Abstract
Ginger roots have been used to treat inflammation and have been reported to inhibit cyclooxygenase (COX). Ultrafiltration liquid chromatography mass spectrometry was used to screen a chloroform partition of a methanol extract of ginger roots for COX-2 ligands, and 10-gingerol, 12-gingerol, 8-shogaol, 10-shogaol, 6-gingerdione, 8-gingerdione, 10-gingerdione, 6-dehydro-10-gingerol, 6-paradol, and 8-paradol bound to the enzyme active site. Purified 10-gingerol, 8-shogaol and 10-shogaol inhibited COX-2 with IC50 values of 32 μM, 17.5 μM and 7.5 μM, respectively. No inhibition of COX-1 was detected. Therefore, 10-gingerol, 8-shogaol and 10-shogaol inhibit COX-2 but not COX-1, which can explain, in part, anti-inflammatory properties of ginger.
Keywords: ginger, Zingiber officinale, cyclooxygenase, gingerol, shogaol, paradol
1. Introduction
Cyclooxygenases (COX)-1 and COX-2 are the targets of widely used nonsteroidal anti-inflammatory drugs (NSAIDs) and are essential for such physiological processes as maintenance of the gastrointestinal tract, renal function and fever. COX-1 is expressed constitutively in all tissues, but COX-2 is induced specifically during inflammatory, degenerative, and neoplastic processes [1]. COX-1 and COX-2 catalyze the conversion of arachidonic acid to the endoperoxide prostaglandin (PG)H2 which can form a variety of prostaglandins, thromboxanes and prostacyclin through catalysis by non-rate limiting enzymes [2] or by non-enzymatic rearrangement. For example, PGD2 and PGE2 can be formed from PGH2 non-enzymatically or through the action of specific synthases. By inhibiting COX-1 and/or COX-2, NSAIDs prevent the enzymatic conversion of arachidonic acid to pro-inflammatory cyclic endoperoxides. Based on the assumption that inhibition of COX-2 but not COX-1 might reduce the side effects observed in older NSAIDs, the discovery of COX-2 selective inhibitors has become an important area of pharmaceutical research.
In vitro investigations of ginger (Zingiber officinalis Roscoe) preparations and some isolated gingerol-related compounds (see structures of gingerols and related compounds in Figure 1) have shown anti-inflammatory effects of ginger including inhibition of COX [3], inhibition of nuclear factor κB [4] and inhibition of 5-lipoxygenase [5]. In vivo studies using animal models of inflammation and clinical trials have confirmed the anti-inflammatory activities of ginger preparations. For example, ginger extracts have been shown to inhibit joint swelling in an animal model of rheumatoid arthritis [6] and reduce knee pain in human subjects suffering from osteoarthritis [7]. Since the anti-inflammatory constituents of ginger are incompletely understood, the present study used a mass spectrometry-based screening assay to help identify inhibitors of one of its pharmacologically important targets, COX-2.
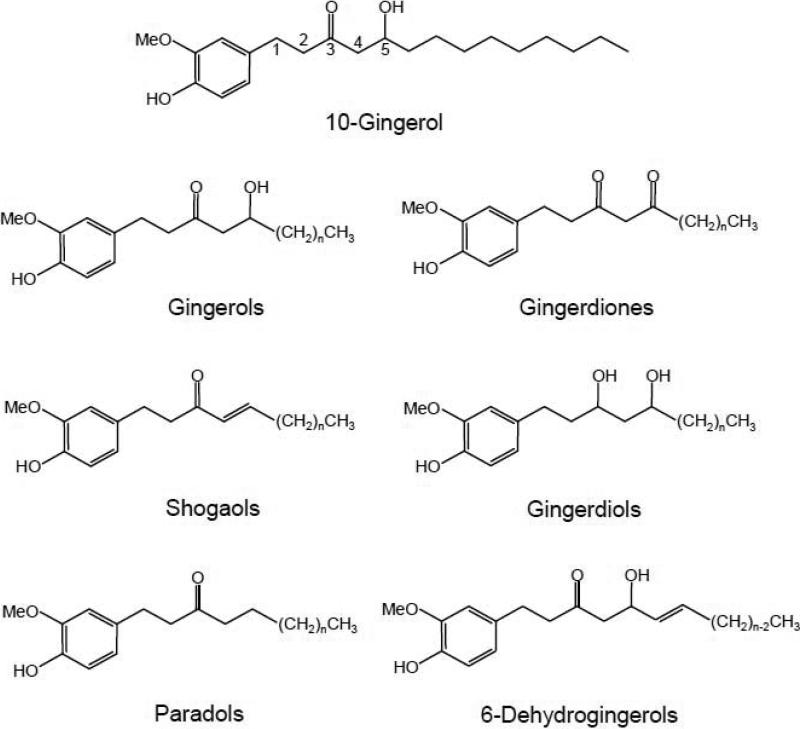
Figure 1.
Chemical structures of 10-gingerol and gingerol-related compounds from ginger root. In each group of compounds, the carbon atom on the alkyl side chain next to the phenyl ring is defined as C1. Using gingerol as an example, if n=4, 6, 8, or 10, then the compound is called 6-, 8-, 10-, or 12-gingerol.
Invented and developed in our laboratory, pulsed ultrafiltration mass spectrometry is an established approach for the screening of complex mixtures such as extracts of botanical dietary supplements, medicinal plants and combinatorial libraries for the discovery of ligands to macromolecular targets such as enzymes [8,9]. Using this approach to screen a chloroform extract of ginger root for ligands to COX-2, ten gingerol-related compounds including gingerols, shogaols, paradols, and gingerdiones were found to be ligands of COX-2 and were ranked according to their relative binding. Three of these compounds, 10-gingerol, 8-shogaol and 10-shogaol, were assayed for inhibition of COX-1 and COX-2 using a functional assay based on liquid chromatography-tandem mass spectrometry (LC-MS-MS), and their IC50 values were determined. These compounds might be responsible, in part, for the anti-inflammatory activity of ginger and may be used as marker compounds for the chemical and biological standardization of ginger dietary supplements.
2. Materials and methods
2.1. Materials
Human recombinant COX-2, ovine COX-1, arachidonic acid, PGE2, d4-PGD2, and d2-PGE2 (labeled with deuterium atoms at positions 3 and 4) were purchased from Cayman Chemicals (Ann Arbor, MI). The cofactors, (-)epinephrine and hematin, were purchased from Sigma-Aldrich (St. Louis, MO). Celecoxib was purchased from 3B PharmaChem International (Wuhan, China). All organic solvents were HPLC grade or better and were purchased from Thermo Fisher (Hanover Park, IL). Formic acid was purchased from EMD Chemicals (San Diego, CA). Purified water was prepared by using a Millipore Milli-Q purification system (Millipore, Billerica, MA). All other chemicals and solvents were ACS reagent grade, unless stated otherwise. 6-Gingerol, 8-gingerol, 10-gingerol, and 6-shogaol were purchased from Chromadex (Santa Ana, CA). 8-Shogaol and 10-shogaol were isolated from powdered ginger root as described previously [10].
Powdered ginger roots (Zingiber officinalis Roscoe) were purchased from General Nutrition Corp. (Pittsburgh, PA; Lot No. 2533EE4596) and extracted as follows. Approximately 5 g of the powder was extracted twice at room temperature with 100 mL methanol using sonication. After evaporation of the solvent under reduced pressure at 35 °C, the residue was suspended in 50 mL water and extracted with chloroform (6 × 50 mL). The chloroform partition was evaporated under reduced pressure at 25 °C to obtain 1.14 g residue. A stock solution (0.1 mg/mL) of this material was prepared in methanol and stored at 4 °C for analysis.
2.2. Pulsed ultrafiltration LC-MS
Screening using pulsed ultrafiltration LC-MS was carried out based on the method of Nikolic et al. [11]. An aliquot of the ginger extract (7 μL) was diluted into 93 μL of 50 mM phosphate buffer (pH 7.4) and mixed with 45 μL buffer containing 150 pmol of human recombinant COX-2. In place of ginger extract, otherwise identical preparations contained a mixture of isolated gingerol-related compounds at a final concentration of 1.3 μM each. Positive controls contained the COX-2 inhibitor celecoxib (1.2 μM, final concentration) in place of the ginger extract; and negative controls contained 45 μL blank buffer in place of the COX-2 buffer solution. After incubation in the dark for 1 h at 37 °C to allow binding to reach equilibrium, each mixture was filtered through a 30,000 Da molecular weight cut-off centrifugal filter, which had been pre-washed with 150 μL phosphate buffer by centrifugation at 10,000 g for 15 min. The samples were rinsed three times with 150 μL portions of 5% acetonitrile in water to remove compounds not bound to COX-2 (for celecoxib, water was used instead of acetonitrile in water).
Each filter was transferred to a new centrifuge tube to remove compounds that had bound non-specifically to the container, and the ligands were dissociated from COX-2 using 400 μL methanol/water (90:10; v/v). After 10 min incubation at room temperature, the dissociated ligands were collected by ultrafiltration with centrifugation at 10,000 g for 10 min. The dissociation process was repeated, and the ultrafiltrates were combined for each sample, dried under a stream of nitrogen gas, and reconstituted in 50 μL of 50% methanol in water immediately prior to analysis using LC-MS and LC-MS-MS.
A Waters (Milford, MA) Quattro II triple quadrupole mass spectrometer equipped with a Waters 2690 Alliance HPLC system and negative ion electrospray was used for the separation and detection of gingerol-related compounds as reported previously [10], except that the solvent system consisted of a linear 20 min gradient from 35% to 90% acetonitrile in water at a flow rate of 250 μL/min for the analysis of mixtures of isolated gingerol-related compounds. Selective detection of gingerol-related compounds was carried out using the tandem mass spectrometric techniques of constant neutral loss and selected reaction monitoring as described by Tao et al. [10]. Briefly, negative ion electrospray and collision-induced dissociation with constant neutral loss tandem mass spectrometry was used to fragment deprotonated molecules of gingerol-related compounds such as gingerols, shogaols, paradols, etc., to form product ions through neutral losses of structural features common to these compounds. Gingerols and 6-dehydrogingerols were detected during constant neutral loss scanning of 194 u, and shogaols, paradols and gingerdiones were detected while scanning for a characteristic neutral loss of 136 u [10].
Collision-induced dissociation with selected reaction monitoring (SRM) was then used to measure unique ion pairs corresponding to deprotonated gingerol-related compounds and their structurally informative fragment ions. The pairs of precursor and product ions used during SRM for each gingerol-related compound are shown in Table 1. Specific binding of each compound to COX-2 was determined by comparing the LC-MS-MS peak area of the compound after release from COX-2 during pulsed ultrafiltration with the area of the corresponding peak for the control incubation containing no COX-2. The relative binding of each ligand to COX-2 was determined by comparing the LC-MS-MS peak area of the compound released from COX-2 after ultrafiltration to the peak area of the unbound compound recovered in the ultrafiltrate.
Table 1.
Pulsed ultrafiltration LC-MS-MS binding and inhibition of cyclooxygenases by gingerol-related compounds
| Compound | m/z | SRM transition m/za | Retention time (min) | Relative binding to COX-2b | IC50 COX-1 μM | IC50 COX-2 μM |
|---|---|---|---|---|---|---|
| ginger extract | 20.0 ± 0.4c | 7.5 ± 0.6c | ||||
| 6-gingerol | 293 | 293 – 99 | 25.1 | 0 | ||
| 8-gingerol | 321 | 321 – 193 | 33.5 | 0 | ||
| 10-gingerol | 349 | 349 – 193 | 40.1 | 0.17 ± 0.03 | >50 | 32.0 ± 1.5 |
| 12-gingerol | 377 | 377 – 193 | 47.5 | 0.23 ± 0.01 | ||
| 6-shogaol | 275 | 275 – 139 | 35.1 | 0 | ||
| 8-shogaol | 303 | 303 – 167 | 42.3 | 0.27 ± 0.02 | >50 | 17.5 ± 2.2 |
| 10-shogaol | 331 | 331 – 195 | 49.7 | 0.35 ± 0.03 | >50 | 7.5 ± 0.6 |
| 12-shogaol | 359 | 359 – 223 | 56.0 | 0 | ||
| 6-paradol | 277 | 277 – 141 | 38.6 | 0.41 ± 0. 01 | ||
| 8-paradol | 305 | 305 – 169 | 45.2 | 0.70 ± 0.02 | ||
| 10-paradol | 333 | 333 – 197 | 52.2 | 0 | ||
| 6-gingerdiol | 295 | 295 – 280 | 24.1 | 0 | ||
| 8-gingerdiol | 323 | 323 – 308 | 32.0 | 0 | ||
| 6-dehydro-6-gingerol | 291 | 291 – 155 | 20.5 | 0 | ||
| 6-dehydro-8-gingerol | 319 | 319 – 183 | 28.3 | 0 | ||
| 6-dehydro-10-gingerol | 347 | 347 – 211 | 35.4 | 0.15 ± 0.01 | ||
| 6-gingerdione | 291 | 291 – 155 | 28.0 | 0.23 ± 0.02 | ||
| 8-gingerdione | 319 | 319 – 183 | 35.2 | 0.20 ± 0.03 | ||
| 10-gingerdione | 347 | 347 – 211 | 42.3 | 0.24 ± 0.05 |
MS-MS transitions using selected reaction monitoring (SRM)
Relative binding = (pulsed ultrafiltration peak area)/(peak area washing buffer)
μg/mL
2.3. COX inhibition assay
Inhibition of COX-1 and COX-2 by the chloroform partition of the ginger extract, by the positive control, celecoxib, and by individual ginger constituents was determined using the method of Cao et al. [12]. Briefly, the concentration of PGE2 formed by COX-1 or COX-2 in the presence of an inhibitor or ginger extract was measured using LC-MS-MS, and the percent of COX inhibition by each test solution was determined by comparing the amount of PGE2 produced in the experiment with that produced in the negative control incubation. For IC50 value determination, 12 different concentrations of each inhibitor were assayed three times. The IC50 value of each inhibitor toward COX-1 or COX-2 was determined by plotting and analyzing the inhibition curve data using Graph Pad Prism 5 software (Mountain View, CA).
3. RESULTS AND DISCUSSION
3.1. Pulsed ultrafiltration MS screening of ginger extract for COX-2 ligands
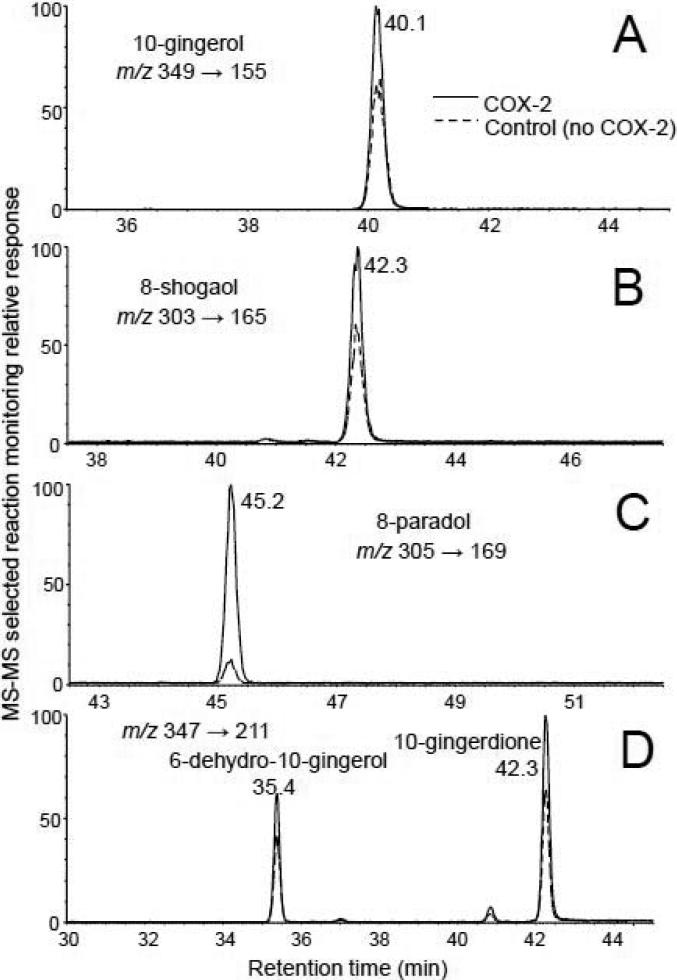
Using pulsed ultrafiltration LC-MS screening [11], the COX-2 selective inhibitor celecoxib showed significant binding to human COX-2 as expected (data not shown), and its IC50 concentration was determined to be 50 nM using a functional enzyme assay [12]. These positive control experiments indicated that these assays were functioning appropriately. The chloroform partition of the methanol extract of ginger roots was then screened using pulsed ultrafiltration LC-MS-MS. Since only gingerol-related compounds (Figure 1) were detected as COX-2 ligands in the ginger preparation, constant neutral loss and selected reaction monitoring LC-MS-MS techniques were used to enhance the signal-to-noise of the target analytes by eliminating signals for non-gingerol derivatives. Examples of pulsed ultrafiltration tandem mass spectrometric analysis for each of the classes of gingerol-related compounds showing specific binding to COX-2 are shown in Figure 2. Determination of specific binding to COX-2 was based on an increase in LCMS-MS peak area after incubation with active COX-2 and ultrafiltration affinity extraction, compared to control experiments without enzyme. Specific binding of gingerol-related compounds to the active site of COX-2 was confirmed by repeating the pulsed ultrafiltration experiment in the presence of the high affinity COX-2 inhibitor celecoxib, which displaced the weaker binding gingerol-related compounds from the active site and reduced their peak areas in the LC-MS-MS chromatograms.
Figure 2.
Pulsed ultrafiltration mass spectrometric screening of a chloroform partition of a methanolic extract of ginger root for ligands to COX-2. LC-MS-MS with selected reaction monitoring (SRM) was used for the selective detection of gingerol-related compounds including A) 10-gingerol; B) 8-shogaol; C) 8-paradol; and D) 10-gingerdione and 6-dehydro-10-gingerol. Specific binding to COX-2 resulted in peak enhancement compared with control incubations without COX-2.
Six classes of gingerol-related compounds were detected during pulsed ultrafiltration including 4 gingerols, 4 shogaols, 3 paradols, 2 gingerdiols, 3 gingerdiones, and 3 dehydrogingerols. Compounds in the ginger preparation were identified by comparison with standards, or by comparison of tandem mass spectra with the literature [10]. The HPLC retention times, masses of the deprotonated molecules, and selected reaction monitoring transitions used for detection and measurement of these compounds are shown in Table 1.
Except for the gingerdiols, at least some members of each class of the gingerol-related compounds showed specific binding to the active site of COX-2. All 3 gingerdiones bound to COX-2, but only 2 gingerols, 2 shogaols, 2 paradols, and 1 dehydrogingerol were COX-2 ligands. Among the gingerols, 10-gingerol and 12-gingerol but not 6-gingerol or 8-gingerol bound to COX-2, 8-shogaol and 10-shogaol were COX-2 ligands but not 6-shogaol or 12-shogaol, and 6-paradol and 8-paradol but not 10-paradol bound to COX-2. The relative affinity of each of these ligands for COX-2 was determined by calculating a bound/free ratio, and these values are shown in Table 1.
The paradols showed the highest affinity for COX-2 followed by the shogaols and then the gingerols and gingerdiones. Of much lower relative affinities for COX-2 were the dehydrogingerols, and lastly, the gingerdiols which showed no binding to COX-2 (Table 1). The carbonyl group at carbon 3 (see numbering scheme in Figure 1) appeared to be essential for binding to COX-2, since the gingerdiols which lack this carbonyl group did not bind to COX-2. Within each gingerol-related class of compounds, an optimum alkyl chain length was observed. For example, 10-shogaol bound to COX-2 with higher affinity than did either 8-shogaol or 12-shogaol, and 8-paradol bound to COX-2 with higher affinity than either 6-paradol or 10-paradol.
3.2. IC50 value determination and quantitative analysis of COX inhibitors
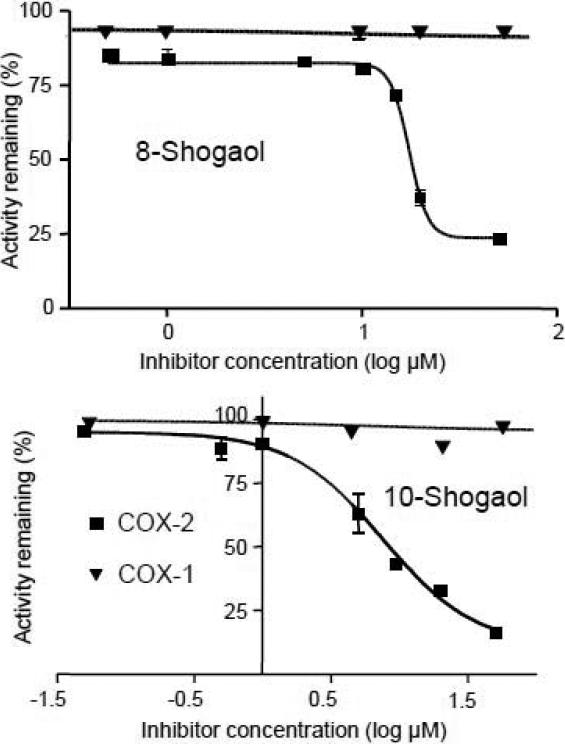
The IC50 values of the ginger extract and the COX-2 ligands 10-gingerol, 8-shogaol and 10-shogaol were determined for both COX-2 and COX-1 (Table 1), and examples of dose-response curves for the determination of these IC50 values are shown in Figure 3. Unfortunately, insufficient quantities of other gingerol-related compounds were available for additional IC50 value measurements. The IC50 determinations confirmed that each of these COX-2 ligands was a COX-2 inhibitor but did not inhibit COX-1, which means that these gingerols and shogaols are COX-2 selective inhibitors. Among these three compounds, the most potent COX-2 inhibitor was 10-shogaol with an IC50 of 7.5 ± 0.6 μM, followed by 8-shogaol (IC50 17.5 ± 2.2 μM) and 10-gingerol (IC50 32.0 ± 1.5 μM). This order of COX-2 inhibitory activity is consistent with the relative binding values determined using pulsed ultrafiltration LC-MS-MS (Table 1) and provides validation for the use of this approach to rank order ligands according to their affinity for a receptor as originally reported by Sun et al. [13].
Figure 3.
Dose-response curves for inhibition of COX-1 and COX-2 by the gingerol-related compounds 8-shogaol and 10-shogaol.
Previously reported IC50 values for the inhibition of COX-2 by 6-shogaol (2.1 μM), 8-shogaol (7.2 μM), 6-gingerol (>50 μM), and 8-gingerol (10 μM) [3] are similar to those observed here with respect to the determination that shogaols are more potent inhibitors of COX-2 than the corresponding gingerols. However, the present study shows that 8-shogaol is a more potent inhibitor, not a weaker inhibitor, of COX-2 than is 6-shogoal with an IC50 17.5 μM and no COX-2 binding activity, respectively). This difference might be the result of our use of purified human recombinant COX-2 instead of the cell-based assay in the cited study [3].
Quantitative analysis of 10-gingerol, 8-shogaol and 10-shogaol in the ginger extract was carried out using LC-MS-MS, and these compounds were determined to be 3.4%, 1.0% and 0.5% (w/w) of the extract, respectively. The chloroform partition of the methanol extract of ginger roots was assayed for COX-1 and COX-2 inhibition, and the IC50 values were 20.0 ± 0.4 μM and 7.5 ± 0.6 μM, respectively (Table 1). This indicates that this ginger preparation exhibits weak COX inhibition, but is a more potent inhibitor of COX-2 than COX-1. These data are consistent with a recent report [6] which found that fractions of ginger root containing substantial amounts of gingerols, and gingerol-related compounds were the most effective at inhibiting the formation of PGE2 which is a measure of COX activity.
4. CONCLUSIONS
Ginger preparations have a long history of human use for their anti-inflammatory properties, however, only recently have some of the compounds responsible for this activity and their mechanisms of action been identified [4]. This study provides information regarding the identification of gingerol-related compounds that have anti-inflammatory activity through the specific inhibition of COX-2. The development of mass spectrometric-based assays for screening botanical extracts for COX-2 ligands [11] and for measuring PGE2 as a means of determining COX-2 inhibition [12] facilitated this investigation.
Although the observed enzyme inhibitory activities are weak, at least some gingerol-related compounds including gingerols and shogaols selectively inhibit the inducible form of cyclooxygenase, COX-2, but not the constitutive form, COX-1. Since inhibition of COX-1 is associated with gastrointestinal irritation, selective inhibition of COX-2 should help minimize this side effect. Overall, the ginger preparation used in this study was found to inhibit COX-2 approximately three-fold more than COX-1, and this property should contribute to the beneficial anti-inflammatory activity of ginger products.
ACKNOWLEDGEMENTS
This research was supported by grant P01 CA48112 from the National Cancer Institute and grant P50 AT00155 from the Office of Dietary Supplements, the National Center for Complementary and Alternative Medicine and the Office on Research in Women's Health.
ABBREVIATIONS
- SRM
selected reaction monitoring
- COX
cyclooxygenase
- NSAIDs
non-steroidal anti-inflammatory drugs
- PG
prostaglandin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. LITERATURE CITED
- 1.Yokoyama C, Tanabe T T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem. Biophys. Res. Commun. 1989;165:888–894. doi: 10.1016/s0006-291x(89)80049-x. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekharan NV, Simmons DL. The cyclooxygenases. Genome Biol. 2004;5:241. doi: 10.1186/gb-2004-5-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tjendraputra E, Tran VH, Biu-Brennan D, Roufogalis BD, Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001;29:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 4.Grzanna R, Lindmark L, Frondoza CG. Ginger—an herbal medicinal product with broad anti-inflammatory actions. J. Med. Food. 2005;8:125–132. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 5.Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diaryl heptanoids. Chem. Pharm. Bull. (Tokyo) 1992;40:387–391. doi: 10.1248/cpb.40.387. [DOI] [PubMed] [Google Scholar]
- 6.Funk JL, Frye JB, Oyarzo JN, Timmermann BN. Comparative effects of two gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. J. Nat. Prod. 2009;72:403–407. doi: 10.1021/np8006183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44:2531–2538. doi: 10.1002/1529-0131(200111)44:11<2531::aid-art433>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Nikolic D, Habibi-Goudarzi S, Corley DG, Gafner S, Pezzuto JM, van Breemen RB. Evaluation of cyclooxygenase-2 inhibitors using pulsed ultrafiltration mass spectrometry. Anal. Chem. 2000;72:3853–3859. doi: 10.1021/ac0000980. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BM, Nikolic D, van Breemen RB. Applications of pulsed ultrafiltration mass spectrometry. Mass Spectrom. Rev. 2002;21:76–86. doi: 10.1002/mas.10020. [DOI] [PubMed] [Google Scholar]
- 10.Tao Y, Li W, Liang W, van Breemen RB. Identification and quantification of gingerols and related compounds in ginger dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2009;57:10014–10021. doi: 10.1021/jf9020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolic D, Habibi-Goudarzi S, Corley DG, Gafner S, Pezzuto JM, van Breemen RB. Evaluation of cyclooxygenase-2 inhibitors using pulsed ultrafiltration mass Spectrometry. Anal. Chem. 2000;72:3853–3859. doi: 10.1021/ac0000980. [DOI] [PubMed] [Google Scholar]
- 12.Cao H, Yu R, Tao Y, Nikolic D, van Breemen RB. Measurement of cyclooxygenase inhibition using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010 doi: 10.1016/j.jpba.2010.08.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Gu C, Liu X, Liang W, Yao P, Bolton JL, van Breemen RB. Ultrafiltration tandem mass spectrometry of estrogens for characterization of structure and affinity for human estrogen receptors. J. Am. Soc. Mass Spectrom. 2005;16:271–279. doi: 10.1016/j.jasms.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]