Abstract
Unregulated production of reactive oxygen species (ROS) is a marker of cellular and organismal aging linked to cognitive decline in humans and rodents. The sources of elevated ROS contributing to cognitive decline are unknown. Because NADPH oxidase (Nox) inhibition may prevent memory decline with age, we hypothesized that Nox and not mitochondrial sources of synaptic ROS production are linked to individual variance in cognitive performance in aged mice. Young (8 mo) and aged (26 mo) mice were tested in the novel object recognition task (NORT). Mitochondrial and Nox ROS production was assayed in isolated synaptosomes using spin-trapping electron paramagnetic resonance (EPR) spectroscopy. Aged mice exhibited variance in NORT performance, with some performing similar to young mice while others exhibited poorer short-term memory. EPR studies indicated that Nox rather than mitochondria was the major ROS source at the synapse, and Nox- but not mitochondrial-induced ROS levels correlated with NORT performance in aged mice. Our findings support the hypothesis that variance in Nox-specific synaptic ROS production may predict short-term memory deficits with age.
Keywords: aging, short-term memory, superoxide, NADPH oxidase, mitochondria, synaptosomes
2. Introduction
Substantial evidence suggests that brain aging is associated with a sustained increase in superoxide and a progressive decline in many cognitive functions (Clausen et al., 2008; Droge and Schipper, 2007). Mitochondria are one source of oxidant production in the brain during aging (Wallace, 2005), but several recent studies suggest that an extra-mitochondrial sources of superoxide may contribute to cognitive deficits in aging (Ali et al., 2006; Hu et al., 2006; Hu et al., 2007). Identifying these other sources of ROS will aid in development of novel treatments for reversing ROS-related neuropathology and cognitive decline associated with age. New data indicate that NADPH oxidase (Nox) enzymes are widely expressed in the CNS, potentially contributing to large range of physiologic functions and to a variety of CNS diseases (Sorce and Krause, 2009). Although Nox2 is found predominantly in professional phagocytes, it is now clear that Nox2 and homologs (Nox1, Nox 3–5, and Duox1, and 2) are expressed in a diverse array of tissues and cell types in a complex manner with Nox2 and Nox4 expressed in neurons in the adult mouse nervous system, reviewed in (Infanger et al., 2006). We recently reported that expression of Nox2 was significantly increased in neurons throughout the forebrain in aged mice (Dugan et al., 2009). Additionally, Nox2 appeared to be the major source of increased neuronal and synaptic superoxide production in old (24 mo) animals. Inhibition of Nox2 prevented age-associated reductions in spatial learning (Dugan et al., 2009), suggesting Nox2 hyper signaling contributes to some forms of cognitive decline with age. Here we tested the hypothesis that individual differences in ROS production from mitochondria and/or NADPH oxidase may predict individual differences in non-spatial short-term memory as measured by novel object recognition. Novelty detection has been shown clinically to predict successful cognitive performance in aged humans (Daffner et al., 2006). To test our hypothesis, we examined the relative contributions of abnormal ROS production from mitochondria and/or NADPH oxidase to aging-related impairments in short-term memory in the novel object recognition task (NORT).
3. Results
3.1 Novel object recognition
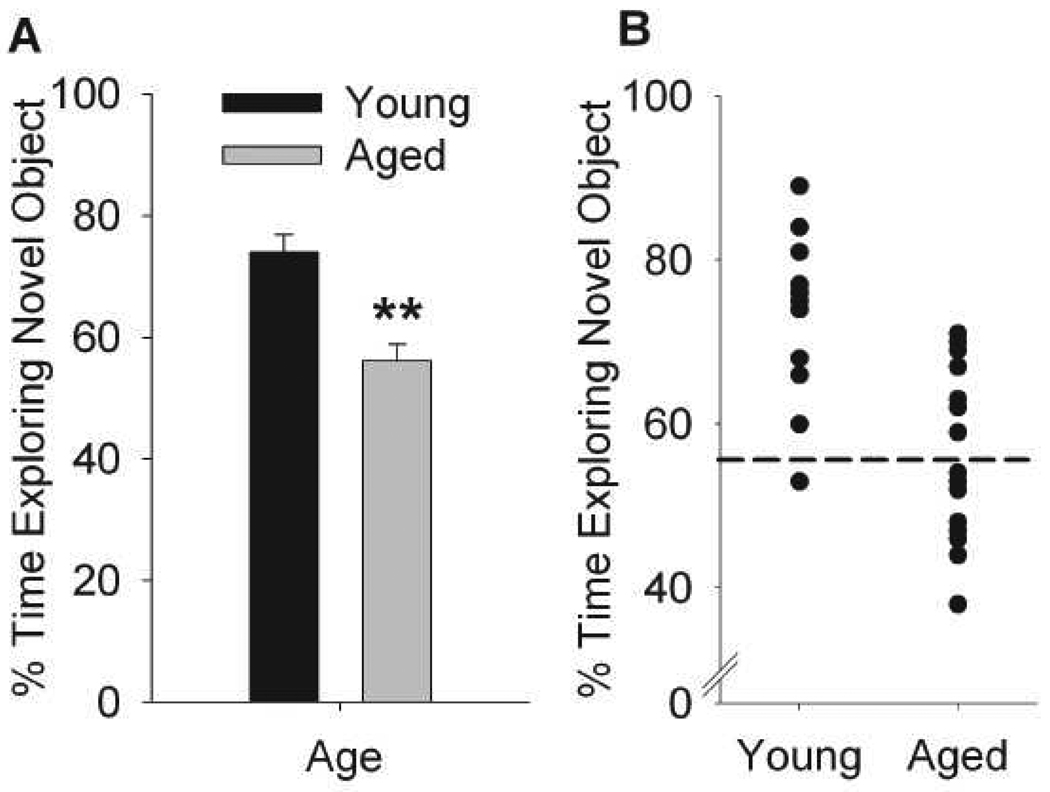
Aged mice exhibited no differences in distance traveled during habituation nor in total object exploration (Mean±SEM sec exploring: Sample Phase: Young: 31.8±4.6, Aged: 27±3.7; Choice Phase: Young 24.8±4.3, Aged: 31.4±3.3). Overall, mice spent more time exploring the novel object compared to the familiar object (F(1,26)=20.8, p<0.0001) although aged mice spent significantly less time exploring the novel object compared to young mice (AgeXObject: F(1,26)=6.49, p<0.05) with significant reductions in %novel object exploration compared to young mice (Figure 1A, F(1,26)=21.14, p<0.0001). As depicted in Figure 1B scatterplot, half of the aged animals fell 2 standard deviations below the young group mean (i.e. below 54%, age-impaired), while half remained above this cutoff and exhibited similar performance as young mice (age-unimpaired).
Figure 1.
Mice exhibit variance in age-related novel object recognition performance. One-trial novel object recognition was assessed across young (8 mo) and aged (26 mo) male C57BL6N mice. (A) Percentage time exploring the novel object compared to total object exploration during choice phase. Data are presented as mean ± SEM, **p<0.001, main effect of age. (B) Individual variance in novel object recognition across aged and young mice. N=13-15/group. Dashed line depicts the cutoff criterion (2 standard deviations below mean performance in young mice) for grouping aged mice into “impaired” and “unimpaired” categories. The mice assigned for EPR spin trapping analysis are depicted using open symbols.
3.2 Nox and mitochondria contributions to synaptosomal superoxide burst
To explore ROS dynamics at the synapse and their effects on age and cognitive performance, we utilized isolated nerve terminals (synaptosomes) which closely mimic a neuronal environment. Synaptosomal preparations contain both pre- and post-synaptic vesicles and are populated with metabolically active mitochondria. We have previously reported that synaptosomal preparations exhibit NADPH-dependent oxygen consumption and quantitative production of superoxide radicals (Behrens et al., 2008). This activity was partially inhibitable by the specific Nox2 inhibitor apocynin, with residual signal likely from Nox4. When malate and pyruvate were subsequently added to synaptosomes, oxygen consumption due to NAD+-linked mitochondrial metabolic activity was triggered (data not shown). This technique enables one to follow both Nox and mitochondrial activities in synaptosomes and quantify resultant superoxide production.
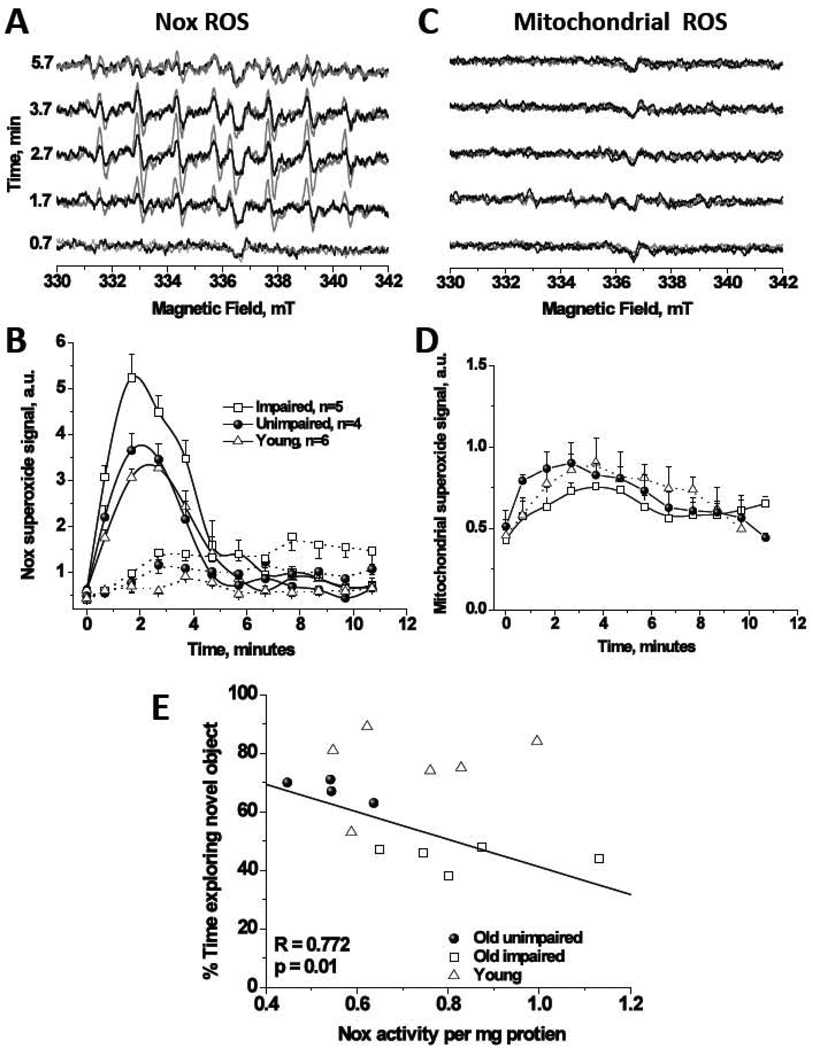
Figures 2A and B show NADPH-induced apocynin-inhibitable synaptosomal ROS bursts using EPR spin-trapping spectroscopy. The EPR signals in Figure 2A are attributable to DEPMPO-hydroxyl radical adduct which originates from superoxide radical anion as this signal was abolished in the presence of superoxide dismutase (200 U/ml, data not shown). Addition of NADPH to synaptosomes resulted in time dependent EPR signals and co-incubation with apocynin (300 µM final concentration) reduced the signal substantially in all groups, consistent with superoxide production from Nox2; Figure 2B. Note that apocynin didn’t completely abolish the NADPH-dependent activity as a residual activity exhibiting different kinetic profile persists. Since apocynin inhibits the translocation of the cytosolic component p47phox of Nox2 but not Nox4 (Barbieri et al., 2004), we suggest that the residual activity is due to Nox4. Mitochondria metabolism, however, was only associated with < 10% of the detected superoxide (compare Figure 2A,B with Figure 2C,D). No agents, e.g. phorbol esters, were used to trigger assembly of Nox enzyme, indicating that the enzyme was already in the active, superoxide-capable state, enabling the correlative studies to be performed.
Figure 2.
EPR spin trapping spectroscopy elucidates that Nox is a major source of superoxide in synaptosomes with minor contribution from synaptosomal mitochondria in young and old mice brains. (A) Representative EPR spectra recorded over ~ 6 minutes from mixing of 5 mM NADPH with 0.2–0.5 mg synaptosomal protein isolated from brain of age-impaired (gray lines) or age-unimpaired (black lines) mice at 37°C with 70 mM DEPMPO. In the incubations, 10 mM DETC was included to inhibit the SOD enzyme resulting in enhanced, and hence, quantifiable EPR signals. (B) Observed superoxide bursts upon adding NADPH in all groups (solid lines) and the effect of inclusion of the Nox2 inhibitor apocynin (300 µM final concentration, dashed lines), n=6, 5, and 4 for young, age-impaired, and age-unimpaired groups, respectively. See methods for details. Data are mean ± SEM. (C) Representative EPR spectra of superoxide production from metabolically active synaptosomal mitochondria in the same preparations under the same conditions except that Nox substrate is replaced by mitochondria ones. State 3 respiration was initiated by the addition of 10 mM malate + 10 mM pyruvate. Addition of ADP was not necessary to initiate state 3 oxygen consumption indicating that synaptosomes contain a pool of the ATPase substrate. The mitochondrial superoxide signals were quantified in panel (D). (E) Novel object recognition performance correlates with the yield Nox-produced superoxide at the synapses in old but not in young brains. Area under superoxide bursts’ EPR signals over the first 6 minutes from activity initiations were collected and normalized by the total synaptosomal protein contents for each group. Linear regression analysis is only applied on the old groups and the solid line represents the least-square best fit for the 9 data points obtained for the two old groups. Statistically significant linear negative correlation was obtained (R = −0.772, SD = 8.64, p = 0.01) with the general trend of higher superoxide yields in old brains from animals with lower recognition performance. Young animals however performed consistently better in the behavioral test independently of the superoxide levels in their synaptosomes.
A two-way ANOVA on the EPR signals, revealed an overall significant dependence of Nox activity on age (F(2,84)=12.64, p<0.001) and on the time from mixing with NADPH (F(6,84)=53.35, p<0.001). Multiple comparisons of all groups showed that the total normalized Nox activity in age-impaired animals was significantly greater than that in age-unimpaired (144.57±6.9%, p<0.001) and young mice (126.9±9.4%, p<0.01). No significant difference in Nox activity was observed, however, between young and age-unimpaired groups (p=0.271). Although mitochondria leaked much smaller amounts of superoxide, two-way ANOVA detected significant dependence of mitochondrial superoxide on age (F(2,84)=3.893, p<0.05). Both age-impaired and age-unimpaired mitochondria released more superoxide relative to young (Figure 2D, % of young±SEM: 122.0±5.2%, p<0.05, age-unimpaired; 114.5±5.0%, p=0.15, age-impaired). When combined data from aged groups were fit using linear regression, a significant negative correlation was obtained between %time spent exploring novel object and Nox activity normalized to protein contents (R=0.77, p=0.01), Fig. 2E. However, young animals performed consistently better in the cognitive test independently of synaptosomal Nox or mitochondria superoxide production. Note that the EPR data for young group, although included in Fig. 2E, are not included in the regression analysis which is only applied on combined aged groups. Similar analyses carried out with distance travelled (habituation phase) and total time spent exploring objects (sample phase) showed no significant relationship between these measures and ROS activity in the aged group (data not shown). Measures of prepulse inhibition, an operational measure of sensorimotor gating, also did not correlate with ROS activity (data not shown).
4. Discussion
Recent data in aged humans have indicated that novelty detection is associated with performance across multiple cognitive tests and may contribute to aging related cognitive decline (Daffner et al., 2001; Daffner et al., 2007). In this study, we examined the association between novel object recognition performance and ROS production at the synapses of young and old mice. Mitochondria slightly contributed to the levels of superoxide (<10%) in synaptosomes, but these measures were not dramatically affected by age. Cognitively, impaired aged animals exhibited significantly larger Nox superoxide signal than unimpaired aged or young animals. Indeed, a significant correlation was observed between NORT performance and Nox2 activity in aged but not young brains. Correlations were not observed between activity or sensorimotor gating performance and ROS production, suggesting that this association is specific to object recognition performance.
ROS are usually described as harmful byproducts of mitochondrial respiratory activity and are implicated in neuronal dysfunctions underlying neurodegenerative diseases and age-associated cognitive deficits (Gruber et al., 2008; Halliwell, 1992; Quick et al., 2008; Wallace, 2005). Recent evidence suggests however, that ROS are also required for long-term potentiation (LTP) and modulate learning and memory (Kamsler and Segal, 2003; Kishida and Klann, 2007). Intracellular Cu,Zn- superoxide dismutase and extracellular (EC)- superoxide dismutase overexpression improves LTP and spatial memory function in old animals, yet causes a decline in the same parameters in young animals (reviewed in (Hu et al., 2007; Kishida and Klann, 2007)). Conversely, cognition was unaffected by overexpression of mitochondrial Mn-SOD in young or aged mice. Taken together with the present data, mitochondrial-ROS does not appear to be linked to declines in cognitive performance with age. However ROS production from other sources like Nox2 may contribute to this form of cognition in an age dependent manner.
We have recently shown that treatment of mice from mid-age with an SOD-mimetic or for as little as 7 days with apocynin, Nox2 inhibitor, prevents decline in spatial memory in old mice (Dugan et al., 2009). Here we provide evidence that ROS levels, and in particular those originated from Nox2, may be correlated with individual variance in novel object recognition in aged but not young mice. The molecular mechanism(s) responsible for age-associated increases in Nox2-induced ROS remains to be explored. For example, it is important to explore region-specific superoxide changes in connection with multiple tests for cognitive performance in old animals. It has been shown however by this group, and others, that aging is associated with a state of an increased brain inflammation (Ali et al., 2006; Dugan et al., 2009). Nox2-induced ROS in prefrontal cortex, CA1 and CA3 regions of the hippocampus were particularly elevated in old brains as revealed by superoxide imaging in these regions. Although direct relation between ROS elevations in these regions and memory deficits is still lacking, general reduction in brain superoxide levels rescued memory decline in aged animals (Quick et al., 2008). Furthermore, the need to identify specific mechanisms by which some aged animals maintain normal Nox2 superoxide production while others show marked increases with age is a potentially important research avenue that could help identify interventions to enhance successful cognitive aging mechanisms. In an important recent study (Bruce-Keller et al., 2010), Nox2 expression in neurons and microglia of Alzheimer Disease (AD) patients was found to correlate with mild cognitive impairment (MCI). It was also found that NOX inhibition prevents neuronal injury by oligomeric amyloid beta peptides which suggests that increases in NOX-associated redox pathways in neurons is an important player in the early pathogenesis of AD. It is therefore conceivable that Nox2-induced amyloid plaques mediate faster cognitive declines in the poor NORT performers.
5. Experimental Procedure
5.1 Subjects
Male C57BL/6N mice from Charles River Laboratories (Wilmington, MA) aged 8 months (n=13) and 26 months (n=15) at time of NORT testing were used. Mice were housed 2/cage in a temperature-controlled room (21–22°C) under a reverse 12h/12h light cycle. All procedures were in accordance with the “Principles of Laboratory Animal Care” NIH guidelines and approved by the University of California San Diego animal care committee. Mice underwent a battery of tests in the following order: prepulse inhibition, attention set shift task, novel object exploration and locomotor exploration (in that order). The novel object recognition task is presented here.
5.2 Novel object recognition task
Procedures were modified from a previously described NORT protocol (Frick and Gresack, 2003). Single trial novel object recognition was performed in an open arena (60 X 60 cm) using 2 object types (Lego pyramid and 50 mL plastic conical tube) affixed to the floor using Velcro tape in opposing corners of the open field 10 cm away from the walls. Each mouse completed one session with three successive trials. In Trial 1, habituation phase, the mouse was placed in the center of the empty open field box and allowed to freely explore. During Trial 2, the sample phase, two of the same objects were placed in opposite corners of the box and the mouse was allowed to explore the objects. Half of the mice were randomly assigned to either starting with the Lego pyramid or the conical tube as the familiar object. In Trial 3, one of the objects was replaced with a new object to assess novel object exploration. Each trial was 5 min with a 3 min inter-trial interval (ITI; this time point was chosen to allow for the largest behavioral window to detect performance variance in aged mice). The mouse was removed from the testing box and placed in a holding cage during the ITI. The box and the objects were carefully cleaned with water between each trial and cleaned with 70% alcohol at the end of each testing session. Locomotor activity was assessed using Ethovision tracking software (Noldus Information Technology, Netherlands). Object exploration was defined as when the mouse’s nose was within 2 cm of the object.
5.3 Synaptosomal ROS measurements
Representative mice from each group, young, age-impaired and age-unimpaired were sacrificed 50–60 days after NORT testing (criterion used to group mice was based on Lee et al. (Lee et al., 2005)). Mice were sacrificed by cervical dislocation and brains were immediately removed and dissected. Frontal cortex was dissected away (for a separate study) and all remaining tissue was placed in an ice cold sucrose isolation buffer (120 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 1.2 mM MgCl2, 25 mM HEPES, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM glucose) until synaptosomes were isolated ~30 minutes later as described previously (Behrens et al., 2007). EPR experiments were carried out as previously described (Behrens et al., 2008); briefly, 0.2–0.5 mg synaptosomal protein was mixed with 70 mM DEPMPO (5-(diethylphosphoryl)-5-methyl-1-pyrroline-N-oxide, Axxora, San Diego, CA) and appropriate combinations of the substrates/inhibitors, the mixture was loaded into 50 µL-glass capillary and introduced into the EPR cavity of a MiniScope MS200 Benchtop spectrometer, which allowed the temperature to be maintained at 37±0.1 °C. We recorded time evolution of the EPR spectra over 11 minutes from triggering Nox or mitochondria activity by adding appropriate combination of substrates (Fig. 2A,C). For correlative analysis, we quantified the signals accumulated over the acquisition time of ~6 minutes; i.e. the area under oxidative burst curves and normalized by the protein concentration (Figure 2E). EPR conditions were as follows: microwave power, 5 mW; modulation amplitude, 2 G; modulation frequency, 100 kHz; MW frequency, 9.49 kHz; sweep width, 150 G centered at 3349.0 G; scan rate, 7.5 G s−1 and each spectrum was the average of 2 scans.
5.4 Data analyses
Behavior
Overall locomotor activity was assessed by a one-way ANOVA with a factor of age across total distance, velocity, and zone entries during the habituation trial. Object exploration during the choice phase was assessed in a 2 way ANOVA with age as a between subject factor and object (novel or familiar) as a within subject factor. To normalize for exploration time, a one way ANOVA with age as a between subject factor was completed on percentage of time (s) spent exploring the novel object compared to the amount of time exploring both objects (time novel object exploration/ (time novel object + familiar object exploration)*100). This score was used as performance criteria for grouping aged mice as “age-impaired” or “age-unimpaired”. Aged mice with %novel object exploration falling 2 standard deviations below the mean of the young group (i.e. 54% or below) were classified as “age impaired”, while those within 2 standard deviations of the young group mean were classified as “age-unimpaired”. Total amount of object exploration was also assessed using a two way ANOVA with age as the between subject factor and session (sample vs. choice) as within subject factors. During the habituation phase, aged mice exhibited no significant differences in locomotor activity as measured by distance traveled and average velocity. Note that aged mice exhibited no differences in distance traveled during habituation (data not shown) nor in total object exploration (Time (sec) exploring: Sample Phase: Young: 31.8±4.6, Aged: 27±3.7; Choice Phase: Young 24.8±4.3, Aged: 31.4±3.3).
EPR Data Analysis
EPR spectra were analyzed using PPBatch Show and kinetic analyses were carried out using KinShow software by Magnettech, Germany. Two EPR spectra were averaged and registered every 30 seconds over 11 minutes and the amplitudes of the recorded EPR signals were then employed as a measure of superoxide radical levels. Two-Way ANOVA was carried out on young versus combined old (age dependence) or for time-dependence of the EPR signal amplitudes in all groups. The total sample size per these comparisons was 105 (7 time points×(4 Old Unimpaired+ 5 Old Impaired+ 6 Young). Multiple comparisons between the three groups were carried out by Tukey Test and differences were considered statistically significant for p < 0.05. Area under oxidative burst curves were calculated by OriginPro Software and employed for correlating synaptosomal ROS in old animals with NORT outcomes.
Research highlights.
Aged mice exhibited performance variance in novel object recognition test (NORT).
Some old mice performed similar to young while others exhibited poorer short-term memory.
EPR spin trapping was used to identify synaptic ROS production by NADPH-oxidase and mitochondria.
Synaptic superoxide from NADPH-oxidase, but not from mitochondria, predicts poor performance.
Nox-induced ROS may predict variance in age-related cognitive decline across individuals.
Acknowledgements
The authors are indebted to Mrs. J. Lucero for synaptosomal isolations. This study was supported by Stein Institute on Research on Aging (VBR, JWY, SSA, DVJ), the Veterans Affairs Center of Excellence for Stress and Mental Health (VBR), and the National Institute of Mental Health R01 MH074697 (VBR, MAG), MH66248 (DVJ), and the National Institute on Aging R21AG030320 (LLD), K25AG026379 (SSA).
Abbreviations
- EPR
electron paramagnetic resonance
- Nox
NADPH oxidase
- NORT
novel object recognition task
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali SS, Xiong C, Lucero J, Behrens MM, Dugan LL, Quick KL. Gender differences in free radical homeostasis during aging: shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell. 2006;5:565–574. doi: 10.1111/j.1474-9726.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med. 2004;37:156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-Induced Loss of Phenotype of Fast-Spiking Interneurons Is Mediated by NADPH-Oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 Mediates the Increase in NADPH-Oxidase in the Ketamine Model of Schizophrenia. J. Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, LeVine H, 3rd, Keller JN, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Rentz DM, Scinto LF, Faust R, Budson AE, Holcomb PJ. Pathophysiology underlying diminished attention to novel events in patients with early AD. Neurology. 2004;56:1377–1383. doi: 10.1212/wnl.56.10.1377. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ. Increased responsiveness to novelty is associated with successful cognitive aging. J Cogn Neurosci. 2006;18:1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Chong H, Riis J, Rentz DM, Wolk DA, Budson AE, Holcomb PJ. Cognitive status impacts age-related changes in attention to novel and target events in normal adults. Neuropsychology. 2007;21:291–300. doi: 10.1037/0894-4105.21.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, Behrens MM. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS ONE. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing--where do we stand? Front Biosci. 2008;13:6554–6579. doi: 10.2741/3174. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Klann E, Thiels E. Superoxide dismutase and hippocampal function: age and isozyme matter. Antioxid Redox Signal. 2007;9:201–210. doi: 10.1089/ars.2007.9.201. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Paradoxical actions of hydrogen peroxide on long-term potentiation in transgenic superoxide dismutase-1 mice. J Neurosci. 2003;23:10359–10367. doi: 10.1523/JNEUROSCI.23-32-10359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging. 2008;29:117–128. doi: 10.1016/j.neurobiolaging.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]