Abstract
Vascular cognitive impairment has been traditionally defined by structural pathology – an accumulation of infarcts -- leading to progressive cognitive decline. Recent evidence, however, suggests that cognitive impairment may be independently mediated by hemodynamic dysfunction including global and hemispheral hypoperfusion and altered cerebral blood flow regulation. In this review we examine evidence for the contribution of hemodynamic impairment to cognitive dysfunction in the setting of large vessel disease, cardiac failure, and microvascular disease. If there is a hemodynamic component of vascular cognitive impairment, then treatments proposed to correct impaired vascular physiology may reasonably be expected to treat the cognitive dysfunction as well.
Introduction
The ancient Romans had an exquisitely engineered aqueduct system to supply water to their cities. Originating in surrounding hills, the system required a descent in elevation of exactly 34cm per Km to keep the water flowing smoothly. Too steep a gradient and the water would overflow; too flat and the system would clog. For hundreds of years the system brought more than 50 million gallons of water per day to the citizens of Rome, for drinking, bathing and irrigation. As with the historical water needs of the citizens of Rome, brain tissue requires a well-regulated system to maintain functional homeostasis. Obstructed conduits, inadequate pumping force, and dysfunctional vessels can reduce flow or alter homeostatic blood flow regulation. Cognitive function, perhaps more than any other brain process, appears to be sensitive to changes in hemodynamic state.1
Structural versus hemodynamic impairment
Vascular cognitive impairment (VCI) has been difficult to characterize because of its multifactorial etiology and poor standardization of measurement. Structural pathology of both Alzheimer's disease (AD) and vascular dementia is reported to be present in a high percentage of cases diagnosed clinically as either one condition or the other; infarcts and white matter lesions are found in brains of 60–90% of Alzheimer's patients, and plaques and tangles are found in more than a third of those diagnosed with vascular dementia2. Among vascular cases, both the accumulation of small infarcts3 and the strategic placement of large infarcts4 are well described to produce cognitive impairment. Although structural pathology is unquestionably important in the development of dementia, there is burgeoning evidence that cerebral hemodynamic impairment may be a direct mediator of cognitive dysfunction and decline.
Hemodynamic abnormalities, specifically cerebral hypoperfusion and impaired cerebral vasomotor reactivity (CVR), are present in a variety of cerebrovascular conditions. Our central thesis for the purposes of this review is that whereas structural pathology may reflect irreversible, end-organ damage leading to permanent cognitive dysfunction, hemodynamic causes of cognitive decline may be reversible and thus a target for treatment. The purpose of this review is to consider the evidence for hemodynamic mediation of cognitive dysfunction. We will examine vascular-related cognitive impairment at three levels of ischemic disease: hemispheral hypoperfusion caused by carotid occlusive disease, global cerebral hypoperfusion produced by cardiac failure, and microvascular pathology leading to loss of vasomotor reactivity and cerebral blood flow (CBF) autoregulation.
Definition of vascular cognitive impairment
The set of clinical elements that defines VCI continues to be debated.5 Although there is an historical emphasis on memory dysfunction in dementia, newer evidence shows that small-vessel disease in the frontal lobes may be associated with disruption of executive function and processing speed in the absence of difficulties in new learning and mnestic function.6 Memory abnormality is therefore not necessary for a diagnosis of VCI, reflected in the recently-established harmonization standards.7 But because AD and VCI can co-occur and interact in manner not identifiable until pathology is verified, establishing “pure cases” of VCI remains problematic. In addition, some cardiovascular conditions are associated with hippocampal atrophy,8 9 contributing directly to memory loss. We therefore take the position that the rubric of vascular cognitive disorders is an umbrella concept, encompassing any vascular condition which is sufficient to produce cognitive change.
An important step forward has been the recognition that vascular dementia, like AD, represents only one end of the severity continuum. At the other end lies mild deficits with minimal impact on daily activities, sometimes referred to Vascular Cognitive Impairment No Dementia (CIND) or non-amnestic MCI.10 These are important to identify because longitudinal studies show that individuals with such early changes are at significant risk for developing more disabling syndromes.11 Mild syndromes were not recognized as VCI initially because of the belief that the end-product of the responsible vascular disease is neuronal death, whether from large territory infarction or lacunar disease. Consequently, a major underlying assumption is that function, like brain tissue, is irretrievably lost. There is evidence, as discussed below, to contradict this assumption. Finally, it is interesting to note parallels between the dynamic nature of cognitive functions such as information processing, attention and working memory, and the dynamic physiology of blood flow: these cognitive functions appear uniquely susceptible to hemodynamic compromise which may help further distinguish cognitive dysfunction originating from vascular causes as opposed to cortical degenerative causes like AD.
Hemispheral hypoperfusion as a reversible pathology for cognitive decline
Perhaps the clearest evidence for a cerebral hemodynamic effect is in large-vessel disease producing hemispheral hypoperfusion. Here, a drop in perfusion pressure to a cerebral hemisphere supplied by a blocked carotid artery induces a series of well-described hemodynamic responses including dilation of cerebral arterioles and increase of oxygen extraction fraction (OEF) to preserve aerobic metabolism.12 Further reduction in perfusion pressure results in ischemia, then frank infarction. These hemodynamic responses, part of the autoregulatory process, allow reversibility of ischemia and, at least under some circumstances, recovery of brain function.13
The association between carotid occlusive disease and dementia was first reported by Miller Fisher in 1954 in a patient with bilateral carotid occlusion and progressive dementia14. Many carotid treatment studies have compared pre-and postoperative cognitive performance, with inconsistent treatment effects on cognition.15–17 Systematic reviews of the impact of carotid disease on cognitive dysfunction have been inconclusive.18–20 A recent review of 32 papers in which cognition was assessed before and after CEA or carotid-artery stenting found that 5 reported cognitive decline, 6 documented no significant change, and in the remaining, percentages of improvement varied across cognitive domains21. Unfortunately, measurement of cognitive impairment varied widely, and none of the studies measured blood flow.
The argument that hypoperfusion is an independent cause for cognitive impairment in carotid-occlusive disease is strengthened by studies reporting cognitive impairment in patients with carotid stenosis in the absence of frank infarction. Bakker et al22 reported cognitive impairment in 39 cases with cerebral or retinal TIAs but no stroke on MRI. Johnston et al examined the relationship between side of carotid stenosis and cognitive impairment among patients with high grade carotid disease but no infarction23. Cognitive dysfunction and cognitive decline, measured by MMSE were correlated with left, but not right ICA stenosis. The authors concluded that because the MMSE is more sensitive to left hemispheral dysfunction, the stenosis was more than a marker of generalized cognitive dysfunction, but a causal factor in the dysfunction. Although the association was statistically significant, cognitive impairment occurred in only 34% of patients with severe stenosis, while the remaining patients with stenosis had normal cognition24. Again, however, hemodynamic measurements were not made.
Blood flow was measured by Tatemichi et al. in a 55 year old man with bilateral ICA occlusions presenting with a subacute onset of severe behavioral and cognitive changes25. Quantitative CBF and PET studies showed a 40–50% reduction in blood flow and metabolism. Following EC-IC bypass, the patient demonstrated neuropsychological improvement accompanied by significant increases in cerebral blood flow and metabolism. In a larger case series, 25 patients with unilateral carotid occlusion and poor neuropsychological performance underwent EC-IC bypass26. Cognitive improvement was associated with increased CBF, increased CVR, and decreased OEF. The possibility that EC-IC bypass can improve cognition or prevent its decline is being formally tested in the NINDS-sponsored Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON) trial27. In this study, PET measurements are taken in all patients with recently symptomatic carotid occlusion. Among those with increased OEF, indicating so-called stage-II hemodynamic failure, half receive EC-IC bypass and half best medical therapy alone. Hemisphere-specific and global cognitive tests are administered at baseline and 2 years after randomization. The differences in 2-year cognitive scores between the surgical and medical groups will determine whether direct cerebral hemodynamic treatment of patients with known hemodynamic failure can benefit from hemispheral reperfusion. Demonstration of a positive effect of revascularization on cognition would constitute important proof of reversible cognitive impairment due to a hemodynamic cause within the context of a randomized clinical trial.
Cognitive impairment in cardiac disease due to global cerebral hemodynamic dysfunction
Congestive heart failure (CHF) is a model for the effects of whole-brain hypoperfusion on cognitive dysfunction. Although cardiac embolism and decreased CBF may both contribute to cognitive impairment,28 as heart failure progresses to more severe stages, reduced CBF correlates with a rising prevalence of cognitive dysfunction.29 Choi et al found that global CBF was 19% less in patients with severe CHF than in controls.30 Unlike embolic stroke which produces focal deficits, low CBF affects more complex cognitive tasks such as memory, attention, and executive skills subserved by distributed brain regions.
Zuccala et al were among the first to show that cognition was affected by cardiac failure.31 Among 57 elderly individuals with chronic CHF, poorer cognition was associated with left ventricular ejection fraction (LVEF) ≤ 30%. With finer-grained neuropsychological assessment, it has become increasingly apparent that not all cognitive functions are equally susceptible to global hemodynamic compromise. Vogels et al studied individuals with NYHA Stage II and III CHF.32 CHF patients had greater deficits in widely-distributed processing skills, whereas focal cognitive functions, such as language, were not different between patients and controls. We studied 116 consecutive candidates for transplant and compared their function to age-matched norms.33 We also found that more-broadly dispersed spheres of cognitive functions were affected in patients with severe heart failure.
The opportunity to confirm further the relationship between cerebral hemodynamics and cognition has come from the restoration of cardiac output through medical or surgical intervention. Zuccala et al studied retrospectively 1,220 older patients with heart failure and found that those starting ACE inhibitors, a drug class known to have positive effects on CBF, had an increased probability of improving cognitive function during hospital stay.34 Other evidence has been derived by comparisons before and after cardiac transplantation. Several recent studies showed cognitive benefit after transplantation.35 36 Mechanistic support for improvement in CBF that would account for concomitant changes in cognition has been provided by blood flow and TCD studies.37 38
Whereas cerebral hypoperfusion seems to mediate cognitive impairment and recovery, there appears also to be a risk to restoring whole-brain blood flow too quickly, similar to reperfusion injury after carotid endarterectomy (CEA) or angioplasty39. We studied 69 consecutive patients who had just undergone implantation with a left ventricular assist device (LVAD) and found that 19 developed neurologic dysfunction, mainly cognitive slowing and inattention. The risk of this condition correlated with an increased cardiac index, the effect attributed to mild cerebral edema. Reduction of LVAD outflow in 16 of the 19 symptomatic patients led to improvement in symptoms in 14 of them.
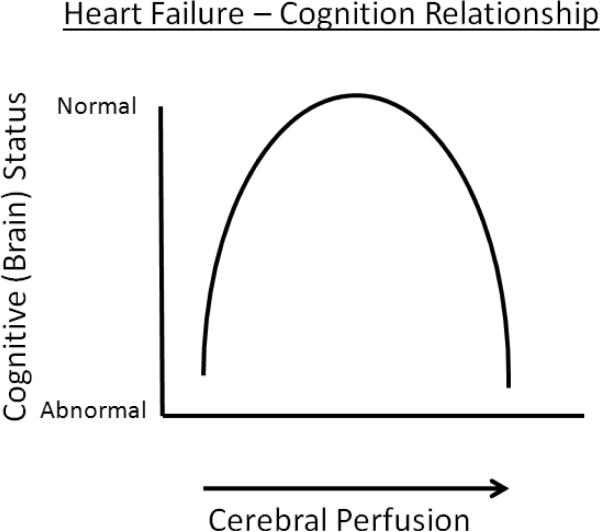
Collectively, the evidence from these heart failure studies suggests that optimization of cognitive function occurs in the middle range of CBF (Figure 1). At the higher and lower ends of the spectrum, there is degradation of function; normalizing CBF reverses the cognitive dysfunction.
Figure 1.
Illustration of cognitive dysfunction occuring with both hypoperfusion and hyperperfusion in the setting of CHF.
Cognitive impairment related to vascular dysfunction in microvascular ischemia
In the absence of chronic hemispheric hypoperfusion caused by large-vessel stenosis or global cerebral hypoperfusion produced by cardiac failure, the cerebral microvasculature may undergo altered hemodynamic function in patients with stroke risk factors. Hypertension, diabetes, and direct effects on the endothelium have been shown to be associated with changes in cerebral blood flow and vasoreactivity in this population40 41. Demonstrating a causal link between these hemodynamic changes and cognitive impairment has been more difficult to prove, however, in part because of the co-occurrence of small vessel infarction. Nonetheless, the association is worth considering because, like global and hemispheral hemodynamic impairment, the potential exists for ameliorating cognitive impairment by reversing abnormal microvascular hemodynamics.
Hypertension has been the most studied risk factor for cognitive impairment because of its effects on vascular function. In one study carotid femoral pulse wave velocity (PWV), a measure of arterial stiffness, correlated with poorer scores on the MMSE among elderly patients with never-treated essential hypertension.42 Silent infarction was not evaluated in this study, however, so it may have been that those with greater arterial stiffness had silent infarcts to explain the cognitive impairment. A similar study in elderly and middle-aged men showed PWV associated with lower processing capacity and executive functioning, and increased intima-medial thickness (IMT), a pre-atherosclerotic marker correlating with memory impairment.43 With no imaging in this study, however, frank infarction as an intermediary between vascular dysfunction and cognitive impairment could not be ruled out. In the Johnston study described in the carotid section above, it was also shown that IMT was associated with cognitive impairment, suggesting that IMT may be a non-lateralizing marker of vascular dysfunction that was contributing to cognitive impairment.
In addition to hypertension, hypotension may also play a hemodynamic role in the development of cognitive impairment44 45. This hypothesis has appeal because CBF is known to be low in patients with dementia46, and cerebral hypoperfusion appears to be associated with cognitive impairment in large vessel disease and cardiac failure as also discussed above. Low CBF imaged in dementia may be a reflection of lower metabolism, or a consequence of the dementing process, however.47
An additional pathophysiological link between hemodynamics and cognitive impairment concerns white matter hyperintensities (WMH). There is evidence that WMHs arise from chronic hypoperfusion48. CVR has also been shown to be lower when WMHs are present.49 Regardless of cause, these pathological changes have been associated with cognitive decline50 51. An association between WMH and impaired hemodynamic function was demonstrated by Fu et al who investigated patients >60 years of age with WMH on MRI but no stroke or significant large-vessel stenosis52. They showed that extent of these lesions correlated inversely with CVR measured by TCD.
Other stroke risk factors have also been linked to impaired cerebral hemodynamics. Giannopoulos et al recently showed an association between metabolic syndrome and impaired CVR in 83 patients in the hemisphere opposite to that supplied by a stenotic carotid artery, suggesting that metabolic syndrome, with its associated endothelial effects53 alters cerebral hemodynamics more broadly than via unilateral hemispheral hypoperfusion. Furthermore, in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarction and Leukoencephalopathy (CADASIL), a condition associated with WMH and early dementia, flow-related vascular dysfunction appears to occur even before vascular smooth muscle degeneration and granular osmophilic material deposition has taken place54.
Given the evidence that cerebral hemodynamic dysfunction appears to have an independent association with cognitive impairment, the question arises whether treatment of the hemodynamic impairment can reverse cognitive impairment. Blood pressure management may be one effective way to reverse the effects of hemodynamic impairment. In hypertensives who develop dementia, the prevention of hypotension has been shown to protect against cognitive decline.55 In this study of vascular dementia patients, those who had hypertension kept below systolic pressure of 135mmHg had deterioration of cognitive function, whereas those kept 135–150 improved. In addition, some have evaluated antihypertensives with known positive effects on CBF. The Cardiovascular Health Cognition sub-study showed that patients taking centrally-acting ACE inhibitors had a 65% per year reduction in decline of MMSE scores compared with others taking non-blood flow related antihypertensives. An ongoing NIH-sponsored, randomized clinical trial is assessing cognitive function, CBF and CVR among 100 elderly, hypertensive patients taking one of 3 antihypertensives with varying effects on cerebral blood flow56.
Conclusions
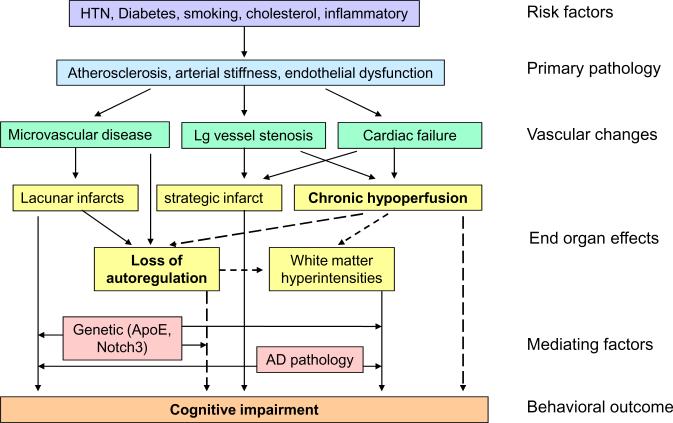
The relationship between vascular pathophysiology, and cognitive impairment is complex, as shown in Figure 2. Stroke risk factors contribute to vascular changes, which in turn cause both structural pathology and chronic hemodynamic failure. These end-organ effects in the brain produce cognitive impairment either directly or through the further influence of autoregulatory loss and accumulation of WMH. Genetic factors and AD pathology may further mediate the effects of vascular dysfunction on cognitive impairment. Although the prevention of structural pathology will remain an important tenet in the treatment of VCI, the evidence for the relationship between reversibility of hemodynamic dysfunction and the reversibility of cognitive impairment appears strong enough to warrant serious clinical attention and continued scientific study.
Figure 2.
Model for the pathophysiology of cognitive impairment. Dotted lines indicate potentially reversible hemodynamic pathways.
Future Directions
Based on the successes and failures of clinical studies discussed in this review, we propose that certain key variables should be present in any future study of the relationship between blood flow and cognition (see Table). First, the study must include a quantifiable measure of cerebral hemodynamics such as blood flow by PET or arterial spin labeling MRI, autoregulation by TCD vasoreactivity57, or hypercapnea-induced Blood Oxygen Level Dependent (BOLD) changes by functional MRI.58 Second, the cognitive assessment must include functions which are sensitive to hypoperfusion (e.g. attention and executive function), and specific to the disease state being studied (e.g. hemisphere-specific for carotid disease, bihemispheral functions for cardiac failure). Third, presence prior strokes, small vessel infarcts, microbleeds,59 and white matter hyperintensities must be examined as covariates. Fourth, depression, renal failure, hepatic dysfunction, pulmonary disease, and other variables which can independently alter cognition, must be controlled for or serve as exclusions. Only with careful study design and thoughtful consideration of potentially relevant variables will the complex relationship between blood flow and cognition be disentangled enough to alter our patients' clinical course and move the field forward.
Table.
Critical variables to include in studies of cerebral hemodynamic impairment and cognitive dysfunction. PET=positron emission tomography, ASL=arterial spin labeling, BOLD=blood oxygen level dependent, FLAIR=fluid attenuated inversion recovery.
| variable | examples |
|---|---|
| Quantifiable hemodynamic measure | PET, ASL MRI, TCD vasoreactivity, fMRIBOLD hypercapnea response |
| Cognitive tests sensitive to blood flow and specific to disease state | Tests of attention and executive function; Hemisphere-specific for carotid disease |
| Structural imaging for territorial stroke, small vessel infarcts, microbleeds, and white matter hyperintensities. | FLAIR, T2-weighted, gradient echo MRI |
| Covariates that can alter cognition | Depression, hepatic disease, renal failure |
Acknowledgments
Sources of Funding: This work was supported by the Doris and Stanley Tananbaum Family Foundation and by NIH/NINDS R01 NS048212 (RSM) and NIH/NHLBI 1P50 HL077096 (RML)
Disclosures: Relevant to this manuscript, Dr. Marshall was supported by NIH/NINDS R01 NS048212, and Dr. Lazar was supported by NIH/NHLBI 1P50 HL077096
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34:335–337. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Prohovnik I. Strategic infarcts in vascular dementia. A clinical and brain imaging experience. Arzneimittelforschung. 1995;45:371–385. [PubMed] [Google Scholar]
- Merino JG, Hachinski V. Historical Perspective. In: Festa JR, Lazar RM, editors. Neurovascular Neuropsychology. Springer; New York: 2009. pp. 1–6. [Google Scholar]
- Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Vogels RL, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail. 2007;9:1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Horstmann A, Frisch S, Jentzsch RT, Muller K, Villringer A, Schroeter ML. Resuscitating the heart but losing the brain: brain atrophy in the aftermath of cardiac arrest. Neurology. 2010;74:306–312. doi: 10.1212/WNL.0b013e3181cbcd6f. [DOI] [PubMed] [Google Scholar]
- Nyenhuis DL, Gorelick PB. Diagnosis and management of vascular cognitive impairment. Curr Atheroscler Rep. 2007;9:326–332. doi: 10.1007/s11883-007-0040-5. [DOI] [PubMed] [Google Scholar]
- Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Lazar RM, Mohr JP, Pile-Spellman J, Hacein-Bey L, Duong DH, Joshi S, Chen X, Levin B, Young WL. Higher cerebral function and hemispheric blood flow during awake carotid artery balloon test occlusions. J Neurol Neurosurg Psychiatry. 1999;66:734–738. doi: 10.1136/jnnp.66.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. Senile Dementia-- A new explanation of its causation. Arch Neurol. 1951;65:1–7. [PMC free article] [PubMed] [Google Scholar]
- Drinkwater JE, Thompson SK, Lumley JS. Cerebral function before and after extra-intracranial carotid bypass. J Neurol Neurosurg Psychiatry. 1984;47:1041–1043. doi: 10.1136/jnnp.47.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H, Hojer-Pedersen E, Gulliksen G, Haase J, Enevoldsen E. Reversible ischemic neurological deficit and minor strokes before and after EC/IC bypass surgery. A neuropsychological study. Acta Neurol Scand. 1986;73:615–618. doi: 10.1111/j.1600-0404.1986.tb04608.x. [DOI] [PubMed] [Google Scholar]
- Binder LM, Tanabe CT, Waller FT, Wooster NE. Behavioral effects of superficial temporal artery to middle cerebral artery bypass surgery: preliminary report. Neurology. 1982;32:422–424. doi: 10.1212/wnl.32.4.422. [DOI] [PubMed] [Google Scholar]
- Lunn S, Crawley F, Harrison MJ, Brown MM, Newman SP. Impact of carotid endarterectomy upon cognitive functioning. A systematic review of the literature. Cerebrovasc Dis. 1999;9:74–81. doi: 10.1159/000015901. [DOI] [PubMed] [Google Scholar]
- Bakker FC, Klijn CJ, Jennekens-Schinkel A, Kappelle LJ. Cognitive disorders in patients with occlusive disease of the carotid artery: a systematic review of the literature. J Neurol. 2000;247:669–676. doi: 10.1007/s004150070108. [DOI] [PubMed] [Google Scholar]
- Rao R. The role of carotid stenosis in vascular cognitive impairment. Eur Neurol. 2001;46:63–69. doi: 10.1159/000050765. [DOI] [PubMed] [Google Scholar]
- De Rango P, Caso V, Leys D, Paciaroni M, Lenti M, Cao P. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke. 2008;39:3116–3127. doi: 10.1161/STROKEAHA.108.518357. [DOI] [PubMed] [Google Scholar]
- Bakker FC, Klijn CJ, Jennekens-Schinkel A, van der Tweel I, Tulleken CA, Kappelle LJ. Cognitive impairment in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. J Neurol. 2003;250:1340–1347. doi: 10.1007/s00415-003-0222-1. [DOI] [PubMed] [Google Scholar]
- Johnston SC, O'Meara ES, Manolio TA, Lefkowitz D, O'Leary DH, Goldstein S, Carlson MC, Fried LP, Longstreth WT., Jr Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med. 2004;140:237–247. doi: 10.7326/0003-4819-140-4-200402170-00005. [DOI] [PubMed] [Google Scholar]
- Barnett HJ. Carotid Disease and Cognitive Dysfunction. Annals of Internal Medicine. 2004;140:303–304. doi: 10.7326/0003-4819-140-4-200402170-00013. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Prohovnik I, Eidelberg D. Dementia associated with bilateral carotid occlusions: neuropsychological and haemodynamic course after extracranial to intracranial bypass surgery. J Neurol Neurosurg Psychiatry. 1995;58:633–636. doi: 10.1136/jnnp.58.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasoh M, Ogasawara K, Kuroda K, Okuguchi T, Terasaki K, Yamadate K, Ogawa A. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol. 2003;59:455–460. doi: 10.1016/s0090-3019(03)00152-6. discussion 460–453. [DOI] [PubMed] [Google Scholar]
- Marshall RS. Study of Carotid Occlusion and Neurocognition. NINDS. 2004–2012 [Google Scholar]
- Pullicino PM, Hart J. Cognitive impairment in congestive heart failure?: Embolism vs hypoperfusion. Neurology. 2001;57:1945–1946. doi: 10.1212/wnl.57.11.1945. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Jarden JO, Godtfredsen J, Vorstrup S. Cerebral blood flow in patients with congestive heart failure treated with captopril. Am J Med. 1984;76:91–95. doi: 10.1016/0002-9343(84)90892-1. [DOI] [PubMed] [Google Scholar]
- Choi BR, Kim JS, Yang YJ, Park KM, Lee CW, Kim YH, Hong MK, Song JK, Park SW, Park SJ, Kim JJ. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97:1365–1369. doi: 10.1016/j.amjcard.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Cattel C, Manes-Gravina E, Di Niro MG, Cocchi A, Bernabei R. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. J Neurol Neurosurg Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels RL, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P, van der Flier WM, Weinstein HC. Transcranial Doppler blood flow assessment in patients with mild heart failure: correlates with neuroimaging and cognitive performance. Congest Heart Fail. 2008;14:61–65. doi: 10.1111/j.1751-7133.2008.07365.x. [DOI] [PubMed] [Google Scholar]
- Festa JR, Shapiro PA, Mancini DM, Lantz ER, Deng MC, Naka Y, Lazar RM. Cognitive impairment in heart failure is not predicted by emotional factors. Journal of Coronary Artery Disease. 2007;7:92–193. [Google Scholar]
- Zuccala G, Onder G, Marzetti E, Monaco MR, Cesari M, Cocchi A, Carbonin P, Bernabei R. Use of angiotensin-converting enzyme inhibitors and variations in cognitive performance among patients with heart failure. Eur Heart J. 2005;26:226–233. doi: 10.1093/eurheartj/ehi058. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Starling RC, Myerowitz PD, Haas GJ. Neuropsychological function in patients with end-stage heart failure before and after cardiac transplantation. Acta Neurol Scand. 1995;91:260–265. doi: 10.1111/j.1600-0404.1995.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Roman DD, Kubo SH, Ormaza S, Francis GS, Bank AJ, Shumway SJ. Memory improvement following cardiac transplantation. J Clin Exp Neuropsychol. 1997;19:692–697. doi: 10.1080/01688639708403754. [DOI] [PubMed] [Google Scholar]
- Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- Massaro AR, Dutra AP, Almeida DR, Diniz RV, Malheiros SM. Transcranial Doppler assessment of cerebral blood flow: effect of cardiac transplantation. Neurology. 2006;66:124–126. doi: 10.1212/01.wnl.0000191397.57244.91. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Yamadate K, Kobayashi M, Endo H, Fukuda T, Yoshida K, Terasaki K, Inoue T, Ogawa A. Postoperative cerebral hyperperfusion associated with impaired cognitive function in patients undergoing carotid endarterectomy. J Neurosurg. 2005;102:38–44. doi: 10.3171/jns.2005.102.1.0038. [DOI] [PubMed] [Google Scholar]
- Kozera GM, Wolnik B, Kunicka KB, Szczyrba S, Wojczal J, Schminke U, Nyka WM, Bieniaszewski L. Cerebrovascular reactivity, intima-media thickness, and nephropathy presence in patients with type 1 diabetes. Diabetes Care. 2009;32:878–882. doi: 10.2337/dc08-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretnar-Oblak J, Sabovic M, Zaletel M. Associations between systemic and cerebral endothelial impairment determined by cerebrovascular reactivity to L-arginine. Endothelium. 2007;14:73–80. doi: 10.1080/10623320701346692. [DOI] [PubMed] [Google Scholar]
- Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, Trivilou P, Zerva L, Stamboulis E, Kremastinos DT. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens. 2009;22:525–530. doi: 10.1038/ajh.2009.35. [DOI] [PubMed] [Google Scholar]
- Muller M, Grobbee DE, Aleman A, Bots M, van der Schouw YT. Cardiovascular disease and cognitive performance in middle-aged and elderly men. Atherosclerosis. 2007;190:143–149. doi: 10.1016/j.atherosclerosis.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag. 2008;4:395–402. doi: 10.2147/vhrm.s2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- Brown WD, Frackowiak RS. Cerebral blood flow and metabolism studies in multi-infarct dementia. Alzheimer Dis Assoc Disord. 1991;5:131–143. doi: 10.1097/00002093-199100520-00010. [DOI] [PubMed] [Google Scholar]
- Gorelick PB. Status of risk factors for dementia associated with stroke. Stroke. 1997;28:459–463. doi: 10.1161/01.str.28.2.459. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Bakker SL, de Leeuw FE, de Groot JC, Hofman A, Koudstaal PJ, Breteler MM. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology. 1999;52:578–583. doi: 10.1212/wnl.52.3.578. [DOI] [PubMed] [Google Scholar]
- De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, Breteler MM. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- Marquine MJ, Attix DK, Goldstein LB, Samsa GP, Payne ME, Chelune GJ, Steffens DC. Differential Patterns of Cognitive Decline in Anterior and Posterior White Matter Hyperintensity Progression. Stroke. 2010 Jul 22; doi: 10.1161/STROKEAHA.110.587717. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JH, Lu CZ, Hong Z, Dong Q, Ding D, Wong KS. Relationship between cerebral vasomotor reactivity and white matter lesions in elderly subjects without large artery occlusive disease. J Neuroimaging. 2006;16:120–125. doi: 10.1111/j.1552-6569.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hirata K, Elkind MS, Jin Z, Rundek T, Miyake Y, Boden-Albala B, Di Tullio MR, Sacco R, Homma S. Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS) Am Heart J. 2008;156:405–410. doi: 10.1016/j.ahj.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubroca C, Lacombe P, Domenga V, Maciazek J, Levy B, Tournier-Lasserve E, Joutel A, Henrion D. Impaired vascular mechanotransduction in a transgenic mouse model of CADASIL arteriopathy. Stroke. 2005;36:113–117. doi: 10.1161/01.STR.0000149949.92854.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Judd BW, Tawaklna T, Rogers RL, Mortel KF. Improved cognition after control of risk factors for multi-infarct dementia. JAMA. 1986;256:2203–2209. [PubMed] [Google Scholar]
- Hajjar I, Hart M, Milberg W, Novak V, Lipsitz L. The rationale and design of the antihypertensives and vascular, endothelial, and cognitive function (AVEC) trial in elderly hypertensives with early cognitive impairment: role of the renin angiotensin system inhibition. BMC Geriatr. 2009;9:48. doi: 10.1186/1471-2318-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS, Rundek T, Sproule DM, Fitzsimmons BF, Schwartz S, Lazar RM. Monitoring of cerebral vasodilatory capacity with transcranial Doppler carbon dioxide inhalation in patients with severe carotid artery disease. Stroke. 2003;34:945–949. doi: 10.1161/01.STR.0000062351.66804.1C. [DOI] [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, Mikulis DJ. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke. 2008;39:2021–2028. doi: 10.1161/STROKEAHA.107.506709. [DOI] [PubMed] [Google Scholar]
- Yakushiji Y, Nishiyama M, Yakushiji S, Hirotsu T, Uchino A, Nakajima J, Eriguchi M, Nanri Y, Hara M, Horikawa E, Kuroda Y. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke. 2008;39:3323–3328. doi: 10.1161/STROKEAHA.108.516112. [DOI] [PubMed] [Google Scholar]