Abstract
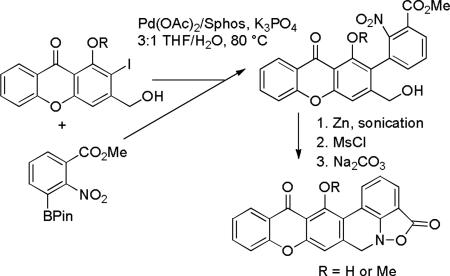
A convergent, practical route to unstable hexacyclic parnafungin A and C models has been developed. Two iodoxanthones were prepared in four or five steps (33-50% overall yield). Suzuki-Miyaura coupling of the iodoxanthones with excess readily available 3-carbomethoxy-2-nitrophenyl pinacol boronate afforded the hindered highly functionalized 2-arylxanthones (53-58%) in the first key step. In the second key step, zinc reduction gave benzisoxazolinones that were treated with MsCl and then base to generate the unstable hexacyclic parnafungin A (13% overall yield for 8 steps) and C (8% overall yield for 9 steps) models. Analogously to the parnafungins, hexacyclic parnafungin C model decomposes to a phenanthridine with a half-life of 2 d in CDCl3.
Introduction
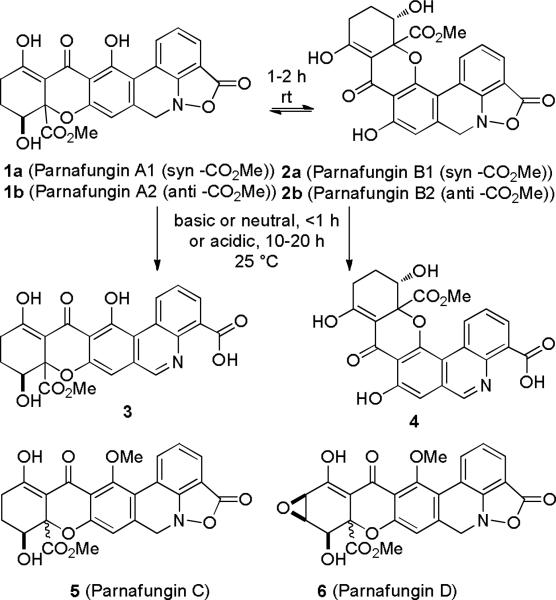
Parnafungins A1 (1a), A2 (1b), B1 (2a), and B2 (2b) are antifungal agents recently isolated from the Fusarium larvarum complex and other Hypocrealean fungi by a Merck group (see Scheme 1).1 They inhibit fungal polyadenosine polymerase (PAP) and show broad spectrum antifungal activity with in vivo efficacy against Candida albicans in a mouse model.1b Affinity selection/mass spectrometry studies indicated that the linear parnafungins A (1) bind preferentially to PAP.1c More recently, the same group isolated two stereoisomers of parnafungin C (5), the O-methylated analogues of parnafungins A1 and A2, and parnafungin D (6) which has an additional epoxide.
SCHEME 1.
Parnafungin Structures and Decomposition Products
Parnafungins A1, A2, B1 and B2 equilibrate readily (1-2 h) by a retro conjugate addition that opens the pyranone ring giving a bisphenol enedione. Conjugate addition then occurs from either face of the enone and either phenol to give a mixture of the four isomers. The two stereoisomers of parnafungin C undergo a similar equilibration, but the O-methyl group prevents the formation of structures analogous to parnafungins B1 and B2. The fused benzisoxazolinone ring of the parnafungins (1, 2, 5, and 6) is unstable. At neutral or basic pH, parnafungins A (1) and B (2) decompose to phenanthridines 3 and 4, respectively in less than 1 h.1 Decomposition at pH 3 is slower, but the same compounds are generated in 10-20 h. Phenanthridines 3 and 4 are unfortunately not biologically active. The synthesis of parnafungins A1, A2, B1, and B 2 that exist as a mixture of four equilibrating isomers with a half-life in solution of less than a day at optimal pH poses a very challenging problem.
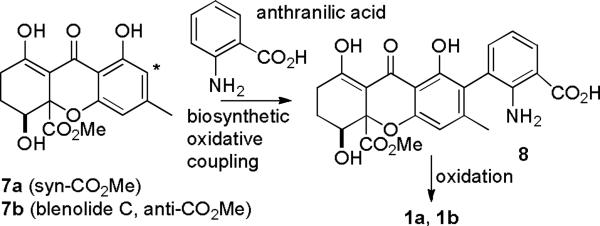
The biosynthesis of the parnafungins might involve the oxidative coupling of 7 with anthranilic acid to give 8 (see Scheme 2). Oxidation of both the benzylic methyl group and the aniline might give rise to parnafungins A1 (1a) and A2 (2). Blenolide C (7b) was recently isolated2 and numerous natural products are known that result from either oxidative dimerization of 7 or oxidative coupling with other components at the asterisk-marked carbon. Bräse3 and Nicolaou4 recently synthesized blenolide C (7b) and related compounds have also been synthesized.5
SCHEME 2.
Possible Biosynthesis of the Parnafungins
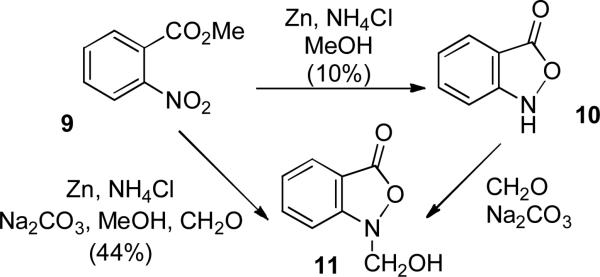
The novel structural feature of the parnafungins is the fused benzisoxazolinone ring, which is necessary for biological activity. We therefore decided to initiate our studies by developing a route to 4H,7H-isoxazolo[4,3,2-de]phenanthridin-4-one (12), which could then be extended to the synthesis of the parnafungins. We were guided by Wierenga's report,6 which was based on earlier work of Bamberger7 and Cohen,8 that reduction of methyl o-nitrobenzoate (9) with zinc, NH4Cl, and Na2CO3 in MeOH containing formaldehyde afforded hydroxymethylbenzisoxazolinone (11) in 44% yield (see Scheme 3). Alcohol 11 was also prepared by the reaction of basic formaldehyde with 10, which was formed in low yield by the zinc reduction of 9.
SCHEME 3.
Wierenga Benzisoxazolinone Synthesis
4H,7H-Isoxazolo[4,3,2-de]phenanthridin-4-one (12) might be accessible by an intramolecular Friedel–Crafts alkylation of 13 (see Scheme 4). Hydroxymethylbenzisoxazolinone 13 will be formed by Wierenga's procedure from biphenylcarboxylate 14, which has been prepared by Liu9 from methyl 3-chloro-2-nitrobenzoate (18) by Suzuki-Miyaura coupling with phenylboronic acid (17, R = H). An alternate approach involves the synthesis of 12 from 15 by an intramolecular SN2 reaction with C–N bond formation. Suzuki-Miyaura coupling of 18 with the appropriate boronic acid 17, R = CH2OH, should form 16, which will be converted to 15 by zinc reduction in the absence of formaldehyde. The Friedel–Crafts route via 13 is appealing because it is highly convergent and uses phenylboronic acid (17, R = H). However, the Friedel-Crafts reaction may not work well with an unactivated aromatic ring. The SN2 route via 15 requires the more complex arylboronic acid 17, R = CH2OH, but may be more versatile.
SCHEME 4.
Retrosynthesis of Tetracycle 12
Results and Discussion
Suzuki-Miyaura coupling of 18 and phenylboronic acid (19a) by Liu's procedure9 afforded 14 in 65% yield (see Scheme 5).10 The yield was improved to 99% using SPhos, Pd(OAc)2, and K3PO4 in wet THF by Buchwald's procedure.11 Reduction of 14 with Zn and NH4Cl with sonication for 30 minutes in THF/MeOH/H2O afforded a 3:1 mixture of the desired benzisoxazolinone 21a and the over reduction product amino ester 22a. Benzisoxazolinone 21a, which decomposed on chromatography, was isolated in 60% yield by washing the mixture of 21a and 22a with 9:1 hexanes/Et2O to remove 22a. The desired hydroxymethylbenzisoxazolinone 13 was obtained in 61% yield by reaction of 21a with formaldehyde and Na2CO3 in aqueous THF. Unfortunately, all attempts to form 12 by intramolecular Friedel-Crafts alkylation on the phenyl ring of 13 were unsuccessful.
SCHEME 5.
Synthesis of Tetracycle 24b
We repeated this sequence with 3,5-dimethoxyphenylboronic acid (19b) to see if the Friedel-Crafts alkylation could be accomplished with an electron rich aromatic ring. Suzuki-Miyaura coupling of 18 with 19b with SPhos proceeded analogously to give 20b in 99% yield. Zinc reduction provided 21b in 66% yield, which was treated with formaldehyde to afford 23b in 76% yield. We were pleased to find that treatment of 23b with 10% TFA in CH2Cl2 afforded 24b in 27% yield, presumably by formation of an iminium cation that added to the aromatic ring. Although this route to 24b provided the first synthesis of a 4H,7H-isoxazolo[4,3,2-de]phenanthridin-4-one, it can't be used for the synthesis of the parnafungins because the Friedel-Crafts reaction would have to occur at a deactivated carbon meta to the oxygen substituents and para to the carbonyl group. We therefore turned to the second route using an SN2 reaction to form the C-N bond.
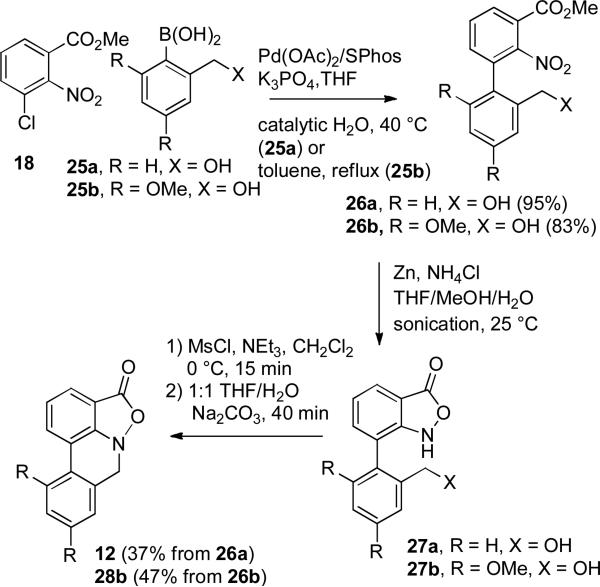
Suzuki-Miyaura coupling of 18 with 1.5 equiv of 2-hydroxymethylphenylboronic acid (25a) using SPhos afforded biphenyl 26a in 95% yield (see Scheme 6). Zinc reduction provided a mixture of benzisoxazolinone 27a and the over reduced amino ester analogous to 22. This mixture was treated with MsCl and Et3N in CH2Cl2 for 15 min at 0 °C to provide the mesylate of 27a. Reaction of the mesylate with Na2CO3 in 1:1 THF/H2O for 40 minutes gave the desired tetracyclic isoxazolo[4,3,2-de]phenanthridinone 12 in 37% overall yield for the three steps from 26a.
SCHEME 6.
Synthesis of Tetracycles 12 and 28c
Suzuki-Miyaura coupling of 18 with 3 equiv of boronic acid 25b12 provided biphenyl 26b (83%) with the oxygen substituents on the same carbons as in the parnafungins. Reduction of 26b with zinc afforded benzisoxazolinone 27b, which was reacted with MsCl and Et3N to give the mesylate. Reaction of the mesylate with Na2CO3 in 1:1 THF/H2O provided 28b in 47% overall yield for the three steps from 26b. This sequence provides a short and efficient route to the unstable tetracyclic isoxazolo[4,3,2-de]phenanthridinone moiety of the parnafungins.
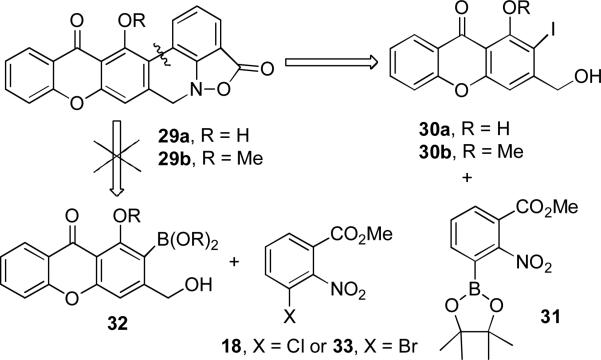
We now turned our attention to the preparation of hexacyclic models 29a and 29b of parnafungins A and C that differ from the natural product only in the substituents on and the oxidation state of the ring furthest from the benzisoxazolinone (see Scheme 7). This could be carried out by Suzuki-Miyaura coupling of chloride 18 or bromide 33 with boronate 32. However, this approach is not appealing because excess boronic acid 32, which requires many steps to prepare, will be needed. A more appealing approach is to use an excess of the simpler boronate 31 with iodides 30a or 30b. The preparation of iodides 30a and 30b will be more straightforward than that of boronic acid 32. Pinacol boronate 31 should also be suitable for coupling with the fully functionalized tricyclic iodide needed for parnafungin synthesis.
SCHEME 7.
Retrosynthesis of Hexacyclic Models 29a and 29b
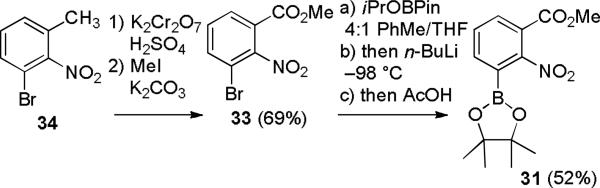
Oxidation of 34 with potassium dichromate and sulfuric acid gave 3-bromo-2-nitrobenzoic acid,13 which was treated with K2CO3 and MeI in DMF to give 33 in 69% overall yield (see Scheme 8). The preparation of pinacol boronate 31 was challenging because of the nitro and ester groups. Reaction of 33 with PinBBPin or HBPin and Pd(OAc)2/SPhos in dioxane at 80 °C resulted in debromination. A similar reaction with neopBBneop gave mainly the biphenyl dimer. Eventually we prepared 31 efficiently by a Barbier-type procedure developed by a Merck group for the synthesis of 3-pyridyl boronic acid.14 A mixture of bromide 33 and 2 equiv of isopropyl pinacol boronate in 4:1 toluene/THF was cooled to – 98 °C and treated very slowly with 2 equiv n-BuLi. Transmetallation occurred readily and the resulting aryllithium was then trapped immediately by the isopropyl pinacol boronate. Quenching with acetic acid gave a precipitate that was removed by filtration. The filtrate was concentrated to give crude 31 that was crystallized from hexanes at –20 °C to give 31 in 52% yield. The yield was lower and some 33 was recovered if less than 2 equiv of both isopropyl pinacol boronate and n-Buli were used.
SCHEME 8.
Synthesis of Pinacol Boronate 31
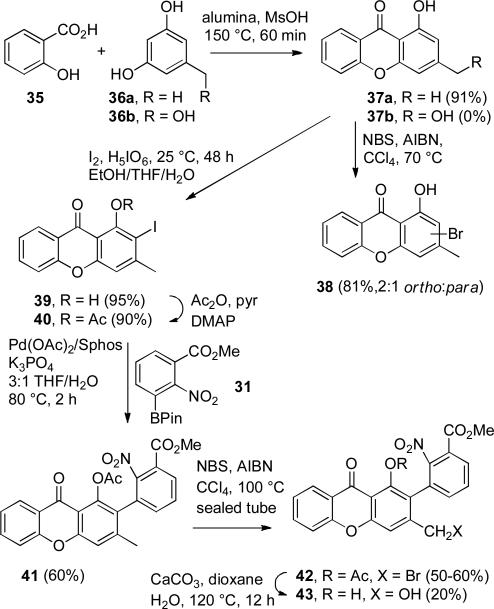
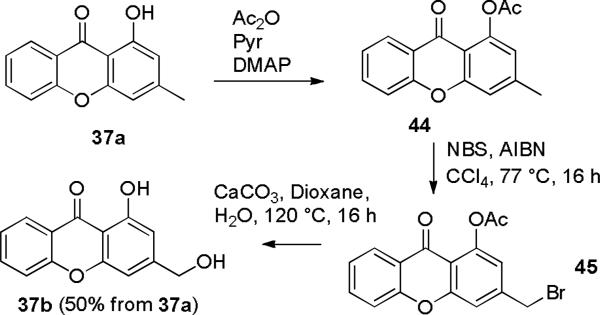
Xanthone 37a was prepared in 91% yield by condensation of salicylic acid (35) with orcinol (36a) using methanesulfonic acid on alumina at 150 °C for 60 min (see Scheme 9).15 The hydroxymethylxanthone 37b,16 rather than 37a, is needed for the construction of iodoxanthone 30, but the analogous condensation with 5-hydroxymethylresorcinol (36b) did not provide 37b. We therefore investigated procedures to oxidize the methyl group of 37a. Attempted benzylic bromination of 37a with NBS and AIBN in CCl4 afforded a 2:1 mixture of ortho and para ring bromination products 38.17,18 We therefore postponed the oxidation of the methyl group of 37a until after the Suzuki-Miyaura coupling. Iodination19 of 37a with iodine and periodic acid in ethanol/THF/H2O afforded the desired ortho-iodophenol 39 in 95% yield that was converted to acetate 40 containing a little inseparable deiodination product 44 in 90% yield on treatment with Ac2O, pyridine, and DMAP in CH2Cl2 for 2 h. Extensive deiodination of 40 occurred at longer reaction times.20
SCHEME 9.
Unsuccessful Route to 43
Suzuki-Miyaura coupling of 40 with 3 equiv of 31 afforded the desired 2-arylxanthone 41 in 60% yield. Benzylic bromination of the hindered methyl group with NBS and AIBN required heating in CCl4 in a sealed tube at 100 °C and gave benzylic bromide 42 in 50-60% yield. Hydrolysis of the acetate and benzylic bromide with CaCO3 in aqueous dioxane at 120 °C proceeded in low yield giving 43 in only 20% yield. We therefore investigated other approaches to functionalize the methyl group of 37a.
Reaction of 37a with Ac2O, DMAP, and pyridine in CH2Cl2 afforded aryl acetate 44 (see Scheme 10). The acetate makes the aromatic ring of 44 less nucleophilic than that of phenol 37a so that benzylic bromination of 44 with NBS and AIBN in CCl4 for 16 h at 77 °C proceeded smoothly to give benzylic bromide 45. Hydrolysis of both the acetate and benzylic bromide of 45 with CaCO3 in aqueous dioxane at 120 °C for 16 h provided hydroxymethylxanthone 37b in 50% overall yield for the three steps from 37a.
SCHEME 10.
Oxidation of 37a to give 37b
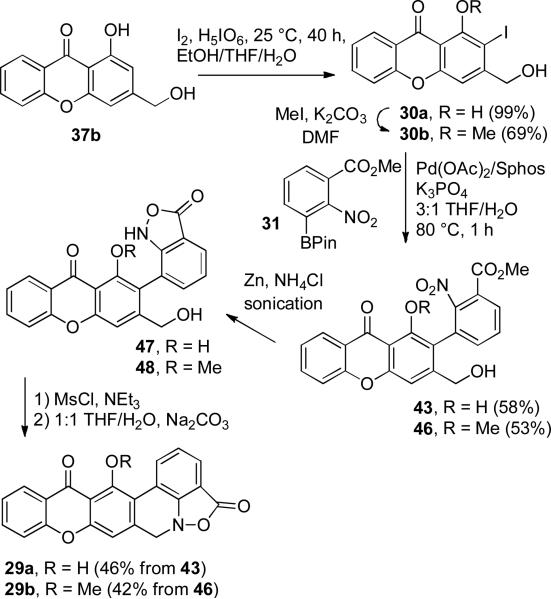
Iodination19 of 37b with iodine and periodic acid in ethanol/THF/H2O afforded the desired ortho-iodophenol 30a in 99% yield that was converted to methyl ether 30b in 69% yield (see Scheme 11). The single regioisomeric iodide was expected to be the desired ortho-iodophenol 30a based on literature precedent.19 However, the position of the iodide could not be confirmed by analysis of the spectral data. We therefore established the structure of 30a by X-ray crystal structure determination (see Supporting Information).
SCHEME 11.
Synthesis of Hexacyclic Parnafungin Models 29a and 29b
Suzuki-Miyaura coupling of 30a with 2.6 equiv of pinacol boronate 31 using SPhos afforded phenol 43 in 58% yield. A similar coupling of 30b with 2.8 equiv of 31 provided methyl ether 46 in 53% yield. These reactions proceed well with 30-50 mol% of both Pd(OAc)2 and SPhos. Use of Pd2(dba)3 instead of Pd(OAc)2 afforded only the deiodination product, methyl 2-nitrobenzoate, and the biphenyl from homo coupling of boronate 31. Use of water as a co-solvent is essential and use of degassed solvents was also important. Under these optimal conditions, hindered, highly functionalized 2-arylxanthone 43 was obtained in reasonable yield without protection of the primary alcohol or phenol.
The three-step sequence developed for the conversion of 26a (26b) to 12 (28b) proceeded uneventfully to complete the synthesis of hexacyclic models 29a and 29b. Reduction of 43 and 46 with Zn and NH4Cl in MeOH/THF/H2O with sonication afforded 47 and 48, respectively. Mesylation with MsCl and Et3N in CH2Cl2 afforded the mesylates, which were treated with Na2CO3 in 1:1 THF/H2O to give hexacyclic parnafungin A model 29a (46% for three steps) and parnafungin C model 29b (43% for three steps), respectively. The 1H NMR spectral data of the three right-hand rings of 29a and 29c correspond closely to those reported for parnafungins A (1) and C (5). The proton para to the phenol of the central ring of 29a absorbs at δ 6.97, whereas the analogous proton absorbs at δ 6.72 and 6.74 in parnafungins A1 and A2. The proton para to the methoxy group of the central ring of 29b absorbs at δ 7.30, which is downfield by 0.33 ppm from that of 29a. The proton para to the methoxy group of parnafungin C absorbs at δ 7.06, which is also downfield by 0.32-0.34 ppm from those of parnafungins A1 and A2.
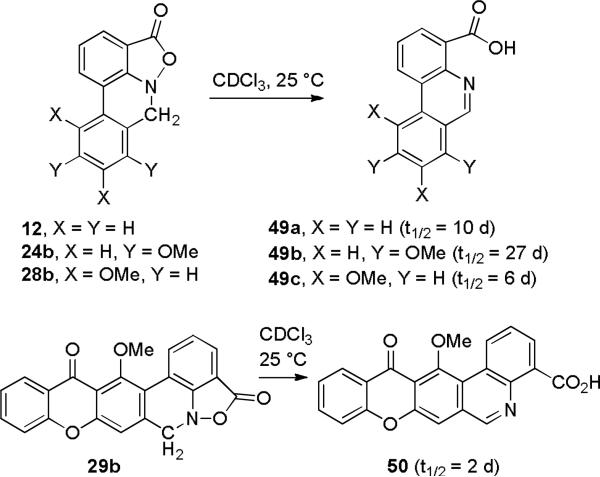
The Merck group proposed that parnafungins A (1) and B (2) decomposed to 3 and 4, respectively, by either an E2 reaction or by hydrolysis of the benzisoxazolinone followed by loss of water. We explored the stability of tetracyclic models 12, 24b, 28b, and hexacyclic parnafungin C model 29b in CDCl3. Hexacyclic parnafungin A model 29a was not sufficiently soluble in CDCl3 or other solvents suitable for NMR spectroscopic analysis. All four benzisoxazolinones decomposed cleanly to the respective phenanthridines 49a,21 49b, 49c, and 50 without any evidence for the formation of an intermediate (see Scheme 12).10,22 This suggests that the reaction proceeds by an E2 elimination unless loss of water is much faster than hydrolysis of the benzisoxazolinone.
SCHEME 12.
Decomposition of Benzisoxazolinones 12, 24b, 28b and 29b
If the reaction occurs by an E2 elimination, the rate of the reaction should be proportional to the kinetic acidity of the methylene protons because deprotonation occurs in the rate determining step, even if protonation of the carbonyl group by adventitious HCl is the initial step. The C-methyl group of m-methoxytoluene is deprotonated twice as fast as that of toluene by lithium amide bases, whereas the C-methyl group of o-methoxytoluene is deprotonated ten times slower than that of toluene.23 The observed rates of decomposition of the four parnafungin models correlate well with expected effect of the methoxy groups on the acidity of the methylene group providing further support for an E2 mechanism. Hexacyclic parnafungins C model 29b with two meta methoxy groups that each double the rate of deprotonation of toluene and a para ketone that should also increase the acidity of the methylene group decomposed most rapidly with a half-life of 2 days at a rate similar to that of parnafungins A-C. Tetracycle 28b with two meta methoxy groups, but lacking the para ketone decomposed next most rapidly with a half-life of 6 days. Tetracycle 12 with no methoxy groups decomposed with a half-life of 10 days and tetracycle 22b with ortho and para methoxy groups that decrease the rate of deprotonation decomposed slowest with a half-life of 27 days.
In conclusion, a convergent, practical route to the unstable hexacyclic parnafungin A and C models 29a and 29b has been developed. Iodoxanthone 30a was prepared in four steps (50% overall yield from the readily available xanthone 37a. Suzuki-Miyaura coupling of 30a and 30b with excess readily available pinacol boronate 31 afforded the hindered highly functionalized 2-arylxanthones 43 (58%) and 46 (53%) in the first key step. In the second key step, zinc reduction of 43 and 46 gave benzisoxazolinones 47 and 48 that were treated with MsCl and then base to generate the unstable hexacyclic parnafungin A and C models 29a (46% from 43, 13% overall yield for 8 steps from 37a) and 29b (42% from 46, 8% overall yield for 9 steps from 37a). Analogously to the parnafungins, hexacyclic parnafungin C model 29b decomposed to 50 with a half-life of 2 d in CDCl3. We are now exploring routes to the iodo hydroxymethyl phenol from blenolide C (7) that will be coupled with 31 to give parnafungins A (1) and B (2).
Experimental Section
General Experimental Methods
Reactions were conducted in flame- or oven-dried glassware under a nitrogen atmosphere and were stirred magnetically. The phrase “concentrated” refers to removal of solvents by means of a rotary evaporator attached to a diaphragm pump (15-60 Torr) followed by removal of residual solvents at < 1 Torr with a vacuum pump. Flash chromatography was performed on silica gel 60 (230-400 mesh). Analytical thin layer chromatography (TLC) was performed using silica gel 60 F-254 pre-coated glass plates (0.25 mm). TLC Plates were analyzed by short wave UV illumination, or by dipping in vanillin stain (27 g of vanillin in 380 mL of EtOH, 50 mL of water and 20 mL of concentrated sulfuric acid) and heating on a hot plate or by spray with permanganate spray (5 g of KMnO4 in 495 mL of water). THF and ether were dried and purified by distillation from sodium/benzophenone. DIPEA, Et3N, MeOH, and benzene were distilled from CaH2. 1H and 13C NMR spectra were obtained on a 400 MHz spectrometer in CDCl3 with tetramethylsilane as internal standard unless otherwise indicated. Chemical shifts are reported in δ (ppm downfield from tetramethylsilane). Coupling constants are reported in Hz with multiplicities denoted as s (singlet), d (doublet), t (triplet), q (quartet), p (pentet), m (multiplet) and br (broad). COSY spectra were recorded for all compounds and used to assign 1H NMR spectra. IR spectra were acquired on an FT-IR spectrometer and are reported in wave numbers (cm-1). High resolution mass spectra were obtained using the following ionization techniques: chemical ionization (CI), electron impact (EI), electrospray ionization analyzed by quadrupole time of flight (QTOF). Activated Zn was prepared by the procedure of Kishi.25
Methyl 2-Nitro-[1,1'-biphenyl]-3-carboxylate (14)
A mixture of methyl 3-chloro-2-nitrobenzoate (18) (101 mg, 0.47 mmol), 2-phenylboronic acid (19a) (86 mg, 0.70 mmol, 1.5 equiv), Pd(OAc)2 (2.1 mg, 0.02 equiv), SPhos (12 mg, 0.06 equiv), and K3PO4 (300 mg, 1.41 mmol, 3 equiv) in THF (1 mL) and H2O (10 μL) was heated at 40 °C under N2 for 4 h.11 The mixture was cooled, diluted with Et2O, and filtered. The filtrate was concentrated to give 179 mg of crude 14. Flash chromatography on silica gel (6:1 hexanes/EtOAc) gave 116 mg (99%) of pure 14, with spectral data identical to those of a sample prepared by the literature procedure:9 mp 110-111 °C; 1H NMR (CDCl3) 8.02 (d, 1, J = 4.8); 7.60 (d, 2, J = 4.8), 7.45-7.39 (m, 3), 7.37-7.31 (m, 2), 3.91 (s, 3); 13C NMR (CDCl3) 163.8, 149.4, 135.4, 135.3, 135.1, 130.0, 129.9, 128. 8, 128.7 (2 C), 128.1 (2 C), 123.1, 53.1; IR 1732, 1543, 1310.
Methyl 3',5'-Dimethoxy-2-nitro-[1,1'-biphenyl]-3-carboxylate (20b)
A mixture of methyl 3-chloro-2-nitrobenzoate (18) (104 mg, 0.48 mmol), 3,5-dimethoxyphenylboronic acid (19b) (132 mg, 0.72 mmol, 1.5 equiv), Pd(OAc)2 (2.1 mg, 0.02 equiv), SPhos (12 mg, 0.06 equiv), and K3PO4 (305 mg, 1.44 mmol, 3 equiv) in THF (1 mL) and H2O (10 μL) was heated at 40 °C under N2 for 4 h. The mixture was cooled, diluted with Et2O, and filtered. The filtrate was concentrated to give 252 mg of crude 20b. Flash chromatography on silica gel (5:1 hexanes/EtOAc) gave 144 mg (99%) of pure 20b: mp 156-157 °C; 1H NMR (CDCl3) 8.02 (dd, 1, J = 7.3, 2.2), 7.65-7.57 (m, 2), 6.50 (t, 1, J = 2.2), 6.47 (d, 2, J = 2.2), 3.92 (s, 3), 3.79 (s, 6); 13C NMR (CDCl3) 163.8, 160.8 (2 C), 149.4 (weak), 137.1, 135.2, 135.1, 130.2, 129.9, 123.1, 106.5 (2 C), 100.9, 55.4 (2 C), 53.1; IR (neat) 1729, 1541, 1344; HRMS (EI) calcd for C16H15NO6 (M+) 317.0899, found 317.0904.
7-Phenyl-2,1-benzisoxazolin-3-one (21a)
A solution of 14 (110 mg, 0.43 mmol) in 5 mL of 2:2:1 THF/MeOH/H2O was treated with activated Zn (160 mg) and NH4Cl (120 mg). The resulting mixture was sonicated at 25 °C for 30 minutes. The mixture was then diluted with Et2O and filtered. The filtrate was washed with brine, dried (Na2SO4), and concentrated to give 90 mg of a 3:1 mixture of benzisoxazolinone 21a and methyl 2-amino-[1,1'-biphenyl]-3-carboxylate (22a)9 as a white solid. The crude product was washed with 10 mL of 9:1 hexanes/Et2O twice, which removed amino ester 22a giving 55 mg (60%) of pure 21a: mp 98 °C (decomposition); 1H NMR (CDCl3) 8.53 (br s, 1, NH), 7.85 (d, 1, J = 8.0), 7.75 (d, 1, J = 8.0), 7.64 (br d, 2, J = 8.0), 7.51 (dd, 2, J = 8.0, 8.0), 7.43 (t, 1, J = 8.0), 7.41 (dd, 1, J = 8.0, 8.0); 13C NMR (CDCl3) 169.0, 153.1, 135.1, 134.2, 129.4 (2 C), 128.8, 127.6 (2 C), 126.4, 125.5, 124.6, 113.2; IR 1734; HRMS (ESI+) calcd for C13H10NO2 (MH+) 212.0712, found 212.0702.
7-(3,5-Dimethoxyphenyl)-2,1-benzisoxazolin-3-one (21b)
A solution of 20b (100 mg, 0.32 mmol) in 5 mL of 2:2:1 THF/MeOH/H2O was treated with activated Zn (56 mg) and NH4Cl (30 mg). The resulting mixture was sonicated at 25 °C for 30 minutes. Additional activated Zn (30 mg) and NH4Cl (34 mg) were added and the reaction was sonicated at 25 °C for another 30 minutes. The mixture was then diluted with EtOAc and filtered. The filtrate was washed with brine, dried (Na2SO4), and concentrated to give 94 mg of a 3:1 mixture of benzisoxazolinone 21b and methyl 2-amino-3',5'-dimethoxy-[1,1'-biphenyl]-3-carboxylate (22b) as a semisolid. The crude product was washed with 10 mL of 3:1 hexanes/EtOAc, which removed the amino ester giving 56 mg (66%) of pure 21b: mp 124 °C (decomposition); 1H NMR (CDCl3) 8.53 (br s, 1, NH), 7.85 (d, 1, J = 7.3), 7.75 (d, 1, J = 7.3), 7.40 (dd, 1, J = 7.3, 7.3), 6.77 (d, 2, J = 2.0), 6.52 (t, 1, J = 2.0), 3.85 (s, 6); 13C NMR (CDCl3) 169.0, 161.5 (2 C), 153.0, 137.0, 134.0, 126.3, 125.4, 124.8, 113.1, 105.7 (2 C), 100.5, 55.5 (2 C); IR (neat) 1742, 1736, 1605, 1156; HRMS (ESI+) calcd for C15H14NO4 (MH+) 272.0923, found 272.0919.
Flash chromatography of the 3:1 mixture of 21b and 22b on silica gel (5:1 hexanes/EtOAc) resulted in the decomposition of 21b. Only 22b was isolated in 15-25% yield: 1H NMR (CDCl3) 7.89 (dd, 1, J = 8.0, 1.8), 7.23 (dd, 1, J = 8.0, 1.2), 6.68 (dd, 1, J = 8.0, 8.0), 6.55 (d, 2, J = 2.0). 6.48 (t, 1, J = 2.), 6.04 (br, 2, w1/2 = 19, NH2), 3. 88 (s, 3), 3.81 (s, 6); 13C NMR (CDCl3) 168.8, 161.2 (2 C), 147.8, 140.4, 134.6, 130.8, 128.6, 115.5, 110.5, 107.1 (2 C), 99.8, 55.4 (2 C), 51.6; HRMS (ESI+) calcd for C16H18NO4 (MH+) 288.1236, found 288.1222.
1-Hydroxymethyl-7-phenyl-2,1-benzisoxazolin-3-one (13)
A solution of 21a (50 mg, 0.23 mmol) in 8 mL of 1:1 THF/H2O was treated with Na2CO3 (60 mg) and 37% aqueous CH2O (0.7 mL). The reaction was stirred at 25 °C for 2.5 h. The solution was then diluted with EtOAc, washed with water and brine, and dried (Na2SO4). Concentration gave 44 mg of crude 13 as a white solid, which was washed with 10:1 hexanes/EtOAc to give 34 mg (61%) of pure 13: mp 119 °C (decomposition); 1H NMR (CDCl3) 7.82 (d, 1, J = 8.0), 7.64 (d, 1, J = 8.0), 7.59 (d, 2, J = 7.2), 7.50 (dd, 2, J = 7.2, 7.2), 7.44 (t, 1, J = 7.2), 7.42 (dd, 1, J = 8.0, 8.0), 4.81 (s, 2), 3.14 (br s, 1, w1/2 = 21.6, OH); 13C NMR (CDCl3) 168.6, 152.1, 136.1, 136.0, 129.2 (2 C), 128.8, 128.2 (2 C), 128.1, 126.0, 124.7, 116.3, 74.1; IR 1764 1739; HRMS (ESI+) calcd for C14H11NO3Na (MNa+) 264.0637, found 264.0636.
7-(3,5-Dimethoxyphenyl)-1-hydroxymethyl-2,1-benzisoxazolin-3-one (23b)
A solution of 21b (51 mg, 0.21 mmol) in 8 mL of 1:1 THF/H2O was treated with Na2CO3 (60 mg) and 37% aqueous CH2O (0.7 mL). The reaction was stirred at 25 °C for 12 h. The solution was then diluted with EtOAc, washed with water and brine, and dried (Na2SO4). Concentration gave 48 mg of white solid crude 23b, which was washed with 5:1 hexanes/EtOAc to give 43 mg (76%) of pure 23b: mp 136 °C (decomposition); 1H NMR (CDCl3) 7.80 (d, 1, J = 8.0), 7.64 (d, 1, J = 8.0), 7.39 (dd, 1, J = 8.0, 8.0), 6.71-6.76 (m, 2), 6.51-6.55 (m, 1), 4.89 (d, 2, J = 8.0), 3.83 (s, 6), 3.50 (t, 1, J = 8.0, OH); 13C NMR (CDCl3) 168.7, 161.2 (2 C), 152.0, 137.9, 135.7, 128.0, 125.8, 124.8, 116.2, 106.2 (2 C), 100.6, 74.3, 55.5(2 C); IR(neat) 3413, 1767, 1746, 1602, 1157; HRMS (ESI+) calcd for C16H15NO5Na (MNa+) 324.0848, found 324.0842.
8,10-Dimethoxy-4H,7H-isoxazolo[4,3,2-de]phenanthridin-4-one (24b)
A solution of 23b (24 mg, 0.08 mmol) in CH2Cl2 (3 mL) was treated with TFA (0.3 mL) in one portion at 0 °C. The reaction was slowly warmed to 25 °C and stirred for 30 minutes. Concentration and flash chromatography of the residue on silica gel (6:2:1 hexanes/EtOAc/CH2Cl2) gave 6 mg (27%) of pure 24b: mp 222 °C (decomposition); 1H NMR (CDCl3) 7.76 (d, 1, J = 7.3), 7.70 (d, 1, J = 8.0), 7.26 (dd, 1, J = 8.0, 7.3), 6.90 (d, 1, J = 1.8), 6.49 (d, 1, J = 1.8), 4.63 (s, 2), 3.90 (s, 3), 3.87 (s, 3); 13C NMR (CDCl3) 168.0, 161.1, 157.8, 156.5, 130.7, 127.1, 124.9, 124.5, 121.6, 112.2, 110.7, 99.3, 99.0, 55.7, 55.6, 48.7; IR (neat) 1762; HRMS (ESI+) calcd for C16H14NO4 (MH+) 284.0923, found 284.0920.
Methyl 2'-Hydroxymethyl-2-nitro-[1,1'-biphenyl]-3-carboxylate (26a)
A mixture of methyl 3-chloro-2-nitrobenzoate (18) (220 mg, 1.0 mmol), 2-(hydroxymethyl)phenylboronic acid (25a) (228 mg, 1.5 mmol, 1.5 equiv), Pd(OAc)2 (7 mg, 0.03 equiv), SPhos (13 mg, 0.03 equiv), and K3PO4 (636 mg, 3.0 mmol, 3 equiv) in THF (1 mL) and H2O (10 μL) was heated at 40 °C under N2 for 4 h. The mixture was cooled, diluted with Et2O, and filtered. The filtrate was concentrated to give 400 mg of crude 26a. Flash chromatography on silica gel (6:1 hexanes/EtOAc) gave 273 mg (95%) of pure 26a: mp 109.5-111 °C; 1H NMR (CDCl3) 8.08 (d, 1, J = 8.0), 7.62 (dd, 1, J = 8.0, 8.0), 7.56 (d, 1, J = 8.0), 7.56 (d, 1, J = 8.0), 7.46 (dd, 1, J = 8.0, 8.0), 7.33 (dd, 1, J = 8.0, 8.0), 7.14 (d, 1, J = 8.0), 4.50 (dd, 1, J = 12.8, 4.4), 4.41 (dd, 1, J = 12.8, 7.6), 3.92 (s, 3), 1.79 (dd, 1, J = 7.6, 4.4, OH); 13C NMR (CDCl3) 163.6, 149.8, 139.0, 135.5, 133.8, 133.4, 130.5, 129.7, 129.6, 129.4, 128.7, 127.7, 123.0, 63.0, 53.1; IR 1730, 1540, 1372, 1440; HRMS (ESI+) calcd for C15H13NO5Na (MNa+) 310.0691, found 310.0703.
4H,7H-Isoxazolo[4,3,2-de]phenanthridin-4-one (12)
A solution of 26a (73 mg, 0.26 mmol) in 5 mL of 2:2:1 MeOH/THF/H2O was treated with activated Zn (80 mg) and NH4Cl (60 mg). The resulting mixture was sonicated at 25 °C for 30 minutes. The reaction was diluted with EtOAc, filtered. The filtrate was washed with H2O and brine, and dried (Na2SO4). Concentration gave 64 mg of a 3:1 mixture of 7-(2-hydroxymethylphenyl)-2,1-benzisoxazolin-3-one (27a) and methyl 2-amino-2'-hydroxymethyl-[1,1'-biphenyl]-3-carboxylate as a solid.
The crude mixture of 27a and the amino ester was dissolved in anhydrous CH2Cl2 (5 mL) and cooled to 0 °C. NEt3 (0.1 mL, 0.75 mmol) and MsCl (40 μL, 0.5 mmol) were added and the resulting reaction was stirred at 0 °C for 15 minutes. The reaction was diluted with Et2O and washed with water and brine. The organic layer was dried (Na2SO4) and concentrated to give 102 mg of crude 7-(2-methanesulfonyloxymethylphenyl)-2,1-benzisoxazolin-3-one.
The crude mesylate was dissolved in 4 mL of 1:1 THF/H2O and Na2CO3 (80 mg) was added at 25 °C. The resulting mixture was stirred at 25 °C for 40 minutes. The reaction was diluted with Et2O and washed with brine. The organic layer was dried (Na2SO4) and concentrated to give 62 mg of crude 12. Flash chromatography (7:1 hexanes/EtOAc) gave 21 mg (37% from 26a) of pure 12: mp 137-138 °C; 1H NMR (CDCl3) 7.83 (d, 1, J = 8.0), 7.81 (d, 1, J = 8.0), 7.71 (d, 1, J = 8.0), 7.46 dd, 1, J = 8.0, 8.0), 7.37 (dd, 1, J = 8.0, 8.0), 7.32 (d, 1, J = 8.0), 7.31 (dd, 1, J = 8.0, 8.0), 4.65 (s, 2); 13C NMR (CDCl3) 167.9, 156.4, 131.1, 129.35, 129.29, 129.2, 128.4, 127.0, 125.4, 124.4, 123.2, 121.7, 111.1, 55.3; IR 1766; HRMS (EI) calcd for C14H9NO2 (M+) 223.0633, found 223.0636.
Methyl 2'-Hydroxymethyl-4',6'-dimethoxy-2-nitro-[1,1'-biphenyl]-3-carboxylate (26b)
Boronic acid 25b was prepared from 886 mg (2.68 mmol) of the THP ether of 2-bromo-3,5-dimethoxybenzyl alcohol by the literature procedure,12 except that the recrystallization step was not carried out. The entire batch of crude 25b was used directly in the Suzuki coupling.
Freshly distilled THF (1 mL) was added to a mixture of Pd(OAc)2 (11.5 mg, 0.07 equiv%) and SPhos (64 mg, 0.21 equiv). The resulting solution was stirred at 25 °C under N2 for 30 minutes to form a Pd stock solution. A mixture of methyl 3-chloro-2-nitrobenzoate (18) (160 mg, 0.74 mmol), crude boronic acid 25b (900 mg, 3 equiv), and K3PO4 (530 mg, 2.5 mmol, 3.4 equiv) were mixed in a flask. The flask was purged with N2 three times. Freshly distilled toluene (3 mL) and the Pd stock solution were then added. The resulting mixture was refluxed for 90 minutes. The mixture was cooled, diluted with Et2O, and filtered through a pad of Celite. The filtrate was concentrated to give 946 mg of crude 26b. Flash chromatography (hexanes/EtOAc 3:1 to 2:1) gave 213 mg (83%) of pure 26b: mp 129-131 °C; 1H NMR (CDCl3) 8.03 (d, 1, J = 8.0), 7.60 (dd, 1, J = 8.0, 7.3), 7.47 (d, 1, J = 7.3), 6.72 (d, 1, J = 1.8), 6.42 (d, 1, J = 1.8), 4.38 (dd, 1, J = 12.8, 3.6), 4.29 (dd, 1, J = 12.8, 7.3), 3.90 (s, 3), 3.85 (s, 3), 3.66 (s, 3), 1.90 (dd, 1, J = 7.3, 3.6, OH); 13C NMR (CDCl3) 164.0, 161.5, 158.0, 150.6, 141.7, 136.7, 130.6, 130.2, 130.0, 123.3, 114.7, 104.2, 98.0, 62.9, 55.8, 55.4, 53.0; IR 1730, 1540, 1370, 1155; HRMS (EI) calcd for C17H17NO7 (M+) 347.1005, found 347.1006.
9,11- Dimethoxy-4H,7H-isoxazolo[4,3,2-de]phenanthridin-4-one (28b)
A solution of 26b (55 mg, 0.16 mmol) in 5 mL MeOH/THF/H2O 2:2:1 was treated with activated Zn (80 mg) and NH4Cl (60 mg). The resulting mixture was sonicated at 25 °C for 25 minutes. The reaction was diluted with EtOAc, and filtered. The filtrate was washed with H2O and brine, and dried (Na2SO4). Concentration gave 44 mg of a 6:1 mixture of 7-(2-hydroxymethyl-4,6-dimethoxyphenyl)-2,1-benzisoxazolin-3-one (27b) and methyl 2-amino-2'-hydroxymethyl-4',6'-dimethoxy-[1,1'-biphenyl]-3-carboxylate.
The crude mixture of 27b and the amino ester was then dissolved in anhydrous CH2Cl2 (5 mL) and cooled to 0 °C. NEt3 (0.12 mL, 0.86 mmol) and MsCl (32 μL, 0.44 mmol) were added and the resulting reaction was stirred at 0 °C for 15 minutes. The reaction was diluted with Et2O and washed with water and brine. The organic layer was dried (Na2SO4) and concentrated to give 88 mg 7-(2-methanesulfonyloxymethyl-4,6-dimethoxyphenyl)-2,1-benzisoxazolin-3-one.
The crude mesylate was dissolved in 4 mL of 1:1 THF/H2O and Na2CO3 (80 mg) was added at 25 °C. The resulting mixture was stirred at 25 °C for 40 minutes. The reaction was diluted with Et2O and washed with brine. The organic layer was dried (Na2SO4) and concentrated to give 65 mg of crude 28b. Flash chromatography (hexanes/EtOAc 3:1) gave 21 mg (47% from 26b) of pure 28b: mp 234 °C (decomposition); 1H NMR (CDCl3) 8.31 (d, 1, J = 7.4), 7.61 (d, 1, J = 8.0), 7.26 (dd, 1, J = 8.0, 7.4), 6.55 (d, 1, J = 1.8), 6.47 (d, 1, J = 1.8), 4.52 (s, 2), 4.00 (s, 3), 3.87 (s, 3); 13C NMR (CDCl3) 168.4, 160.9, 158.9, 156.3, 134.1, 131.0, 125.5, 122.4, 120.4, 111.3, 110.5, 105.2, 99.0, 56.1, 55.64, 55.56; IR 1761; HRMS (EI) calcd for C16H13NO4 (M+) 283.0845, found 283.0844.
Methyl 3-Bromo-2-nitrobenzoate (33)
A suspension of 3-bromo-2-nitrotoluene (34, 3.55 g, 16.4 mmol) and potassium dichromate (9.0 g, 30.5 mmol) in 15 mL of water was prepared in a 100 mL two-neck flask. The mixture was treated with 26.5 mL of conc H2SO4 through an addition funnel and a thermometer was used to monitor the internal temperature. The temperature was kept between 50 and 56 °C during the addition using an ice bath during for cooling if needed. After the addition was complete (15 min), the reaction was stirred for 15 min and then heated at 65 °C for 3 h. The reaction was cooled and poured into 100 mL of ice-water. The resulting mixture was stirred for 5 min and filtered. The solid obtained was added to 30 mL of 2 M Na2CO3 solution and the resulting suspension was filtered. The filtrate was cooled to 0 °C and 21 mL of 25% aqueous HCl was added dropwise. The resulting slurry was extracted twice with EtOAc. The water layer was saturated with NaCl and extracted with EtOAc. The combined EtOAc layers were concentrated to give 3.42 g of crude 3-bromo-2-nitrobenzoic acid.13
The crude acid was dissolved in 10 mL of anhydrous DMF and the resulting solution was treated with 10 g (72 mmol) of K2CO3 and 4 mL (64 mmol) of methyl iodide. The reaction was stirred at 25 °C for 12 h. The mixture was then diluted with 100 mL of water and extracted with EtOAc (3 ×). The combined EtOAc layers were concentrated to give 4.13 g of crude 33. Flash chromatography (6:1 hexanes/EtOAc) gave 2.96 g (69%) of pure 33:13c Rf = 0.41 (3:1 hexanes/EtOAc); mp 116-117 °C; 1H NMR (CDCl3) 8.04 (d, 1, J = 8.0), 7,88 (d, 1, J = 8.0), 7.46 (dd, 1, J = 8.0, 8.0), 3.92 (s, 3); 13C NMR (CDCl3) 162.4, 150.6, 137.9, 130.9, 130.3, 124.4, 114.4, 53.3; IR 1732, 1542, 1439, 1368, 1284.
Methyl 2-Nitro-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoate (31)
A solution of 33 (262 mg, 1.0 mmol) and isopropyl pinacol borate (400 μL, 2.0 mmol) in 5 mL of 4:1 PhMe/THF was cooled in a MeOH/Liq N2 bath (–97 °C). The solution turned milky and the resulting mixture was treated with 1.45 M n-BuLi (1.38 mL, 2.0 mmol) dropwise over 1 h. After the addition was complete, the reaction became yellow brown. This reaction was warmed to –78 °C and stirred at that temperature for 1 h. The reaction was then warmed to –20 °C over 10 min and AcOH (0.12 mL, 2.1 mmol) was added. The reaction was further warmed to 25 °C, diluted with EtOAc, and filtered. The filtrate was washed with 1:1 saturated NH4Cl solution/H2O (twice), saturated NaHCO3 solution, and brine and dried (Na2SO4). Concentration gave 401 mg of crude 31 that contained toluene. The product was taken up in hexanes (0.5 mL) and the resulting solution was cooled at –20 °C for 30 min resulting in the crystallization of 31. The crystals were washed with 10:1 hexanes/EtOAc twice and air-dried to give 166 mg (52%) of pure 31. This compound has an extremely weak UV absorption at 254 nm because of the twisted conformation and visualizes poorly with TLC stains: 1H NMR (CDCl3) 7.94 (d, 1, J = 7.2), 7.89 (d, 1, J = 7.2), 7.59 (dd, 1, J = 7.2, 7.2), 3.90 (s, 3), 1.35 (s, 12); 13C NMR (CDCl3) 164.7, 153.4, 138.4, 135.0, 132.5, 130.5, 124.3, 85.0 (2 C), 53.1, 24.5 (4 C); IR 1735, 1546, 1358, 1270, 1139; HRMS (ESI+) calcd for C14H18NO6BNa (MNa+) 330.1125, found 330.1118.
1-Hydroxy-3-hydroxymethyl-9H-xanthen-9-one (37b)
1-Hydroxy-3-methyl-9H-xanthen-9-one (37a)15 (511 mg, 2.26 mmol) in 10 mL of anhydrous CH2Cl2 was treated with acetic anhydride (0.64 mL, 6.78 mmol), pyridine (1.10 mL, 13.6 mmol) and DMAP (55 mg, 0.45 mmol) and the resulting solution was stirred at 25 °C for 4 h. 10 mL of H2O was added to the reaction and the mixture was stirred for another 30 min to hydrolyze the excess Ac2O. The reaction was then diluted with CH2Cl2, washed with 5% aqueous HCl, saturated NaHCO3 solution, and brine, and dried (Na2SO4). Concentration gave 610 mg of crude 1-acetoxy-3-methyl-9H-xanthen-9-one (44) that was used for the next step without purification. An analytical sample was prepared by recrystallization from hexanes/EtOAc: Rf = 0.37 (3:1 hexanes/EtOAc); 1H NMR (CDCl3) 8.24 (d, 1, J = 8.0), 7.69 (dd, 1, J = 8.0, 8.0), 7.43 (d, 1, J = 8.0), 7.34 (dd, 1, J = 8.0, 8.0), 7.21 (s, 1), 6.83 (s, 1), 2.49 (s, 3), 2.49 (s, 3); 13C NMR (CDCl3) 175.6, 170.0, 157.2, 155.3, 149.7, 146.3, 134.6, 126.5, 124.0, 122.2, 119.4, 117.6, 116.1, 112.7, 21.9, 21.2; IR 1770, 1655, 1630, 1609, 1192. The spectral data are identical to those previously reported.24
The crude acetate was transferred to a resealable tube and 5 mL of CCl4 was added. The mixture was heated to 77 °C, and AIBN (74 mg, 0.45 mmol) and NBS (480 mg, 2.70 mmol) were then added. The tube was sealed and heated at that temperature for 16 h. The reaction was cooled, diluted with CH2Cl2, washed with water, 10% aqueous hydrochloric acid, and brine, and dried (Na2SO4). Concentration gave 913 mg of crude product, which was a 7:4:2 mixture of 1-acetoxy-3-bromomethyl-9H-xanthen-9-one (45), 1-acetoxy-3-dibromomethyl-9H-xanthen-9-one and recovered 1-acetoxy-3-methyl-9H-xanthen-9-one (44) that was used for the next step. An analytical sample of 45 was prepared by flash chromatography (6:1 to 3:1 hexanes/EtOAc) to remove 1-acetoxy-3-methyl-9H-xanthen-9-one (44) and recrystallization from hexanes/EtOAc: Rf = 0.35 (3:1 hexanes/EtOAc); 1HNMR (CDCl3) 8.24 (d, 1, J = 8.0), 7.72 (dd, 1, J = 8.0, 8.0), 7.46 (d, 1, J = 8.0), 7.45 (s, 1), 7.37 (dd, 1, J = 8.0, 8.0), 7.04 (s, 1), 4.51 (s, 2), 2.50 (s, 3); 13C NMR (CDCl3) 175.4, 169.7, 157.2, 155.3, 150.3, 144.7, 135.3, 126.6, 124.3, 122.2, 118.8, 117.6, 116.4, 114.6, 30.9, 21.2; IR 1772, 1657, 1632, 1610, 1194.
Crude benzyl bromide 45 and CaCO3 (500 mg, 5 mmol) in 6 mL of 1:1 1,4-dioxane/H2O in a sealed tube were heated at 120 °C for 16 h. The reaction was cooled and K2CO3 (500 mg, 3.62 mmol) was added. The reaction was stirred at 25 °C for 2 h. The reaction was diluted with EtOAc, washed with water and brine, and dried (Na2SO4). Concentration gave 743 mg of crude 37b. Flash chromatography (3:1 to 2:1 hexanes/EtOAc) gave 275 mg (50%) of pure 37b: Rf = 0.38 (1:1 hexanes/EtOAc); mp 149-150 °C; 1H NMR (CDCl3) 12.63 (s, 1, ArOH), 8.27 (d, 1, J = 8.0), 7.76 (dd, 1, J = 8.0, 8.0), 7.47 (d, 1, J = 8.0), 7.40 (dd, 1, J = 8.0, 8.0), 6.98 (s, 1), 6.77 (s, 1), 4.79 (d, 2, J = 5.6), 1.93 (t, 1, J = 5.6, OH); 13C NMR (CDCl3) 182.0, 161.9, 156.5, 156.2, 151.3, 135.5, 125.9, 124.1, 120.6, 117.9, 108.0, 107.8, 104.4, 64.5; IR 3501 (br), 1655, 1609; HRMS (EI+) calcd for C14H10O4 (M+) 242.0579, found 242.0582.
1-Hydroxy-3-hydroxymethyl-2-iodo-9H-xanthen-9-one (30a)
A solution of benzyl alcohol 37b (106 mg 0.44 mmol), I2 (89 mg, 0.35 mmol, 0.8 equiv), and H5IO6 (40 mg, 0.18 mmol, 0.4 equiv) in 7.5 mL of 2:2:1 THF/EtOH/H2O was stirred at 25 °C for 40 h. The reaction was quenched with 5 mL of 10% Na2S2O3 solution, and diluted with EtOAc. The mixture was washed with water and brine, and dried (Na2SO4). Concentration gave 159 mg (99%) of crude 30a, which can be used directly without further purification: Rf = 0.56 (1:1 hexanes/EtOAc); mp 220-221 °C (darkens at 199 °C); 1H NMR (DMSO-d6) 13.51 (s, 1, ArOH), 8.18 (d, 1, J = 8.0), 7.93 (dd, 1, J = 8.0, 8.0), 7.69 (d, 1, J = 8.0), 7.52 (dd, 1, J = 8.0, 8.0), 7.23 (s, 1), 5.83 (t, 1, J = 5.6, OH), 4.50 (d, 2, J = 5.6); 13C NMR (DMSO-d6) 180.8, 159.3, 155.9, 155.6, 154.1, 136.6, 125.6, 124.8, 119.5, 118.2, 107.0, 105.9, 77.5, 67.9; IR 3445 (br), 1638, 1602, 1571, 1267; HRMS (EI+) calcd for C14H9IO4 (M+) 367.9546, found 367.9545. A sample for X-ray crystallography was prepared by recrystallization from THF.
3-Hydroxymethyl-2-iodo-1-methoxy-9H-xanthen-9-one (30b)
Iodo-9H-xanthen-9-one 30a (100 mg, 0.27 mmol) in anhydrous DMF (4 mL) was treated with MeI (0.05 mL, 2.7 mmol, 10 equiv) and K2CO3 (373 mg, 2.7 mmol, 10 equiv). The reaction was stirred at 25 °C for 12 h. The reaction was filtered and the filtrate was concentrated. The solid was re-dissolved in a minimum amount of THF and silica gel (6 g) was added to the solution. The solvent was removed and 30b absorbed on silica gel was purified by flash chromatography (2:1 hexanes/EtOAc) to give 72 mg (69%) of pure 30b: Rf = 0.54 (1:1 hexanes/EtOAc); mp 216-217 °C (darkens at 214 °C); 1H NMR (DMSO-d6) 8.18 (d, 1, J = 8.0, 1), 7.85 (dd, 1, J = 8.0, 8.0); 7.64 (d, 1, J = 8.0), 7.51 (s, 1), 7.47 (dd, 1, J = 8.0, 8.0), 5.86 (t, 1, J = 5.6, OH), 4.51 (d, 2, J = 5.6), 3.84 (s, 3); 13C NMR (DMSO-d6) 173.9, 158.3, 157.4, 154.6, 152.2, 135.2, 126.2, 124.4, 121.8, 117.8, 114.6, 112.2, 89.7, 67.8, 61.3; IR 3467 (br) 1656, 1600; HRMS (EI+) calcd for C15H11IO4 (M+) 381.9702, found 381.9695.
1-Hydroxy-3-hydroxymethyl-2-(2-nitro-3-methoxycarbonylphenyl)-9H-xanthen-9-one (43)
Iodo-9H-xanthen-9-one 30a (30 mg, 81 μM), boronate 31 (65 mg, 211 μM, 2.6 equiv), Pd(OAc)2 (5.5 mg, 24 μM, 0.30 equiv) and SPhos (9.6 mg, 24 μM, 0.30 equiv) were added to a sealed tube. The tube was evacuated and backfilled with N2 three times. Freshly distilled THF (1.5 mL) and degassed water (freeze-thaw cycle three times) (0.5 mL) were then added to the mixture. The reaction was heated at 80 °C for 2 h. The reaction was cooled, diluted with EtOAc, washed with H2O and brine, and dried (Na2SO4). Concentration gave 90 mg of crude 43. Flash chromatography (2:1 hexanes/EtOAc) gave 20 mg (58%) of pure 43: Rf = 0.28 (1:1 hexanes/EtOAc); H1 NMR (DMSO-d6) 12.77 (s, 1, ArOH), 8.20 (d, 1, J = 8.0), 8.10 (d, 1, J = 8.0), 7.96 (dd, 1, J = 8.0, 8.0), 7.88 (dd, 1, J = 8.0, 8.0), 7.77 (d, 1, J = 8.0), 7.74 (d, 1, J = 8.0), 7.54 (dd, 1, J = 8.0, 8.0), 7.29 (s, 1), 5.64 (t, 1, J = 5.6, OH), 4.33 (dd, 1, J = 15.6, 5.6), 4.26 (dd, 1, J = 15.6, 5.6), 3.87 (s, 3); 13C NMR (DMSO-d6) 181.5, 163.8, 157.9, 156.1, 155.8, 152.2, 149.5, 136.8, 136.7, 131.8, 130.8, 127.9, 125.4, 124.9, 123.8, 119.9, 118.2, 113.8, 106.6, 104.4, 60.7, 53.3; IR 3543 (br), 3416 (br), 1727, 1649, 1612, 1543, 1472, 1434, 1283; HRMS (ESI+) calcd for C22H16NO8 (MH+) 422.0876, found 422.0867.
3-Hydroxymethyl-1-methoxy-2-(2-nitro-3-methoxycarbonylphenyl)-9H-xanthen-9-one (46)
Iodide 30b (20 mg, 52 μM), boronate 31 (45 mg, 147 μM, 2.8 equiv), Pd(OAc)2 (5.6 mg, 25 μM, 0.50 equiv) and SPhos (11 mg, 27 μM, 0.55 equiv) were added to a sealed tube. The tube was evacuated and backfilled with N2 for three times. Freshly distilled THF (0.6 mL) and degassed water (freeze-thaw cycle three times) (0.2 mL) were then added to the mixture. The reaction was heated at 80 °C for 2 h. The reaction was cooled, diluted with EtOAc, washed with H2O and brine, and dried (Na2SO4). Concentration gave 51 mg of crude 46. Flash chromatography (1:1 hexanes/EtOAc) gave 12 mg (53%) of pure 46: Rf = 0.18 (1:1 hexanes/EtOAc); 1H NMR (DMSO-d6) 8.16 (d, 1, J = 8.0), 8.10 (d, 1, J = 8.0), 7.88 (dd, 1, J = 8.0, 8.0), 7.87 (d, 1, J = 8.0, 8.0), 7.81 (d, 1, J = 8.0), 7.67 (d, 1, J = 8.0), 7.56 (s, 1), 7.48 (dd, 1, J = 8.0, 8.0), 5.64 (t, 1, J = 5.6, OH), 4.26 (d, 2, J = 5.6), 3.87 (s, 3), 3.57 (s, 3); 13C NMR (DMSO-d6) 174.5, 163.8, 157.6, 157.0, 154.6, 150.2, 149.0, 136.3, 135.3, 131.5, 130.8, 128.5, 126.0, 124.5, 123.9, 122.4, 122.1, 117.8, 113.7, 110.7, 62,1 60.5, 53.3; IR 3440 (br), 1733, 1657, 1605, 1541, 1468, 1416, 1290, HRMS (ESI+) calcd for C23H17NO8Na (MNa+) 458.0852, found 458.0850.
4,7-Dihydro-15-hydroxy-[1]benzopyrano[2,3-j]isoxazolo[4,3,2-de]phenanthridine-4,14-dione (29a)
A solution of Suzuki-Miyaura coupling product 43 (18 mg, 49 μmol), Zn (40 mg, 0.61 mmol), and NH4Cl (60 mg, 1.12 mmol) in 5 mL of 2:2:1 MeOH/THF/H2O was sonicated at 25 °C for 15 min. The reaction was diluted with 5 mL of THF and filtered. The filtrate was further diluted with 10 mL of EtOAc, and the resulting solution was washed with brine and dried (MgSO4). Concentration gave 20 mg of crude hydroxymethyl benzisoxazolinone 47.
A suspension of the crude hydroxymethyl benzisoxazolinone 47 in 5 mL of anhydrous CH2Cl2 was cooled to 0 °C. NEt3 (24 μL, 4 equiv) and MsCl (10 μL, 3 equiv) were added. The reaction was stirred at 0 °C for 15 min and warmed to 25 °C and stirred for another 45 min. The solid dissolved as the reaction proceeded. The solution was diluted with CH2Cl2, washed with water and brine, and dried (Na2SO4). Concentration gave 24 mg of the benzisoxazolinone mesylate.
A solution of the crude mesylate in 6 mL of 1:1 THF/H2O was cooled to 0 °C. Na2CO3 (106 mg, 1 mmol) was added to this solution. The reaction was stirred at 0 °C for 5 min and 25 °C for another 55 min. A precipitate formed as the reaction proceeded. The reaction was diluted with 20 mL of THF to dissolve the precipitate and the resulting aqueous THF solution was washed with brine and dried (MgSO4). Concentration gave 7.5 mg of crude orange 29a. Crude 29a was washed twice with chloroform and air dried to give 7 mg (46%) of pure 29a as a poorly soluble yellow-orange solid that was too insoluble in all common NMR solvents for a 13C NMR spectrum to be obtained: 1H NMR (CDCl3) 13.84 (s, 1, ArOH), 8.62 (d, 1, J = 8.0), 8.34 (d, 1, J = 8.0), 7.83 (dd, 1, J = 8.0, 8.0), 7.72 (d, 1, J = 8.0), 7.54 (d, 1, J = 8.0), 7.48 (dd, 1, J = 8.0, 8.0), 7.38 (dd, 1, J = 8.0, 8.0), 6.99 (s, 1), 4.69 (s, 2); HRMS (ESI+) calcd for C21H12NO5 (MH+) 358.0715, found 358.0707.
4,7-Dihydro-15-methoxy-[1]benzopyrano[2,3-j]isoxazolo[4,3,2-de]phenanthridine-4,14-dione (29b)
A solution of Suzuki-Miyaura coupling product 46 (14 mg, 32 μmol), Zn (34 mg, 0.55 mmol) and NH4Cl (54 mg, 1 mmol) in 5 mL of 2:2:1 MeOH/THF/H2O was sonicated at 25 °C for 15 min. The reaction was diluted with EtOAc, and filtered. The filtrate was washed with brine, and dried (Na2SO4). Concentration gave 18 mg of crude hydroxymethyl benzisoxazolinone 48.
A solution of hydroxymethyl benzisoxazolinone 48 in 3 mL of anhydrous CH2Cl2 was cooled to 0 °C and treated with NEt3 (18 μL, 4 equiv) and MsCl (8 μL, 3 equiv). The reaction was stirred at 0 °C for 15 min and warmed to 25 °C and stirred for another 45 min. The solution was diluted with CH2Cl2, washed with water and brine, and dried (Na2SO4). Concentration gave 18 mg of the benzisoxazolinone mesylate.
A solution of the crude mesylate in 6 mL of 1:1 THF/H2O solution was cooled to 0 °C. Na2CO3 (106 mg, 1 mmol) was added to this solution. The reaction was stirred at 0 °C for 5 min and 25 °C for another 55 min. The resulting mixture was diluted with EtOAc, washed with water and brine, and dried (Na2SO4). Concentration gave 12 mg of crude 29b. Flash chromatography (2:1 hexanes/EtOAc) gave 5 mg (42%) of pure 29b: Rf = 0.59 (1:1 hexanes/EtOAc); 1H NMR (CDCl3) 8.62 (d, 1, J = 8.0), 8.34 (d, 1, J = 8.0), 7.77 (d, 1, J = 8.0), 7.76 (dd, 1, J = 8.0, 8.0), 7.48 (d, 1, J = 8.0), 7.45 (dd, 1, J = 8.0, 8.0), 7.40 (dd, 1, J = 8.0, 8.0), 7.30 (s, 1), 4.66 (s, 2), 4.02 (s, 3); 13C NMR (CDCl3) 175.5, 167.9, 158.9, 157.4, 156.8, 155.0, 139.1, 135.0, 132.2, 126.8, 126.4, 124.57, 124.55, 122.7, 119.09, 119.07, 117.5, 116.8, 114.1, 111.3, 61.8, 56.0; IR 1765, 1652, 1609, 1470, 1415; HRMS (ESI+) calcd for C22H14NO5 (MH+) 372.0872, found 372.0868.
Decomposition of 4H,7H-Isoxazolo[4,3,2-de]phenanthridinones 12, 24b and 28b in CDCl3
A sample (3 mg) of each compound was dissolved in 0.5 mL of CDCl3 in an NMR tube at 25 °C. The decomposition was monitored by 1H NMR spectroscopy. The reaction formed only the phenanthridine-4-carboxylic acids 49a, 49b, and 49c, respectively. No intermediates were observed.
Data for phenanthridine-4-carboxylic acid (49a): 1H NMR (CDCl3) 9.33 (s, 1), 8.86 (d, 1, J = 8.0), 8.82 (d, 1, J = 8.0), 8.72 (d, 1, J = 8.0), 8.21 (d, 1, J = 8.0), 8.05 (dd, 1, J = 8.0, 8.0), 7.90 (dd, 1, J = 8.0, 8.0), 7.87 (dd, 1, J = 8.0, 8.0). An authentic sample of 49a was prepared by the literature procedure.21b The 1H NMR spectrum of the authentic sample of 49a is identical to that of the product of decomposition of 12 in CDCl3.
The data for the mixture of 24b and 49b matched those of authentic samples. An authentic sample of 49b was prepared from 22b as previously described.10
Data for 8,10-dimethoxyphenanthridine-4-carboxylic acid (49c):1H NMR (CDCl3) 9.60 (dd, 1, J = 8.0, 1.3), 9.12 (s, 1), 8.70 (dd, 1, J = 8.0, 1.3), 7.78 (dd, 1, J = 8.0, 8.0), 7.08 (d, 1, J = 2.2), 7.05 (d, 1, J = 2.2), 4.16 (s, 3), 4.03 (s, 3); HRMS (ESI+) calcd for C16H14NO4 (MH+) 284.0923, found 284.0910. The 10-methoxy group deshields H-1 which absorbs far downfield at δ 9.60.26
Decomposition of 4,7-Dihydro-15-methoxy-[1]benzopyrano[2,3-j]isoxazolo[4,3,2-de]phenanthridine-4,14-dione (29b) in CDCl3
A solution of benzisoxazolinone 29b in CDCl3 was monitored at 25 °C. The compound slowly rearranged with a half life of 2 days to give acid 14-hydroxy-13-oxo-13H-[1]benzopyrano[2,3-j]phenanthridine-4-carboxylic acid (50) as the major product: 1H NMR (CDCl3) 9.83 (d, 1, J = 8.0), 9.34 (s, 1), 8.82 (d, 1, J = 8.0), 8.39 (d, 1, J = 8.0), 8.06 (s, 1), 7.95 (dd, 1, J = 8.0, 8.0), 7.81 (dd, 1, J = 8.0, 8.0), 7.54 (d, 1, J = 8.0, 1), 7.46 (dd, 1, J = 8.0, 8.0), 4.15 (s, 3).
X-Ray Data Collection, Solution, and Refinement for 1-Hydroxy-2-iodo-3-hydroxymethyl-9H-xanthen-9-one (30a)
All operations were performed on a Bruker-Nonius Kappa Apex2 diffractometer, using graphite-monochromated MoKα radiation. All diffractometer manipulations, including data collection, integration, scaling, and absorption corrections were carried out using the Bruker Apex2 software.27 Preliminary cell constants were obtained from three sets of 12 frames. Data collection was carried out at 120K, using a frame time of 10 sec and a detector distance of 60 mm. The optimized strategy used for data collection consisted of 4 phi and 4 omega scan sets, with 0.5° steps in phi or omega; completeness was 100.0%. A total of 2870 frames were collected. Final cell constants were obtained from the xyz centroids of 5788 reflections after integration.
From the systematic absences, the observed metric constants and intensity statistics, space group P21/c was chosen initially; subsequent solution and refinement confirmed the correctness of this choice. The structure was solved using Superflip,28 and refined (full-matrix-least squares) using the Oxford University Crystals for Windows program.29 All ordered non-hydrogen atoms were refined using anisotropic displacement parameters; ordered hydrogen atoms were first regularized with the use of restraints and subsequently allowed to ride on the corresponding carbon atoms. Compound 30a exhibited disorder of the H atom attached to atom O(4) (–CH2OH group). After location of the two disordered atoms by a combination of Fourier and geometric considerations, each H atom (H41 and H42) was assigned an occupancy of 0.5, and allowed to ride on atom O(4). The final least-squares refinement converged to R1 = 0.0593 (I > 2σ(I), 3102 data) and wR2 = 0.1234 (F2, 3488 data, 172 parameters). The final CIF is available as supporting material.
Supplementary Material
Acknowledgment
We are grateful to the National Institutes of Health (GM-50151) for support of this work. We thank the National Science Foundation for partial support of this work through grant CHE-0521047 for the purchase of a new X-ray diffractometer. We thank Professor Stephen Buchwald, Massachusetts Institute of Technology, for advice on Suzuki-Miyaura couplings.
Footnotes
Supporting Information Available: A figure showing the crystal structure of 30a, X-ray crystallographic data for 30a (CIF), and copies of 1H and 13C NMR spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.a Parish CA, Smith SK, Calati K, Zink D, Wilson K, Roemer T, Jiang B, Xu D, Bills G, Platas G, Peláez F, Díez MT, Tsou N, McKeown AE, Ball RG, Powles MA, Yeung L, Liberator P, Harris G. J. Am. Chem. Soc. 2008;130:7060–7066. doi: 10.1021/ja711209p. [DOI] [PubMed] [Google Scholar]; b Jiang B, Xu D, Allocco J, Parish C, Davison J, Veillette K, Sillaots S, Hu W, Rodriguez-Suarez R, Trosok S, Zhang L, Li Y, Rahkhoodaee F, Ransom T, Martel N, Wang H, Gauvin D, Wiltsie J, Wisniewski D, Salowe S, Kahn JN, Hsu M-J, Giacobbe R, Abruzzo G, Flattery A, Gill C, Youngman P, Wilson K, Bills G, Platas G, Pelaez F, Diez MT, Kauffman S, Becker J, Harris G, Liberator P, Roemer T. Chem. Biol. 2008;15:363–374. doi: 10.1016/j.chembiol.2008.02.016. [DOI] [PubMed] [Google Scholar]; c Adam GC, Parish CA, Wisniewski D, Meng J, Liu M, Calati K, Stein BD, Athanasopoulos J, Liberator P, Roemer T, Harris G, Chapman KT. J. Am. Chem. Soc. 2008;130:16704–16710. doi: 10.1021/ja805531w. [DOI] [PubMed] [Google Scholar]; d Overy D, Calati K, Kahn JN, Hsu M-J, Martín J, Collado J, Roemer T, Harris G, Parish CA. Bioorg. Med. Chem. Lett. 2009;19:1224–1227. doi: 10.1016/j.bmcl.2008.12.081. [DOI] [PubMed] [Google Scholar]; e Bills GF, Platas G, Overy DP, Collado J, Fillola A, Jiménez MR, Martín J, González del Val A, Vicente F, Tormo JR, Peláez F, Calati K, Harris G, Parish C, Xu D, Roemer T. Mycologia. 2009;101:449–472. doi: 10.3852/08-163. [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Krohn K, Zia-Ullah, Flörke U, Pescitelli G, Di Bari L, Antus S, Kurtán T, Rheinheimer J, Draeger S, Schulz B. Chem. Eur. J. 2008;14:4913–4923. doi: 10.1002/chem.200800035. [DOI] [PubMed] [Google Scholar]
- 3.a Nising CF, Ohnemüller (née Schmid) UK, Bräse S. Angew. Chem. Int. Ed. 2006;45:307–309. doi: 10.1002/anie.200502913. [DOI] [PubMed] [Google Scholar]; b Gérard EMC, Bräse S. Chem. Eur. J. 2008;14:8086–8089. doi: 10.1002/chem.200801507. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaou KC, Li A. Angew. Chem. Int. Ed. 2008;47:6579–6582. doi: 10.1002/anie.200802632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a Frank B, Stöckigt J, Zeidler U, Franckowiak G. Chem. Ber. 1973;106:1198–1220. [Google Scholar]; b Tatsuta K, Yoshihara S, Hattori N, Yoshida S, Hosokawa S. J. Antibiot. 2009;62:469–470. doi: 10.1038/ja.2009.52. [DOI] [PubMed] [Google Scholar]
- 6.a Wierenga W, Harrison AW, Evans BR, Chidester CG. J. Org. Chem. 1984;49:438–442. [Google Scholar]; b Wierenga W, Evans BR, Zurenko GE. J. Med. Chem. 1984;27:1212–1215. doi: 10.1021/jm00375a022. [DOI] [PubMed] [Google Scholar]
- 7.Bamberger E, Pyman FL. Ber. 1909;42:2297–2330. [Google Scholar]
- 8.Cohen T, Gray WF. J. Org. Chem. 1972;37:741–744. [Google Scholar]
- 9.Liu B, Moffett KK, Joseph RW, Dorsey BD. Tetrahedron Lett. 2005;46:1779–1782. [Google Scholar]
- 10.A portion of this work was published in preliminary form: Zhou Q, Snider BB. Org. Lett. 2009;11:2936–2939. doi: 10.1021/ol901054r.
- 11.a Walker SD, Barder TE, Martinelli JR, Buchwald SL. Angew. Chem. Int. Ed. 2004;43:1871–1876. doi: 10.1002/anie.200353615. [DOI] [PubMed] [Google Scholar]; b Barder TE, Walker SD, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 12.Tan Y-L, White AJP, Widdowson DA, Wilhelm R, Williams DJ. J. Chem. Soc., Perkin Trans. 1. 2001:3269–3280. [Google Scholar]
- 13.a Burger A, Avakian S. J. Org. Chem. 1940;5:606–609. [Google Scholar]; b Erickson JLE, Dechary JM, Pullig TR. J. Am. Chem. Soc. 1952;74:5621–5623. [Google Scholar]; c Kasahara A, Izumi T, Murakami S, Miyamoto K, Hino T. J. Heterocycl. Chem. 1989;26:1405–1413. [Google Scholar]
- 14.Li W, Nelson DP, Jensen MS, Hoerrner RS, Cai D, Larsen RD, Reider PJ. J. Org. Chem. 2002;67:5394–5397. doi: 10.1021/jo025792p. See also: Kristensen JL, Lysén M, Vedsø P, Begtrup M. Org. Synth. 2005;81:134–139.
- 15.Yari A, Darvishi L, Shamsipur M. Anal. Chim. Acta. 2006;555:329–335. [Google Scholar]
- 16.Hydroxymethylxanthone 37b has been previously prepared by degradation of sydowinin A: Hamasaki T, Sato Y, Hatsuda Y. Agr. Biol. Chem. 1975;39:2341–2345.
- 17.Reaction of xanthone 37a with pyridinium perbromide in acetic acid also gave a similar mixture of ortho and para bromides in our hands although it was claimed to give only the ortho bromide: Patel GN, Verma RS, Pardasani RT, Trivedi KN. Pol. J. Chem. 1988;62:409–416.
- 18.Bromination of related xanthone methyl ethers with NBS occurs only on the aromatic ring with 2% dibenzoyl peroxide (DBP) as initiator. With 10% DBP both methyl bromination (45%) and ring bromination (22%) products are obtained: Goissis G. Tetrahedron Lett. 1982;23:4821–4822.
- 19.a Rodighiero P, Manzini P, Pastorini G, Bordin F, Guiotto A. J. Het. Chem. 1987;24:485–488. [Google Scholar]; b Suzuki H, Nakamura K, Goto R. Bull. Chem. Soc. Jpn. 1966;39:128–131. [Google Scholar]; c Fatiadi AJ. In: Synthetic Reagents. Pizey JS, editor. Vol. 4. Halsted Press, Wiley; New York: 1981. pp. 184–187. [Google Scholar]; d Stavber S, Jereb M, Zupan M. Synthesis. 2008:1487–1513. [Google Scholar]
- 20.Talekar RS, Chen GS, Lai S-Y, Chern J-W. J. Org. Chem. 2005;70:8590–8593. doi: 10.1021/jo051191x. [DOI] [PubMed] [Google Scholar]
- 21.a Sengupta D, Anand N. Ind. J. Chem. 1985;24B:923–927. [Google Scholar]; b Atwell GJ, Baguley BC, Denny WA. J. Med. Chem. 1988;31:774–779. doi: 10.1021/jm00399a015. [DOI] [PubMed] [Google Scholar]
- 22.The decomposition of 12 under basic condition with NaOD in CD3CN/D2O is much more complex giving a mixture of 49a, the corresponding N-oxide, and other products.
- 23.a Streitwieser A, Jr., Koch HF. J. Am. Chem. Soc. 1964;86:404–409. [Google Scholar]; b Schlosser M, Maccaroni P, Marzi E. Tetrahedron. 1998;54:2763–2770. [Google Scholar]
- 24.Fonteneau N, Martin P, Mondon M, Ficheux H, Gesson J-P. Tetrahedron. 2001;57:9131–9135. [Google Scholar]
- 25.Hannick SM, Kishi YJ. Org. Chem. 1983;48:3833–3835. [Google Scholar]
- 26.H-1 of 6-phenyl-10-methoxyphenanthridine absorbs at δ 9.54. H-1 and H-10 of 6-phenylphenanthridine absorb at δ 8.63 and 8.71: Youn SW, Bihn JH. Tetrahedron Lett. 2009;50:4598–4601.
- 27.Apex2, Version 2 User Manual, M86-E01078, Bruker Analytical X-ray Systems, Madison, WI, June 2006.
- 28.Palatinus L, Chapuis GJ. Appl. Cryst. 2007;40:786–790. [Google Scholar]
- 29.Betteridge PW, Carruthers JR, Cooper RI, Prout K, Watkin DJ. J. Appl. Cryst. 2003;36:1487. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.