DOI] [PubMed] [Google Scholar]
- Bulgheroni P, Bulgheroni MV, Andrini L, Guffanti P, Giughello A. Gait patterns after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1997;5:14–21. doi: 10.1007/s001670050018. [DOI] [PubMed] [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994;72:431–42. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- Cram JR, Kasman GS, Holtz J. Introduction to surface electromyography. Gaithersburg: Aspen Publishers Inc; 1998. [Google Scholar]
- Di Fabio RP, Graf B, Badke MB, Breunig A, Jensen K. Effect of knee joint laxity on long-loop postural reflexes: evidence for a human capsular-hamstring reflex. Exp Brain Res. 1992;90:189–200. doi: 10.1007/BF00229271. [DOI] [PubMed] [Google Scholar]
- Elmqvist LG, Lorentzon R, Johansson C, Langstrom M, Fagerlund M, Fugl-Meyer AR. Knee extensor muscle function before and after reconstruction of anterior cruciate ligament tear. Scand J Rehabil Med. 1989;21:131–9. [PubMed] [Google Scholar]
- Ferber R, Osternig LR, Woollacott MH, Wasielewski NJ, Lee JH. Gait perturbation response in chronic anterior cruciate ligament deficiency and repair. Clinical Biomechanics. 2003;18:132–41. doi: 10.1016/s0268-0033(02)00182-1. [DOI] [PubMed] [Google Scholar]
- Fremerey RW, Lobenhoffer P, Zeichen J, Skutek M, Bosch U, Tscherne H. Proprioception after rehabilitation and reconstruction in knees with deficiency of the anterior cruciate ligament: a prospective, longitudinal study. J Bone Joint Surg Br. 2000;82:801–6. doi: 10.1302/0301-620x.82b6.10306. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Campbell KR, Hawthorne DL, Hawkins DA. Electromyographic study of the anterior cruciate ligament-hamstrings synergy during isometric knee extension. J Orthop Res. 1989;7:152–5. doi: 10.1002/jor.1100070122. [DOI] [PubMed] [Google Scholar]
- Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–50. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Heroux ME, Trenblay F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Knee Surgery Sports Traumatology Arthroscopy. 2006;14:823–33. doi: 10.1007/s00167-006-0063-4. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am J Sports Med. 2006;34:299–311. doi: 10.1177/0363546505284183. [DOI] [PubMed] [Google Scholar]
- Horak FB, Diener HC, Nashner LM. Influence of Central Set on Human Postural Responses. Journal of Neurophysiology. 1989;62:841–53. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80:1132–45. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Programmed and triggered actions to rapid load changes during precision grip. Exp Brain Res. 1988;71:72–86. doi: 10.1007/BF00247523. [DOI] [PubMed] [Google Scholar]
- Knoll Z, Kocsis L, Kiss RM. Gait patterns before and after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12:7–14. doi: 10.1007/s00167-003-0440-1. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Fukubayashi T, Takeshita D. Mechanism of quadriceps femoris muscle weakness in patients with anterior cruciate ligament reconstruction. Scandinavian Journal of Medicine & Science in Sports. 2002;12:371–5. doi: 10.1034/j.1600-0838.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ, MacKinnon CD. The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle. Exp Brain Res. 2005;163:361–9. doi: 10.1007/s00221-004-2182-9. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Polych MA, Byblow WD. Proposed cortical and sub-cortical contributions to the long-latency stretch reflex in the forearm. Exp Brain Res. 2004;156:72–9. doi: 10.1007/s00221-003-1767-z. [DOI] [PubMed] [Google Scholar]
- Livengood AL, DiMattia MA, Uhl TL. “Dynamic Trendlenburg”: Single-Leg-Squat Test for Gluteus Medius Strength. Athetic Therapy Today. 2004;9:24–25. [Google Scholar]
- Lorentzon R, Elmqvist LG, Sjostrom M, Fagerlund M, Fuglmeyer AR. Thigh musculature in relation to chronic anterior cruciate ligament tear: muscle size, morphology, and mechanical output before reconstruction. Am J Sports Med. 1989;17:423–9. doi: 10.1177/036354658901700318. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Shields RK. Weight-bearing exercise accuracy influences muscle activation strategies of the knee. J Neurol Phys Ther. 2007;31:12–9. doi: 10.1097/01.npt.0000260569.69863.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan S, Shields RK. Influence of age on neuromuscular control during a dynamic weight-bearing task. Journal of Aging and Physical Activity. 2009;17:327–43. doi: 10.1123/japa.17.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC, Day BL. Long-Latency Automatic Responses to Muscle Stretch in Man: Origin and Function. In: Desmedt JE, editor. Motor Control Mechanisms in Health and Disease. New York: Raven Press; 1983. pp. 509–39. [PubMed] [Google Scholar]
- Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–8. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. The human stretch reflex and the motor cortex. Trends Neurosci. 1991;14:87–91. doi: 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Grey MJ, Sinkjaer T. Evidence for a supraspinal contribution to the human quadriceps long-latency stretch reflex. Exp Brain Res. 2006;168:529–40. doi: 10.1007/s00221-005-0120-0. [DOI] [PubMed] [Google Scholar]
- Oeffinger DJ, Shapiro R, Nyland J, Pienkowski D, Caborn DN. Delayed gastrocnemius muscle response to sudden perturbation in rehabilitated patients with anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2001;9:19–27. doi: 10.1007/s001670000152. [DOI] [PubMed] [Google Scholar]
- Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat. 1998;13:1–119. [PubMed] [Google Scholar]
- Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34:2006–12. doi: 10.1177/0363546506290403. [DOI] [PubMed] [Google Scholar]
- Petersen N, Christensen LO, Morita H, Sinkjaer T, Nielsen J. Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man. J Physiol. 1998;512 ( Pt 1):267–76. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:1323–9. doi: 10.1016/j.arthro.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR. Does anterior cruciate ligament reconstruction restore normal knee kinematics?: A prospective MRI analysis over two years. J Bone Joint Surg Br. 2006;88:324–30. doi: 10.1302/0301-620X.88B3.16787. [DOI] [PubMed] [Google Scholar]
- Shields R, Madhavan S. Neuromusuclar Control of the Knee during a Resisted Single Limb Squat Exercise. American Journal of Sports Medicine. 2005 doi: 10.1177/0363546504274150. Online July 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995;77:1166–73. doi: 10.2106/00004623-199508000-00004. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Krogsgaard M. Sensorimotor control of knee stability. A review. Scand J Med Sci Sports. 2001;11:64–80. doi: 10.1034/j.1600-0838.2001.011002064.x. [DOI] [PubMed] [Google Scholar]
- Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985:43–9. [PubMed] [Google Scholar]
- Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris a prospective twitch interpolation study. J Bone Joint Surg Br. 2001;83:1104–10. doi: 10.1302/0301-620x.83b8.11618. [DOI] [PubMed] [Google Scholar]
- van Doornik J, Masakado Y, Sinkjaer T, Nielsen JB. The suppression of the long-latency stretch reflex in the human tibialis anterior muscle by transcranial magnetic stimulation. Exp Brain Res. 2004;157:403–6. doi: 10.1007/s00221-004-1966-2. [DOI] [PubMed] [Google Scholar]
- Ware JE. Manual and Interpretation Guide. Nimrod Press; 1993. SF-36 Health Survey. [Google Scholar]
- Wojtys EM, Huston LJ. Longitudinal effects of anterior cruciate ligament injury and patellar tendon autograft reconstruction on neuromuscular performance. American Journal of Sports Medicine. 2000;28:336–44. doi: 10.1177/03635465000280030901. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Segal RL. Reducing human biceps brachii spinal stretch reflex magnitude. J Neurophysiol. 1996;75:1637–46. doi: 10.1152/jn.1996.75.4.1637. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Carp JS. Adaptive plasticity in spinal cord. Adv Neurol. 1993;59:163–74. [PubMed] [Google Scholar]
- Zeller BL, McCrory JL, Kibler WB, Uhl TL. Differences in kinematics and electromyographic activity between men and women during the single-legged squat. American Journal of Sports Medicine. 2003;31:449–56. doi: 10.1177/03635465030310032101. [DOI] [PubMed] [Google Scholar]