Abstract
Human molecular cytogenetics integrates the knowledge on chromosome and genome organization at the molecular and cellular levels in health and disease. Molecular cytogenetic diagnosis is an integral part of current genomic medicine and is the standard of care in medical genetics and cytogenetics, reproductive medicine, pediatrics, neuropsychiatry and oncology. Regardless numerous advances in this field made throughout the last two decades, researchers and practitioners who apply molecular cytogenetic techniques may encounter several problems that are extremely difficult to solve. One of them is undoubtedly the occurrence of somatic genome and chromosome variations, leading to genomic and chromosomal mosaicism, which are related but not limited to technological and evaluative limitations as well as multiplicity of interpretations. More dramatically, current biomedical literature almost lacks descriptions, guidelines or solutions of these problems. The present article overviews all these problems and gathers those exclusive data acquired from studies of genome and chromosome instability that is relevant to identification and interpretations of this fairly common cause of somatic genomic variations and chromosomal mosaicism. Although the way to define pathogenic value of all the intercellular variations of the human genome is far from being completely understood, it is possible to propose recommendations on molecular cytogenetic diagnosis and management of somatic genome variations in clinical population.
Keywords: Molecular cytogenetics, somatic genome variations, molecular diagnosis, chromosome instability, genomic instability, mosaicism.
INTRODUCTION
It is hard to find a cytogeneticist who has not encountered the problem of interpreting mosaicism. It seems to be relatively simple when the majority of cells are abnormal, but is extremely difficult in low-level mosaics or in cases of detecting cryptic, complex or dynamic mosaic cell populations. With the introduction of molecular cytogenetic techniques into basic research and diagnostic practice, this has become not only a problem of practicing cytogeneticists, but a major focus of biomedicine encompassing genomics, medical genetics, neuropsychiatry, aging research and reproductive medicine [1]. Since postulating the idea that somatic genome variations (SGV) can be a source of human interindividual diversity in health and disease [2, 3], it became evident that current diagnostic research cannot leave aside this issue.
There are two main problems surrounding SGV detection during molecular cytogenetic diagnosis. The first one is technological. The majority of the researchers’ audience is unfamiliar with techniques providing for the highest resolution of SGV evaluation, even though such technologies do exist [4]. Additionally, no criteria have been delineated for definition of mosaic genome variations [1-4]. The second problem is related to interpretation of data on SGV. Succeeding in detecting mosaicism (or SGV) does not necessarily mean diagnostic success. It is usually hard to come to definite conclusion about results of scoring such a rare event as low-level mosaicism. The problem is even more complicated because there are no data on benign rates of SGV in humans. Therefore, to provide for opportunities regarding molecular cytogenetic diagnosis of SGV (mosaicism), additional large-scale studies and case-reports performed with thorough phenotype evaluations and high-resolution single-cell genome analyses are required [1-3].
This article overviews both problems in the light of new developments in molecular cytogenetics. Further, we have attempted to define the way it can be managed in molecular cytogenetic diagnosis of chromosome imbalances in normal and clinical population on the basis of rare data on SGV. Additionally, associations between this phenomenon and disease are described in order to simplify interpretations of mosaicism. Finally, brief troubleshooting and perspectives of SGV research in this extent are given to assist researchers in molecular diagnosis of mosaicism.
THE ESTIMATED IMPACT OF SGV ON MOLECULAR CYTOGENETIC DIAGNOSIS
To get a view of SGV impact on molecular cytogenetic diagnosis, one has to refer to the diseases that are associated with. These are malignant [5, 6], brain [3, 7] and autoimmune diseases [8] as well as a number of hereditary and chromosomal syndromes that are associated with genome or chromosome instability [1-3, 7, 9, 10]. Numerous morbid conditions are also found to associate with SGV or chromosomal mosaicism: spontaneous fetal loss [11, 12], stillbirth, idiopathic congenital malformations or some clinical features of hereditary polygenic disorders [1-3, 11, 13]. Finally, cases of complex chromosomal and genomic rearrangements (CCRs), copy number variations (CNVs) and monogenic syndromes can exhibit SGV [14-18]. Together, it makes an appreciable contribution to human morbidity.
Molecular cytogenetic diagnosis has become an integral part of modern medical care being required for numerous fields of medicine [1-4, 19-21]. Molecular cytogenetic techniques are not equally applied for each aforementioned condition. All types of chromosomal imbalances manifesting at chromosomal or subchromsomal level (i.e. chromosomal syndromes, almost all malignant and some brain diseases, fetal losses and idiopathic congenital malformations) strongly require molecular cytogenetic techniques in contrast to genome/chromosome instability syndromes that are caused by gene mutations [2-4, 19-35]. The resolution or application need of molecular cytogenetic techniques for diagnosis is determined by DNA sequences that are involved in genomic rearrangements (for more details see [2-4, 19-21]). The molecular cytogenetic platforms that have to be mentioned in this context are FISH (fluorescence in situ hybridization) and CGH (comparative genomic hybridization) or, more precisely, array CGH. However, the most applicable methods for detection of SGV are based on FISH (for review see [2, 4]). Table 1 gives an overview of conditions associated with SGV that requires molecular cytogenetic diagnosis.
Table 1.
Diseases and Morbid Conditions Associated with SGV Requiring Molecular Cytogenetic Diagnosis
| Disease/Morbid Condition | Platforms for Diagnosis | Necessity of Molecular Cytogenetic Diagnosis* | Key Refs |
|---|---|---|---|
| Spontaneous abortions (10-15 wks) | FISH | +++ | [12] |
| Abnormal prenatal development (prenatal diagnosis) | FISH | +++ | [11, 22-24] |
| CGH** | |||
| Chromosomal syndromes/non-specific causal abnormalities | FISH | +++ | [1-4, 11, 19-21] |
| CGH | |||
| Diseases caused by CNVs | CGH | +++ | [25] |
| FISH | |||
| Idiopathic congenital malformations | FISH | +++ | [2, 11, 19-21] |
| Developmental delays | CGH | ||
| Idiopathic learning disability | FISH | +++ | [7, 11, 26] |
| CGH | |||
| Cancer | FISH | +++ | [2, 4-6, 19-21] |
| CGH | |||
| Autism | FISH | ++ | [7, 27-30] |
| CGH | |||
| Schizophrenia | FISH | + | [31-33] |
| CGH | |||
| Autoimmune diseases | FISH | + | [8] |
| Monogenic syndromes | Fiber FISH | + | [34, 35] |
–the level of necessity is defined as indispensable
–array CGH included.
rarely applied (+);
method of choice
As one can see, morbid conditions, which are in need of molecular cytogenetics, are either those caused by subtle genomic rearrangements (detected at molecular level) or those associated with mosaicism in a proportion of cases. These are more commonly manifested as cases of chromosomal mosaicism [1]. Syndromes of chromosome instability exhibit changes in chromosomal numbers and/or structure in somatic cells, which can be defined as SGV or somatic chromosomal mosaicism [10, 36, 37]. However, being of monogenic nature, many of these diseases are usually diagnosed via molecular genetic techniques (sequencing, PCR). Therefore, a molecular cytogenetic technique applied in this instance has to provide simultaneously for high resolution and for single-cell analysis [4]. The next part of our brief review addresses this as well as other issues concerning technical aspects of SGV detection by molecular cytogenetic techniques.
THE FIRST PROBLEM: TECHNICAL POTENTIAL OF MOLECULAR CYTOGENETICS FOR SGV DETECTION
Recently, a series of reviews has addressed the level of excellence in molecular cytogenetics with respect to identification of SGV [1-4, 21, 38]. It allows us to skip wide discussions about technological aspects of molecular cytogenetic analyses of human chromosomes for detection of mosaicism/SGV. Nevertheless, we would like to point some aspects relevant to the diagnosis. Firstly, we have to mention that it is really possible to achieve the resolution as high as 1% of cells affected by SGV or even lower [4, 28, 36-42]. The scoring of such a rare event as low-mosaicism is granted by approaches that include several molecular cytogenetic FISH-based techniques. These analyses possess the potential to embrace large cell populations. The latter means such assays to be more likely interphase than metaphase ones. For analyzing the majority of cell types, this is the unique possibility. Here, it is to note the existence of numerous drawbacks of the classical interphase FISH protocols: numerous genome behavioral peculiarities (replication, high-ordered chromosome organization in interphase nuclei) mimic chromosome number/structure variations (SGV), chromosomal heteromorphisms, or simply lack of visualization of whole chromosomes [4, 36, 43-47]. To get rid of these technical problems, it has been proposed to use in parallel multiprobe interphase FISH (simultaneous multitarget), digital analyses of FISH data (QFISH) and interphase multicolor banding technique for visualization of whole chromosomes in non-dividing cells [3, 12, 29, 36-44, 46, 47]. Although the approach has been exclusively applied to basic research, it has the potential to be a powerful diagnostic tool [1-4, 21].
Regardless developments in molecular cytogenetics that allow to analyze either interphase nuclei or DNA isolated from any cell type, the major part of cases requiring cytogenetic evaluation are firstly assessed through metaphase-analysis-based techniques [4, 21, 48]. This is the source of another, yet unresolved, problem — discrepancies between metaphase and interphase molecular cytogenetic analyses. To date, no clear explanation has been found to understand the basis and meaning of such differences. Eventually, one can see it looking through those few papers describing recommendations concerning detection of mosaicism in clinical cytogenetic practice or basic SGV research [2, 3, 12, 29, 36-42, 49-51]. Despite of the same ultimate aim referred to uncovering the presence of mosaicism, these guidelines differ significantly by technological requirements and the ways of the development. Taking into account the lack of major technological impediments, the problem of “mosaicism guidelines” correlation is the essential technical one.
THE SECOND PROBLEM: WHAT DOES SGV MEAN?
As previously noted, the confirmation of mosaicism (especially, low-level mosaicism) in an individual leads to the problem of the interpretation. In other words, pathogenicity of mosaicism or SGV is always a matter of conjecture. It seems to be easier when abnormal cell lines are prevalent, but the borderline between pathogenic condition and benign variation is undetermined [3]. This is further complicated inasmuch as SGV is more commonly observed in a clinical population and is known to be associated with malignization [1-15, 25-29, 31, 32, 35-38, 42, 48]. Due to a higher frequency, the best studied in this context is aneuploidy or aneuploidization of somatic tissues [1-4]. Analyses of aneuploidy effect on transcriptional activity of the whole cellular genome yielded contradictory results, showing, however, that aneuploidization should possess a global negative effect on cellular physiology via transcriptional changes [52, 53]. Studies implicating clinical population, aneuploidy syndromes or chromosomally instable tissues (disease models) have shown that deleterious effects are only produced in cases of either chromosome-specific increase of aneuploidy rates or a global rise of spontaneous chromosomal mutations. Background (sporadic) aneuploidy is more likely to be a result of natural (benign) SGV without appreciable effects [2, 3, 7, 10, 27-29, 32, 36-42, 54].
Since the data on SGV were rarely confirmed to be pathogenic, it is better to consider such genomic variations rather a neutral or a susceptibility factor than a causative genetic abnormality. Unless repeatedly described in the literature, low-level mosaics or SGV cannot be defined as disease-causing mutations. It does not mean that some disease with strong genetic background do not require molecular cytogenetic diagnosis respecting the presence of SGV, but suggests more critical assessment as to constitutional genomic rearrangements. In summary, even though several lines of evidence suggest an association between low-level mosaics, SGV and an abnormal phenotype, their clinical interpretation and diagnostic value seem to require additional case-control studies.
SOLUTIONS AND RECOMMENDATIONS
Molecular cytogenetic diagnosis of SGV means: (i) scoring of rare events; (ii) possible lack of reproducibility; (iii) different approaches may not yield similar results; (iv) detection of SGV does not imply guaranteed diagnostic success. Together, it makes an impression that the solution of all the difficulties surrounding this task is hardly possible. Notwithstanding, either separate or sequential application of numerous molecular cytogenetic techniques gives extremely high resolution for scoring single-cell rare events [1-3, 12, 36-42]. The irreproducibility is essentially produced by analysis of cells cultivated in vitro (scoring of insufficient number of metaphase cells or combined metaphase/interphase analyses) [4]. Therefore, application of interphase molecular cytogenetic approaches using uncultured cell populations can help to escape from such situations. This is partially confirmed by analysis of SGV in neuropsychiatric diseases [27-29, 32, 36, 37, 40-47]. These studies as well as the analysis of unaffected somatic tissues have also generated an integrated approach towards uncovering SGV affecting 1% of cells or lower [2-4, 7, 29, 36, 37, 40-47]. Thus, SGV detection meets no more technological problems than those associated with selecting benchmarks for evaluations of mosaicism in a given cell population. Although it still does not answer the question concerning the pathogenicity, the application of specified guidelines allows to make evaluations of mosaicism occurrence in large cohorts with respect to its biomedical meaning. Moreover, these data would give the possibility to determine the real amount of abnormal cells in an individual regardless any effect it can produce. Table 2 summarizes experimentally tested recommendations for detection of mosaicism (SGV).
Table 2.
Recommendations for Detection of Chromosomal Mosaicism and SGV
| Source | Area of Application | Detection Rate | Amount of Cells to Score |
|---|---|---|---|
| Hsu et al., 1992 [49] | Prenatal diagnosis by cytogenetic techniques | 0.5% (1 cell)* | 200 |
| Caudill et al., 2005 [50] | Prenatal diagnosis by cytogenetic techniques | 15% | 15-30 |
| Vorsanova et al., 2005 [12] | Fetal aneuploidy by molecular cytogenetic techniques | <5% | 300-500 |
| Yurov et al., 2005 [39]; 2007 [29, 41]; 2008 [42]; Iourov et al., 2006 [3]; 2009 [38]. | Chromosome abnormalities or SGV by molecular cytogenetic techniques | <0.1% | 1000-10000 |
| Iourov et al., 2009 [36, 37] | Chromosome instability or SGV by molecular cytogenetic techniques | 0.1-1% | 1000-10000 |
| Iourov et al., 2006 [40]; 2009 [38] | Chromosome abnormalities or SGV by molecular cytogenetic techniques | <0.5% (1 cell)* | >100 |
| Wiktor et al., 2009 [51] | Sex chromosome aneuploidy by cytogenetic techniques | >3% (1 cell) | 20-30 |
– pseudomosaicism.
The recommendations presented in Table 2 are unlikely to be combined for producing unified guidelines. The latter is mainly caused by different techniques applied for the development and area of application. Therefore, to solve the problem, a large-scale study of several cell populations acquired from different somatic human tissues appears to be strongly required. Nonetheless, before such data will be available, one has to follow these recommendations or to develop new ones on basis of original molecular cytogenetic investigation.
Despite of difficulties in interpreting results, there are positive associations between SGV and several morbid conditions [1-3, 7, 12, 29, 36, 37, 40-47, 54]. Since no other data is available to solve the problem of interpretation multiplicity, we found pertinent to mention all the pathologic conditions associated with SGV.
SGV manifesting as chromosome-specific mosaicism or instability are common among spontaneous abortions in the first trimester [12]; chromosomal, microdeletion/duplication and CNV-associated syndromes [3, 7, 11, 13, 25, 45, 48, 54]; chromosome instability syndromes (ataxia-telangiectasia) [37]; supernumerary marker chromosomes [55-57]; idiopathic learning disability with/without congenital malformations [1-4, 7, 58, 59]; autism [27-29]; schizophrenia [31, 32, 42]; autoimmune diseases [8]; Alzheimer’s disease [36]. This type of SGV is also a susceptibility factor for female germline aneuploidization leading to trisomic conceptuses (trisomy of chromosome 21) [60] and a “license to live” for males with X-linked dominant diseases (i.e. Rett syndrome) who need an additional chromosome X to escape intrauterine lethality [61]. CCRs are primarily associated with reproduction problems [14, 15, 17] and more rarely with learning disability and congenital malformations [17]. SGV manifesting as increased levels of genome/chromosome instability, spontaneous chromosomal mutations or aneuploidization are observed in cancers or cancer-predisposition syndromes [2, 5, 6, 9, 10, 13]; chromosome instability syndromes (ataxia-telangiectasia) [9, 10, 36, 37]; idiopathic learning disability with/without congenital malformations [1-4, 7, 58, 59]; schizophrenia [31, 32, 42]; diseases of pathological/accelerating aging [10]. Another important diagnostic problem related to molecular cytogenetics of SGV is dynamic mosaicism. The latter is indeed relatively easy to solve: a series of high-resolution metaphase/interphase molecular cytogenetic techniques has to be used [62]. Finally, it is supposed that numerous other diseases could be associated with SGV [1-4].
THE UPDATED SCHEME FOR MOLECULAR CYTOGENETIC DIAGNOSIS
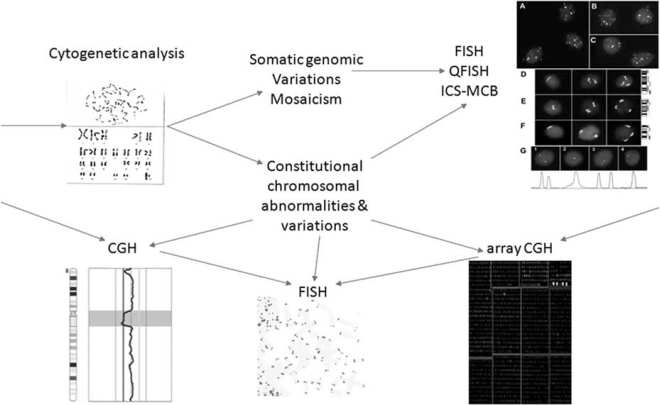
Taking into account all the developments in the field of molecular cytogenetics as well as main characteristics of more popular FISH-, CGH- and array-CGH-based techniques [1-4, 19-21, 26, 34-47, 63, 64], we have made an attempt to propose an original diagnostic scheme that include the analyses of mosaicism and SGV. Fig. (1) depicts the proposed scheme of molecular cytogenetic diagnosis taking into account all the points discussed herein. It is noteworthy that previous schemes of molecular diagnosis in the available biomedical literature have never addressed the detection of SGV manifested at chromosomal or subchromosomal level (chromosomal mosaicism). Together, one can get an idea of the huge amount of “diagnostic work” that has to be performed for high-resolution diagnosis of the mosaicism. Additionally, “starting techniques” have to be those providing for whole genome screen (i.e. cytogenetic banding analyses, standard (high-resolution) CGH or array CGH). However, it is to keep in mind that CGH-based techniques are poorly applicable for low-level mosaicism and SGV detection in large cell populations [4]. FISH-based techniques (multiprobe FISH and quantitative FISH) allow targeted single-cell analysis of genomic loci in huge cell populations and only multicolor banding analysis depicts whole chromosomes in non-dividing cells (for more details see Fig. 1 and [1-4, 21, 38]). We suggest our proposal to lead to more effective molecular cytogenetic diagnosis in cases of mosaicism or SGV.
Fig. (1).
A proposed scheme of molecular cytogenetic diagnosis including detection of chromosomal mosacism or SGV manifesting at chromosomal (subchromosomal) level. Following arrows, one can get a sequence of analyses to be performed for high-resolution molecular cytogenetic diagnosis (arrows from outside of the figure indicate those techniques that provide for whole genome analysis having, thereby, potential to be applied as starting ones). The sequence, however, is not mandatory and is usually defined according to laboratory experience or case singularities. Part of the figure symbolizing “FISH QFISH ICS-MCB” is a reproduction of a figure from Yurov et al. [41], an openaccess article distributed under the terms of the Creative Commons Attribution License. ICS-MCB — interphase chromosome-specific multicolor banding (for details see [40, 47]).
CONCLUDING REMARKS
It is hard not to recognize that current biomedical diagnostic research and practice are more focused on constitutional genomic rearrangements that are easier to interpret and to use for disease-association studies [63, 64]. As a result, numerous human morbid conditions are postulated to require molecular cytogenetic diagnosis by array-CGH-based techniques [17, 20, 24-26, 30, 33, 54, 63, 64], which operate with total DNA isolated from a pool of cells and are inapplicable for studying the majority of mosaicism types and SGV [1-4, 7, 19, 21, 23, 27-29, 36-42, 47, 54, 57, 62]. Therefore, high-resolution whole genome screen is not the complete diagnostic solution for current molecular diagnosis. SGV require a specific set of molecular cytogenetic techniques to be diagnosed (Fig. 1). Thus, to provide a highly technological medical care (at least, in diagnostic terms), these have to be introduced.
The problems that surround diagnosis of SGV and mosaicism remain to be thoroughly addressed by large-scale forthcoming studies targeted at definition of causative amount of abnormal cells per analysis, local effect on cellular/tissular physiology with respect to potential effects on the whole organism, correlations between metaphase and interphase analyses in cases of molecular cytogenetic diagnosis of chromosome abnormalities. To this end, we would like to point out that current molecular cytogenetics possess tools for high-resolution detection of SGV, which have to be used for uncovering the biomedical significance of this common and, probably, most mysterious type of genomic variations.
Hopefully, further research will be able to solve this complex and widely relevant problem.
ACKNOWLEDGEMENTS
The authors are supported by Philip Morris USA Inc.
REFERENCES
- 1.Iourov IY, Vorsanova SG, Yurov YB. Chromosomal mosaicism goes global. Mol. Cytogenet. 2008;1:26. doi: 10.1186/1755-8166-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iourov IY, Vorsanova SG, urov YB. Intercellular genomic (chromosomal) variations resulting in somatic mosaicism: mechanisms and consequences. Curr. Genomics. 2006;7:435–446. [Google Scholar]
- 3.Iourov IY, Vorsanova SG, urov YB. Chromosomal variation in mammalian neuronal cells: known facts and attractive hypotheses. Int. Rev. Cytol. 2006;249:143–191. doi: 10.1016/S0074-7696(06)49003-3. [DOI] [PubMed] [Google Scholar]
- 4.Vorsanova SG, Yurov YB, Iourov IY. Human interphase chromosomes: a review of available molecular cytogenetic technologies. Mol. Cytogenet. 2010;3:1. doi: 10.1186/1755-8166-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duesberg P, Fabarius A, Hehlmann R. Aneuploidy, the primary cause of the multilateral genomic instability of neoplastic and preneoplastic cells. IUBMB Life. 2004;56:65–81. doi: 10.1080/15216540410001667902. [DOI] [PubMed] [Google Scholar]
- 6.Li L, McCormack AA, Nicholson JM, Fabarius A, Hehlmann R, Sachs RK, Duesberg PH. Cancer-causing karyotypes: chromosomal equilibria between destabilizing aneuploidy and stabilizing selection for oncogenic function. Cancer Genet. Cytogenet. 2009;188:1–25. doi: 10.1016/j.cancergencyto.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Iourov IY, Vorsanova SG, Yurov YB. Molecular cytogenetics and cytogenomics of brain diseases. Curr. Genomics. 2008;9:452–465. doi: 10.2174/138920208786241216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati PM, Zuin M, Lucchi S, Meroni PL, Marasini B, Zeni S, Watnik M, Grati FR, Simoni G, Gershwin ME, Podda M. X chromosome monosomy: a common mechanism for autoimmune diseases. J. Immunol. 2005;175:575–578. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 9.Gollin SM. Mechanisms leading to chromosomal instability. Sem. Cancer Biol. 2005;15:33–42. doi: 10.1016/j.semcancer.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Yurov YB, Vorsanova SG, Iourov IY. GIN'n'CIN hypothesis of brain aging: deciphering the role of somatic genetic instabilities and neural aneuploidy during ontogeny. Mol. Cytogenet. 2009;2:23. doi: 10.1186/1755-8166-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schinzel A, editor. Catalogue of Unbalanced Chromosome Aberrations in Man. 2nd. Berlin, New York: de Gruyter; 2001. [Google Scholar]
- 12.Vorsanova SG, Kolotii AD, Iourov IY, Monakhov VV, Kirillova EA, Soloviev IV, Yurov YB. Evidence for high frequency of chromosomal mosaicism in spontaneous abortions revealed by interphase FISH analysis. J. Histochem. Cytochem. 2005;53:375–380. doi: 10.1369/jhc.4A6424.2005. [DOI] [PubMed] [Google Scholar]
- 13.Youssoufian H, Pyeritz RE. Mechanisms and consequences of somatic mosaicism in humans. Nat. Rev. Genet. 2002;3:749–758. doi: 10.1038/nrg906. [DOI] [PubMed] [Google Scholar]
- 14.Karadeniz N, Mrasek K, Weise A. Further delineation of complex chromosomal rearrangements in fertile male using multicolor banding. Mol. Cytogenet. 2008;1:17. doi: 10.1186/1755-8166-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasakyan S, Lohmann L, Aboura A, Quimsiyeh M, Menezo Y, Tachdjian G, Benkhalifa M. De novo complex intra chromosomal rearrangement after ICSI: characterization by BACs micro array-CGH. Mol. Cytogenet. 2008;1:27. doi: 10.1186/1755-8166-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piotrowski A, Bruder CE, Andersson R, de Ståhl TD, Menzel U, Sandgren J, Poplawski A, von Tell D, Crasto C, Bogdan A, Bartoszewski R, Bebok Z, Krzyzanowski M, Jankowski Z, Partridge EC, Komorowski J, Dumanski JP. Somatic mosaicism for copy number variation in differentiated human tissues. Hum. Mutat. 2008;29:1118–1124. doi: 10.1002/humu.20815. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Carvalho CM, Lupski JR. Complex human chromosomal and genomic rearrangements. Trends Genet. 2009;25:298–307. doi: 10.1016/j.tig.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlieb B, Beitel LK, Alvarado C, Trifiro MA. Selection and mutation in the "new" genetics: an emerging hypothesis. Hum. Genet. 2010 doi: 10.1007/s00439-010-0792-9. (in press) [DOI] [PubMed] [Google Scholar]
- 19.Liehr T, Claussen U. Multicolor-FISH approaches for the characterization of human chromosomes in clinical genetics and tumor cytogenetics. Curr. Genomics. 2002;3:213–235. [Google Scholar]
- 20.Emanuel BS, Saitta SC. From microscopes to microarrays: dissecting recurrent chromosomal rearrangements. Nat. Rev. Genet. 2007;8:869–883. doi: 10.1038/nrg2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iourov IY, Vorsanova SG, Yurov YB. Recent patents on molecular cytogenetics. Recent Pat. DNA Gene Seq. 2008;2:6–15. doi: 10.2174/187221508783406585. [DOI] [PubMed] [Google Scholar]
- 22.Hultén MA, Dhanjal S, Pertl B. Rapid and simple prenatal diagnosis of common chromosome disorders: advantages and disadvantages of the molecular methods FISH and QF-PCR. Reproduction. 2003;126:279–297. doi: 10.1530/rep.0.1260279. [DOI] [PubMed] [Google Scholar]
- 23.Liehr T, Ziegler M. Rapid prenatal diagnostics in the interphase nucleus: procedure and cut-off rates. J. Histochem. Cytochem. 2005;53:289–291. doi: 10.1369/jhc.4B6394.2005. [DOI] [PubMed] [Google Scholar]
- 24.Kleeman L, Bianchi DW, Shaffer LG, Rorem E, Cowan J, Craigo SD, Tighiouart H, Wilkins-Haug LE. Use of array comparative genomic hybridization for prenatal diagnosis of fetuses with sonographic anomalies and normal metaphase karyotype. Prenat. Diagn. 2009;29:1213–1217. doi: 10.1002/pd.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Notini AJ, Craig JM, White SJ. Copy number variation and mosaicism. Cytogenet. Genome Res. 2008;123:270–277. doi: 10.1159/000184717. [DOI] [PubMed] [Google Scholar]
- 26.Knight SJ, Regan R. Idiopathic learning disability and genome imbalance. Cytogenet. Genome. Res. 2006;115:215–224. doi: 10.1159/000095917. [DOI] [PubMed] [Google Scholar]
- 27.Vorsanova SG, Iurov IIu, Demidova IA, Voinova-Ulas V Iu, Kravets VS, Solov'ev IV, Gorbachevskaia NL, Iurov IuB. Variations of heterochromatic chromosomal regions and chromosome abnormalities in children with autism: identification of genetic markers in autistic spectrum disorders. Zh. Nevrol. Psikhiatr. Im S S Korsakova. 2006;106:52–57. [PubMed] [Google Scholar]
- 28.Vorsanova SG, Yurov IY, Demidova IA, Voinova-Ulas VY, Kravets VS, Solov'ev IV, Gorbachevskaya NL, Yurov YB. Variability in the heterochromatin regions of the chromosomes and chromosomal anomalies in children with autism: identification of genetic markers of autistic spectrum disorders. Neurosci. Behav. Physiol. 2007;37:553–558. doi: 10.1007/s11055-007-0052-1. [DOI] [PubMed] [Google Scholar]
- 29.Yurov YB, Vorsanova SG, Iourov IY, Demidova IA, Beresheva AK, Kravetz VS, Monakhov VV, Kolotii AD, Voinova-Ulas VY, Gorbachevskaya NL. Unexplained autism is frequently associated with low-level mosaic aneuploidy. J. Med. Genet. 2007;44:521–525. doi: 10.1136/jmg.2007.049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusenda M, Sebat J. The role of rare structural variants in the genetics of autism spectrum disorders. Cytogenet. Genome Res. 2008;123:36–43. doi: 10.1159/000184690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassett AS, Chow EW, Weksberg R. Chromosomal abnormalities and schizophrenia. Am. J. Med. Genet. 2000;97:45–51. doi: 10.1002/(sici)1096-8628(200021)97:1<45::aid-ajmg6>3.0.co;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yurov YB, Vostrikov VM, Vorsanova SG, Monakhov VV, Iourov IY. Multicolor fluorescent in situ hybridization on post-mortem brain in schizophrenia as an approach for identification of low-level chromosomal aneuploidy in neuropsychiatric diseases. Brain. Dev. 2001;23(Suppl 1):S186–190. doi: 10.1016/s0387-7604(01)00363-1. [DOI] [PubMed] [Google Scholar]
- 33.Sebat J, Levy DL, McCarthy SE. Rare structural variants in schizophrenia: one disorder, multiple mutations; one mutation, multiple disorders. Trends Genet. 2009;25:528–535. doi: 10.1016/j.tig.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greulich-Bode KM, Wang M, Rhein AP, Weier JF, Weier HU. Validation of DNA probes for molecular cytogenetics by mapping onto immobilized circular DNA. Mol. Cytogenet. 2008;1:28. doi: 10.1186/1755-8166-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu CM, Wang M, Greulich-Bode K, Weier JF, Weier HU. Quantitative DNA fiber mapping. In: Liehr T, editor. Fluorescence in situ hybridization (FISH) – Application guide. Berlin, Heidelberg: Springer Verlag; 2009. pp. 269–291. [Google Scholar]
- 36.Iourov IY, Vorsanova SG, Liehr T, Yurov YB. Aneuploidy in the normal, Alzheimer's disease and ataxia-telangiectasia brain: differential expression and pathological meaning. Neurobiol. Dis. 2009;34:212–220. doi: 10.1016/j.nbd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Yurov YB. Increased chromosome instability dramatically disrupts neural genome integrity and mediates cerebellar degeneration in the ataxia-telangiectasia brain. Hum. Mol. Genet. 2009;18:2656–2669. doi: 10.1093/hmg/ddp207. [DOI] [PubMed] [Google Scholar]
- 38.Iourov IY, Vorsanova SG, Soloviev IV, Yurov YB. Interphase FISH: detection of intercellular genomic variations and somatic chromosomal mosaicism. In: Liehr T, editor. Fluorescence in situ hybridization (FISH) – Application guide. Berlin, Heidelberg: Springer Verlag; 2009. pp. 301–311. [Google Scholar]
- 39.Yurov YB, Iourov IY, Monakhov VV, Soloviev IV, Vostrikov VM, Vorsanova SG. The variation of aneuploidy frequency in the developing and adult human brain revealed by an interphase FISH study. J. Histochem. Cytochem. 2005;53:385–390. doi: 10.1369/jhc.4A6430.2005. [DOI] [PubMed] [Google Scholar]
- 40.Iourov IY, Liehr T, Vorsanova SG, Kolotii AD, Yurov YB. Visualization of interphase chromosomes in postmitotic cells of the human brain by multicolour banding (MCB) Chromosome Res. 2006;14:223–229. doi: 10.1007/s10577-006-1037-6. [DOI] [PubMed] [Google Scholar]
- 41.Yurov YB, Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Kutsev SI, Pellestor F, Beresheva AK, Demidova IA, Kravets VS, Monakhov VV, Soloviev IV. Aneuploidy and confined chromosomal mosaicism in the developing human brain. PLoS ONE. 2007;2:e558. doi: 10.1371/journal.pone.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yurov YB, Iourov IY, Vorsanova SG, Demidova IA, Kravets VS, Beresheva AK, Kolotii AD, Monakhov VV, Uranova NA, Vostrikov VM, Soloviev IV, Liehr T. The schizophrenia brain exhibits low-level aneuploidy involving chromosome 1. Schizophr. Res. 2008;98:139–147. doi: 10.1016/j.schres.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 43.Soloviev IV, Yurov YB, Vorsanova SG, Fayet F, Roizes G, Malet P. Prenatal diagnosis of trisomy 21 using interphase fluorescence in situ hybridization of postreplicated cells with sitespecific cosmid and cosmid contig probes. Prenat. Diagn. 1995;15:237–248. doi: 10.1002/pd.1970150307. [DOI] [PubMed] [Google Scholar]
- 44.Yurov YB, Soloviev IV, Vorsanova SG, Marcais B, Roizes G, Lewis R. High resolution fluorescence in situ hybridization using cyanine and fluorescein dyes: ultra-rapid chromosome detection by directly fluorescently labeled alphoid DNA probes. Hum. Genet. 1996;97:390–398. doi: 10.1007/BF02185780. [DOI] [PubMed] [Google Scholar]
- 45.Vorsanova SG, Iourov IY, Beresheva AK, Demidova IA, Monakhov VV, Kravets VS, Bartseva OB, Goyko EA, So-loviev IV, Yurov YB. Non-disjunction of chromosome 21, alphoid DNA variation, and sociogenetic features of Down syndrome. Tsitol. Genet. 2005;39(6):30–36. [PubMed] [Google Scholar]
- 46.Iourov IY, Soloviev IV, Vorsanova SG, Monakhov VV, Yurov YB. An approach for quantitative assessment of fluorescence in situ hybridization (FISH) signals for applied human molecular cytogenetics. J. Histochem. Cytochem. 2005;53:401–408. doi: 10.1369/jhc.4A6419.2005. [DOI] [PubMed] [Google Scholar]
- 47.Iourov IY, Liehr T, Vorsanova SG, Yurov YB. Interphase chromosome-specific multicolor banding (ICS-MCB): a new tool for analysis of interphase chromosomes in their integrity. Biomol. Eng. 2007;24:415–417. doi: 10.1016/j.bioeng.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Gersen SL, Keagle MB. The principles of clinical cytogenetics. 2nd. Totowa, NJ: Humana Press; 2005. [Google Scholar]
- 49.Hsu LYF, Kaffe S, Jenkins EC, Alonso L, Benn PA, David K, Hirschhorn K, Lieber E, Shanske A, Shapiro LR, Schutta E, Warburton D. Proposed guidelines for diagnosis of chromosome mosaicism in amniocytes based on data derived from chromosome mosaicism and pseudomosaicism studies. Prenat. Diagn. 1992;12:555–573. doi: 10.1002/pd.1970120702. [DOI] [PubMed] [Google Scholar]
- 50.Caudill SP, Van Dyke DL, Chen AT, Reidy JA, Ing PS, Schwartz S, Vance GH. Evaluating current policy for detecting mosaicism in amniotic fluid cultures: implications for current cell counting practices. Stat. Med. 2005;24:615–622. doi: 10.1002/sim.2040. [DOI] [PubMed] [Google Scholar]
- 51.Wiktor AE, Bender G, Van Dyke DL. Identification of sex chromosome mosaicism: is analysis of 20 metaphase cells sufficient? Am. J. Med. Genet. A. 2009;149A:257–259. doi: 10.1002/ajmg.a.32625. [DOI] [PubMed] [Google Scholar]
- 52.FitzPatrick DR, Ramsay J, McGill NI, Shade M, Carothers AD, Hastie ND. Transcriptome analysis of human autosomal trisomy. Hum. Mol. Genet. 2002;11:3249–3256. doi: 10.1093/hmg/11.26.3249. [DOI] [PubMed] [Google Scholar]
- 53.Altug-Teber O, Bonin M, Walter M, Mau-Holzmann UA, Dufke A, Stappert H, Tekesin I, Heilbronner H, Nieselt K, Riess O. Specific transcriptional changes in human fetuses with autosomal trisomies. Cytogenet. Genome Res. 2007;119:171–184. doi: 10.1159/000112058. [DOI] [PubMed] [Google Scholar]
- 54.Dierssen M, Herault Y, Estivill X. Aneuploidy: from a physiological mechanism of variance to Down syndrome. Physiol. Rev. 2009;89:887–920. doi: 10.1152/physrev.00032.2007. [DOI] [PubMed] [Google Scholar]
- 55.Liehr T, Mrasek K, Weise A, Kuechler A, von Eggeling F, Claussen U, Starke H. Characterization of small supernumerary marker chromosomes (sSMC) in human. Curr. Genomics. 2004;5:279–286. [Google Scholar]
- 56.Liehr T, Mrasek K, Weise A, Dufke A, Rodríguez L, Martínez Guardia N, Sanchís A, Vermeesch JR, Ramel C, Polityko A, Haas OA, Anderson J, Claussen U, von Eggeling F, Starke H. Small supernumerary marker chromosomes--progress towards a genotype-phenotype correlation. Cytogenet. Genome Res. 2006;112:23–34. doi: 10.1159/000087510. [DOI] [PubMed] [Google Scholar]
- 57.Fickelscher I, Starke H, Schulze E, Ernst G, Kosyakova N, Mkrtchyan H, MacDermont K, Sebire N, Liehr T. A further case with a small supernumerary marker chromosome (sSMC) derived from chromosome 1 — evidence for high variability in mosaicism in different tissues of sSMC carriers. Prenat. Diagn. 2007;27:783–785. doi: 10.1002/pd.1776. [DOI] [PubMed] [Google Scholar]
- 58.Vorsanova SG, Iurov IuB. Molecular cytogenetic pre- and postnatal diagnosis of chromosomal abnormalities. Vest. Ross. Akad. Med. Nauk. 1999;11:12–15. [PubMed] [Google Scholar]
- 59.Vorsanova SG, Iurov IuB, Solov'ev IV, Demidova IA, Sharonin VO, Male R, Zhiollant M, Beresheva AK, Kolotii AD, Kravets VS, Ruazes Zh. Current methods of molecular cytogenetics in pre- and postnatal diagnosis of chromosome aberrations. Klin. Lab. Diagn. 2000;8:36–39. [PubMed] [Google Scholar]
- 60.Hulten MA, Patel SD, Tankimanova M, Westgren M, Papa-dogiannakis N, Johnson AM, Iwarsson E. On the origin of trisomy 21 Down syndrome. Mol. Cytogenet. 2008;1:21. doi: 10.1186/1755-8166-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vorsanova SG, Yurov YB, Ulas VY, Demidova IA, Kolotii AD, Gorbatchevskaia NL, Beresheva AK, Soloviev IV. Cytogenetic and molecular-cytogenetic studies of Rett syndrome (RTT): a retrospective analysis of a Russian cohort of RTT patients (the investigation of 57 girls and three boys) Brain Dev. 2001;23(Suppl 1):S196–201. doi: 10.1016/s0387-7604(01)00347-3. [DOI] [PubMed] [Google Scholar]
- 62.Iourov IY, Vorsanova SG, Liehr T, Monakhov VV, Soloviev IV, Yurov YB. Dynamic mosaicism manifesting as loss, gain and rearrangement of an isodicentric Y chromosome in a male child with growth retardation and abnormal external genitalia. Cytogenet. Genome Res. 2008;121:302–306. doi: 10.1159/000138903. [DOI] [PubMed] [Google Scholar]
- 63.Bejjani BA, Shaffer LG. Clinical utility of contemporary molecular cytogenetics. Annu. Rev. Genomics Hum. Genet. 2008;9:71–86. doi: 10.1146/annurev.genom.9.081307.164207. [DOI] [PubMed] [Google Scholar]
- 64.Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annu. Rev. Med. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]