Abstract
Emission from field burning of crop residue, a common practice in many parts of the world today, has potential effects on air quality, atmosphere and climate. This study provides a comprehensive size and compositional characterization of particulate matter (PM) emission from rice straw (RS) burning using both in situ experiments (11 spread field burning) and laboratory hood experiments (3 pile and 6 spread burning) that were conducted during 2003–2006 in Thailand. The carbon balance and emission ratio method was used to determine PM emission factors (EF) in the field experiments. The obtained EFs varied from field to hood experiments reflecting multiple factors affecting combustion and emission. In the hood experiments, EFs were found to be depending on the burning types (spread or pile), moisture content and the combustion efficiency. In addition, in the field experiments, burning rate and EF were also influenced by weather conditions, i.e. wind. Hood pile burning produced significantly higher EF (20±8 g kg−1 RS) than hood spread burning (4.7±2.2 g kg−1 RS). The majority of PM emitted from the field burning was PM2.5 with EF of 5.1±0.7 g m−2 or 8.3±2.7 g kg−1 RS burned. The coarse PM fraction (PM10-2.5) was mainly generated by fire attention activities and was relatively small, hence the resulting EF of PM10 (9.4±3.5 g kg−1 RS) was not significantly higher than PM2.5. PM size distribution was measured across 8 size ranges (from <0.4 μm to >9.0 μm). The largest fractions of PM, EC and OC were associated with PM1.1. The most significant components in PM2.5 and PM10 include OC, water soluble ions and levoglucosan. Relative abundance of some methoxyphenols (e.g., acetylsyringone), PAHs (e.g., fluoranthene and pyrene), organochlorine pesticides and PCBs may also serve as additional signatures for the PM emission. Presence of these toxic compounds in PM of burning smoke increases the potential toxic effects of the emission. For illustration, an estimation of the annual RS field burning in Thailand was made using the obtained in situ field burning EFs and preliminary burning activity data.
Keywords: Rice straw field burning, pile burning, PM composition, levoglucosan, methoxyphenols, semi-VOC
1. Introduction
Rice is cultivated globally and is by far the most popular crop in Asia (Kadam et al., 2000) where the annual production is rapidly increasing to meet the domestic and export demand. With improved living conditions in rural areas, farmers tend to rely more on commercial fuel and less on crop residue for domestic cooking, which leads to even more field burning of rice straw (RS). This inexpensive method of crop residue disposal is practiced in many parts of the world to clear the surface biomass from land for faster crop rotation; to control undesirable weeds, pests and diseases; and to return some nutrients to the soil. This activity emits a large amount of toxic air pollutants (particles, and inorganic and organic gases) and greenhouse gases (Andreae and Merlet, 2001; Reid et al, 2004). Agricultural field burning activities are linked to elevated air pollution levels in USA (Jiminez et al., 2007), Europe (Viana et al., 2008), and Asia, e.g. Taiwan (Yang et al., 2006) and Thailand (Tipayarom and Kim Oanh, 2007). In Asia, intensive open burning activities commonly take place during the dry season when stagnant atmospheric conditions are normally observed, hence can lead to high air pollution build-up in adjacent urban areas (Tipayarom and Kim Oanh, 2007). Overall, emissions from field burning activities deteriorate local air quality and have been reported to cause high personal exposure (Wu et al., 2006) and result in adverse health effects (Torigoe et al., 2000). Crop residue field burning is an important type of open biomass burning in Asia that is a leading cause of regional atmospheric phenomena with strong climate implications such as Atmospheric Brown Clouds (UNEP & C4, 2002; UNEP, 2008). Fine particles, one of the major pollutants emitted from the field burning of RS, are a major cause of concern due to their harmful effects on human health (Pope et al., 2009) and the earth’s climate (Bond et al., 2004). Atmospheric particulate matter (PM) is itself a complex mixture and its potential environmental and health effects depend on particle size and composition. PM also carry signatures of contributing emission sources that can be used to indirectly quantify the source contribution using receptor models (Gordon, 1988). These models are especially applicable when the emission inventory data are not available. This is even more important for the crop residue field burning, as the direct method of quantification based upon emission inventories is complex because of the distributed nature and short time spans of burning events.
Despite its potential high contribution to air pollution in Asian cities (Kim Oanh et al., 2006), this important field burning emission source is often overlooked in air quality management practices, mainly due to the lack of reliable information on the magnitude and composition of the emissions. A few RS burning experiments have been conducted for the purposes of emission characterization, but most were from outside of Asia. A number of these studies compared levels of atmospheric species measured in ambient air during burning episodes and non-burning (Viana et al. 2008; Yang et al., 2006; Lee et al., 2008) and have indicated enrichment of several air pollution species in RS burning smoke that can serve as useful markers for this source. These studies, however, have not reported emission factors (EF) which are required for the emission inventory purpose. EF have mainly been derived from laboratory experiments which simulated burning conditions, such as a large enclosure (Hays et al. 2005), a wind tunnel (Jenkins et al., 1996), or burning RS fuel in a wood stove (Sheesley et al., 2003). Recently, EF of selected air pollutants (particle number and gaseous pollutants) from RS burning in a stove were reported for China (Zhang et al. 2008). Nevertheless, EF and PM emission characterization for in situ field RS burning in Asia have not been found in the literature.
Understandably, the random nature of combustion and many other factors may cause variations in pollutant EF and source emission profiles of RS burning. These include moisture content of RS, moisture content of field surface soil, biomass loading (which varies between rice species and cultivation practices), spreading or pile burning, fertilizers and pesticide treatments, burning time-of-day (drier in the afternoon) and weather conditions. It is expected that a wide range of EF and emission characteristics may be observed in both in situ field burning and controlled laboratory experiments. To reduce the uncertainty and to recommend appropriate ranges of pollutant EF and emission profiles for Asia, more experimental studies should be conducted under conditions representative to the common practices in the region.
The present study carried out a series of in situ RS paddy field burning experiments and simulated burning in a laboratory fume hood. PM EF, physical and chemical characterization were accomplished for in situ field burning, hood spread burning and hood pile burning, and are described herein.
2. Experimental design
Experiments were conducted during the RS burning seasons (December to April next year) in the period of 2003–2006 with 11 field (in situ burning of harvested paddies in Pathumthani of Thailand) and 9 hood experiments (laboratory experiments for spread and pile burning) at the Asian Institute of Technology (AIT). Detailed information for both field and hood experiments are presented in Table A1, supplementary information (SI).
2.1 Field burning and smoke sampling
Field experiments took place directly in harvested paddies and followed the local RS burning practices, in order to provide a representative source emission characterization. The experimental fields were harvested by combine harvesters which cut the upper part of RS and spread it in windrows while leaving the lower part virtually untouched (standing part). Fire was started at the upwind edge of a selected paddy, i.e. following the head burning method. We observed that spread RS was burned properly when fire swept over the paddy, but the standing part in many cases were only partially burned - especially when RS moisture was high. Our survey of 110 local farmer households of 7 districts in Pathumthani shows that RS from the major crop (harvested in Nov-Dec) and secondary crop (harvested mainly in March-April) is always burned in field. The burning takes place 2–7 days following the harvest of a paddy and generally in the afternoon, between 11:00–17:00 (Tipayarom and Kim Oanh, 2007).
Rice paddies selected for the experiments were located in the middle of large agricultural fields and far away from local roads and houses. Each field experiment was conducted on one selected paddy of a size up to a few thousand m2 (Table 1A, SI), and both background (BG) sampling and burning smoke air sampling were collected. The BG sampling was conducted prior to burning and lasted 2–4 hours. After the completion of BG sampling the fire was started in the experimental paddy. RS burning smoke sampling commenced immediately after the fire became stable and continued until the fire stopped. The smoke sampling period varied between experiments and lasted from about 30 minutes to 1 hour. Both samplings were conducted on the same day, BG sampling started at about 9:00–10:00 am and the smoke sampling ended around 17:00. The difference between pollutant concentrations in the smoke and BG samples collected in a field experiment was considered as the net contribution from the RS burning smoke. All sampling equipment was placed at a fixed downwind site in each experimental paddy, about 5 m away from the downwind edge of the paddy, to avoid damage from the flame and heat. All sampling inlets were positioned at 1.5 m above ground. A Hi-Vol TSP sampler (collecting TSP and semi-VOC) and 2 dichot samplers (collecting PM2.5 and PM10-2.5) were located at about 1–1.5 m from each other (almost in a triangle arrangement). At this distance, the samplers were considered to be close enough to catch the same part of a smoke plume and far away enough to minimize the inlet flow disturbance. Continuous field measurements were recorded for wind (Mechanical wind recorder), temperature and humidity (Thermo-Hydrometer, Delta TRAK®), and CO and CO2 (IAQ-CALC™, model 8760/876). The equipment and an 8-stage cascade impactor (TISCH) were located close to the middle of the triangle so that they were also measuring the same part of the smoke plume as the PM samplers. Continuous data recorded (every 5 seconds) from the CO/CO2 meter was used for EF estimation using emission ratio. Another CO/CO2 meter was used to measure the gas concentrations near the fire, and these data were mainly used to determine the combustion efficiency. The second CO/CO2 meter was hung on a tip of a 2m-long stick and kept near the flame at all times. A summary of the equipment and analytical methods used is presented in Table A2, SI. All equipment was calibrated before use.
2.2 Hood burning and stack sampling
Hood experiments were conducted to study emission from both spread burning (6 experiments) and pile burning (3 experiments). Isokinetic smoke samples were collected using a semi-volatile sampling train (Auto 5™, AST® Sampler) following US EPA modified Method 5. The hood and the source sampling method used for these experiments were similar to that previously reported by Kim Oanh et al. (2002). The sampling period covered the whole burning cycle from the moment of stable firing to the end of the burning process. A 20 liter Tedlar bag connected to a calibrated personal pump was used to collect the bulk flue gas for subsequent measurements of gaseous pollutants (CO and CO2 were used for estimation of combustion efficiency reported in this paper, Table 3).
Table 3.
SPM EF and detail of hood experiments
| Hood exp. | Hood 1 | Hood 2 | Hood 3 | Hood 4 | Hood 5 | Hood 6 | Hood 7 | Hood 8 | Hood 9 |
|---|---|---|---|---|---|---|---|---|---|
| Burning type | pile | spread | spread | spread | spread | spread | spread | pile | pile |
| MCEa | na | na | na | na | 0.88 | 0.93 | 0.93 | 0.92 | 0.92 |
| RS moist., % | na | 5 | 22 | 30 | 46 | 36 | 30 | 27 | 27 |
| DAHb | 1 | 7 | 4 | 2 | na | 5 | na | 3 | 3 |
| EF, g kg−1 RS | 27.3 | 0.5 | 2.9 | 7.7 | 24.4 | 3.2 | 5.1 | 21.1 | 12.0 |
| EF, g m−2 | - | 0.2 | 1.7 | 4.4 | 15.9 | 1.4 | 1.9 | - | - |
-MCE: modified combustion efficiency; na: not available
- DAH (day after harvest) is the number of days between the crop harvest and the field burn experiment, hood burning took place 1 day after field burning
na – not available; - EF in g m−2 is not applicable for pile burning
RS samples used for the hood experiments were collected together with a surface soil layer of about 10 cm thickness. Three 1-m2 plots of representative unburned paddy were collected from each experimental field and were used for the hood burning. For spread burning hood experiments, first the RS in a sampled part (of a paddy) was evenly distributed, then the samples (1-m2 plots) were randomly taken from within this part. These samples contained both spreading RS and standing RS. In 3 pile burning experiments, RS samples were mostly taken from windrows with a biomass density of about 1.5–2 times higher than the evenly spreading RS fields. Each 1-m2 sampled plot was kept in a separate air tight plastic bag (to minimize moisture loss) and transported to AIT where the hood experiment was done on the following day. For burning in the hood, the RS paddy sample plot was further segregated into 25×25 cm2 sub-plots. Each sub-plot was placed in a 30×30 cm2 tray and the trays were continuously fed, one after another, into the chamber in a conveyer-like set up. RS sample in each tray was ignited and burned with free air supply (from the open bottom of the hood). The set-up was made with the intention to imitate as closely as possible the in situ field burning. However, there were many variables that may affect the smoke emission from both field and hood experiments hence differences are expected. Specifically, meteorological conditions in the field may affect the emission results as discussed later.
2.3 Sample analysis
PM samples
For the field burning experiments, fine (PM2.5) and coarse particles (PM10-2.5) were collected by two dichot samplers (Anderson series 214) on mixed cellulose (plus some Teflon) and pre-baked quartz filters. PM samples collected on pre-baked quartz filters on 8 cascade impactor stages (ranging from <0.4 μm to >9 μm) were used for further size distribution analysis. PM filters (pre-baked glass fiber filter, GFF) collected with the Andersen Hi-Vol sampler (225 L min−1) were used for analyses of the particulate phase of semi-volatile organic compounds (semi-VOC). The latter included polycyclic aromatic hydrocarbons (PAH), organochlorine pesticides (OCP) and polychlorinated biphenyls (PCBs). During transportation between the field locations and the AIT laboratory the sampling materials (filters and XAD-2, PUF) were properly wrapped and placed in an ice box. Each filter was put in a separate Petri dish that was kept in an airtight plastic bag both during transportation and refrigeration in the laboratory. Field blanks were treated in the same way and used for correction of concentration results.
The stack samples collected in hood experiments included suspended particle samples on pre-baked GFF, without size segregation (SPM), and the gaseous phase compounds (semi-VOC) collected on XAD-2. The isokinetic rates of the stack sampling were 90±10% which enabled capture of representative SPM samples.
All PM samples were analyzed for mass, water soluble ions (IC) and elements (PIXE or ICP-OES) following the methods described in Kim Oanh et al. (2006). Coarse and fine PM collected on pre-baked quartz filters were analyzed for EC/OC (Sunset analyzer, TOT, NIOSH 5040) at the University of Illinois at Urbana-Champaign.
Field Hi-Vol TSP samples were segregated into 2 halves. One half was analyzed for 16 US EPA priority PAHs, following Method TO13A (US EPA, 1999a) with the detail presented in Kim Oanh et al. (2002). The analyzed PAHs included naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fth), pyrene (Pyr), benz(a)anthracene (BaA), chrysene (Chry), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenz(a,h)anthracene (DahA), benz(g,h,i)perylene (BghiP) and ideno(1,2,3-c,d)pyrene (IcdP). The other half of each TSP filter was Soxhlet extracted, using a solvent mixture of 90% hexane and 10% diethyl ether, and subsequently analyzed for PCBs and organochlorine pesticides (OCPs) using a GC/ECD, following Method TO-4A (US EPA, 1999b), as described by Pentamwa and Kim Oanh (2008). In total, 14 PCBs (PCB 18, PCB 31, PCB 28, PCB 52, PCB 44, PCB 101, PCB118, PCB138, PCB149, PCB 153, PCB 180, PCB 170, PCB 194 and PCB 209), and 16 OCPs (α-BHC, β-BHC, γ-BHC, δ-BHC, heptachlor, aldrin, heptachlor epoxide, endosulfan I, DDT, DDE, DDD, dieldrin, endrin, endrin aldehyde, endosulfan II, and endosulfan sulfate) were quantified.
For hood SPM GFF samples, one half of each filter was analyzed for PAHs (no PCBs and OCPs) and the other half was analyzed for ions and elements. One set of field experiment PM2.5 and PM10-2.5 samples collected on Teflon filters was analyzed for levoglucosan and methoxyphenols at the University of Washington in Seattle, following the method described previously (Simpson et al., 2004; 2005). The 21 methoxyphenols analyzed include guaiacol (GU), ethyl-guaiacol (ETGU), syringol (SY), eugenol (EUG), propyl-guaiacol (PRGU), vanillin (VAN), cis-isoeugenol (CISOEU), methyl-syringol (MESY), trans-isoeugenol (TISOEU), aceto-vanillone (ACETV), ethyl-syringol (ETSY), guaiacyl-acetone (GUACE), allyl-syringol (ALLSY), propyl-syringol (PRSY), syring-aldehyde (SYALD), acetylsyringone (ACETSY), coniferyl-aldehyde (CONIF), propionyl-syringone (PRSYON), butyryl-syringone (BUSYON), sinapyl-aldehyde (SINAP). Samples for levoglucosan and other semi-VOC were transported in ice boxes and stored in refrigerators for a maximum period of 1 month before the analyses.
Sampling and analysis for biomass density and C content
Prior to field burning, a composite above ground RS sample was collected from each experimental paddy for subsequent analyses of biomass density and RS moisture content (oven drying). The carbon content (C) was also analyzed for the last 4 field experiments. This composite sample was formed by 4 randomly selected 25×25 cm2 paddy plots (making a total sampled area of 1 m2). Similarly, after burning a leftover above ground biomass sample on a 1-m2 composite paddy sample was also collected for analysis of unburned C in the last 4 field experiments. Prior to a pile burning hood experiment, a sub-sample was taken from the collected paddy sample for determination of the actual biomass density of the pile.
Composite surface soil samples were collected from the paddy before and after burning for analysis of soil carbon content. A composite soil sample was prepared from 5 subsamples that were randomly collected using a core sampler. Each subsample was of 5 cm diameter and 10 cm depth. The soil samples taken after burning also contained ash. Carbon in soil was analyzed using a CS – 200 Carbon/Sulfur Analyzer while C in RS was determined using a Leco ® CHN – 1000 analyzer at the Ministry of Science and Technology of Thailand following the ASTM standard D5373-02 method (ASTM, 2008).
2.4 Emission Factor Determination
Emission factors (EF) are presented as g m−2 of paddy burned and g kg−1 of dry RS burned for the field (in situ) and hood spread burning, which are convertible using the actual dry RS biomass density (kg m−2 paddy). For hood pile burning, the area based EF is not applicable hence only the EF in g kg−1 of dry RS is reported.
EF for the hood experiments was directly determined based on the measurements of flue gas volume and pollutant mass concentration using Equation 1.
| (Equation 1) |
Concentration of SPM was first determined using the blank-corrected mass of SPM collected and total sampled volume (provided by Auto 5™ sampler). Other data (flowrate and sampling time) were also automatically recorded by the sampler. For the spread hood burning, the area burned was 3 m2. Thus the EF was expressed in g SPM per m2 paddy and then converted to g SPM per kg RS. For the pile burning experiments, the “RS burned” was the mass of RS available for burning (kg) in the collected pile samples and obtained EF were directly expressed in g SPM per kg RS.
EF for the field experiments was determined using method involving the carbon balance and emission ratio, that is commonly used in biomass open burning studies (Zarate et al., 2000; Dhammapala et al. 2007; Li et al, 2008; and references therein). This method calculates the carbon emitted with carbon containing species, i.e. CO2, CO, CH4, NMHC and particulate C, based on the difference in C measured before and after burning (Li et al., 2008). The EF of other species are then determined using the concentration ratio of the interested species to a reference species, either CO2 or CO, measured simultaneously in the emission plume. Reid et al. (2004) recommends to use CO2 as the reference species if the modified combustion efficiency (MCE=CO2/(CO+CO2) for the flaming combustion phase is above 0.9.
In our study, the C analysis results for the above ground biomass and paddy soil samples, taken before burning and after burning, were available for field experiments 8–11. There was no significant difference in soil C before burning (3.2%) and after burning (3.1%), as seen in Table 3A (SI). Therefore, the difference in C content in the above ground biomass samples alone was used to estimate the amount of C oxidized (and released in the emission) using Equation 2.
| (Equation 2) |
Where,
Coxidized: amount of C released
CBeforeBurnt: amount of C available in above ground biomass sample before burning
CAfterBurnt: amount of C remained in above ground biomass after burning
Other carbon containing species (than CO2 and CO) were not measured in this study therefore we followed the suggestion by Reid et al. (2004) to assume that 90% of C released was in the forms of CO2 and CO (Equation 3).
| (Equation 3) |
This study estimated Coxidized based on 1 m2 of the rice paddy hence CO2 and CO in Eq. 3 are the EFCO2 and EFCO, respectively, on 1 m2 of paddy basis. These can further be converted to the EF on 1 kg of RS basis using the biomass loading, kg m−2, determined in each experiment. To solve Eq. 3, the ratios of net CO to CO2 measured near fire were used to close the system. These were the average ratios (Table 3A, SI) calculated for each experiment using the net CO (COnear fire – CObackground) and net CO2, both in mg m−3, obtained every 5 seconds.
Subsequently, the PM EF was determined using the emission ratio method. In our field experiments the values of MCE, estimated from the measurements made near the fire, were all above 0.92 (0.93±0.01, see Table 1) which justifies the use of CO2 as the reference species (Reid et al., 2004). Thus, the PM EF is determined using Equation 4.
Table 1.
PM EF (dichot samples) of field burning based on carbon balance and emission ratios to CO2 (n=4)
| Field exp. | EF, g m −2 paddy | EF, g kg−1 of dry RS burned | RS Moist., % | MCE | Rice species | Dry mass, kg m−2 | Wind m s−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM2.5 | PM10 | CO2 | CO | PM2.5 | PM10 | CO2 | CO | ||||||
| Field 8 | 4.85 | 4.93 | 902 | 62 | 6.66 | 6.77 | 1238 | 85 | 33 | 0.940 | Pit 2 | 0.73 | 1.1 (N) |
| Field 9 | 4.25 | 4.86 | 1049 | 78 | 5.43 | 6.21 | 1339 | 100 | 26 | 0.937 | Path 1 | 0.78 | 3 (SE) |
| Field 10 | 5.75 | 6.02 | 533 | 50 | 10.85 | 11.35 | 1005 | 102 | 22 | 0.920 | Sup 90 | 0.53 | 1.2 (SW) |
| Field 11 | 5.40 | 7.03 | 533 | 50 | 10.19 | 13.27 | 1005 | 102 | 22 | 0.920 | Sup 90 | 0.53 | 1.2 (SW) |
| Average | 5.1±0.7 | 5.7±1.0 | 754±263 | 62±10 | 8.3±2.7 | 9.4±3.5 | 1147±169 | 97±8 | 26±5 | 0.93±0.01 | - | 0.64±0.13a | - |
EFCO2 estimated based on carbon were available only for the last 4 field experiments (8–11)
- Average dry biomass for all 11 fields is 0.58±0.14 kg m−2
Pit 2: Pisanulok 2; Path1: Pathumthani 1; Sup90: Supanburi 90
MCE: modified combustion efficiency; na: not available.
| (Equation 4) |
Where, EFCO2 is emission factor of CO2 (g m−2 paddy or g kg−1 RS). The emission ratio between PM and CO2 (ERPM/CO2) is calculated using Equation 5.
| (Equation 5) |
Where, ΔPM and ΔCO2 are the net contribution from the burning smoke. The delta was the difference between the concentration in the smoke plume (PMP, CO2,P) and that of background sampling (PMB, CO2,B), namely:
Note that the ΔCO2 here was estimated using the CO2 concentrations recorded at the downwind site (CO2,P) where the PM samplers were located.
3. Results and discussion
3.1 Carbon oxidation efficiency and CO2 EF
Average carbon content in bone dry RS in this study was 49.0±2.7% by weight which appears to be similar to that of general biomass of around 50% (Reid et al., 2004) but higher than the reported value for California RS of 38% (Jenkins et al., 1996). Carbon content was determined for 4 field experiments (8, 9, and combined 10 & 11). The oxidation efficiency (percent Coxidized per total available carbon) varied between the experiments. The average carbon oxidation of >90% was obtained for field 8 and 9 when standing RS was also mostly burned, but was only 69% for field 10 and 11 (Table 3A, SI) when the standing RS stem was mostly unburned. This variation reflects the actual field burning practices observed. The obtained CO2 EF was in the range of 1.15±0.17 kg per kg RS (available/subjected to) burning (Table 1). This is lower than the expected theoretical value because of the unburned C discussed above. Our results for CO2 EF are higher than the reported value of around 0.8 kg per kg RS in a laboratory study in China (Zhang et al. 2008). Similarly, our CO EF results (97±8 g kg−1 RS) are also higher than the value of around 64 g kg−1 RS by Zhang et al. (2008). Note that the average RS dry biomass density in this study for all field and hood experiments was (0.58±0.14) kg m−2 that is comparable with the reported value for USA rice of 0.7 kg m−2 (Hays et al. 2005).
3.2 PM EF from field experiments
PM concentration
Levels measured at the fixed downwind sites of the burning paddies were highly fluctuating (Table 2). This was mainly caused by the varying wind directions observed during the field experiments that deflected the plume away from the equipment from time to time. Thus, average dilution factors of the smoke plume that reached the measurement equipment changed from one experiment to another. In fact, 2 field experiments failed to catch the burning plume due to abrupt changes of wind direction; hence they were excluded from further data analysis. In total, only 11 successful experiments are reported in this paper. For the purpose of this study, the ratios between pollutants (e.g. PM and CO2) and the PM composition are more important than the measured absolute pollutant concentrations. High observed ratios of PM2.5/PM10 in the smoke, above 0.9 (Table 2), suggest the dominance of fine particles in the emission. The coarse particles (PM10-2.5) contributed only a minor fraction of PM10 and the contribution was higher in those experiments when there were intensive fire suppression activities performed to prevent the fire from spreading to surrounding paddies. These activities, which included beating at the edge of the fire with green tree branches, obviously stirred up and suspended coarse ash and soil particles.
Table 2.
PM concentrations (μg m−3) in the field burning experiments (n=11)
| Parameter | PM2.5 | PM10-2.5 | PM10 | PM2.5/PM10 |
|---|---|---|---|---|
| Plume | 1089±1477 | 160±143 | 1168±1611 | 0.92±0.08 |
| Background | 23±14 | 20±16 | 36±28 | 0.57±0.10 |
| Net burning smoke | 1067±1479 | 142±143 | 1134±1614 | 0.93±0.07 |
All concentrations are reported at 25°C, 1 atmosphere
PM emission factor
PM EF values obtained from in situ field burning experiments using the emission ratio are presented in Table 1 only for the last 4 experiments (8 to 11) due to the absence of the C data in the first experiments. The fluctuations in the measured EF between experiments reflects the variations in burning conditions and other parameters including RS moisture, surface soil moisture, biomass density and weather conditions. Field 9, for example, was conducted in rather higher wind conditions (~3m s−1) making combustion more efficient which may be a reason for the lower PM EF. Field 9 was also the experimental paddy with the highest biomass density, and yielded the highest CO2 EF. Lower combustion efficiency in field experiments 10 and 11 plus intensive fire suppression activities may be a reason for the high PM EF in these 2 experiments. Overall, a larger data set is still required to show clear associations between EF, fuel moisture, fuel loading or meteorological conditions.
PM size distribution
The EF results (Table 1, based on dichot samples) show that majority of PM10 was PM2.5. The PM2.5 EF in g m−2, averaged 5.1±0.7, was less variable than the EF in g kg−1 RS, 8.3±2.7. Larger variations in the latter were most probably caused by the fluctuations in biomass density, which is a function of not only rice species but also farming practices. The ratio between PM2.5 EF and PM10 EF, estimated based on the dichot sampling results, is 0.90±0.10.
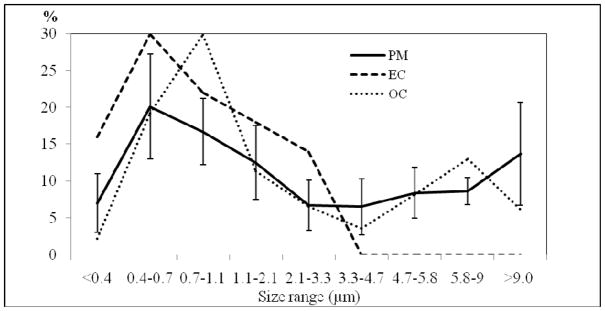
PM EF of different size ranges based on the cascade impactor samples are presented Figure 1. Small particles of the size 0.4–1.1 μm contributed the largest mass fraction of PM, EC and OC. The contribution from PM1.1 and PM2.1 was estimated at 44% and 56% of the SPM mass, respectively. PM1.1 thus contributed about 78% to PM2.1. The estimated EF of the PM2.1 fraction was 6.0±2.6 g m−2 and 9.4±5.1 g kg−1 RS, which is comparable with the PM2.5 EF shown in Table 1 (more detail of EF of different size ranges is given in Table 4A, SI). EF results based on the cascade impactor samples were more variable with higher values for coarse fraction compared to EFs based on the dichot samples. We did not use a pre-separator, therefore PM larger than 10 μm should also be present in the first stage of the cascade impactor. In fact, a high mass of coarse PM was observed on the first stage of the cascade impactor, especially for those samples when intensive fire suppression activities took place.
Figure 1.
Percentage size distribution based on smoke samples by Cascade impactor of PM (averaged for 4 field experiments) and EC/OC (for one experiment: Field 7, Dec 21, 2003, total EC: 40 μg m−3, total OC: 243 μg m−3)
3.3 PM EF from hood experiments
Effects of moisture and MCE
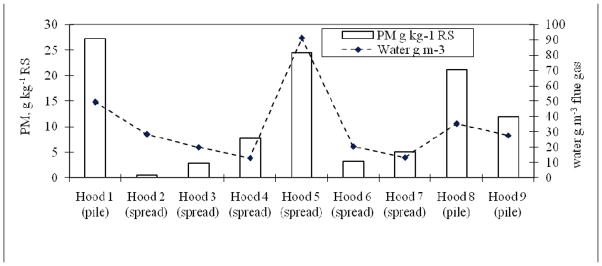
EF fluctuated considerably among 9 hood experiments and, in general, higher values were obtained for the pile burning. Overall, PM EF correlated with the flue gas moisture content (Figure 2). The latter was observed to reflect not only the moisture content of RS but also that of the surface paddy soil. The linear relationship between water content in the flue gas (y) and PM EF, g kg−1 dry RS (x), was y = 1.98x + 12.03 with R2 = 0.59. The high moisture content of RS of Hood 5 (Table 3) is a distinct feature that linked to the high EF for this experiment, even for the spread burning type. An increased period between crop harvest and RS field sampling (7 days) in Hood 2 yielded a lower moisture content in the RS, resulting in more efficient combustion and lower EF.
Figure 2.
SPM emission factors vs. water content in flue gas in hood experiments
Both moisture content and burning types (pile or spread) affect the combustion efficiency. Averaged MCE for Hood 5 was the lowest 0.88, i.e. smoldering phase (Reid et al., 2004), that resulted in higher PM EF, as mentioned above. A linear relationship between the MCE (y) and PM EF (x) was obtained (y = −0.0023x + 0.9462; R2 = 0.76 for hood experiments alone; and y = −0.0022x + 0.9504; R2 = 0.66 for all hood and field experiments). The negative slopes in these equations confirm that more complete combustion with higher MCE would result in lower PM emission.
Pile, spread vs. field burning
With biomass loads of around 1.0–1.3 kg m−2, i.e. 1.5–2.0 times the biomass load for the spread hood burning experiments, the pile burning (Hood 1, 8 and 9) produced higher PM EF (than the spread burning) even when the RS moisture content was in the same range (Table 3). PM EF in g kg−1 RS for hood experiments were estimated assuming an oxidation efficiency of 90%, which is the average carbon oxidation efficiency when standing RS was also partially burned, i.e. similar to Field 8 and 9 (Table 3A, SI). The average EF of hood pile burning was 20±8 g kg−1 RS. It was observed that pile burning SPM samples may also contain coarse fraction (ash) carried up with a high flame. The hood spread burning with typical RS moisture content of that in paddies, i.e. of 22–35% (except for Hood 2 and Hood 5) yielded PM EF of 4.7±2.2 g kg−1 RS or 2.4±1.5 g m−2 which are lower than the EF of PM2.5 obtained in the field experiments (8.3±2.7 g kg−1). The burning in the hood spread experiments was with a low flame and no large ash particles were seen in the collected SPM samples. The PM size range in the hood spread burning experiments is therefore likely to be comparable with the PM2.5 in field experiments, assuming that all PM generated from agricultural biomass open burning would be in the submicron size (Hays et al. 2005). However, the PM EF from the hood spread experiments were unlikely to have been influenced by the coarse particles generated by fire suppression activities that influenced the PM EF for some of the field experiments. Furthermore, the evenly spread RS paddy samples used in these hood experiments would have biomass density less than the RS windrows but higher than the RS standing rows present in the field burning samples. Overall, the RS in the paddy samples prepared for hood spread burning experiments may have been more evenly distributed compared to the experimental fields, and may have burned more efficiently and yielded lower EF. These, together with other factors including the random nature of combustion, may be the causes of the differences observed for the hood spread burning experiments compared to the field spread burning experiments.
A few EF values extending over a wide range have been previously reported in the literature. Overall, our PM2.5 EF for field experiments (8.3±2.7 g kg−1) and SPM EF for hood spread burning (4.7±2.2 g kg−1 are lower than the EF obtained for simulated field burning conditions in an enclosure of 13±0.3 g kg−1 (Hays et al., 2005), whereas our values are higher than the value reported for RS burning in a wind tunnel of 3.9 g kg−1 (Jenkins et al., 1996). The EF obtained for a domestic woodstove burning RS fuel of 6.2±1.0 g kg−1 (Sheesley et al., 2003) is in between our field and hood spread burning values.
Air pollution EF from field burning activities depend upon many variables, hence caution should be taken when applying a specific range of values for the purpose of developing emission inventory. The PM EF obtained in this study for the field experiments would be more relevant to the places where combine harvesters are used (e.g. Thailand), whereas those obtained for hood pile burning may be applicable for places where the manual harvesting technique is common (e.g. many regions of Vietnam or Indonesia, where RS after harvest is piled up at a paddy corner and burned largely under smoldering conditions). However, these real piles would have higher biomass loads than the piles in our hood experiments. If fire is started at the pile base the combustion is even more smoldering hence resulting in larger EF than those obtained in our hood pile experiments.
3.4 PM composition
PM composition is presented for field (PM2.5, PM10) and hood pile burning (SPM) emissions (Table 4). Due to the predominant presence of the analytes in PM2.5, and also that the coarse fraction (PM10-2.5) contributed only a minor fraction (<10%) of the PM10 mass, the fractional compositions of PM2.5 and PM10 of field burning smoke are quite similar, except for some elements. The compositional characterization was more complete for the field experiments compared to hood experiments, hence, in this section we focus the discussion on the field experiment results. Hood samples were analyzed for ions, elements and PAHs in SPM, with no size segregation, therefore the obtained profiles may not be fully comparable with PM2.5 and PM10 profiles obtained from the field experiments.
Table 4.
PM emission factors (g kg−1 RS) and composition (mg g−1 PM)
| Field, PM2.5 | Field, PM10 | Hood pile, SPM | ||
|---|---|---|---|---|
| EF PM, g kg−1 RS burned | 8.3±2.7 (n=4) | 9.4±3.5 (n=4) | 20.1±7.7 (n=3) | |
| PM composition, mg g−1 PM (part per thousand) | ||||
| (n=9) | (n=9) | (n=3) | ||
| EC/OC | EC | 57.7±27.9 | 56.9±25.3 | |
| OC | 335.4±88.0 | 328.6±84.7 | ||
| TC | 393.0±64.0 | 385.5±63.3 | ||
| Ions | Na+ | 2.56±2.77 | 3.53±3.48 | 0.12±0.24 |
| K+ | 50.0±34.0 | 47.3±33.6 | 7.2±10.5 | |
| NH4+ | 23.8±11.7 | 22.8±9.2 | 0.54±0.88 | |
| Mg2+ | 0.11±0.22 | 0.18±0.28 | nd | |
| Ca2+ | 0.15±0.41 | 1.08±1.06 | 0.75±0.90 | |
| F− | 2.45±3.28 | 2.48±3.17 | 0.81±1.36 | |
| Cl− | 69.3±31.6 | 68.6±30.7 | 14.6±17.8 | |
| NO3− | 2.93±3.72 | 2.91±3.20 | 0.56±0.88 | |
| SO42− | 9.82±7.21 | 15.53±13.06 | 3.5±4.1 | |
| Total | 161.2±24.5 | 164.4±24.3 | 28.1±4.9 | |
| Elementa | Fe | 0.038±0.108 | 0.105±0.306 | 0.040±0.025 |
| Ni | 0.036±0.068 | 0.047±0.070 | ||
| Pb | 0.045±0.094 | 0.115±0.197 | 0.049±0.015 | |
| Sr | 0.013±0.029 | 0.016±0.028 | ||
| Ti | 0.015±0.046 | 0.016±0.043 | 0.005±0.002 | |
| V | 0.091±0.130 | 0.140±0.191 | 0.004±0.002 | |
| Zn | 0.036±0.099 | 0.066±0.155 | 0.26±0.15 | |
| Si | 0.14±0.34 | 0.185±0.364 | 5.89±2.88 | |
| Al | 0.18±0.33 | 2.53±2.47 | 0.26±0.04 | |
| Ca | 0.85±1.33 | 2.65±3.40 | 0.50±0.12 | |
| Cd | 0.009±0.023 | 0.009±0.022 | ||
| Cr | 0.080±0.169 | 0.163±0.292 | 0.003±0.002 | |
| Cu | 0.171±0.409 | 0.162±0.391 | ||
| Mg | 0.906±1.710 | 1.739±1.783 | 0.065±0.012 | |
| Mn | 0.005±0.015 | 0.010±0.028 | 0.025±0.026 | |
| S | 1.174±0.404 | 1.24±0.24 | ||
| As | 0.003±0.003 | 0.003±0.004 | ||
| Se | 0.0001±0.0002 | 0.0005±0.0004 | ||
| Br | 0.047±0.011 | 0.044±0.013 | ||
| Rb | 0.036±0.013 | 0.035±0.013 | ||
| Zr | 4E-05±7E-05 | 0.0002±0.0003 | ||
| Ag | 0.004±0.005 | 0.005±0.005 | ||
| Sn | nd | 0.002±0.003 | ||
| Sb | nd | 0.001±0.001 | ||
| Ba | 0.016±0.022 | 0.015±0.016 | ||
| Bi | nd | 0.001±0.001 | ||
| Total | 2.1±0.2 | 4.9±0.5 | 6.5±1.8 | |
| PAHb | Naph | 0.42±0.57 | 0.39±0.53 | 0.012±0.025 |
| Acy | nd | nd | nd | |
| Ace | 0.02±0.07 | 0.02±0.05 | 0.001±0.002 | |
| Flu | nd | nd | 0.005±0.011 | |
| Phe | 0.03±0.07 | 0.02±0.05 | 0.004±0.008 | |
| Ant | 0.02±0.01 | 0.01±0.01 | 0.0003±0.0007 | |
| Fth | 0.50±0.75 | 0.49±0.75 | 0.015±0.029 | |
| Pyr | 0.29±0.33 | 0.26±0.27 | 0.01±0.02 | |
| BaA | 0.12±0.18 | 0.11±0.14 | 0.012±0.024 | |
| Chry | 0.17±0.21 | 0.15±0.17 | 0.01±0.02 | |
| BbF | 0.13±0.12 | 0.12±0.11 | 0.021±0.037 | |
| BkF | 0.05±0.04 | 0.05±0.04 | 0.011±0.022 | |
| BaP | 0.13±0.15 | 0.11±0.13 | 0.013±0.025 | |
| DahA | 0.08±0.10 | 0.08±0.10 | nd | |
| BghiP | 0.02±0.03 | 0.02±0.03 | nd | |
| Ideno | nd | nd | nd | |
| Total PAH | 2.0±0.2 | 1.8±0.1 | 0.11±0.01 | |
| Levoglucosan Methoxy- phenols c | LG | 56.2 | 57.4 | |
| ETGU | 0.0019 | 0.0018 | ||
| EUG | 0.0050 | 0.0047 | ||
| VAN | 0.0294 | 0.0278 | ||
| TISOEU | 0.0034 | 0.0032 | ||
| ACETV | 0.0073 | 0.0069 | ||
| GUACE | 0.0172 | 0.0163 | ||
| ALLSY | 0.0037 | 0.0035 | ||
| PRSY | 0.0029 | 0.0028 | ||
| SYALD | 0.0835 | 0.0797 | ||
| ACETSY | 0.3880 | 0.3715 | ||
| CONIF | nd | 0.0058 | ||
| PRSYON | 0.0622 | 0.0597 | ||
| SINAP | 0.0100 | 0.0109 | ||
| Total meth. | 0.62 | 0.60 | ||
| Total all | 614±42 | 613±41 | ||
nd: not detected; blank cells: not analyzed (for SPM in hood experiments)
EC/OC data available for 2 hood spread burning experiments, not presented in Table 4 (see the text).
- Elements analyzed by PIXE and ICP-OES: Be, Co, Li, Mo, Tl were not detected in any sample
- The detection limits of the PAHs were in the order of 1 ng mL−1 sample extract.
- only 13 detected among 21 analyzed methoxyphenols are presented in Table 4. Detection limits for the methoxyphenols were in the range of 1 – 30 ng mL−1 of sample extract.
Carbon speciation, ions and elements
The largest contribution comes from OC, (average 330 mg g−1 PM), followed by EC (57 mg g−1 PM) and levoglucosan (56–57 mg g−1 PM). The ratio of EC to TC (total carbon) of 0.15 obtained for PM emitted from RS burning was much lower than the value of above 0.70 obtained for PM emitted from diesel vehicles (Kim Oanh et al. 2010) which is a remarkable feature to distinguish between these two source categories.
The majority of EC was found in fine particles. In fact, the cascade impactor samples show that all EC was associated with PM3.3. PM1.1 contained 68% of total EC and 52% of total OC in SPM (Figure 1). The sum of water soluble ionic species for PM2.5 and PM10 were about 160 mg g−1 of PM mass. The relatively high concentrations of these ionic markers are similar to those reported in literature (Hays et al. 2005). However our results for Cl−, K+, SO42− and NH4+ are higher than Hays et al., which may be linked to the application of fertilizers (Potash and urea-based) to the paddy fields by local farmers. Elements typically associated with soil minerals, such as Ca, Al, Mg, Cr and Fe, were higher in PM10 than in PM2.5. For other elements the fractional compositions in PM2.5 and PM10 were mostly similar. The sum of analyzed elements contribute 2.1±0.2 mg g−1 for PM2.5 and 4.9±0.5 mg g−1 for PM10.
Levoglucosan and methoxyphenols
These two classes of organic compounds have been suggested as tracers for PM derived from biomass combustion (Jiminez et al., 2007; Oros and Simoneit, 1999; Kjallstrand et al., 2000; Schauer and Cass, 2000). Levoglucosan is formed via pyrolysis of the wood polymer cellulose, whereas methoxyphenols are formed via pyrolysis of the wood polymer lignan (Jiminez et al., 2007). Therefore, these chemicals can serve as unique tracers for the contribution of biomass combustion to ambient PM. The levoglucosan abundance in PM found in our study (~55 mg g−1 PM) is intermediate between values previously reported by Sheeslay et al. (2003) (~18 mg g−1 PM2.5) and Hays et al. (2005) (~87 mg g−1 PM2.5). Our results are consistent with previous studies of biomass tracers (measured in ambient PM samples impacted by residential wood combustion) which also found that levoglucosan was present almost exclusively in the fine fraction of PM (e.g. Simpson et al., 2004).
Among the 21 analyzed methoxyphenols, four compounds (GU, MEGU, PRGU, MESY) were not quantified and four (SY, CISOEU, ETSY, BUSYON) were not detected in any sample. Table 4 presents only the 13 detected compounds. Acetylsyringone was the most abundant (~0.4 mg g−1 PM) while others were detected at lower concentrations. In a study of RS combustion, Hays et al. (2005) also found that acetylsyringone was the most abundant particle-bound methoxyphenol (0.8 mg g−1 PM2.5). Sheesley et al. (2003) reported much higher concentrations of acetylsyringone (1.5 mg g−1 PM2.5), and other low molecular weight syringols were present in even higher concentrations (e.g. syringol, 5.5 mg g−1 PM2.5). Note that the methoxyphenol measurements in the current study, and those of Hays et al. (2005) and Sheesley et al. (2003), were done only for the particulate phase. Previous studies have shown that methoxyphenols are semi-VOC and distributed between the gaseous and particulate phases in smoke, and low molecular weight compound such as guaiacol and syringol exist almost exclusively in the gas phase under typical ambient temperatures and particle concentrations (Hawthorne et al., 1989; Schauer et al., 2001).
Semi-VOC
Particulate phase concentrations of 16 PAHs, 14 PCBs and 16 OCPs are discussed in this paper. The source profiles of these semi-VOC compounds in the field experiments were estimated assuming that their presence was entirely in the fine fractions (PM2.5). Thus, to estimate the profiles, the mass of a compound found in TSP samples was divided by the mass of PM2.5 and PM10 samples, respectively, which were collected simultaneously with TSP in each field experiment. The 16 PAHs contributed only around 2 mg g−1 PM2.5 and PM10, in the field burning and only 0.11 mg g−1 SPM from the hood pile burning. Beside naphthalene, the most abundant PAHs detected are fluoranthene and pyrene. Other 4 and 5-ring compounds (from BaA to BaP) were also relatively abundant while of the 6-ring compounds BghiP was detected at a low level and IcdP was not detected. The relative abundance of the light PAHs in PM found in this study is similar to other studies for RS burning (Jenkins et al., 1996; Sheesley et al., 2003) and other biomass smoke (Oros and Simoneit, 1999). The ratios of individual PAH to PM found in our study, mostly 0.1–0.2 mg g−1 PM for 4–5 benzene ring compounds, are also similar to those reported by Jenkins et al. (1996) but higher than the ratio for 5 ring PAHs reported by Sheesley et al. (2003) of 0.03–0.04 mg g−1 PM. Fluoranthene, the most abundant PAH in our study, has a higher contribution, averaging 0.5 mg g−1 PM, but varying significantly among the field experiments.
OCP and PCB
Out of 16 OCPs 6 were detected in the filter samples (Table 5). Although the levels found in the smoke PM samples (gas phase not included) were less than 0.5 ng m−3 for individual OCP, their presence certainly increases toxicity of the PM emitted from the field burning activities. In Thailand, by about 2002 the use of all the OCPs analyzed in this study was banned (FDA, 2007). However, remaining OCPs in paddy soil from the over 40 years of past intensive applications may be re-emitted during the burning. For PCB, out of 14 analyzed compounds 9 (plus overlapped peaks of PCB149 and PCB118 with endosulfan II) were detected in particles, generally at levels less than 1 ng m−3. PCBs were found to be ubiquitous in soil, water and air in the region (Iwata et al., 1994) and specifically in airborne particles in Thailand (Pentamwa and Kim Oanh, 2008). PCBs deposited in soil may also be re-emitted during the paddy burning. In addition, we cannot exclude the possibility that some of the chlorinated semi-VOC we detected may be formed during the combustion of RS.
Table 5.
OCP and PCB in PM emitted from field burning (mg g−1 of PM)
| Compounds | Field PM2.5 (n=10) | Field PM10 (n=10) |
|---|---|---|
| OCP | ||
| α-BHC | 0.00011±0.00013 | 0.00010±0.00011 |
| γ-BHC | 3.E-06 ±5.E-06 | 2.E-06±5.E-06 |
| Heptachlor | 0.00013±0.00025 | 0.00011±0.00020 |
| Aldrin | 0.00034±0.00051 | 0.00029±0.00039 |
| Heptachlor epoxide | 0.00056±0.00125 | 0.00049±0.00109 |
| Endosulfan I | 0.0001±0.0003 | 0.00008±0.00023 |
| PCB | ||
| PCB 18 | 0.00002±0.00004 | 0.00002±0.00004 |
| PCB 31 | 0.0044±0.0053 | 0.0040±0.0049 |
| PCB 28 | 0.00015±0.00020 | 0.00013±0.00018 |
| PCB 52 | 0.00033±0.00058 | 0.00027±0.00046 |
| PCB 44 | 0.00029±0.00035 | 0.00025±0.00029 |
| PCB 101 | 0.00005±0.00014 | 0.00004±0.00011 |
| PCB149, 118 & Endo II | 0.00001±0.00004 | 0.00001±0.00003 |
| PCB 153 | nd | 2E-06±5E-06 |
| PCB 180 | 0.00021±0.00043 | 0.00018±0.00036 |
| PCB 209 | 0.00020±0.00046 | 0.00020±0.00045 |
Detection limits (DL) of 11 analyzed PCB were in the range of 0.195–0.3 ng mL−1 while DL of 16 OCP was 0.06–0.35 ng mL−1 (total sample extract was 1.5mL). Only detected compounds are presented in Table 5.
PCB 149, 118 & Endo II: overlapped peaks of PCB149, PCB118, and Endosulfan II
The average reconstructed mass (sum of all analytes minus the double counted elements) is 61–62% of PM2.5 and PM10 mass. There is some double counting of levoglucosan (6%), PAHs (0.3%) and methoxyphenols (0.06%) with OC. If the organic matter (OM) is estimated based on the OC value using a scaling factor of 2.0 (suggested range of 1.4–2.1 by Turpin and Lim, 2001) then the mass closure would be 85–90%. Further speciation of this primary OC may help to improve mass closure and provide additional source signatures.
Hood burning SPM profiles
No detailed composition data are available for hood spread burning experiments except for EC and OC data for Hood 2 and Hood 3 (not presented in Table 4). Higher EC (75 vs. 28 mg g−1 SPM) and lower OC (290 mg g−1 vs. 470 mg g−1 SPM) were obtained in Hood 2 burning (dry RS, 5% moisture) than Hood 3 burning (RS of 22% moisture). The EC/OC content in SPM of these 2 spread burning hood experiments are in the same range of those in PM2.5 and PM10 of the field experiments (Table 4).
For the 3 hood pile burning experiments presented in Table 4 no EC/OC data were available. Available composition data show fractional compositions of PAHs and water soluble ions were substantially lower in SPM from hood pile burns compared to those (in PM2.5 and PM10) obtained from the field (spread burning) experiments (Table 4). Data for the elements/ions was less consistent – some elements (e.g. Si and Zn) were much more abundant in SPM from the hood pile burning, whereas other elements (e.g. Ti and V) were much less abundant in SPM from the hood pile burning. We observed (visually) that SPM in the hood burning samples contained a significant amount of ash and unburned biomass particles which would contribute a high mass fraction but little PAH and elements/ions. This could lead to the lower emission ratios we observed for PAHs, water soluble ions and several of the elements.
3.5 Preliminary estimation of RS burning emission
We used the obtained EF from in situ field burning experiments to roughly estimate the emission from RS field burning in Thailand using only preliminary burning activity data available. OAE (2007) reports that the annual harvesting area of rice paddies in Thailand was 10,227,870 ha, which accounts for both the major crop and the secondary crop. There is also a third rice crop per year cultivated in some areas in the central part of Thailand, but it is harvested in the rainy season and we assumed, for simplicity, that no field burning was done for this crop. Simply assuming that all RS of these two crops are subjected to field burning and using the area based EF (g m−2) for in situ field burning presented above, the RS field burning activity in Thailand would generate, 0.52±0.07 Tg PM2.5, 77±27 Tg CO2 and 6.3±1.0 Tg CO annually. The corresponding EC and OC emitted with PM2.5 would be 0.03±0.01 and 0.18±0.05 Tg, respectively.
Note that the presented values are most likely to be the high estimates of the total RS burning emission in the country. Further investigation is required to obtain realistic burning activities, e.g. the portion of the RS burned in the field, for better emission estimation. Nevertheless, non-burning alternatives or efficient energy recovery from this crop residue would help to relieve the atmosphere from this pollution burden.
The EF obtained in this study can be used to estimate the emission from RS burning in other countries in Asia where comparable conditions exist. The use of the area based EF (g m−2) much simplified the emission calculation as the plantation area is generally known. However, if biomass loadings are not in the same range presented in this study (0.58±0.14 kg RS m−2) the mass based EF (g kg−1) should be used instead.
4. Conclusions
PM EF from RS burning in the field and hood experiments varied considerably depending on a range of factors such as moisture content, meteorological conditions, and fire control activities, which all affected the combustion process and hence the emission. There is a clear relationship between SPM and moisture content of flue gas emitted from the hood experiments. Hood pile burning produced the highest PM EF, especially for the RS and surface soil samples with high moisture content. PM2.5 contributed the majority (about 90%) of PM10 emission. Among the 8 cascade impactor size ranges, the first 2 stages or PM1.1 contributed the largest fraction of SPM mass (44%), EC (68%) and OC (52%). The most abundant components in PM2.5 and PM10 were OC, water soluble ions and levoglucosan. Characteristics of PM emissions from RS burning include EC/TC ratio (~0.15) as well as the high relative abundance of K+, NH4+, Cl−, SO42−, acetylsyringone, fluoranthene and pyrene. These features may serve as potential markers for RS PM. The presence of some OCPs and PCBs in the emitted PM may not only serve as useful signatures for RS PM in source apportionment studies but also emphasize the additional toxicity of the emission. Further investigation on the potential formation and presence of toxic compounds in RS burning smoke would provide useful information for source identification and health risk analysis. A wide range of EF obtained in our study suggests that care should be taken to select the most appropriate EF values, based on fuel properties (especially moisture) and dominant regional burn practices (spread vs. pile burning), when calculating emission inventories.
Supplementary Material
Acknowledgments
The authors would like to thank the Swedish International Development Agency for providing financial support to the research through the AIRPET project coordinated by AIT. Further acknowledgement is extended to the NIH Research Grant # D43 TW0642 funded by the Fogarty International Center, National Institutes on Environmental Health Services, NIOSH, and the Agency for Toxic Substances and Disease Registry, that made levoglucosan and methoxyphenols analyses possible. Collaborators for EC/OC and carbon analysis are duly thanked. The students and staff of the air quality research group at AIT and other partners are acknowledged for the field sampling activities and cooperation in various capacities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreae MO, Merlet P. Emission of trace gases and aerosols from biomass burning. Global Biogeochemical cycles. 2001;15(4):955–966. [Google Scholar]
- ASTM standard D5373-02. Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Laboratory Samples of Coal. ASTM International; West Conshohocken, PA: 2008. Available on http://www.astm.org. [Google Scholar]
- Bond TC, Streets DG, Yarber KF, Nelson SM, Woo J-H, Klimont ZA. Technology-based global inventory of black and organic carbon emissions from combustion. Journal of Geophysical Research. 2004;109(D14203) doi: 10.1029/2003JD003697. [DOI] [Google Scholar]
- Dhammapala R, Claiborn C, Simpson C, Jimenez J. Emission factors from wheat and Kentucky bluegrass stubble burning: Comparison of field and simulated burn experiments. Atmospheric Environment. 2007;41:1512–1520. doi: 10.1021/es062039v. [DOI] [PubMed] [Google Scholar]
- FDA. Thailand’s National Chemicals Management Profile (2005) Chemical Safety Group Thailand Focal Point for WHO/IPCS, and IFCS, Food and Drug Administration, Ministry of Public Health of Thailand. 2007 available on http://ipcs.fda.moph.go.th/e_ipcs/profile/2005/summary.
- Gordon GE. Receptor models. Environmental Science and Technology. 1988;22(10):1132–1142. doi: 10.1021/es00175a002. [DOI] [PubMed] [Google Scholar]
- Hawthorne SB, Krieger MS, Miller DJ, Mathiason MB. Collection and quantification of methoxylated phenol tracers for atmospheric pollution from residential wood stoves. Environmental Science and Technology. 1989;23:470–475. [Google Scholar]
- Hays MD, Fine PM, Geron CD, Kleeman MJ, Gullett BK. Open burning of agricultural biomass: Physical and chemical properties of particle-phase emissions. Atmospheric Environment. 2005;39:6747–6764. [Google Scholar]
- Iwata H, Tanabe S, Sakai N. Geographic distribution of persistent organochlorines in air, water and sediment from Asia and Oceania and their implications for global redistribution from lower latitudes. Environment Pollutions. 1994;85:15–33. doi: 10.1016/0269-7491(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Jenkins BM, Jones AD, Turn SQ, Williams RB. Particle Concentrations, Gas–particle Partitioning, and Species Intercorrelations for Polycyclic Aromatic Hydrocarbons (PAH) Emitted during Biomass Burning. Atmospheric Environment. 1996;30(22):3825–3835. [Google Scholar]
- Jiminez J, Claiborn C, Dhammapala R, Simpson CD. Developing a source fingerprint for burning of wheat and Kentucky bluegrass stubble in Eastern Washington and Northern Idaho. Environmental Science and Technology. 2007;41(22):7824–7829. doi: 10.1021/es062039v. [DOI] [PubMed] [Google Scholar]
- Kadam KL, Forrest LH, Jacobson WA. Rice straw as a lignocellulosic resource: collection, processing, transportation and environmental aspects. Biomass and Bioenergy. 2000;18:369–389. [Google Scholar]
- Kim Oanh NT, Nghiem LH, Phyu YL. Emission of Polycyclic Aromatic Hydrocarbons, Toxicity, and Mutagenicity from domestic cooking using sawdust briquettes, wood and kerosene. Environmental Science & Technology. 2002;36:833–839. doi: 10.1021/es011060n. [DOI] [PubMed] [Google Scholar]
- Kim Oanh NT, Upadhyay N, Zhuang YH, Hao ZP, Murthy DVS, Lestari P, Villarin JT, Chengchua K, Co HX, Dung NT, Lindgren ES. Particulate air pollution in six Asian cities: spatial and temporal distributions, and associated sources. Atmospheric environment. 2006;40:3367–3380. [Google Scholar]
- Kim Oanh NT, Thiansathit W, Bond TC, Subramanian R, Winijkul E, Ittipol Pawarmart I. Compositional characterization of PM2.5 emitted from in-use diesel vehicles. Atmospheric Environment. 2010;44:15–22. [Google Scholar]
- Kjallstrand J, Ramnas O, Peterson G. Methoxyphenols from burning of Scandinavian forest plant materials. Chemosphere. 2000;4:735–741. doi: 10.1016/s0045-6535(99)00427-0. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Engling G, Candice Lung SC, Lee KY. Particle size characteristics of levoglucosan in ambient aerosols from rice straw burning. Atmospheric Environment. 2008;42:8300–8308. [Google Scholar]
- Li XH, Wang SX, Duan L, Hao JM, Li C, Chen YS, Yang L. Particulate and trace gas emissions from open burning of wheat straw and corn stover in China. Environmental Science and Technology. 2007;41:6052–6058. doi: 10.1021/es0705137. [DOI] [PubMed] [Google Scholar]
- OAE (Office of Agriculture Economics) Thailand Agricultural Statistic Yearbook 2007. 2007 available on http://www.oae.go.th/statistic/yearbook50/
- Oros DR, Simoneit BRT. Identification of molecular tracers in organic aerosols from temperate climate vegetation subjected to biomass burning. Aerosol Science and Technology. 1999;31:433–445. [Google Scholar]
- Ortiz de Zarate I, Ezcurra A, Lacaux JP, Dinh PV. Emission factor estimates of cereal waste burning in Spain. Atmospheric Environment. 2000;34:3183–3193. [Google Scholar]
- Pentamwa P, Kim Oanh NT. Annals of the New York Academy of Sciences, 1140, Environmental Challenges in the Pacific Basin. Vol. 1140. 2008. Levels of Pesticides and PCBs in Selected Homes in Bangkok Metropolitan Region, Thailand; pp. 91–112. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Ezzati M, Dockery DW. Fine-Particulate Air Pollution and Life Expectancy in the United States. The New England Journal of Medicine. 2009;360:376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JS, Koppmann R, Eck TF, Eleuterio DP. A review of biomass burning emissions, part II: Intensive physical properties of biomass burning particles. Atmospheric Chemistry and Physics Discussions. 2004;4:5135–5200. [Google Scholar]
- Schauer JJ, Cass GR. Source apportionment of wintertime gas-phase and particle-phase air pollutants using organic compounds as tracers. Environmental Science and Technology. 2000;34:1821–1822. [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BR. Measurement of emissions from air pollution sources. 3. C1-C29 organic compounds from fireplace combustion of wood. Environmental Science & Technology. 2001;35:1716–1728. doi: 10.1021/es001331e. [DOI] [PubMed] [Google Scholar]
- Sheesley RJ, Schauer JJ, Chowdhury Z, Cass GR, Simoneit BRT. Characterization of organic aerosols emitted from the combustion of biomass indigenous to South Asia. Journal of Geophysical Research. 2003;108(D9):4285. [Google Scholar]
- Simpson CD, Katz BS, Paulsen M, Dills RL, Kalman DA. Determination of levoglucosan in atmospheric fine particulate matter. Journal of Air Waste Management Association. 2004;54:689–694. doi: 10.1080/10473289.2004.10470945. [DOI] [PubMed] [Google Scholar]
- Simpson CD, Paulsen M, Dills RL, Liu LJS, Kalman DA. Determination of methoxyphenols in ambient atmospheric particulate: tracers for wood combustion. Environmental Science and Technology. 2005;39(2):631–637. doi: 10.1021/es0486871. [DOI] [PubMed] [Google Scholar]
- Tipayarom D, Kim Oanh NT. Effects from Open Rice Straw Burning Emission on Air Quality in the Bangkok Metropolitan Region. Journal of Science Asia. 2007;33(3):339–345. [Google Scholar]
- Torigoe K, Hasegawa S, Numata O, Yazaki S, Matsunaga M, Boku N, Hiura M, Ino H. Influence of emission from rice straw burning on bronchial asthma in children. Pediatrics. 2000;42:143–150. doi: 10.1046/j.1442-200x.2000.01196.x. [DOI] [PubMed] [Google Scholar]
- Turpin BJ, Lim HJ. Species Contributions to PM2.5 Mass Concentrations: Revising Common Assumptions for Estimating Organic Mass. Aerosol Science and Technology. 2001;35:602–610. [Google Scholar]
- US EPA. Compendium Method TO-13A. U.S Environmental Protection Agency, Center for Environmental Research Information. Office of Research and Development; Cincinnati, OH 45268: 1999a. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Air Using Gas Chromatography/Mass Spectrometry (GC/MS) [Google Scholar]
- US EPA. U.S Environmental Protection Agency, Center for Environmental Research Information. Office of Research and Development; Cincinnati, OH 45268: 1999b. Compendium Method TO-4A: Determination of Pesticides and Polychlorinated Biphenyls in Ambient Air Using High Volume Polyurethane Foam (PUF) Sampling Followed by Gas Chromatographic/Multi-Detector Detection (GC/MD) available on http://www.docstoc.com/docs/7827128/EPA-R. [Google Scholar]
- UNEP and C4. The Asian Brown Cloud: Climate and Other Environmental Impacts. United Nations environment programme (UNEP); Nairobi, Kenya: 2002. [DOI] [PubMed] [Google Scholar]
- UNEP. Atmospheric Brown Clouds: Regional Assessment Report with Focus on Asia. United Nations environment programme (UNEP); Nairobi, Kenya: 2008. [Google Scholar]
- Viana M, López JM, Querol X, Alastuey A, García-Gacio D, Blanco-Heras G, López-Mahía P, Piñeiro-Iglesias M, Sanz MJ, Sanz F, Chi X, Maenhaut W. Tracers and impact of open burning of rice straw residues on PM in Eastern Spain. Atmospheric Environment. 2008;42:1941–1957. [Google Scholar]
- Wu CF, Jimenez J, Claiborn C, Gould T, Simpson CD, Larson T, Sally Liu LJ. Agricultural burning smoke in eastern Washington: Part II: Exposure Assessment. Atmospheric Environment. 2006;40:639–650. [Google Scholar]
- Yang HH, Tsai C-H, Chao MR, Su YL, Chien SM. Source identification and size distribution of atmospheric polycyclic aromatic hydrocarbons during rice straw burning period. Atmospheric Environment. 2006;40:1266–1274. [Google Scholar]
- Zhang H, Ye X, Cheng J, Yang X, Wang L, Zhang R. A laboratory study of agricultural crop residue combustion in China: Emission Factors and Emission Inventory. Atmospheric Environment. 2008;42:8432–8441. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.