Abstract
Circulating glucose levels are tightly regulated. To identify novel glycemic loci, we performed meta-analyses of 21 genome-wide associations studies informative for fasting glucose (FG), fasting insulin (FI) and indices of β-cell function (HOMA-B) and insulin resistance (HOMA-IR) in up to 46,186 non-diabetic participants. Follow-up of 25 loci in up to 76,558 additional subjects identified 16 loci associated with FG/HOMA-B and two associated with FI/HOMA-IR. These include nine new FG loci (in or near ADCY5, MADD, ADRA2A, CRY2, FADS1, GLIS3, SLC2A2, PROX1 and FAM148B) and one influencing FI/HOMA-IR (near IGF1). We also demonstrated association of ADCY5, PROX1, GCK, GCKR and DGKB/TMEM195 with type 2 diabetes (T2D). Within these loci, likely biological candidate genes influence signal transduction, cell proliferation, development, glucose-sensing and circadian regulation. Our results demonstrate that genetic studies of glycemic traits can identify T2D risk loci, as well as loci that elevate FG modestly, but do not cause overt diabetes.
Impaired β-cell function and insulin resistance are key determinants of type 2 diabetes (T2D). Hyperglycemia in the fasting state is one of the criteria that define the disease1, can predict hard clinical endpoints in non-diabetic individuals2,3 and, when corrected in patients with T2D, may help prevent microvascular4,5 and long-term macrovascular6,7 complications. To date, there are nearly 20 published loci reproducibly associated with T2D8, with most of them also associated with decreased insulin secretion9 due to defective β-cell function or mass. Association studies for diabetes-related quantitative traits in non-diabetic participants have also identified loci influencing fasting glucose (FG) levels, whose effects appear to be mediated by impairment of the glucose-sensing machinery in β-cells10–17.
We recently formed the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) to conduct large-scale meta-analyses of genome-wide data for continuous diabetes-related traits in non-diabetic participants15. We aimed to identify additional loci that influence glycemic traits in persons free of diabetes, and investigate their impact on related metabolic phenotypes. We were also interested in understanding variation in the physiological range and evaluating the extent to which the same variants influence pathological FG variation and T2D risk. The initial MAGIC collaboration identified the FG/T2D-associated locus MTNR1B15, which was also reported by others16,17; this finding demonstrated that studies of continuous glycemic phenotypes in non-diabetic individuals can complement the genetic analyses of diabetes as a dichotomous trait, and improve our understanding of the mechanisms involved in β-cell function and glucose homeostasis. Here, we extend our previous approach by performing meta-analyses of ~2.5M directly genotyped or imputed autosomal SNPs from 21 genome-wide association studies (GWAS). These 21 cohorts include up to 46,186 non-diabetic participants of European descent informative for FG, and 20 GWAS including up to 38,238 non-diabetic individuals informative for fasting insulin (FI), as well as the surrogate estimates of β-cell function (HOMA-B) and insulin resistance (HOMA-IR) derived from fasting variables by homeostasis model assessment18. Follow-up of 25 lead SNPs in up to 76,558 additional individuals of European ancestry identified nine novel genome-wide significant associations (empirically determined as P<5×10−8)19 with FG and one with FI/HOMA-IR. Five of these novel loci also demonstrated genome-wide significant evidence for association between the glucose-raising allele and T2D risk in up to 40,655 cases and 87,022 non-diabetic controls.
The wealth of novel FG and HOMA-B loci contrast with the sole FI/HOMA-IR novel finding and suggests a different genetic architecture for β-cell function and insulin resistance. Furthermore, our data support the hypothesis that not all loci that influence glycemia within the physiological range are also associated with pathological levels of glucose and T2D risk.
RESULTS
Genome wide association meta-analysis of glycemic traits (Stage 1)
We conducted a two-stage association study in individuals of European descent (Online Methods, Supplementary Figure 1, Supplementary Tables 1a and b). Because we sought to identify variants that influence FG in the normal population, hyperglycemia in the diabetic range exerts deleterious effects on β-cell function20,21, and treatment can confound glucose and insulin measurements, we excluded individuals with known diabetes, on anti-diabetic treatment, or with FG ≥7 mmol/L. We combined data from 21 Stage 1 discovery GWAS for FG (N=46,186) and 20 GWAS for FI (N=38,238), HOMA-B (N=36,466) and HOMA-IR (N=37,037), and analyzed associations for ~2.5M autosomal SNPs directly genotyped and imputed22,23 from HapMap CEU sample data assuming an additive genetic effect for each of the four traits.
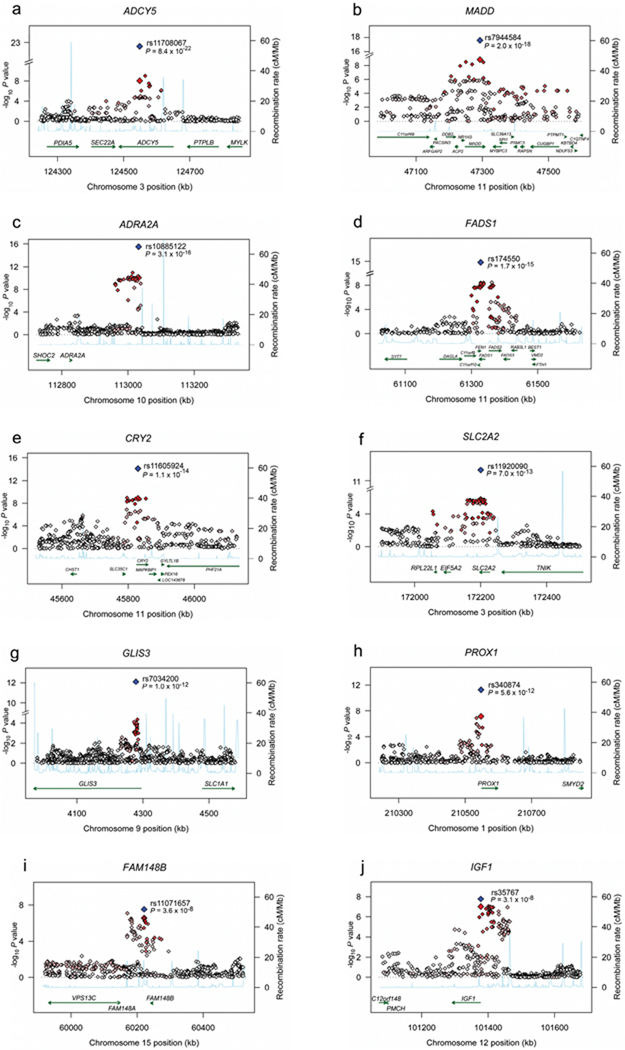
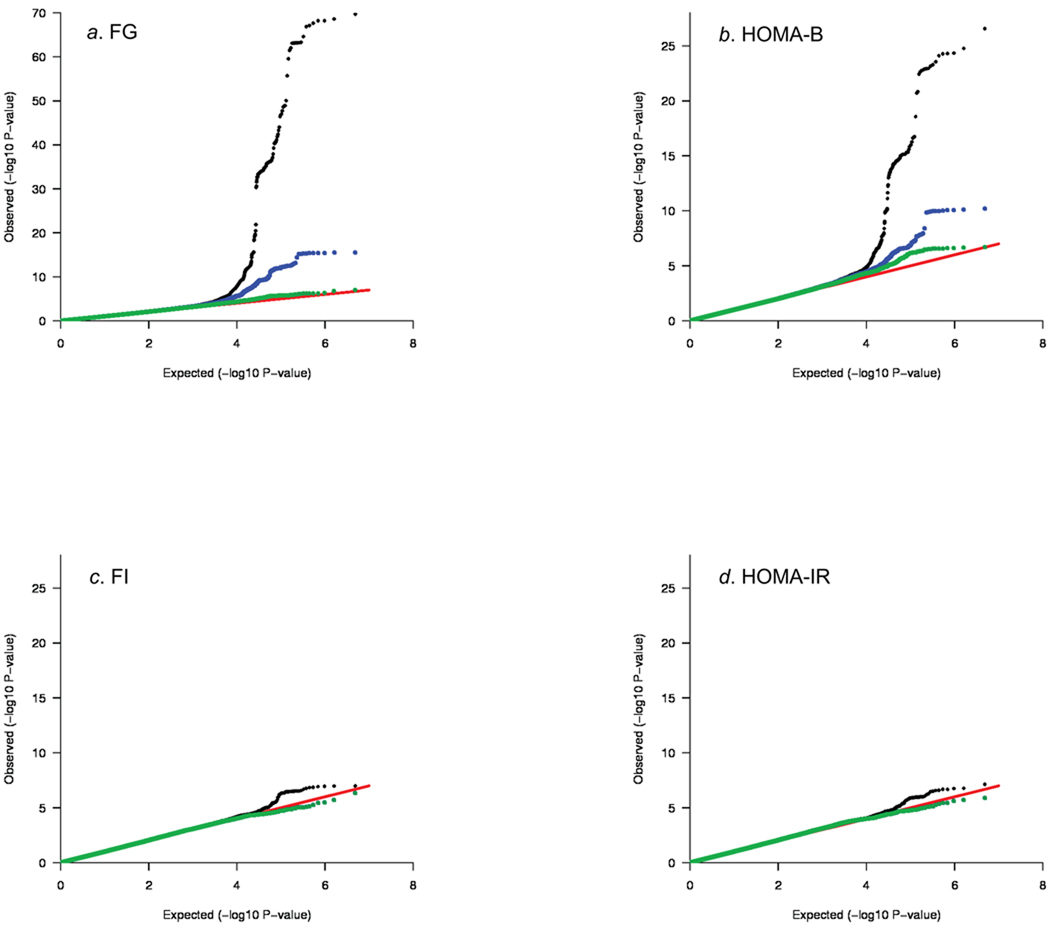
Inverse variance weighted meta-analyses revealed 12 independent loci associated with FG and/or HOMA-B at genome-wide significance levels (Table 1, Supplementary Table 2, Supplementary Figure 2a–b). These included five novel associations for loci in or near ADCY5, MADD, ADRA2A, CRY2 and FADS1 (Table 1, Figure 1a–j); four previously reported FG-associated loci in or near GCK, GCKR, G6PC2, and MTNR1B; the recently reported DGKB/TMEM19524; and two loci in T2D susceptibility genes TCF7L2 (rs4506565, r2=0.92 with the previously reported SNP rs7903146) and SLC30A8 (rs11558471, r2=0.96 with the previously reported SNP rs13266634). Seven additional loci had reproducible evidence for association with FG and/or HOMA-B across studies at the arbitrary summary threshold of P<2×10−5 chosen to prioritize SNPs for follow-up (Table 1, Supplementary Table 2). After excluding SNPs within the four previously genome-wide significant FG loci GCK, GCKR, G6PC2 and MTNR1B, we still observed an excess of small P-values compared to a distribution expected under the null hypothesis (Figure 2a–b), suggesting that some of these additional loci are likely to represent novel FG and/or HOMA-B loci that merit additional investigation.
Table 1.
SNPs associated with fasting glucose-related or insulin-related traits at genome-wide significance levels
| Glucose/HOMA-B selected SNPs | Fasting glucose | HOMA-B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Nearest gene(s) | Alleles (effect/ other) |
Freq | Discovery P value |
I2 estimate (P value) |
Global P value |
Joint analysis N |
Discovery P value |
I2 estimate (P value) |
Global P value |
Joint analysis N |
| rs560887 | G6PC2 | C/T | 0.70 | 4.4×10−75 | 0.31 (0.18) | 8.7×10−218 | 119,169 | 2.0×10−28 | 0.54 (0.01) | 1.5×10−66 | 94,839 |
| rs10830963 | MTNR1B | G/C | 0.30 | 1.2×10−68 | 0.00 (1.00) | 5.8×10−175 | 112,844 | 1.8×10−22 | 0.45 (0.03) | 2.7×10−43 | 90,364 |
| rs4607517 | GCK | A/G | 0.16 | 4.5×10−36 | 0.19 (0.46) | 6.5×10−92 | 118,500 | 7.5×10−8 | 0.36 (0.12) | 1.8×10−16 | 94,112 |
| rs2191349 | DGKB/TMEM195 | T/G | 0.52 | 7.8×10−17 | 0.10 (0.68) | 3.0×10−44 | 122,743 | 5.4×10−11 | 0.09 (0.71) | 2.8×10−17 | 98,372 |
| rs780094 | GCKR | C/T | 0.62 | 2.5×10−12 | 0.00 (1.00) | 5.6×10−38 | 118,032 | 0.25 | 0.32 (0.18) | 3.2×10−4 | 93,990 |
| rs11708067 | ADCY5 | A/G | 0.78 | 8.7×10−9 | 0.04 (0.89) | 7.1×10−22 | 118,475 | 2.2×10−4 | 0.37 (0.10) | 2.5×10−12 | 94,212 |
| rs7944584 | MADD | A/T | 0.75 | 1.5×10−9 | 0.00 (1.00) | 2.0×10−18 | 118,741 | 1.1×10−4 | 0.16 (0.51) | 3.5×10−5 | 94,408 |
| rs10885122 | ADRA2A | G/T | 0.87 | 8.4×10−11 | 0.00 (1.00) | 2.9×10−16 | 118,410 | 3.7×10−6 | 0.11 (0.66) | 2.0×10−6 | 94,128 |
| rs174550 | FADS1 | T/C | 0.64 | 1.5×10−8 | 0.00 (1.00) | 1.7×10−15 | 118,908 | 4.5×10−5 | 0.01 (0.99) | 5.2×10−13 | 94,536 |
| rs11605924 | CRY2 | A/C | 0.49 | 1.5×10−9 | 0.00 (1.00) | 1.0×10−14 | 116,479 | 5.2×10−6 | 0.03 (0.94) | 3.2×10−5 | 92,326 |
| rs11920090 | SLC2A2 | T/A | 0.87 | 1.9×10−6 | 0.00 (1.00) | 8.1×10−13 | 119,024 | 1.4×10−4 | 0.36 (0.11) | 4.5×10−6 | 94,629 |
| rs7034200 | GLIS3 | A/C | 0.49 | 1.2×10−4 | 0.00 (1.00) | 1.0×10−12 | 106,250 | 1.9×10−6 | 0.19 (0.46) | 1.2×10−13 | 83,759 |
| rs340874 | PROX1 | C/T | 0.52 | 7.1×10−8 | 0.00 (1.00) | 6.6×10−12 | 116,882 | 3.7×10−5 | 0.00 (1.00) | 5.3×10−6 | 92,942 |
| rs11071657 | FAM148B | A/G | 0.63 | 2.8×10−7 | 0.00 (1.00) | 3.6×10−8 | 114,454 | 0.23 | 0.08 (0.73) | 0.002 | 90,675 |
| rs11558471 | SLC30A8 | A/G | 0.68 | 2.6×10−11 | - | - | 45,996 | 1.4×10−6 | - | - | 36,283 |
| rs4506565 | TCF7L2 | T/A | 0.31 | 1.2×10−8 | - | - | 46,181 | 1.4×10−6 | - | - | 36,461 |
| Insulin/HOMA-IR selected SNPs | Fasting insulin | HOMA-IR | |||||||||
| rs780094 | GCKR | C/T | 0.62 | 1.1×10−4 | 0.14 (0.57) | 3.6×10−20 | 96,126 | 9.9×10−7 | 0.25 (0.32) | 3.0×10−24 | 94,636 |
| rs35767 | IGF1 | G/A | 0.85 | 1.0×10−7 | 0.17 (0.50) | 3.3×10−8 | 94,590 | 7.8×10−8 | 0.26 (0.28) | 2.2×10−9 | 93,141 |
Directly genotyped and imputed SNPs were tested for association with fasting glucose, fasting insulin and homeostasis model assessment of β-cell function (HOMA-B) and insulin resistance (HOMA-IR). Twenty one discovery cohorts with genome-wide data were meta-analyzed (Stage 1 discovery) and 25 SNPs were promoted for replication of the same trait in a set of 33 additional cohorts with in silico (N=7) or de novo (N=26) genotype data (N=31 for fasting insulin, HOMA-B and HOMA-IR; for Stage 2 replication P-values and effect sizes see Table 2). A joint analysis was then performed (global). Heterogeneity in the discovery sample was assessed using the I2 index48. Replication was not attempted for SNPs in two known type 2 diabetes genes (SLC30A8 and TCF7L2) which achieved genome-wide significance for FG in Stage 1. Freq denotes the allele frequency of the glucose-raising allele. N=sample size. Note that the previously reported GCKR SNP has associations with glucose-related and insulin-related traits.
Figure 1.
Regional plots of ten novel genome-wide significant associations. For each of the ADCY5 (a), MADD (b), ADRA2A (c), FADS1 (d), CRY2 (e), SLC2A2 (f), GLIS3 (g), PROX1 (h), FAM148B (i) and IGF1 (j) regions, directly genotyped and imputed SNPs are plotted with their meta-analysis P values (as –log10 values) as a function of genomic position (NCBI Build 35). In each panel, the Stage 1 discovery SNP taken forward to Stage 2 replication is represented by a blue diamond (with global meta-analysis P value), with its Stage 1 discovery P value denoted by a red diamond. Estimated recombination rates (taken from HapMap) are plotted to reflect the local LD structure around the associated SNPs and their correlated proxies (according to a white to red scale from r2 = 0 to 1, based on pairwise r2 values from HapMap CEU). Gene annotations were taken from the University of California Santa Cruz genome browser.
Figure 2.
Quantile-quantile (Q-Q) plots for fasting glucose (FG) (a), β-cell function by homeostasis model assessment (HOMA-B) (b), fasting insulin (FI) (c), and insulin resistance by homeostasis model assessment (HOMA-IR) (d). In each plot, the expected null distribution is plotted along the red diagonal, the entire distribution of observed P values is plotted in black, and a distribution that excludes the ten novel findings in Figure 1 is plotted in green. For FG and HOMA-B, the distribution that excludes the four genome-wide significant FG-associated loci (GCK, GCKR, G6PC2 and MTNR1B) is plotted in blue. A comparison of the observed P values for each trait shows that FG/HOMA-B associations are much more likely to be detected than FI/HOMA-IR associations.
Stage 1 analyses of FI and HOMA-IR revealed no loci that reached genome-wide significance, but there were six loci with consistent evidence for association across study samples at P<2×10−5 (Table 1, Supplementary Table 2, Supplementary Figure 2c–d). Comparison of the observed P-values with the distribution expected under the null hypothesis demonstrated an excess of small P-values which warrant further investigation (Figure 2c–d).
Replication studies (Stage 2) and global (Stage 1 + Stage 2) meta-analysis for 25 loci
We carried forward to Stage 2 all independent loci with association with any of the four traits at P<2×10−5, except for SNPs in the known T2D genes TCF7L2 and SLC30A8 for which no further validation was sought (Table 1, Supplementary Table 2). We also included the nominally associated top SNP from a strong biological candidate (IRS1, P=10−4 for HOMA-IR) and a locus with P values that approached genome-wide significance in several Stage 1 discovery cohorts (PLXDC2/NEBL), even though their overall Stage 1 P-values were >2×10−5 (Table 1, Supplementary Table 2). In total, 25 loci were chosen for replication.
We directly genotyped 25 variants in 26 additional Stage 2 studies with up to 63,850 non-diabetic participants of European ancestry for FG, and 25 studies and up to 52,892 participants for FI, HOMA-IR and HOMA-B (Supplementary Table 1b, Online Methods). We also obtained in silico replication data for 12,708 additional individuals from seven studies for FG (9,372 participants and five studies for FI, HOMA-IR and HOMA-B), for a total of up to 76,558 individuals for FG and 62,264 for FI, HOMA-IR and HOMA-B in Stage 2 association analyses.
Our combined Stage 1 and 2 meta-analysis, including a total of up to 122,743 participants for FG (98,372 for FI, HOMA-IR and HOMA-B) established genome-wide significant associations for nine novel loci for FG and/or HOMA-B (ADCY5, MADD, CRY2, ADRA2A, FADS1, PROX1, SLC2A2, GLIS3, FAM148B) and one for FI and HOMA-IR (IGF1) (Table 1 and Figure 1a–j). We hereby replicate the recently reported loci DGKB/TMEM195 (FG)24 and GCKR (FG, FI and HOMA-IR)11,12,25 at levels that exceed genome-wide significance. Loci that had previously achieved genome-wide significant associations with FG (G6PC2, MTNR1B and GCK) were also confirmed (Table 1).
We further conducted a global meta-analysis of cohort results adjusted for body mass index (BMI), to test whether these diabetes-related quantitative trait associations may be mediated by associations with adiposity. The adjustment for BMI did not materially affect the strength of the associations with any of the traits (data not shown).
Effect size estimates for genome-wide significant loci
We restricted our effect size estimates (Table 2, Supplementary Table 2) to the Stage 2 replication samples (up to N=76,558) to avoid inflation introduced by the discovery cohorts (“winner’s curse”26). The previously identified loci G6PC2, MTNR1B and GCK showed the largest effects on FG (0.075, 0.067 and 0.062 mmol/L per allele, respectively), with the remaining loci showing smaller effects (0.008 to 0.030 mmol/L per allele, Table 2). The proportion of variance in FG explained by the 14 FG loci with replication data (i.e. all FG loci except for TCF7L2 and SLC30A8) ranged from 3.2–4.4% in six replication studies providing this information. Because results from our largest unselected community-based cohort (Framingham) were on the lower bound of these estimates (3.2%), we felt reassured that the winner’s curse is not a major concern in this instance, and selected it to estimate the proportion of heritability explained and the genotype score. With a heritability estimate of 30.4% in Framingham, these 14 loci explain a substantial proportion (~10%) of the inherited variation in FG. If these same loci harbor additional independent variants (e.g. those due to low frequency alleles not captured by this analysis) that also influence FG27, this estimate of the heritability attributable to these loci is likely to be conservative.
Table 2.
Association of novel SNPs with glycemic traits in MAGIC and type 2 diabetes replication meta-analyses
| SNP | Nearest gene(s) | Alleles (effect/ other) |
Fasting glucose (mmol/L) |
HOMA-B | Fasting insulin (pmol/L) |
HOMA-IR | HbA1c (%) | 2-hr glucose (mmol/L) |
2-hr insulin (pmol/L) |
Type 2 diabetes^ |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs560887 | G6PC2 | C/T | Effect* | 0.075 (0.003) | −0.042 (0.004) | −0.007 (0.004) | 0.006 (0.004) | 0.032 (0.004) | 0.017 (0.020) | −0.031 (0.013) | 0.97 (0.95–0.99) |
| P-value | 8.5 × 10−122 | 7.6 × 10−29 | 0.11 | 0.16 | 1.0 × 10−17 | 0.41 | 0.01 | 0.012 | |||
| rs10830963 | MTNR1B | G/C | Effect* | 0.067 (0.003) | −0.034 (0.004) | −0.006 (0.004) | 0.004 (0.004) | 0.024 (0.004) | 0.056 (0.022) | 0.034 (0.015) | 1.09 (1.06–1.12) |
| P-value | 1.1 × 10−102 | 1.1 × 10−22 | 0.14 | 0.37 | 3.0 × 10−9 | 0.01 | 0.02 | 8.0 × 10−13 | |||
| rs4607517 | GCK | A/G | Effect* | 0.062 (0.004) | −0.025 (0.005) | 0.004 (0.006) | 0.015 (0.006) | 0.041 (0.005) | 0.097 (0.026) | −0.012 (0.015) | 1.07 (1.05–1.10) |
| P-value | 1.2 × 10−44 | 1.2 × 10−6 | 0.46 | 0.01 | 6.3 × 10−19 | 2.0 × 10−4 | 0.42 | 5.0 × 10−8 | |||
| rs2191349 | DGKB/TMEM195 | T/G | Effect* | 0.030 (0.003) | −0.017 (0.003) | −0.002 (0.003) | 0.002 (0.004) | 0.008 (0.003) | 0.000 (0.019) | −0.006 (0.012) | 1.06 (1.04–1.08) |
| P-value | 5.3 × 10−29 | 6.4 × 10−8 | 0.48 | 0.61 | 0.01 | 0.98 | 0.60 | 1.1 × 10−8 | |||
| rs780094 | GCKR | C/T | Effect* | 0.029 (0.003) | 0.014 (0.003) | 0.032 (0.004) | 0.035 (0.004) | 0.004 (0.004) | −0.091 (0.019) | 0.000 (0.011) | 1.06 (1.04–1.08) |
| P-value | 1.7 × 10−24 | 1.4 × 10−5 | 3.6 × 10−19 | 5.0 × 10−20 | 0.32 | 1.4 × 10−6 | 1.00 | 1.3 × 10−9 | |||
| rs11708067 | ADCY5 | A/G | Effect* | 0.027 (0.003) | −0.023 (0.004) | −0.011 (0.004) | 0.006 (0.005) | 0.015 (0.004) | 0.094 (0.023) | 0.008 (0.015) | 1.12 (1.09–1.15) |
| P-value | 1.7 × 10−14 | 3.6 × 10−8 | 0.01 | 0.16 | 5.1 × 10−4 | 6.6 × 10−5 | 0.60 | 9.9 × 10−21 | |||
| rs7944584 | MADD | A/T | Effect* | 0.021 (0.003) | −0.007 (0.004) | 0.002 (0.004) | 0.005 (0.004) | 0.001 (0.004) | −0.017 (0.022) | −0.019 (0.013) | 1.01 (0.99–1.03) |
| P-value | 5.1 × 10−11 | 0.07 | 0.60 | 0.26 | 0.84 | 0.44 | 0.15 | 0.30 | |||
| rs10885122 | ADRA2A | G/T | Effect* | 0.022 (0.004) | −0.010 (0.005) | 0.001 (0.005) | 0.004 (0.005) | 0.007 (0.005) | 0.004 (0.030) | −0.051 (0.019) | 1.04 (1.01–1.07) |
| P-value | 9.7 × 10−8 | 0.03 | 0.90 | 0.47 | 0.21 | 0.89 | 0.007 | 0.020 | |||
| rs174550 | FADS1 | T/C | Effect* | 0.017 (0.003) | −0.020 (0.003) | −0.011 (0.004) | 0.008 (0.004) | 0.007 (0.004) | 0.013 (0.019) | −0.003 (0.012) | 1.04 (1.02–1.06) |
| P-value | 8.3 × 10−9 | 5.3 × 10−10 | 2.7 × 10−3 | 0.03 | 0.053 | 0.49 | 0.82 | 2.3 × 10−4 | |||
| rs11605924 | CRY2 | A/C | Effect* | 0.015 (0.003) | −0.005 (0.003) | 0.001 (0.004) | 0.003 (0.004) | 0.001 (0.003) | 0.023 (0.018) | 0.006 (0.011) | 1.04 (1.02–1.06) |
| P-value | 8.1 × 10−8 | 0.13 | 0.73 | 0.34 | 0.72 | 0.20 | 0.62 | 1.7 × 10−4 | |||
| rs11920090 | SLC2A2 | T/A | Effect* | 0.020 (0.004) | −0.012 (0.005) | 0.002 (0.005) | 0.005 (0.005) | 0.017 (0.005) | 0.015 (0.027) | −0.022 (0.016) | 1.01 (0.99–1.04) |
| P-value | 3.3 × 10−6 | 0.02 | 0.77 | 0.37 | 5.8 × 10−4 | 0.58 | 0.19 | 0.34 | |||
| rs7034200 | GLIS3 | A/C | Effect* | 0.018 (0.003) | −0.020 (0.004) | −0.014 (0.004) | 0.011 (0.004) | 0.003 (0.003) | 0.037 (0.018) | 0.010 (0.011) | 1.03 (1.01–1.05) |
| P-value | 1.2 × 10−9 | 8.9 × 10−9 | 2.7 × 10−4 | 4.6 × 10−3 | 0.32 | 0.04 | 0.36 | 1.3 × 10−3 | |||
| rs340874 | PROX1 | C/T | Effect* | 0.013 (0.003) | −0.008 (0.003) | −0.002 (0.004) | 0.001 (0.004) | 0.009 (0.004) | 0.030 (0.020) | −0.007 (0.012) | 1.07 (1.05–1.09) |
| P-value | 6.6 × 10−6 | 0.02 | 0.68 | 0.74 | 9.5 × 10−3 | 0.13 | 0.56 | 7.2 × 10−10 | |||
| rs11071657 | FAM148B | A/G | Effect* | 0.008 (0.003) | −0.013 (0.004) | −0.009 (0.004) | 0.008 (0.004) | 0.001 (0.004) | −0.065 (0.020) | −0.006 (0.013) | 1.03 (1.01–1.05) |
| P-value | 0.01 | 8.1 × 10−4 | 0.03 | 0.07 | 0.79 | 0.001 | 0.65 | 2.9 × 10−3 | |||
| rs13266634 | SLC30A8 | C/T | Effect* | 0.027 (0.004) | −0.016 (0.004) | −0.004 (0.005) | 0.0002 (0.005) | 0.016 (0.004) | 0.093 (0.022) | −0.011 (0.015) | 1.15 (1.10–1.21)# |
| P-value | 5.5 × 10−10 | 2.4 × 10−5 | 0.44 | 0.97 | 3.3 × 10−5 | 2.0 × 10−5 | 0.47 | 1.5 × 10−8 | |||
| rs7903146 | TCF7L2 | T/C | Effect* | 0.023 (0.004) | −0.02 (0.004) | −0.012 (0.004) | 0.010 (0.005) | 0.013 (0.003) | 0.118 (0.021) | 0.010 (0.013) | 1.40 (1.34–1.46)# |
| P-value | 2.8 × 10−8 | 1.4 × 10−7 | 0.004 | 0.03 | 1.8 × 10−4 | 2.6 × 10−8 | 0.42 | 2.2 × 10−51 | |||
| rs35767 | IGF1 | G/A | Effect* | 0.012 (0.005) | 0.009 (0.005) | 0.010 (0.006) | 0.013 (0.006) | 0.010 (0.005) | 0.027 (0.025) | 0.015 (0.016) | 1.04 (1.01–1.07) |
| P-value | 0.01 | 0.09 | 0.10 | 0.04 | 0.050 | 0.28 | 0.33 | 6.6 × 10−3 | |||
| Sample size for each trait | 45,049–76,558 | 35,435–61,907 | 37,199–62,264 | 35,901–62,001 | 33,718–44,856 | 15,221–15,234 | 7,051–7,062 | 40,655 cases/87,022 controls | |||
Per-allele effect (SE) for quantitative traits was estimated from Stage 2 replication samples for fasting glucose, homeostasis model assessment of β-cell function (HOMA-B), fasting insulin, and homeostasis model assessment of insulin resistance (HOMA-IR), and from discovery meta-analyses of MAGIC GWAS for glycated hemoglobin (HbA1c), 2-hour glucose after an oral glucose tolerance test (BMI-adjusted) and 2-hour insulin (BMI-adjusted). For the first four traits, the regression coefficients are obtained from the replication cohorts so as to avoid an overestimate of the effect size caused by the “winner’s curse”. Results from replication samples were unavailable for rs7903146 and rs13266634, thus discovery meta-analysis results are shown for both SNPs for fasting glucose (N=45,049–45,051), HOMA-B (N=35,435–35,437), fasting insulin (N=37,199–37,201) and HOMA-IR (N=35,901–35,903).
Replication genotyping was undertaken in 27 independent type 2 diabetes (T2D) case/control samples for all except the TCF7L2 and SLC30A8 signals.
Association with T2D for SNPs in TCF7L2 and SLC30A8 loci was estimated from the DIAGRAM+ meta-analysis for a total of 8,130 cases/38,987 controls. For these loci we have included data on the most commonly associated SNPs with T2D in previously published data.
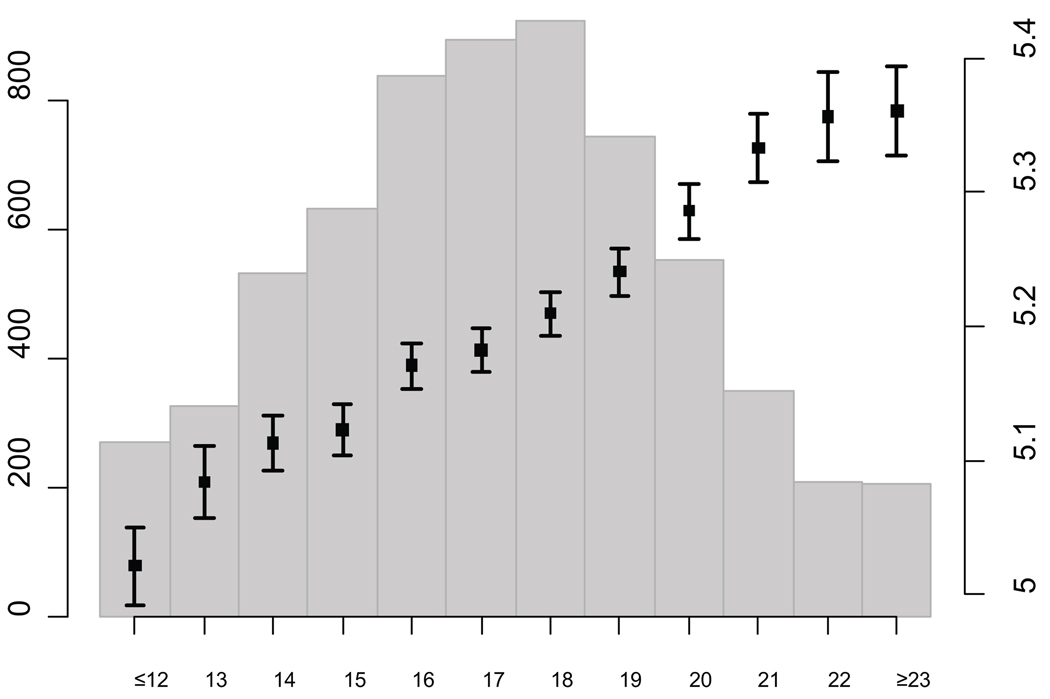
We estimated the combined impact of the 16 loci associated with FG (the 14 loci included in the genotype score plus TCF7L2 and SLC30A8) in some of the largest cohorts (Framingham, NFBC 1966 and ARIC) by constructing a genotype score equal to the sum of the expected number of risk alleles at each SNP weighted by their effect sizes (see Online Methods). FG levels were higher in individuals with higher genotype scores (Figure 3), with mean differences of ~0.4 mmol/L (5.93 vs 5.51 mmol/L in NFBC 1966; 5.36 vs 5.03 mmol/L in Framingham; 5.70 vs 5.29 mmol/L in ARIC) comparing individuals with a score of 23 or higher (5.6% of the sample) to those with a genotype score of 12 or lower (2.9% of the sample). The 0.4 mmol/L (7.2 mg/dl) difference between the two tails of the distribution of risk score in the population (top 5.6% vs bottom 2.9%) is of clinical relevance, as it represents a shift of approximately 25 centile points in the distribution of FG. Prospective evidence has shown that a difference of this magnitude in FG is associated with a relative risk of 1.54–1.73 for future T2D, accounting for other risk factors28. The impact of individual SNPs on FG in the combined discovery and replication samples is shown in Supplementary Figure 3a–p.
Figure 3.
Variation in levels of fasting glucose depending on the number of risk alleles at novel loci, weighted by effect size in an aggregate genotype score for the Framingham Heart Study. The bar plots show the average and standard error of fasting glucose in mmol/L for each value of the genotype score based on the regression coefficient (right Y axis), and the histogram denotes the number of individuals in each genotype score category (left Y axis). Comparable results were obtained for the NFBC 1966 and ARIC cohorts. On average, the range spans ~0.4 mmol/L (~7.2 mg/dl) from low to high genotype score.
We also analyzed data from 1,602 white European children aged 5.9–17.2 from two studies. Though directionally consistent with observations in adults, some effect size estimates were of smaller magnitude (data not shown). As in adults, the largest effect sizes were observed for risk alleles in GCK (beta=0.085; P=1.2×10−5; N=1,602), G6PC2 (beta=0.062; P=1.9×10−4; N=1,582) and MTNR1B (beta=0.033; P=0.058; N=1,309).
Impact of reproducibly associated loci on additional glycemic traits
We sought to investigate all 17 loci associated with FG/HOMA-B or FI/HOMA-IR at genome-wide significance for their effects on other continuous glycemic traits. While most of the 16 loci associated with FG are also strongly associated with HOMA-B (Tables 1 and 2), the associations between FG loci and FI were at best weak; GCKR is the only locus reaching genome-wide significant associations for both FG/HOMA-B and FI/HOMA-IR, with the glucose-raising C allele being associated with increased FI (global P=3.6×10−20) and HOMA-IR (global P=3.0×10−24). These patterns are consistent with the gross trait correlations obtained in Framingham for FG and HOMA-B (r=−0.43) and for FG and FI (r=0.25).
Impairment of glucose homeostasis may be characterized by elevated FG or FI, elevated 2-hour glucose or 2-hour insulin post-oral glucose tolerance test (OGTT), or elevated glycated hemoglobin (HbA1c). We tested associations of each of the 17 loci in a subset of MAGIC cohorts with GWAS data informative for these traits. Since HbA1c is a measure of average glycemia over the preceding 2–3 months, we hypothesized that if an association with additional traits was present it should be directionally consistent. The three loci with the largest effect sizes on FG (G6PC2, MTNR1B and GCK) all showed genome-wide significant and directionally consistent associations with HbA1c, with DGKB/TMEM195, ADCY5, SLC2A2, PROX1, SLC30A8 and TCF7L2 showing nominal (P<0.05) evidence of directionally consistent association (Table 2). The FG-raising alleles at TCF7L2, SLC30A8, GCK and ADCY5 were associated (P<0.0002) with increased 2-hour glucose (Table 2); a parallel MAGIC project reports the genome-wide significant association with 2-hour glucose of another ADCY5 SNP in strong linkage disequilibrium (LD) with our lead SNP (r2=0.82)29. Consistent with previous reports that the FG-raising allele is associated with greater insulin release during OGTT11,12,30, the FG-raising allele in GCKR was associated with lower 2-hour glucose.
Testing of these loci for association with T2D as a dichotomous trait in up to 40,655 cases and 87,022 non-diabetic controls demonstrated that the FG-raising alleles at seven loci (ADCY5, PROX1, GCK, GCKR and DGKB/TMEM195 and the known T2D genes TCF7L2 and SLC30A8), are robustly associated (P<5×10−8) with increased risk of T2D (Table 2); the association of a highly correlated SNP in ADCY5 with T2D in partially overlapping samples is reported by our companion manuscript29. We found less significant T2D associations (P<5×10−3) for variants in or near CRY2, FADS1, GLIS3 and FAM148B (Table 2). These data clearly show that loci with very similar FG effect sizes may have very different T2D risk effects (see for example ADCY5 and MADD in Table 2).
Given that several alleles associated with higher FG levels were also associated with increased T2D risk and that the T2D genes TCF7L2 and SLC30A8 showed association with FG, we systematically investigated association of all established T2D loci with the same four fasting diabetes-related quantitative traits. We found directionally consistent nominal associations (P<0.05) of T2D risk alleles with higher FG for 11 of 18 established T2D loci, including MTNR1B (Supplementary Table 3). These data demonstrate that a large T2D effect size does not always translate to an equivalently large FG effect in non-diabetic persons, as clearly highlighted when contrasting the remarkably small effects of TCF7L2 on FG compared to MTNR1B (Table 2).
Impact of reproducibly associated loci on additional metabolic traits
Next, we used available GWAS results for additional metabolic phenotypes (BMI from GIANT31, blood pressure from Global BPGen32, and lipids from ENGAGE33), to assess the impact of the newly discovered glycemic loci on these traits. None of the novel loci had significant (P<0.01) associations with BMI or blood pressure (Table 3). Interestingly, the FADS1 glucose-raising allele was associated with increased total cholesterol (P=2.5×10−6), low-density lipoprotein cholesterol (P=8.5×10−6) and high-density lipoprotein cholesterol (P=2.9×10−5), but lower triglyceride levels (P=1.9×10−6) (Table 3); a consistent association of this locus with lipid levels has been previously reported34. The FG-associated variant in MADD was not associated with lipid levels and is not in LD (r2<0.1) with a previously reported high-density lipoprotein cholesterol SNP (rs7395662)33, suggesting two independent signals within the same locus, one affecting lipid levels and the other FG levels (Table 3).
Table 3.
Association of novel SNPs with related metabolic traits in other GWAS datasets
| SNP | Nearest gene |
Alleles (effect/ other) |
BMI (kg/m2) | Diastolic blood pressure (mm Hg) |
Systolic blood pressure (mm Hg) |
Hypertension | HDL | LDL | Total cholesterol |
Triglycerides | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs560887 | G6PC2 | C/T | Effect* | −0.013 (0.010) | −0.146 (0.091) | −0.105 (0.135) | −0.023 (0.028) | −0.004 (0.004) | 0.01 (0.011) | 0.019 (0.011) | 0.004 (0.005) |
| P-value | 0.18 | 0.12 | 0.46 | 0.41 | 0.32 | 0.35 | 0.10 | 0.52 | |||
| rs10830963 | MTNR1B | G/C | Effect* | 0.002 (0.010) | 0.034 (0.098) | 0.088 (0.146) | −0.003 (0.030) | 0.005 (0.004) | −0.015 (0.013) | 0.002 (0.014) | −0.004 (0.007) |
| P-value | 0.86 | 0.74 | 0.56 | 0.91 | 0.26 | 0.25 | 0.88 | 0.58 | |||
| rs4607517 | GCK | A/G | Effect* | 0.004 (0.011) | −0.136 (0.111) | −0.128 (0.165) | −0.013 (0.033) | −0.006 (0.005) | 0.012 (0.014) | −0.002 (0.015) | 0.013 (0.007) |
| P-value | 0.75 | 0.23 | 0.45 | 0.70 | 0.21 | 0.38 | 0.87 | 0.054 | |||
| rs2191349 |
DGKB/ TMEM195 |
T/G | Effect* | 0.001 (0.009) | −0.075 (0.082) | −0.046 (0.122) | 0.007 (0.025) | 0.002 (0.003) | 0.009 (0.01) | 0.015 (0.011) | 0.004 (0.005) |
| P-value | 0.95 | 0.37 | 0.71 | 0.79 | 0.64 | 0.40 | 0.18 | 0.44 | |||
| rs780094 | GCKR | C/T | Effect* | 0.012 (0.009) | 0.052 (0.084) | 0.006 (0.124) | 0.020 (0.025) | 0.009 (0.003) | 0.007 (0.01) | −0.019 (0.011) | −0.055 (0.005) |
| P-value | 0.17 | 0.55 | 0.96 | 0.45 | 8.7×10−3 | 0.51 | 0.08 | 9.6 × 10−27 | |||
| rs11708067 | ADCY5 | A/G | Effect* | −0.010 (0.011) | −0.056 (0.104) | 0.047 (0.156) | 0.028 (0.031) | 0.0004(0.004) | −0.014 (0.013) | −0.013 (0.013) | −0.003 (0.006) |
| P-value | 0.35 | 0.60 | 0.77 | 0.37 | 0.92 | 0.26 | 0.32 | 0.62 | |||
| rs7944584 | MADD | A/T | Effect* | 0.023 (0.010) | −0.208 (0.093) | −0.170 (0.140) | −0.038 (0.028) | 0.007 (0.004) | −0.013 (0.012) | −0.016 (0.012) | −0.007 (0.006) |
| P-value | 0.02 | 0.03 | 0.24 | 0.18 | 0.06 | 0.27 | 0.18 | 0.26 | |||
| rs10885122 | ADRA2A | G/T | Effect* | −0.021 (0.014) | −0.079 (0.131) | 0.168 (0.193) | 0.073 (0.039) | 0.01 (0.007) | −0.019 (0.02) | −0.02 (0.021) | −0.02 (0.01) |
| P-value | 0.14 | 0.56 | 0.40 | 0.07 | 0.15 | 0.34 | 0.33 | 0.04 | |||
| rs174550 | FADS1 | T/C | Effect* | 0.003 (0.009) | −0.208 (0.086) | −0.108 (0.128) | 0.013 (0.026) | 0.014 (0.003) | 0.046 (0.010) | 0.052 (0.011) | −0.025 (0.005) |
| P-value | 0.73 | 0.02 | 0.42 | 0.62 | 2.9 × 10−5 | 8.5 × 10−6 | 2.5 × 10−6 | 1.9 × 10−6 | |||
| rs11605924 | CRY2 | A/C | Effect* | 0.011 (0.009) | 0.123 (0.082) | −0.003 (0.123) | 0.004 (0.025) | 0.005 (0.004) | 0.005 (0.011) | 0.008 (0.011) | −0.009 (0.005) |
| P-value | 0.21 | 0.15 | 0.98 | 0.87 | 0.13 | 0.62 | 0.46 | 0.10 | |||
| rs11920090 | SLC2A2 | T/A | Effect* | 0.010 (0.012) | −0.034 (0.117) | −0.023 (0.174) | −0.030 (0.036) | 0.003 (0.005) | −0.004 (0.014) | −0.009 (0.015) | −0.015 (0.007) |
| P-value | 0.42 | 0.78 | 0.90 | 0.41 | 0.60 | 0.81 | 0.57 | 0.04 | |||
| rs7034200 | GLIS3 | A/C | Effect* | −0.002 (0.009) | 0.093 (0.082) | 0.087 (0.122) | 0.006 (0.025) | 0.0002(0.003) | 0.015 (0.01) | 0.028 (0.011) | 0.005 (0.005) |
| P-value | 0.86 | 0.27 | 0.49 | 0.80 | 0.94 | 0.15 | 8.3 × 10−3 | 0.37 | |||
| rs340874 | PROX1 | C/T | Effect* | −0.007 (0.009) | 0.113 (0.085) | 0.093 (0.127) | 0.029 (0.026) | −0.007 (0.003) | 0.009 (0.01) | 0.003 (0.011) | 0.007 (0.005) |
| P-value | 0.46 | 0.20 | 0.48 | 0.27 | 0.04 | 0.39 | 0.81 | 0.19 | |||
| rs11071657 | FAM148B | A/G | Effect* | −0.006 (0.010) | 0.132 (0.091) | −0.007 (0.135) | 0.020 (0.028) | −0.004 (0.004) | 0.012 (0.011) | 0.002 (0.011) | 0.006 (0.005) |
| P-value | 0.54 | 0.16 | 0.96 | 0.49 | 0.22 | 0.28 | 0.86 | 0.30 | |||
| rs13266634 | SLC30A8 | C/T | Effect* | −0.026 (0.011) | −0.081 (0.094) | −0.072 (0.139) | 0.010 (0.029) | 0.003 (0.004) | 0.016 (0.011) | 0.013 (0.011) | 0.005 (0.005) |
| P-value | 0.01 | 0.40 | 0.62 | 0.74 | 0.47 | 0.13 | 0.24 | 0.33 | |||
| rs7903146 | TCF7L2 | T/C | Effect* | −0.033 (0.009) | 0.026 (0.091) | 0.025 (0.137) | 0.003 (0.028) | 0.005 (0.004) | 0.007 (0.012) | 0.007 (0.012) | −0.006 (0.006) |
| P-value | 4.4 × 10−4 | 0.78 | 0.86 | 0.92 | 0.22 | 0.53 | 0.55 | 0.31 | |||
| rs35767 | IGF1 | G/A | Effect* | 0.003 (0.012) | −0.102 (0.113) | −0.078 (0.167) | −0.005 (0.034) | 0.003 (0.005) | −0.009 (0.015) | −0.012 (0.015) | −0.002 (0.007) |
| P-value | 0.81 | 0.38 | 0.65 | 0.87 | 0.556 | 0.519 | 0.425 | 0.839 | |||
| N | 28,225– 32,530 |
28,591–34,130 | 28,557– 34,135 |
8145–9553 cases 8175–9749 controls |
21,045 | 17,521 | 17,529 | 21,104 |
Potential functional roles of novel associated loci
We investigated the likely functional role of genes mapping closest to the lead SNPs using several sources of data, including human disease databases, evidence from animal models and bioinformatic analyses (see Box, Online Methods and Supplementary Table 4). The newly discovered and established glycemic loci represent various biological functions: signal transduction (DGKB/TMEM195, ADCY5, FADS1, ADRA2A, SLC2A2, GCK, GCKR, G6PC2, IGF1); cell proliferation and development (GLIS3, MADD, PROX1); glucose transport and sensing (SLC2A2, GCK, GCKR, G6PC2); and circadian rhythm regulation (MTNR1B, CRY2). All of these pathways represent further avenues for physiological characterization and possible therapeutic intervention. However, we note that other genes could be causal (Box and Supplementary Table 4) and further experimental evidence will be needed to link unequivocally specific genes with phenotypes.
BOX: GENES NEAREST TO LOCI ASSOCIATED WITH FASTING DIABETES-RELATED QUANTITATIVE TRAITS
DGKB/TMEM195 – This locus was recently reported to be associated with FG24; here we report genome-wide significant replication of that finding and evaluate the genes mapping closest to the lead SNP in further detail. DGKB encodes the β (1 of 10) isotype of the catalytic domain of diacylglycerol kinase, which regulates the intracellular concentration of the second messenger diacylglycerol. In rat pancreatic islets glucose increases diacylglycerol49, which activates protein kinase C (PKC) and thus potentiates insulin secretion50. TMEM195 encodes transmembrane protein 195, an integral membrane phosphoprotein highly expressed in liver.
ADCY5 encodes adenylate cyclase 5, which catalyzes the generation of cAMP. Upon binding to its receptor in pancreatic β cells, glucagon-like peptide 1 (GLP-1) induces cAMP-mediated activation of protein kinase A, transcription of the proinsulin gene and stimulation of insulin secretory processes51.
MADD encodes mitogen-activated protein kinase (MAPK) activating death domain, an adaptor protein that interacts with the tumor necrosis factor α receptor to activate MAPK. Both PKC and MAPK have been implicated in the proliferation of β cells induced by GLP-151, suggesting that DGKB and MADD may contribute to β-cell mass and insulin secretion in this manner as well. Also in this region, SLC39A13 encodes a putative zinc transporter required for connective tissue development and BMP/TGF-β signaling52. NR1H3 encodes the liver X receptor alpha (LXRA) protein, which contains the retinoid response element. Glucose stimulates the transcriptional activity of LXR, which acts as a molecular switch that integrates hepatic glucose metabolism and fatty acid synthesis53.
ADRA2A encodes the α2A adrenergic receptor, which is expressed in β cells and whose activation leads to an outward potassium current independent of the islet KATP channel, thus possibly modifying insulin release54. Mice with null mutations display abnormal glucose homeostasis in addition to cardiac hypertrophy and abnormal heart rate and blood pressure.
FADS1 encodes fatty acid desaturase 1, which catalyzes the biosynthesis of highly unsaturated fatty acids from precursor essential polyunsaturated fatty acids. One such product is arachidonic acid; in rodent β cells, arachidonic acid liberated by phospholipase A2 augments glucose-mediated insulin release55. Two other members of the same family, FADS2 and FADS3, also reside in this region. By directing fatty acids down this metabolic pathway, increased activity of these enzymes may lower circulating triglyceride concentrations.
CRY2 encodes cryptochrome 2, an integral component of the mammalian circadian pacemaker56. Mice with null mutations in this gene present with abnormal circadian rhythmicity and several metabolic abnormalities including impaired glucose tolerance, increased insulin sensitivity, decreased body weight and adipose tissue, and abnormal heart rate. Together with MTNR1B15–17 this is the second circadian gene associated with FG in humans, contributing further evidence to this emerging pathway regulating glucose homeostasis57. In the same region, MAPK8IP1 encodes the scaffolding protein JIP1. Cross-talk between JIP1 and JIP3 has been implicated in the regulation of ASK1-SEK1-JNK signaling during glucose deprivation58. A missense mutation in this gene (S59N) segregates with diabetes in one family affected with a Mendelian form of the disease59.
SLC2A2 encodes the GLUT2 transporter responsible for transport of glucose into β cells and triggering the glucose-mediated insulin secretion cascade. In humans, recessive mutations in this gene lead to Fanconi-Bickel Syndrome, a rare disorder characterized by hepatorenal glycogen accumulation, proximal renal tubular dysfunction and impaired utilization of glucose and galactose60; mouse mutants also display hyperglycemia and abnormal glucose homeostasis61.
GLIS3 encodes the transcription factor GLIS family zinc finger 3 isoform, a Krüppel-like zinc finger protein that both activates and represses transcription and participates in β-cell ontogeny62,63. Functional mutations in this gene cause a syndrome of neonatal diabetes and congenital hypothyroidism63. Polymorphisms within this gene have recently been associated with type 1 diabetes risk (t1dgc.org).
PROX1 encodes the prospero homeobox protein 1, a novel co-repressor of hepatocyte nuclear factor 4α64 that plays a crucial role in β-cell development; mutations in its target gene HNF4A cause maturity-onset diabetes of the young, type 165.
FAM148B encodes the nuclear localized factor 2 (NLF2). It is expressed in endothelial cells and up-regulated by pro-inflammatory cytokines66. As shown here, it has a high level of expression in the pancreas, although its putative molecular connection with glucose homeostasis is presently unclear.
IGF1 encodes the insulin-like growth factor 1, the sole genome-wide significant locus associated with HOMA-IR in our study. Humans and mice null for igf1 display abnormal glucose homeostasis, with insulin resistance, increased circulating insulin and insensitivity to growth hormone67.
Expression analyses
We measured expression of the genes mapping closest to our lead SNPs (in DGKB/TMEM195, ADCY5, MADD, its neighboring gene SLC39A13 [a member of a family of zinc transporters mapping ~45 kb from the MADD lead SNP], ADRA2A, FADS1, CRY2, SLC2A2, GLIS3, PROX1 and FAM148B) in human pancreas and other metabolically relevant tissues (Supplementary Figure 4a). While there was evidence of expression in human islets for nearly all genes tested (with the sole exception of TMEM195), we found that DGKB and MADD were most strongly expressed in brain, SLC2A2, FADS1, TMEM195 and PROX1 in liver and ADCY5 in heart, while SLC39A13, ADRA2A and CRY2 were broadly expressed. Strikingly, FAM148B was highly expressed in the whole pancreas with lower levels in isolated islets, suggesting that it is also present in exocrine cells. A duplicate experiment in a different laboratory obtained similar results (Supplementary Figure 4b). We further examined expression of these transcripts in flow-sorted human β-cells from two separate individuals and documented β-cell expression for all but TMEM195, with SLC39A13, CRY2, GLIS3 and PROX1 being particularly highly expressed in these cells (Supplementary Figure 4c). Expression levels in metabolically relevant tissues for DGKB (β cells) and TMEM195 (liver) provide equally credible evidence for their respective candidacies as the causal gene at these loci. Furthermore, based on its relatively high expression levels in the β cell, SLC39A13 (neighboring gene to MADD) constitutes an intriguing candidate gene that may merit further investigation.
Potential causal variants, eQTLs and copy number variants
Our results interrogate only a fraction of the common variants in any given genomic region; we therefore expect that for the majority of the loci here described the underlying causal variant has yet to be identified. Nevertheless for some there are intriguing possible SNP candidates: in SLC2A2 the lead SNP (rs11920090) is in perfect LD (r2 = 1.0) with rs5400 (Stage 1 discovery association P=5.9×10−6), which codes for the amino acid substitution T110I, predicted to be “possibly damaging” by PolyPhen35 and PANTHER (Pdel= 0.92)36. In GCKR the lead SNP is in strong LD (r2=0.93) with rs1260326, encoding P446L, a non-synomymous variant previously associated with the same traits30 and predicted by PolyPhen to be “probably damaging”. A recent functional study has demonstrated that this variant indirectly leads to increased GCK activity, resulting in the observed effects on FG and triglyceride levels37. Both SLC2A2 T110I and GCKR P446L were predicted “tolerated” by SIFT38, highlighting the difficulties in obtaining consensus functional predictions from different informatic approaches.
We used publicly available eQTL datasets for liver39, cortex40 and Epstein-Barr virus-transformed lymphoblastoid cell lines41 to explore additional possible causal mechanisms testing for association between replicated loci and mRNA expression levels of nearby genes (Online Methods). The lead SNP in FADS1, rs174550, is in strong LD (r2=0.80) and close proximity (130 bp) to rs174548, a SNP highly associated with FADS1 mRNA expression levels in liver (P=1.7×10−5) and with FADS2 mRNA expression levels in lymphoblastoid cells (P=3.1×10−4). SNP rs174548 has also been associated (up to P=4.5×10−8) with a number of serum glycerophospholipid concentrations in a GWAS investigating metabolomic profiles42 and rs174550 also demonstrated strong associations (P<5.2×10−7) with the same metabolites (data not shown). These results are substantiated by previous work associating SNPs in this region with the fatty acid composition of phospholipids43. The latter suggest the minor allele variant of rs174550 results in a reduced efficiency of the fatty acid delta-5 desaturase reaction42. Finally, bioinformatic analysis identifies a perfect proxy, rs174545 (r2=1 with rs174550), whose glucose-raising allele abolishes a predicted miR-124 target site (see Online Methods). Taken together, these data support the hypothesis that not only fatty acid levels, but also their precise composition and degree of desaturation, may influence glucose homeostasis.
Although our study was not designed to explicitly investigate the impact of copy number variation on glycemic traits, we took advantage of existing data44 to investigate whether any of our lead SNPs are in LD with common, diallelic copy number polymorphisms (CNPs) mapping within a 1Mb window. Of the FG loci, only DGKB/TMEM195 has a validated, common CNP affecting sequence within 1 Mb of the index SNP44. Despite the proximity of this CNP to the associated SNP (~25 kb), the CNP is essentially uncorrelated with the index SNP (r2=0.01 in HapMap CEU) and is therefore unlikely to explain the observed FG association.
DISCUSSION
In this meta-analysis of 21 Stage 1 discovery GWAS cohorts followed by targeted Stage 2 replication of 25 loci in 33 additional cohorts (totaling up to 122,743 non-diabetic participants), we report the novel genome-wide significant associations of SNPs in or near ADCY5, MADD, ADRA2A, CRY2, FADS1, GLIS3, SLC2A2, PROX1 and FAM148B with FG and one SNP near IGF1 with FI and HOMA-IR. We have also confirmed associations of variants in GCK, GCKR, G6PC2 and MTNR1B with FG, and achieved genome-wide significance for the recently reported DGKB/TMEM195 locus24 and for variants in the known T2D-associated TCF7L2 and SLC30A8. All of the FG-associated SNPs demonstrate consistent nominal associations with HOMA-B; and those in GCK, G6PC2, MTNR1B, DGKB/TMEM195, ADCY5, FADS1 and GLIS3 do so at genome-wide significant levels. As previously reported11,12,30, GCKR is also associated with FI and HOMA-IR.
Importantly, in addition to the established T2D-associated loci TCF7L2, SLC30A8 and MTNR1B, five of the loci that are associated with elevated FG levels in non-diabetic individuals (ADCY5, GCK, GCKR, PROX1 and DGKB/TMEM195) also increase the risk of T2D in separate T2D case-control studies. However, this overlap is incomplete and highlights that the magnitude of the effect on FG is not predictive of the effect on T2D risk, as shown when comparing FG and T2D effect sizes for MTNR1B and TCF7L2, or for ADCY5 and MADD (Table 2). The latter two loci have similar effect sizes on FG and similar allele frequencies, and yet the former is robustly associated with T2D risk (OR 1.12, P=5.5×10−21) while the latter is not (OR 1.01, P=0.3) in the same samples. This suggests that not all loci associated with FG within the “physiological” range are also associated with “pathological” FG levels and T2D risk. Thus, variation in FG in healthy individuals is not necessarily an endophenotype for T2D, which posits the hypothesis that the mechanism by which glucose is raised, rather than a mere elevation in fasting glucose levels, is a key contributor to disease progression. On the other hand, we cannot rule out the existence of protective variants in loci where elevated FG does not progress to manifest T2D, or the effect of cohort selection in the detection of the loci with variable effects on FG and T2D risk. Nevertheless, this work shows that targeting quantitative traits in GWAS searches can help identify genetic determinants of overt disease.
With regard to insulin resistance, our analyses resulted in only one novel genome-wide significant locus associated with FI and HOMA-IR. The associated SNP rs35767 is 1.2 kb upstream of IGF1, raising the possibility that it may influence IGF1 expression levels (we have found no direct support for this notion in the limited eQTL data available). Although not reaching genome-wide-significance, we note that SNP rs4675095 in the insulin receptor substrate-1 gene (IRS1) was also associated with HOMA-IR (P=4.6×10−3), which given IRS1’s excellent biological credentials will warrant further investigation. This SNP is not in LD with the widely studied missense SNP G972R (rs1801278) nor with the newly discovered T2D SNP rs294364145, whose C risk allele was only nominally associated with increased FI (P=0.02) and HOMA-IR (P=0.04) in our discovery dataset. The previously reported associations of SNPs in PANK1 with fasting insulin24 did not receive strong support in our discovery cohorts (P=0.04 and 0.17 for rs11185790 and rs1075374, respectively).
Notably, our large-scale meta-analyses produced more than a dozen robust associations with FG and only two with FI/HOMA-IR (GCKR and IGF1). Although the somewhat smaller sample size for insulin may have contributed to this discrepancy, a comparison of the similarly-powered HOMA-B and HOMA-IR analyses reveals associations with HOMA-B several orders of magnitude more significant than those seen with HOMA-IR (Figure 2). Because insulin itself is a component of the numerator in both measures, one cannot attribute this discrepancy to technical differences in insulin measurements across cohorts. Similarly, because the QQ plots are very similar for FI and HOMA-IR, we do not believe that the use of a mathematical formula (HOMA-IR) rather than a direct measurement (FI) has affected our analyses substantially. HOMA-B and HOMA-IR have comparable heritability estimates (0.26 and 0.27 in Framingham respectively) and their correlation is significant (r=0.55 in Framingham). Thus, not only there may be a difference in the identity of specific genetic determinants for each trait46, but rather the genetic architecture may be distinct for each trait, with more modest effects, fewer loci, rarer variants or a stronger environmental modification underlying HOMA-IR. In addition, HOMA-IR (which is composed of fasting values) is an imperfect estimate of global insulin resistance, as it addresses mostly hepatic sensitivity to insulin and is partially affected by β-cell function: its heritability is lower than insulin sensitivity derived from the minimal model47. Exploration of gene × environment interactions and analysis of datasets that include 2-hour glucose and insulin values may reveal other genetic factors that increase insulin resistance in humans29.
In conclusion, a large-scale meta-analysis of GWAS has identified ten novel loci associated with glycemic traits whose in-depth physiological investigation should further our understanding of glucose homeostasis in humans and may reveal novel pathways for diabetes therapeutics.
Supplementary Material
REFERENCES
- 1.American Diabetes Association. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 3.Meigs JB, Nathan DM, D'Agostino RB, Sr, Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845–1850. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 4.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 5.Patel A, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 7.Ray KK, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 8.Prokopenko I, McCarthy MI, Lindgren CM. Type 2 diabetes: new genes, new understanding. Trends in Genetics. 2008;24:613–621. doi: 10.1016/j.tig.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florez JC. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: Where are the insulin resistance genes? Diabetologia. 2008;51:1100–1110. doi: 10.1007/s00125-008-1025-9. [DOI] [PubMed] [Google Scholar]
- 10.Weedon MN, et al. A common haplotype of the glucokinase gene alters fasting glucose and birth weight: association in six studies and population-genetics analyses. Am J Hum Genet. 2006;79:991–1001. doi: 10.1086/509517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparso T, et al. The GCKR rs780094 polymorphism is associated with elevated fasting serum triacylglycerol, reduced fasting and OGTT-related insulinaemia, and reduced risk of type 2 diabetes. Diabetologia. 2008;51:70–75. doi: 10.1007/s00125-007-0865-z. [DOI] [PubMed] [Google Scholar]
- 12.Orho-Melander M, et al. A common missense variant in the glucokinase regulatory protein gene (GCKR) is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes. 2008;57:3112–3121. doi: 10.2337/db08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouatia-Naji N, et al. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science. 2008;320:1085–1088. doi: 10.1126/science.1156849. [DOI] [PubMed] [Google Scholar]
- 14.Chen W-M, et al. Association studies in Caucasians identify variants in the G6PC2/ABCB11 region regulating fasting glucose levels. J Clin Invest. 2008;118:2620–2628. doi: 10.1172/JCI34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prokopenko I, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyssenko V, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouatia-Naji N, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Diabetologia. 1985;28:412–419. [Google Scholar]
- 19.Pe'er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32:381–385. doi: 10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
- 20.Brunzell JD, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976;42:222–229. doi: 10.1210/jcem-42-2-222. [DOI] [PubMed] [Google Scholar]
- 21.Weir GC, Bonner-Weir S. Five stages of evolving b-cell dysfunction during progression to diabetes. Diabetes. 2004;53:S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- 22.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Abecasis GR. Mach 1.0: Rapid haplotype reconstruction and missing genotype inference. Am J Hum Genet. 2006;S79:2290. [Google Scholar]
- 24.Sabatti C, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University and Novartis Institutes for BioMedical Research. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 26.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- 27.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009 doi: 10.1126/science.1167728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirosh A, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- 29.Saxena R, et al. Genetic variation in gastric inhibitory polypeptide receptor (GIPR) impacts the glucose and insulin responses to an oral glucose challenge. Nat Genet. doi: 10.1038/ng.521. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaxillaire M, et al. The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes. 2008;57:2253–2257. doi: 10.2337/db07-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willer CJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton-Cheh C, et al. Eight blood pressure loci identified by genomewide association study of 34,433 people of European ancestry. Nature Genetics. 2009 online [Epub ahead of print] [Google Scholar]
- 33.Aulchenko YS, et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kathiresan S, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunyaev S, et al. Prediction of deleterious human alleles. Hum Mol Genet. 2001;10:591–597. doi: 10.1093/hmg/10.6.591. [DOI] [PubMed] [Google Scholar]
- 36.Thomas PD, et al. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 2006;34:W645–W650. doi: 10.1093/nar/gkl229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beer NL, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp357. online [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schadt EE, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biology. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers AJ, et al. A survey of genetic human cortical gene expression. Nat Genet. 2007;39:1494–1499. doi: 10.1038/ng.2007.16. [DOI] [PubMed] [Google Scholar]
- 41.Dixon AL, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 42.Gieger C, et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008;4:e1000282. doi: 10.1371/journal.pgen.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaeffer L, et al. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 44.McCarroll SA, et al. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet. 2008;40:1166–1174. doi: 10.1038/ng.238. [DOI] [PubMed] [Google Scholar]
- 45.Rung J, et al. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009 doi: 10.1038/ng.443. online [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Doria A, Patti M-E, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metabolism. 2008;8:186–200. doi: 10.1016/j.cmet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergman RN, et al. Minimal model-based insulin sensitivity has greater heritability and a different genetic basis than homeostasis model assessment or fasting insulin. Diabetes. 2003;52:2168–2174. doi: 10.2337/diabetes.52.8.2168. [DOI] [PubMed] [Google Scholar]
- 48.Higgins JP, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 49.Peter-Riesch B, Fathi M, Schlegel W, Wollheim CB. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest. 1988;81:1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol. Rev. 1987;67:1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 51.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukada T, et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE. 2008;3:e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitro N, et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445:219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 54.Rorsman P, et al. Activation by adrenaline of a low-conductance G protein-dependent K+ channel in mouse pancreatic B cells. Nature. 1991;349:77–79. doi: 10.1038/349077a0. [DOI] [PubMed] [Google Scholar]
- 55.Keane D, Newsholme P. Saturated and unsaturated (including arachidonic acid) non-esterified fatty acid modulation of insulin secretion from pancreatic beta-cells. Biochem Soc Trans. 2008;36:955–958. doi: 10.1042/BST0360955. [DOI] [PubMed] [Google Scholar]
- 56.Kume K, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 57.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biology. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song JJ, Lee YJ. Cross-talk between JIP3 and JIP1 during glucose deprivation: SEK1-JNK2 and Akt1 act as mediators. J Biol Chem. 2005;280:26845–26855. doi: 10.1074/jbc.M502318200. [DOI] [PubMed] [Google Scholar]
- 59.Waeber G, et al. The gene MAPK8IP1, encoding islet-brain-1, is a candidate for type 2 diabetes. Nat Genet. 2000;24:291–295. doi: 10.1038/73523. [DOI] [PubMed] [Google Scholar]
- 60.Santer R, et al. Mutations in GLUT2, the gene for the liver-type glucose transporter, in patients with Fanconi-Bickel syndrome. Nat Genet. 1997;17:324–326. doi: 10.1038/ng1197-324. [DOI] [PubMed] [Google Scholar]
- 61.Guillam MT, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 62.Kim Y-S, Nakanishi G, Lewandoski M, Jetten AM. GLIS3, a novel member of the GLIS subfamily of Kruppel-like zinc finger proteins with repressor and activation functions. Nucl. Acids Res. 2003;31:5513–5525. doi: 10.1093/nar/gkg776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Senee V, et al. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38:682–687. doi: 10.1038/ng1802. [DOI] [PubMed] [Google Scholar]
- 64.Song K-H, Li T, Chiang JYL. A prospero-related homeodomain protein is a novel co-regulator of hepatocyte nuclear factor 4α that regulates the cholesterol 7α-hydroxylase gene. J. Biol. Chem. 2006;281:10081–10088. doi: 10.1074/jbc.M513420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamagata K, et al. Mutations in the hepatocyte nuclear factor-4α gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 66.Warton K, Foster NC, Gold WA, Stanley KK. A novel gene family induced by acute inflammation in endothelial cells. Gene. 2004;342:85–95. doi: 10.1016/j.gene.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 67.Clemmons DR. Role of insulin-like growth factor in maintaining normal glucose homeostasis. Horm Res. 2004;62(Suppl 1):77–82. doi: 10.1159/000080763. [DOI] [PubMed] [Google Scholar]
- 68.Servin B, Stephens M. Imputation-based analysis of association studies: candidate regions and quantitative traits. PLoS Genet. 2007;3:e114. doi: 10.1371/journal.pgen.0030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- 70.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 71.Petitti DB. Statistical methods in meta-analysis. In: Petitti DB, editor. Meta-analysis, decision analysis, and cost-effectiveness analysis. New York, NY: Oxford University Press; 2000. pp. 94–118. [Google Scholar]
- 72.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 73.Lukowiak B, et al. Identification and purification of functional human beta-cells by a new specific zinc-fluorescent probe. J Histochem Cytochem. 2001;49:519–528. doi: 10.1177/002215540104900412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.