Table 2.
Binding Constants of SaDHNA and Site-Directed Mutantsa
| SaDHNA | SaE22A | SaE74A | SaK100A | SaK100Q | ||
|---|---|---|---|---|---|---|
| NP | Kd (μM) | 18±2 | 13±0.6 | > 4000 | 6.9±0.7 | 11±0.3 |
| k1 (M−1s−1) | (2.4±0.1)×105 | (2.9±0.1)×105 | n.d.b | (2.2±0.1)×105 | (1.9±0.1)×105 | |
| k−1 (s−1) | 4.5±0.1 | 4.0±0.1 | n.d.b | 1.2±0.1 | 2.0±0.1 | |
| MP | Kd (μM) | 13±1 | 11±0.9 | >3000 | 9.1±0.6 | 13±1 |
| k1 (M−1s−1) | (2.9±0.2)×105 | (3.1±0.1)×105 | n.d.b | (2.7±0.1)×105 | (2.8±0.1) ×105 | |
| k−1 (s−1) | 4.2±0.2 | 4.0±0.1 | n.d.b | 2.1±0.1 | 4.3±0.1 | |
| HPO | Kd (μM) | 24±0.2 | 17±2 | >6000 | 6.0±0.1 | 8.8±0.4 |
| k1 (M−1s−1) | (4.5±0.2)×105 | (5.6±0.3)×105 | n.d.b | (6.4±0.3)×105 | (6.8±0.4)×105 | |
| k−1 (s−1) | 10±0.5 | 10±0.2 | n.d.b | 5.8±0.3 | 5.8±0.1 | |
Both the wild-type SaDHNA and mutants have a His-tag (MHHHHHH) at the N-terminus. We have shown previously that the His-tag has no effects on the binding and catalytic properties of the enzyme. The chemical structures of the measured compounds are as follows.
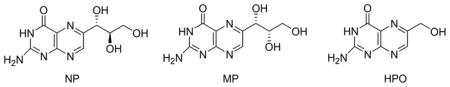
n.d.: not determined.
