Abstract
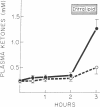
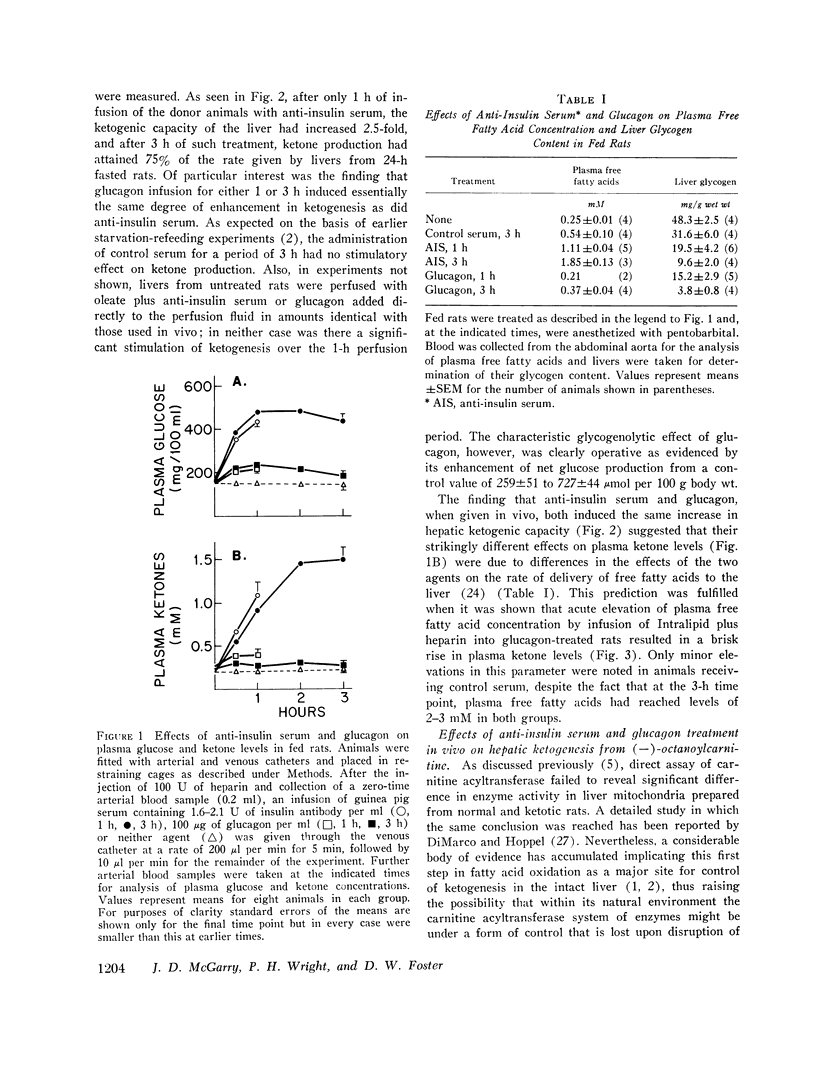
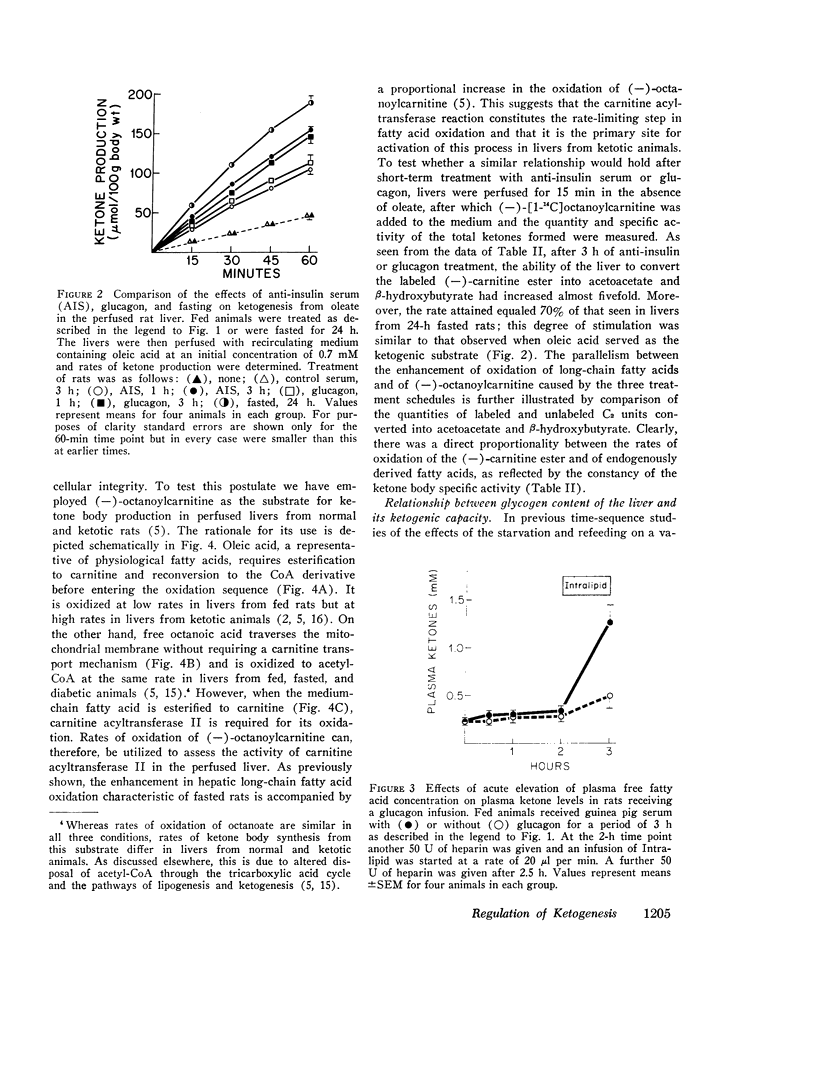
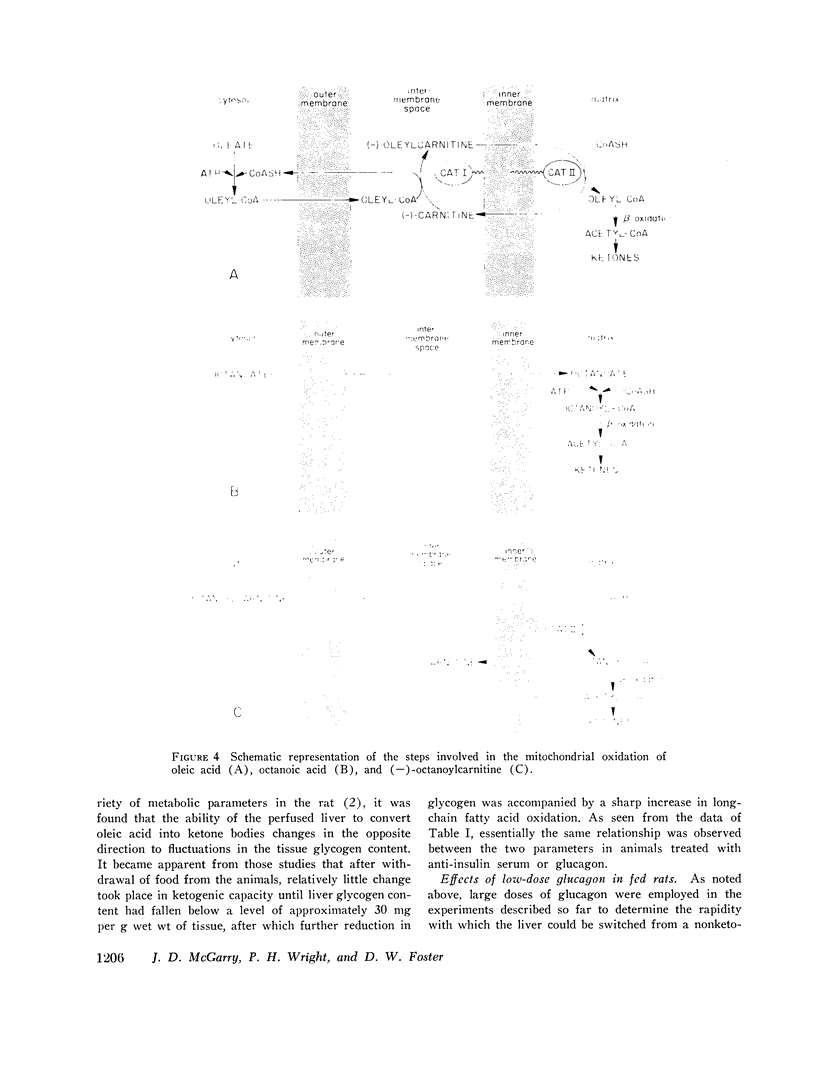
The enhanced capacity for long-chain fatty acid oxidation and ketogenesis that develops in the rat liver between 6 and 9 h after the onset of starvation was shown to be inducible much more rapidly by administration of anti-insulin serum or glucagon to fed rats. After only 1 h of treatment with either agent, the liver had clearly switched from a "nonketogenic" to a "ketogenic" profile, as determined by rates of acetoacetate and b-hydroxybutyrate production on perfusion with oleic acid. As was the case after starvation, the administration of insulin antibodies or glucagon resulted in depletion of hepatic glycogen stores and a proportional increase in the ability of the liver to oxidize long-chain fatty acids and (-)-octanoylcarnitine, suggesting that all three treatment schedules activated the carnitine acyltransferase system of enzymes. In contrast to anti-insulin serum, which produced marked elevations in plasma glucose, free fatty acid, and ketone body concentrations, glucagon treatment had little effect on any of these parameters, presumably due to enhanced insulin secretion after the initial stimulation of glycogenolysis. Thus, after treatment with glucagon alone, it was possible to obtain a "ketogenic" liver from a nonketotic animal. The results are consistent with the possibility that the activity of carnitine acyltransferase, and thus ketogenic capacity, is subject to bihormonal control through the relative blood concentrations of insulin and glucagon, as also appears to be the case with hepatic carbohydrate metabolism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMIN J., GRANT R. T., WRIGHT P. H. Acute insulin deficiency provoked by single injections of anti-insulin serum. J Physiol. 1960 Aug;153:131–145. doi: 10.1113/jphysiol.1960.sp006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda A., Bláquez E., Herrera E. Liver components, blood glucose and ketone bodies in fed and starved suckling rats. Horm Metab Res. 1973 Sep;5(5):350–355. doi: 10.1055/s-0028-1093922. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Bier D. M., Havel R. J. Early effects of anti-insulin serum on hepatic metabolism of plasma free fatty acids in dogs. Diabetes. 1972 May;21(5):280–288. doi: 10.2337/diab.21.5.280. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Cherrington A. D., Kawamori R., Pek S., Vranic M. Arginine infusion in dogs. Model for the roles of insulin and glucagon in regulating glucose turnover and free fatty acid levels. Diabetes. 1974 Oct;23(10):805–815. doi: 10.2337/diab.23.10.805. [DOI] [PubMed] [Google Scholar]
- DiMarco J. P., Hoppel C. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. J Clin Invest. 1975 Jun;55(6):1237–1244. doi: 10.1172/JCI108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Park C. R. Control of gluconeogenesis in liver. IV. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. J Biol Chem. 1969 Aug 10;244(15):4095–4102. [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- Kopec B., Fritz I. B. Comparison of properties of carnitine palmitoyltransferase I with those of carnitine palmitoyltransferase II, and preparation of antibodies to carnitine palmitoyltransferases. J Biol Chem. 1973 Jun 10;248(11):4069–4074. [PubMed] [Google Scholar]
- LOSSOW W. J., BROWN G. W., Jr, CHAIKOFF I. L. The action of insulin in sparing fatty acid oxidation: a study with palmitic acid-1-C14 and octanoate-1-C14. J Biol Chem. 1956 Jun;220(2):839–849. [PubMed] [Google Scholar]
- LOSSOW W. J., CHAIKOFF I. L. Carbohydrate sparing of fatty acid oxidation. I. The relation of fatty acid chain length to the degree of sparing. II. The mechanism by which carbohydrate spares the oxidation of palmitic acid. Arch Biochem Biophys. 1955 Jul;57(1):23–40. doi: 10.1016/0003-9861(55)90173-9. [DOI] [PubMed] [Google Scholar]
- Lee L. P., Fritz I. B. Hepatic ketogenesis during development. Can J Biochem. 1971 May;49(5):599–605. doi: 10.1139/o71-086. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Bomboy J. D., Lewis S. B., Sinclair-Smith B. C., Felts P. W., Lacy W. W., Crofford O. B., Liddle G. W. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. J Clin Invest. 1974 Jan;53(1):190–197. doi: 10.1172/JCI107537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood E. A., Bailey E. The course of ketosis and the activity of key enzymes of ketogenesis and ketone-body utilization during development of the postnatal rat. Biochem J. 1971 Aug;124(1):249–254. doi: 10.1042/bj1240249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackrell D. J., Sokal J. E. Antagonism between the effects of insulin and glucagon on the isolated liver. Diabetes. 1969 Nov;18(11):724–732. doi: 10.2337/diab.18.11.724. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Acute reversal of experimental diabetic ketoacidosis in the rat with (+)-decanoylcarnitine. J Clin Invest. 1973 Apr;52(4):877–884. doi: 10.1172/JCI107252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of ketogenesis and clinical aspects of the ketotic state. Metabolism. 1972 May;21(5):471–489. doi: 10.1016/0026-0495(72)90059-5. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The metabolism of (minus)-octanoylcarnitine in perfused livers from fed and fasted rats. Evidence for a possible regulatory role of carnitine acyltransferase in the control of ketogenesis. J Biol Chem. 1974 Dec 25;249(24):7984–7990. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem. 1971 Feb 25;246(4):1149–1159. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from oleic acid and the influence of antiketogenic agents. J Biol Chem. 1971 Oct 25;246(20):6247–6253. [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- Meier J. M., McGarry J. D., Faloona G. R., Unger R. H., Foster D. W. Studies of the development of diabetic ketosis in the rat. J Lipid Res. 1972 Mar;13(2):228–233. [PubMed] [Google Scholar]
- Parrilla R., Goodman M. N., Toews C. J. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes. 1974 Sep;23(9):725–731. doi: 10.2337/diab.23.9.725. [DOI] [PubMed] [Google Scholar]
- Raskin P., McGarry J. D., Foster D. W. Independence of cholesterol and fatty acid biosynthesis from cyclic adenosine monophosphate concentration in the perfused rat liver. J Biol Chem. 1974 Oct 10;249(19):6029–6032. [PubMed] [Google Scholar]
- Sakurai H., Dobbs R., Unger R. H. Somatostatin-induced changes in insulin and glucagon secretion in normal and diabetic dogs. J Clin Invest. 1974 Dec;54(6):1395–1402. doi: 10.1172/JCI107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg H. E. Carnitine octanoyltransferase. Evidence for a new enzyme in mitochondria. FEBS Lett. 1971 Jan 12;12(3):134–136. doi: 10.1016/0014-5793(71)80051-0. [DOI] [PubMed] [Google Scholar]
- TARRANT M. E., MAHLER R., ASHMORE J. STUDIES IN EXPERIMENTAL DIABETES. IV. FREE FATTY ACID MOBILIZATION. J Biol Chem. 1964 Jun;239:1714–1719. [PubMed] [Google Scholar]
- TARRANT M. E., THOMPSON R. H., WRIGHT P. H. Some aspects of lipid metabolism in rats treated with anti-insulin serum. Biochem J. 1962 Jul;84:6–10. doi: 10.1042/bj0840006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Alpha- and beta-cell interrelationships in health and disease. Metabolism. 1974 Jun;23(6):581–593. doi: 10.1016/0026-0495(74)90086-9. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975 Jan 4;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- WEINHOUSE S., MILLINGTON R. H., FRIEDMAN B. The effect of carbohydrate on the oxidation of fatty acids by liver slices. J Biol Chem. 1949 Dec;181(2):489–498. [PubMed] [Google Scholar]
- Whitty A. J., Shima K., Trubow M., Foà P. P. Effect of glucagon and of insulin on serum free fatty acids in normal and depancreatized dogs. Proc Soc Exp Biol Med. 1969 Jan;130(1):55–61. doi: 10.3181/00379727-130-33487. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Effects of fatty acids, glucagon and anti-insulin serum on the control of gluconeogenesis and ketogenesis in rat liver. Adv Enzyme Regul. 1967;5:229–255. doi: 10.1016/0065-2571(67)90019-2. [DOI] [PubMed] [Google Scholar]
- Wright P. H., Makulu D. R., Posey I. J. Guinea pig anti-insulin serum. Adjuvant effect of H. pertussis vaccine. Diabetes. 1968 Aug;17(8):513–516. doi: 10.2337/diab.17.8.513. [DOI] [PubMed] [Google Scholar]