Abstract
Social recognition (SR) enables rodents to distinguish between familiar and novel conspecifics, largely through individual odor cues. SR tasks utilize the tendency for a male to sniff and interact with a novel individual more than a familiar individual. Many paradigms have been used to study the roles of the neuropeptides oxytocin and vasopressin in SR. However, inconsistencies in results have arisen within similar mouse strains, and across different paradigms and laboratories, making reliable testing of social recognition difficult. The current protocol details a novel approach that is replicable across investigators and in different strains of mice. We created a protocol that utilizes gonadally intact, singly housed females presented within corrals to group-housed males. Housing females singly prior to testing is particularly important for reliable discrimination. This methodology will be useful for studying short-term social memory in rodents, and may also be applicable for longer-term studies.
INTRODUCTION
Across species, the ability to distinguish familiar from novel conspecifics (social recognition) is imperative for display of appropriate social behaviors1. In rodents, information about individuals is primarily passed through volatile and pheromonal cues2,3, which are individually distinct after postnatal days 21–284. Processing of these olfactory cues occurs primarily in the main and accessory olfactory systems, with projections to the lateral entorhinal cortex5. Long-term storage of these cues (one week or longer6) allows the rodent to form a ‘social memory’ wherein a memory of a recently encountered individual is retained for some duration of time1. This allows the animal to display the appropriate behavior(s) upon encountering the individual in the future.
Social memory is commonly examined in rodents through a variety of social recognition tasks that utilize the innate preference by adult rodents to spend more time with novel over familiar conspecifics, usually of the opposite sex. The roles of the neuropeptides oxytocin (Oxt) and vasopressin (Avp) in social memory, one of our research interests, have been investigated using three paradigms: two-trial social recognition, habituation-dishabituation, and social discrimination. In the two-trial social recognition paradigm, a subject animal is exposed to a stimulus animal and after a predetermined period of time, such as 30 minutes, is either re-exposed to the same stimulus animal or to a novel stimulus animal. Typically, the subject spends a greater amount of time investigating the novel animal7,8,9,10. In the habituation-dishabituation task, a subject is exposed to the same “stimulus” animal over repeated trials, and demonstrates a decrease in investigation, or habituation. On the final trial, a novel animal is presented, which normally results in an increase in investigation time, or dishabituation8,10,11,12,13,14,15. The third paradigm of social discrimination is similar to two-trial social recognition, except that on the re-exposure trial both the same and novel stimulus animals are presented simultaneously, allowing the subject animal to choose between the two9,14,16.
Three problems are common across the three test paradigms. First, in both mice8,10,11,12,13,14,15,16,17,18,19 and rats9,20,21,22,23,24, testing occurs in an individually housed subject’s home cage, with the stimulus animals acting as “intruders” in the cage. For those studies that use individually housed male subjects, the stimulus animals are typically either juvenile males6,20,21,22,23 (to decrease risk of aggressive attacks25), or ovariectomized females8,10,11,12,13,14,15,16,17,18,19 (to decrease sexual interest in the female). The use of individually housed subject males, the most commonly used approach, is not optimum. A lack of cage mates for the singly housed subject contributes to an aggressive response to the intruder male26,27,28 and increases attempts to mount when presented with a stimulus female29. For the latter situation, it is necessary to extinguish sex behavior over a period of days11,15 so that the male will engage in investigatory behavior, not just sexual behavior. Social isolation also significantly increases stress-like responses (e.g., heart rate and blood pressure) in response to common procedures (e.g., cage changing, restraint, injection) in rats30. The use of group-housed subject males (isolated only for the duration of the test) has been reported to maintain heightened social recognition responses6.
Second, in all studies discussed above, stimulus animals (male and female) were group-housed littermates. Discriminations can be made between siblings in hamsters31, rats32, and between oxytocin and estrogen knockout and wildtype littermates in mice33. However, hamsters have a difficult time distinguishing between flank gland odors of unrelated sibling littermates31. Additionally, a major component of individual scent information are mouse urinary proteins (MUPs)34 which have recently been shown to be necessary for recognition of individuals3,35,36,37. With group-housing conditions, individual MUPs may be transferred between animals. The use of group-housed stimulus males or females may make distinguishing between and recognition of individuals’ odors more difficult.
Third, allowing the subject and stimulus animals to interact freely in the test cage permits the subject animal to deposit its own scent onto the stimulus animals38. Therefore, a decrease in investigation of the “familiar” female could simply be due to the subject’s recognition of its own familiar scent, and not the scent of the female. Presenting the stimulus mice inside of a corral reduces direct contact between the subject and stimulus animals and their excretions, while still allowing reception of visual and olfactory cues and eliciting high interest and investigation by the subject13,39. In this protocol, we advocate the use of the same two corrals throughout testing (see PROCEDURE, Step 6). With habituation to the corrals (see PROCEDURE, Steps 4–5), the subject male will cease to be interested in them as novel objects and will investigate the female contained within instead. By using the same corrals during Trial 1 and Trial 2, the need to re-habituate the subjects to the corrals is eliminated. Additionally, presenting the stimulus females within a corral prevents the need to extinguish sex behavior prior to the test, as well as allows use of gonadally-intact females, which elicit higher interest from males than ovariectomized females when contact is prevented40.
Gonadally-intact females presents the investigator with a possible confound of estrous state influencing male investigation. However, the day of estrous cycle has previously not been shown to greatly affect investigation by males40. Sexually-naïve males (as we recommend subject males to be) indicate no preference for receptive females’, over non-receptive females’, odors41. Females in all states of estrus elicit higher investigation from males than do ovariecomized females42. Furthermore, subjects from all groups tested would be exposed to the various estrous states, thereby eliminating possible group x estrous condition interactions. Recently, we have published data using gonadally-intact females as stimulus animals and, in testing subjects from all groups over a 4–5 day period, found no influence of estrous cycle on male investigation43.
Social recognition is regulated by Oxt and Avp44 which are synthesized in the magnocellular cells of the supraoptic and paraventricular nuclei of the hypothalamus, as well as in a variety of parvocellular neurons (e.g., bed nucleus of the stria terminalis, paraventricular nucleus of the hypothalamus, and medial amygdala)1,44,45,46. However, not all studies agree on how important the relative contributions of both peptides and their receptors are to social recognition. While studies using transgenic mice indicate that social recognition is dependent upon Oxt10,11,13 and the Oxt receptor15,17, pharmacological studies in rats indicate that at high doses, Oxt inhibits social recognition20,47. Furthermore, while lack of the Avp receptor subtype 1b (Avpr1b) has consistently been found to impair social memory in male12 and female45 mice, conflicting findings have been reported for Avpr1a knockout (KO) mice14,16,48. Furthermore, within our partial forebrain-specific Oxtr KO line (OxtrFB/FB), different social recognition tasks (two-trial and habituation/dishabituation) have given different results15, indicating that the tasks may not test the same aspects of social recognition.
With these problems in mind, we developed a social discrimination task that addresses the above concerns43 while still retaining the advantages of the existing social recognition paradigms (i.e., a simple measure of individual recognition that does not require task learning, and can be repeated with presentation of novel stimulus animals49). A reliable test of social recognition would be consistent across experimenters and labs when tested with the same strain/line of mice, and provide consistent results amongst all control animals. Through rigorous testing of variables of interest (housing conditions of subject and stimulus animals; number of corrals used in each trial; type of stimulus animals used), we developed such a task, and validated its use for testing social recognition in Oxt and Oxtr knockout mice43. Specifically, we used the task to further investigate the role of Oxt and the Oxtr in social recognition, as well as demonstrated that wildtype (WT) mice from three different lines (total Oxt KO, total Oxtr KO, and OxtrFB/FB) have highly consistent social discrimination abilities across testing, spending approximately 65–75% of the test time investigating novel females43. This data, as well as a detailed description of methodologies and possible issues with testing, are contained within this protocol.
MATERIALS
REAGENTS
- Laboratory mice (e.g., C57Bl/6J, Balb/C, Swiss-Webster). Most strains of mice (such as those found in the Mouse Phenome Project: http://www.jax.org/phenome), as well as transgenic or knockout mouse lines (such as those in the Induced Mutant Resource: http://www.jax.org/resources/documents/imr/)50 should be suitable, although investigators should remember that difference in anxiety-like behaviors51,52 and/or sociability53,54 between strains could impact the results. CAUTION Experiments must follow all national and institutional guidelines for care and use of laboratory animals.
EQUIPMENT
Standard mouse cage with bedding
Wire corrals
Light meter
Stopwatch for timing sessions
Video camera and DVDs or tapes
70% ethanol to clean the corrals
Paper and pen to label cages
Scale to weigh animals
EQUIPMENT SETUP
Standard mouse cage with bedding
Testing takes place in a novel, clean, standard rectangular mouse cage (27 cm length × 17 cm width × 12 cm high). Only a thin covering of bedding (hard woodchip; Quality Lab Products, Elkridge, MD) should be on the floor of the cage (≤ 1/8 inch). This will prevent introduction of competing behaviors (e.g., excessive digging), as well as prevent stimulus females from escaping the corrals. The cage should be well-cleaned, and covered with a well-cleaned, well-fitting lid to prevent the animal from jumping out of the cage.
Wire corrals
Corrals can be obtained from www.kitchen-plus.com (item # 31570) and are 4 ¼″ high × 4″ diameter. The space between the wire bars is approx 6mm; large enough for the subject animal to push his nose between (see PROCEDURE, Step 6), allowing the subjects to obtain visual and olfactory information from the stimulus females13,39. The corrals take up a large portion of the cage when two are present but do not seem to prevent free movement of the subject animal around the cage (see Supplementary movie online). CRITICAL STEP Corrals can only be used once per day to prevent transmission of odor; two wire corrals will be needed for each subject animal tested (see PROCEDURE, Step 5). Therefore, a minimum of 10 corrals is recommended, so at least 5 animals can be tested in one day.
Light meter
As a brightly lit environment can be anxiety-provoking55, we recommend decreasing the light in the testing room to approximately 30–40 lux at the cages. To ensure accurate luminosity, we use a light meter (Fisher Scientific, Pittsburgh; Cat #02-401-4).
Video equipment
In addition to live scoring, we recommend that both Trials 1 and 2 (see PROCEDURE) be videotaped. This will allow a permanent archive of testing, as well as later analysis of other possible behaviors of interest (e.g., time spent grooming or rearing) and reliability assessments between observers (see PROCEDURE, Step 9). Cameras should be placed on a tripod and angled so that the entirety of the long side of the cage is in view, the subject animal can be seen in all corners of the cage, and the animal ID tag on the lid (see PROCEDURE, step 4) can be seen (see Supplementary Video 1 online).
Computer program for automated scoring
If videotapes are used, a suitable computer program is needed to allow for scoring investigation times by the subject animals of the stimulus animals. One that we regularly use with good results is the Observer VideoPro 5.0 (Noldus, Wageningen, The Netherlands)56. The program allows the assignment of any key on the keyboard to represent a behavior. For example, “investigation time” of the familiar stimulus animal can be assigned to the letter “f”, “investigation time” of the novel stimulus animal can be assigned to the letter “n”, time spent grooming can be assigned to the letter “g”, etc.
PROCEDURE
Acclimation
-
1
If animals are obtained from a company (e.g., Jackson Laboratory of Bar Harbor, ME, USA or Harlan, Inc., of Indianapolis, IN, USA), allow them to acclimate to the colony undisturbed for 1–2 weeks prior to testing. Mice should be at least 8 weeks old before testing. CAUTION Do not use subject animals older than 6 months of age for baseline studies, as age-dependent decrements in social memory have been observed57. CAUTION Use gonadally-intact females between 3–5 months old as stimulus females, as the highest likelihood of regular estrous cycling occurs at this age58.
-
2
One week prior to testing, singly house stimulus females. Keep subject males group-housed (3–5 per cage) until day of testing (see step 4).
-
3
Transport animals on day of testing from the animal room to the testing room 1 hour prior to testing. Lower the lights in the testing room to approximately 40 lux as measured on the light meter (see Equipment Setup). CRITICAL STEP Experimenter(s) should be blind to treatment and/or genotype throughout the experiment.
-
4
Place each animal into a well-cleaned standard mouse cage (see Equipment Setup). If the cage has any internal features (such as an air vent on the rear), face all cages in the same direction. Mark the lid of each cage with the animal’s identification number. CRITICAL STEP Allow the animal to remain undisturbed, alone, in the new cage for 30 minutes.
-
5
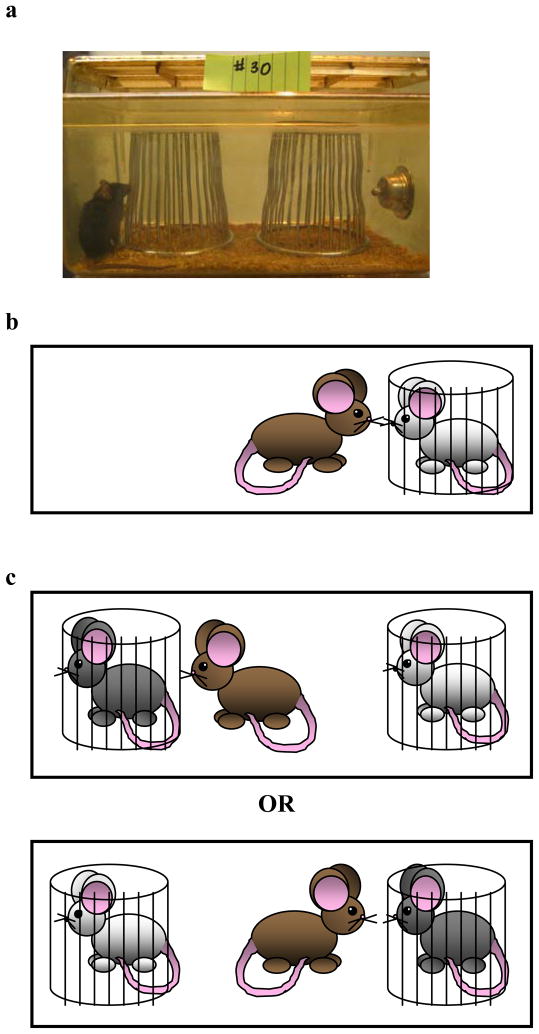
After 30 minutes, place two clean corrals (see Equipment Setup) into the cage; one on the left and one on the right (see Fig 1a). Allow sufficient space for the animal to move completely around each corral. CRITICAL STEP Allow the animal 30 minutes to explore both corrals. CAUTION Place the two corrals in line with one another, using any features of the cage (such as interior vents) to preserve spatial arrangement of the corrals. This will prevent asymmetry in investigation due to spatial novelty.
Figure 1.
The social discrimination task. (a) Photograph depicting placement of corrals within the test cage. (b) A cartoon representation of Trial 1, which consists of exposure to a single corralled female. (c) A cartoon representation of Trial 2, which consists of simultaneous presentation of the “familiar” female (white) and exposure to a second “novel” female (gray). This occurs after a pre-determined delay between Trial 1 and Trial 2.
Social discrimination test
-
6
Social discrimination consists of two trials. To perform an investigation of female 1, follow option A. To perform an investigation of 2 females, one familiar and one novel, perform option A followed by option B.
(A) Trial 1: investigation of female 1 (“same”)
Remove the lid from the test cage and take out one corral; set the empty corral aside. Turn on the video recording equipment. Place a stimulus female in the remaining corral, and return the corral containing the female to the cage (see Fig 1b). Replace the lid. CAUTION Transfer as little bedding and/or nesting material as possible from the female’s home cage. CAUTION Remember to remove the second empty corral, as we have found that when the empty corral remains within the cage during Trial 1, investigation of the female decreases, which could impact memory for the female’s odor (see ANTICIPATED RESULTS).
Observe investigation of the stimulus female by the male for the pre-determined observation time (e.g., 5 minutes). During observations, remain still and sit approximately 18–24” from the cage. Whether scoring live or via pre-recorded video, it is imperative that only actual investigation of the female is scored. As the male cannot physically reach the female through the corral to a great extent, we have defined “investigation” as any time in which the male inserts his nose and/or forepaw through the bars of the corral (similar to13; see Supplemental movie). Start the timer whenever the male does the following: (i) makes direct contact with the female with either the nose or the forepaw, inserted through the bars of the corral; (ii) continues sniffing and/or reaching towards any area within the corral after the female has moved away; or (iii) sniffs any portion of the female that is outside the corral (e.g., her tail). Stop the timer when the male ceases investigation by removing his nose and/or forepaw from the corral or female. CAUTION Only attempts by the male to reach the female are counted as investigation. Do not score the following as investigation: (i) sniffing and/or gnawing the external bars of the corral; (ii) sniffing any fecal matter from himself or the female; (iii) rearing up on hind paws and sniffing the empty top portion of the corral; (iv) climbing on top of the corral; (v) running around the outside of the corral without pausing to investigate; (vi) attempts by the female to reach the male. CRITICAL STEP Be sure to use two separate timers (one for “familiar” female; one for “novel” female) on Trial 2. CAUTION Be consistent in what is recorded as investigation time.
After the observation time is ended, turn off the video recording equipment, take off the lid, and remove female 1 from the corral. Place the female back in her home cage. CRITICAL STEP Be sure to place both corrals back into the male’s cage, noting which cage contained the female.
(B) Trial 2: investigation of female 1 (“familiar”) and female 2 (“novel”)
To test for social discrimination after the pre-determined inter-trial delay (e.g., 30 minutes; see TROUBLE SHOOTING), place female 1 (“familiar” female) in the same corral as during trial 1; place female 2 (“novel” female) in the second, previously empty corral (see Fig 1c and Supplementary movie). CRITICAL STEP Be sure to place the “familiar” female in the same corral as during trial 1 to avoid mixing female odors during trial 2. However, we recommend random placement of the “familiar” female and corral (left or right side of cage) across all subjects, to ensure that investigation is not driven by a place preference (although we have not observed that confound in direct testing). TROUBLESHOOTING
Turn on video recording equipment and carry out the observation as described in Step 6A(i–iii). For this trial, two stopwatches will be used for live scoring: one for the “familiar” female, one for the “novel” female.
Post-testing cleanup
-
7
After all subjects have been tested, remove the corrals from the testing cages. CRITICAL STEP Prior to next use, thoroughly wash corrals in hot water and spray with 70% ethanol to remove each individual female’s odor from the corrals.
-
8
If bodyweight could impact your study, weigh the males, then return them to their group-housed home cage. CAUTION We have noticed that upon return to their cage mates, the males engage in rough-and-tumble play that can become aggressive. Monitor the males’ cages for a few minutes to determine the level of aggression. If fighting continues or an injury occurs, separate the males and/or remove and treat the injured animal.
Scoring
-
9
Assess scoring reliability. To do so, score a random sampling of both Trial 1 and Trial 2 from video. This should be done by a second observer also naïve to group assignment. The reliability between observers can be assessed via statistical software (such as SPSS: SPSS, Inc, Chicago, IL, USA) with a Pearson’s product-moment correlation coefficient (Pearson’s r; see ANTICIPATED RESULTS).
-
10
Using the video, assess any further behaviours of interest. As stated above (Equipment Setup), videotaping both Trial 1 and Trial 2 allows for assessment of behaviors other than investigation of the female that could indirectly influence social discrimination (see TROUBLESHOOTING). These behaviors include, but are not limited to: latency to approach female 1; latency to approach female 2; time spent grooming; number of approaches to each female; and time engaged in “non-social” behaviors.
Data analysis
-
11
If two groups are tested, use either the paired-samples students t-test (for normally distributed data) or the Mann-Whitney U-test (for non-parametric data) to compare time spent during Trial 2 with “familiar” and “novel” females. Data can be analyzed in one of three ways: 1) a direct comparison of time investigating the “familiar” and “novel” conspecfics12; 2) as a difference score (investigation of “novel” female – investigation of “familiar” female)20; or 3) as a relative duration of investigation ratio (investigation of “novel” female/investigation of “familiar” female)11. For more than two groups, use an ANOVA, followed by a post-hoc test.
TIMING
Steps 1 & 2 (acclimation to colony and single housing): 7–14 days
Steps 3 – 5 (acclimation to test apparatus): 1 hour for cohort of experimental animals
Step 6 (testing): 40–50 minutes per animal (5–10 minutes for both Trial 1 and 2; 30 minute inter-trial delay)
Steps 7–8 (clean up): 5–10 minutes
Step 9 (scoring): 10–20 minutes per animal if only female interaction time is scored on testing day with 2 stop watches
Step 10 (scoring): varies depending upon how many other behaviors are scored
Step 11 (data analysis): 1–2 days per experiment
TROUBLESHOOTING
The most likely problems are (i) lack of overall investigation of females and (ii) lack of discrimination between familiar and novel females by the control group. Advice/solutions on troubleshooting these problems can be found in Table 1.
Table 1.
Troubleshooting table
| Step | Problem | Possible Reason | Potential Solution |
|---|---|---|---|
| 6 | Subject spends more time exploring corrals than females | Insufficient habituation to corrals | Allow full 30 minutes with the corrals prior to testing to make them less novel and therefore less interesting |
| Previous female(s) odor still present | Thoroughly wash corrals between test days | ||
| 6 | Investigation time by males very low | Subject has poor olfactory abilities | Prior to testing, examine olfactory abilities to ensure anosmia does not exist in subjects |
| Male unable to reach female(s) | Use corrals with holes/bars at least 4–6mm wide | ||
| Females inappropriate for male | Use younger females (approx 8 weeks old) | ||
| Use stimulus females of a different strain. Strain differences in anxiety, aggression, or sociability can affect the females’ behavior | |||
| Equipment setup/location interferes with task | Place test cages in low-traffic area and sit further away from cage during trials | ||
| 6 | Female escapes from corral | Too much bedding in test cage | Remove some bedding from test cage (leave sufficient to thinly cover the floor of the test cage) |
| 6 | Subject does not discriminate between females | Insufficient habituation to novel environment | Allow full 1 hour in new environment to habituation to prevent impact on preferences for familiar and novel stimuli |
| Exposure procedure interferes with discrimination | Increase investigation time during trials 1 and 2 | ||
| “Novel” female has been placed into “familiar” female corral from trial 1 | Be sure to place the “familiar” female into the same corral as during trial 1 to avoid cross-contamination of female odors. | ||
| 6 | All subjects (control and experimental) discriminate between females | Insufficient inter-trial delay | Increase inter-trial delay to make discrimination more difficult |
| Initial investigation time too long | Decrease the initial investigation time to make acquisition of the social memory more difficult | ||
| 6 | High variability of responses within group | Stress in animal facility | Carefully control husbandry and conditions of testing environment (lighting, temperature, humidity, soundproofing) |
| Inaccurate scoring | Check to be sure investigation time is being scored accurately | ||
| Correlate investigation time between two experimenters for all subjects to discover inaccuracies | |||
| New knockout line or strain is being tested and above problem occurs | New line differs from ‘established’ lines/strains | Strain has baseline differences in sociability that need to be determined prior to testing for social memory | |
| Examine sensory (e.g. anosmia) and/or motor differences that could interfere with testing | |||
| Conduct pilot research in the new line/strain on relevant variables; i.e. investigation time; inter-trial delay length; best stimulus female strain to use |
ANTICIPATED RESULTS
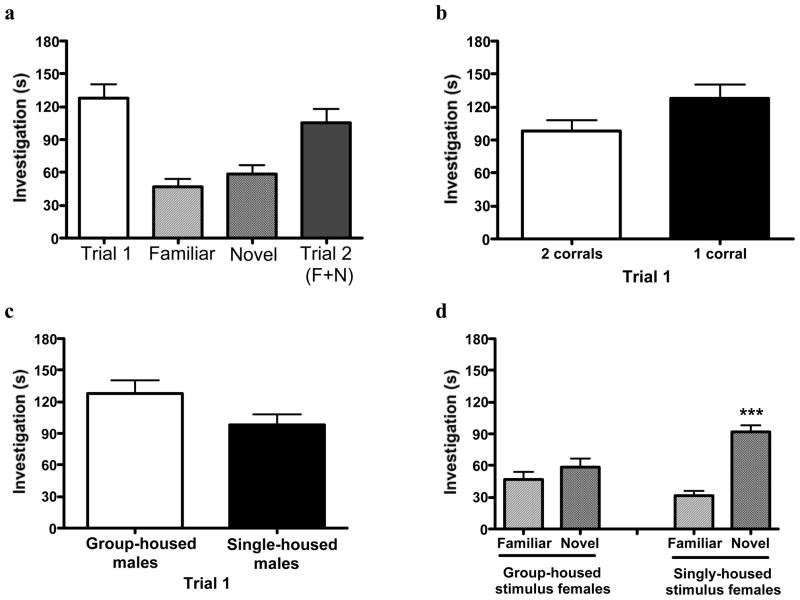
The social discrimination paradigm described above appears to be a reliable test of social recognition. Corralling the stimulus females does not interfere with investigation, as investigation times are high (Fig 2a), and generally similar to a social discrimination task in which direct investigation is permitted19. Furthermore, presenting the subject with two females simultaneously during Trial 2 does not result in a decrease in overall exploration from Trial 1 to Trial 2 (Fig 2a). Therefore, the use of corralled stimulus females is 1) possible without loss of investigation; 2) eliminates the need for extinguishing sex behavior; and 3) allows the use of non-ovariectomized females. However, investigation of the single female during Trial 1 may decrease (although not significantly) when the empty corral remains within the test environment (Fig 2b), suggesting prudence in removng the empty corral during this stage of testing.
Fig 2.
Results using different permutations of the social discrimination task. (a) Representative data of investigation times of corralled C57Bl/6J females by group-housed C57Bl/6J males. (b) Investigation time during Trial 1 (single female in one corral) when the empty corral remains in the test cage (left) or is removed during testing (right). (c) Investigation time during Trial 1 (single female in one corral) by group-housed and single-housed males (p = 0.06 between bars). (d) Significant discrimination occurred between “familiar” and “novel” females by group housed males only when singly-housed stimulus females were used (right); ***p < 0.001 between familiar and novel via paired-samples t-test. For all graphs, data are mean ± SEM; n = 10 per group.
Our protocol addresses the possible negative effects of individual housing on male social recognition6, as we observe that overall investigation of corralled females may be higher (although not significantly so) using group-housed compared to singly-housed males (Fig 2c). Additionally, subject males are best able to discriminate between “familiar” and “novel” females when the stimulus females are singly housed (Fig 2d), likely due to reduced contamination of the individual’s odor.
When two different investigators score the social discrimination task, the Pearsons’ product-moment correlation coefficient (r) reveals high correlations for amount of time with the “same” female (r = 0.991; p < 0.01) and the “novel” female (r = 0.992; p < 0.01). Therefore, with appropriate training and exposure to the task, scoring is consistent across investigators. Additionally, we obtain highly consistent performance from WT animals within our laboratory. WT animals from three lines of mice (on either a C57Bl/6J (Oxt) or a mixed C57Bl/6J:120Sv (Oxtr, OxtrFB/FB) background) spend approximately 65–75% of the time investigating the novel female every time the task is administered (Table 2)43. Furthermore, performance of all WT mice is highly consistent regardless of stimulus females’ strain (C57Bl/6J, Balb/C, and Swiss-Webster), indicating the robustness of the task. Future testing utilizing the novel arrangement of parameters of this social recognition task will show whether different laboratories are able to replicate results using the mice of the same line and/or with the same background.
Table 2.
Social discrimination of different strains by wildtype mice.
| Subject Line | Female Strain | Trial 2: Discrimination % investigation of Novel female [Novel/(Novel + Familiar) × 100] | |
|---|---|---|---|
| Oxt+/+ | n = 10 | Balb/c | 74.3 ± 5.6% ** |
| n = 10 | C57Bl/6J | 64.9 ± 4.2% ** | |
| n = 7 | Swiss Webster | 72.6 ± 4.5% ** | |
| Oxtr±/± | n = 7 | Balb/c | 73.8 ± 4.4% * |
| n = 9 | C57Bl/6J | 64.3 ± 4.2% *** | |
| n = 9 | Swiss Webster | 63.9 ± 5.0% * | |
| Oxtr±/± (FB) | n = 8 | Balb/c | 72.1 ± 5.7% ** |
| n = 9 | C57Bl/6J | 67.9 ± 5.1% ** | |
| n = 9 | Swiss Webster | 63.8 ± 4.8% * | |
p < 0.05;
p < 0.01,
p < 0.001 difference from chance (50%) via one-sample t-test
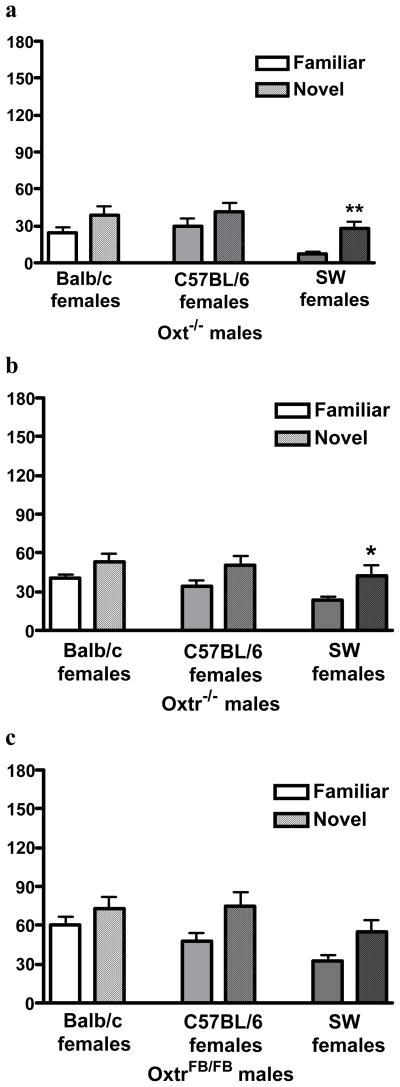
We also demonstrated the usefulness of this task for testing social recognition abilities in Oxt and Oxtr KO males43. KO males from all three lines tested are unable to discriminate between familiar and novel C57Bl/6J and Balb/C females (Figure 3), indicating similar performance when stimulus females from inbred strains are used. Performance differs when presented with Swiss-Webster females: OxtrFB/FB males remain unable to discriminate between familiar and novel females (Figure 3c), whereas Oxt−/− and Oxtr−/− males are (Figure 3a, b). These results indicate that the use of inbred or outbred stimulus females can impact social recognition abilities in Oxt and Oxtr KO males, so care should be taken with choice of stimulus females’ strains. We recommend assessing social recognition abilities using both inbred and outbred stimulus females, to fully elucidate any social recognition deficits.
Fig 3.
Performance on the social discrimination task in three lines of knockout (−/−) mice. Data are mean ± SEM for investigation of a familiar and a novel mouse. Each line was tested three times, with 2 weeks between tests. Differences in exploration of familiar and novel females were assessed via paired samples t test (a) Oxt−/− males and (b) Oxtr−/− males discriminated only between familiar and novel Swiss-Webster (SW) females. (c) OxtrFB/FB males did not discriminate between any of the three strains of stimulus females presented. *p < 0.05; **p < 0.01. Adapted from Macbeth et al., 200943.
Indeed, if a knockout mouse line and/or strain is being tested for social discrimination for the first time, conclusions about possible social memory deficits should not be made before alternative explanations have been assessed (see Table 1 for suggestions). For example, the olfactory abilities of the line and/or strain should be known, as anosmia in the strain will significantly affect performance on the task. We recommend testing a new line and/or strain with at least an olfactory discrimination test15,59 prior to undergoing social recognition testing. Additionally, while corralling the female does control for her behavior, a highly aggressive or non-social female could still influence the male’s willingness to investigate her. Before determining that your line/strain of mice cannot discriminate, consider changing the strain of stimulus animal. We urge experimenters to fully evaluate all variables in this task prior to making any assessments on usefulness in a novel strain of mice, or in rats, as lack of discrimination could be due to external factors such as those listed in Table 1 (e.g., investigation time, inter-trial delay), or physical differences (e.g., olfactory ability), and not due to an inability to discriminate between familiar and novel conspecifics.
This task has not yet been applied to the Avpr KO lines. However, our Avpr1a and Avpr1b KO lines are also on a C57Bl/6J background, so we anticipate similar investigatory abilities of WT males from these lines as was seen in Oxt and Oxtr lines. We are currently undertaking studies to assess the usefulness of this task in further examining the role of the Avpr in social recognition abilities. Furthermore, while we believe this task should be useful in assessing female social recognition, this has not yet been validated. If using this task for female subjects, be sure to take stimulus animal strain, sex, and presentation into consideration.
Supplementary Material
Supplementary Movie: Investigation of females during the social discrimination task. Note the insertion of the subject male’s nose between the bars of both corrals in order to investigate both stimulus females. This can be observed when the male is on the near and far side of the corrals. The beeping heard in the background is the timer used by the experimenter during live scoring; the noise does not seem to adversely affect the subject male’s behavior.
Acknowledgments
We thank Dr. Scott Wersinger for his helpful review of an early version of this manuscript. The authors appreciate the excellent technical support provided by Emily Shepard, James Heath, Anna Brownstein, and the Building 49 animal facility. This research was supported by the NIMH Intramural Research Program (Z01-MH-002498-20)
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
References
- 1.Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 2.Spehr M, et al. Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell Mol Life Sci. 2006;63:1476–1484. doi: 10.1007/s00018-006-6109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Philos Trans R Soc Lond B Biol Sci. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateo JM. Development of individually distinct recognition cues. Dev Psychobiol. 2006;48:508–519. doi: 10.1002/dev.20156. [DOI] [PubMed] [Google Scholar]
- 5.Petrulis A, Alvarez P, Eichenbaum H. Neural correlates of social odor recognition and the representation of individual distinctive social odors within entorhinal cortex and ventral subiculum. Neuroscience. 2005;130:259–274. doi: 10.1016/j.neuroscience.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 8.Winslow JT, Camacho F. Cholinergic modulation of a decrement in social investigation following repeated contacts between mice. Psychopharmacology (Berl) 1995;121:164–172. doi: 10.1007/BF02245626. [DOI] [PubMed] [Google Scholar]
- 9.Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 13.Choleris E, et al. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wersinger SR, et al. Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A Conditional Knockout Mouse Line of the Oxytocin Receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 17.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin D, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 19.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Benelli A, et al. Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides. 1995;28:251–255. doi: 10.1016/0143-4179(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 21.Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol. 1991;1:555–560. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- 22.Popik P, Vetulani J, Van Ree JM. Facilitation and attenuation of social recognition in rats by different oxytocin-related peptides. Eur J Pharmacol. 1996;308:113–116. doi: 10.1016/0014-2999(96)00215-4. [DOI] [PubMed] [Google Scholar]
- 23.Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 24.Bluthe RM, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535:301–304. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- 25.Taylor GT. Urinary odors and size protect juvenile laboratory mice from adult male attack. Dev Psychobiol. 1982;15:171–186. doi: 10.1002/dev.420150209. [DOI] [PubMed] [Google Scholar]
- 26.Connor JL, Lynds PG. Mouse aggression and the intruder-familiarity effect: evidence for multiple-factor determination c57bl. J Comp Physiol Psychol. 1977;91:270–280. doi: 10.1037/h0077318. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell V, Blanchard RJ, Blanchard DC. Mouse aggression increases after 24 hours of isolation or housing with females. Behav Neural Biol. 1981;32:89–103. doi: 10.1016/s0163-1047(81)90317-4. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Kikusui T, Takeuchi Y, Mori Y. The critical role of familiar urine odor in diminishing territorial aggression toward a castrated intruder in mice. Physiol Behav. 2007;90:512–517. doi: 10.1016/j.physbeh.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 29.de Catanzaro D, Gorzalka BB. Isolation-induced facilitation of male sexual behavior in mice. J Comp Physiol Psychol. 1979;93:211–222. doi: 10.1037/h0077550. [DOI] [PubMed] [Google Scholar]
- 30.Sharp JL, Zammit TG, Azar TA, Lawson DM. Stress-like responses to common procedures in male rats housed alone or with other rats. Contemp Top Lab Anim Sci. 2002;41:8–14. [PubMed] [Google Scholar]
- 31.Todrank J, Heth G, Johnston RE. Kin recognition in golden hamsters: evidence for kinship odours. Anim Behav. 1998;55:377–386. doi: 10.1006/anbe.1997.0611. [DOI] [PubMed] [Google Scholar]
- 32.Hopp SL, Owren MJ, Marion JR. Olfactory discrimination of individual littermates in rats (Rattus norvegicus) J Comp Psychol. 1985;99:248–251. [PubMed] [Google Scholar]
- 33.Kavaliers M, et al. Oxytocin and estrogen receptor alpha and beta knockout mice provide discriminably different odor cues in behavioral assays. Genes Brain Behav. 2004;3:189–195. doi: 10.1111/j.1601-183x.2004.00068.x. [DOI] [PubMed] [Google Scholar]
- 34.Beynon RJ, Hurst JL. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides. 2004;25:1553–1563. doi: 10.1016/j.peptides.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Hurst JL, Thom MD, Nevison CM, Humphries RE, Beynon RJ. MHC odours are not required or sufficient for recognition of individual scent owners. Proc Biol Sci. 2005;272:715–724. doi: 10.1098/rspb.2004.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheetham SA, et al. The genetic basis of individual-recognition signals in the mouse. Curr Biol. 2007;17:1771–1777. doi: 10.1016/j.cub.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Sherborne AL, et al. The genetic basis of inbreeding avoidance in house mice. Curr Biol. 2007;17:2061–2066. doi: 10.1016/j.cub.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 39.Kudryavtseva NN, Bondar NP, Avgustinovich DF. Association between experience of aggression and anxiety in male mice. Behav Brain Res. 2002;133:83–93. doi: 10.1016/s0166-4328(01)00443-0. [DOI] [PubMed] [Google Scholar]
- 40.Muroi Y, Ishii T, Komori S, Nishimura M. A competitive effect of androgen signaling on male mouse attraction to volatile female mouse odors. Physiol Behav. 2006;87:199–205. doi: 10.1016/j.physbeh.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Carr WJ, Loeb LS, Dissinger ML. Responses of Rats to Sex Odors. J Comp Physiol Psychol. 1965;59:370–377. doi: 10.1037/h0022036. [DOI] [PubMed] [Google Scholar]
- 42.Ingersoll DW, Weinhold LL. Modulation of male mouse sniff, attack, and mount behaviors by estrous cycle-dependent urinary cues. Behav Neural Biol. 1987;48:24–42. doi: 10.1016/s0163-1047(87)90544-9. [DOI] [PubMed] [Google Scholar]
- 43.Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav. 2009 doi: 10.1111/j.1601-183X.2009.00506.x. Epub Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 45.Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldwell HK, Young WS. In: Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Proteins and Peptides. 3. Lim R, editor. Springer; 2006. pp. 573–607. [Google Scholar]
- 47.Popik P, Vetulani J. Opposite action of oxytocin and its peptide antagonists on social memory in rats. Neuropeptides. 1991;18:23–27. doi: 10.1016/0143-4179(91)90159-g. [DOI] [PubMed] [Google Scholar]
- 48.Wersinger SR, Temple JL, Caldwell HK, Young WS., 3rd Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus) Endocrinology. 2008;149:116–121. doi: 10.1210/en.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winslow JT. Mouse social recognition and preference. Curr Protoc Neurosci. 2003;8(Unit 8.16) doi: 10.1002/0471142301.ns0816s22. [DOI] [PubMed] [Google Scholar]
- 50.Anagnostopoulos AV, Mobraaten LE, Sharp JJ, Davisson MT. Transgenic and knockout databases: behavioral profiles of mouse mutants. Physiol Behav. 2001;73:675–689. doi: 10.1016/s0031-9384(01)00525-x. [DOI] [PubMed] [Google Scholar]
- 51.Ennaceur A, Michalikova S, Chazot PL. Models of anxiety: responses of rats to novelty in an open space and an enclosed space. Behav Brain Res. 2006;171:26–49. doi: 10.1016/j.bbr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Brooks SP, Pask T, Jones L, Dunnett SB. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Genes Brain Behav. 2005;4:307–317. doi: 10.1111/j.1601-183X.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- 53.Moy SS, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 54.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 55.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 56.Noldus LP, Trienes RJ, Hendriksen AH, Jansen H, Jansen RG. The Observer Video-Pro: new software for the collection, management, and presentation of time-structured data from videotapes and digital media files. Behav Res Methods Instrum Comput. 2000;32:197–206. doi: 10.3758/bf03200802. [DOI] [PubMed] [Google Scholar]
- 57.Guan X, Dluzen DE. Age related changes of social memory/recognition in male Fischer 344 rats. Behav Brain Res. 1994;61:87–90. doi: 10.1016/0166-4328(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 58.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 59.Crawley JN, et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie: Investigation of females during the social discrimination task. Note the insertion of the subject male’s nose between the bars of both corrals in order to investigate both stimulus females. This can be observed when the male is on the near and far side of the corrals. The beeping heard in the background is the timer used by the experimenter during live scoring; the noise does not seem to adversely affect the subject male’s behavior.