ROS-mediated amplification of AKT/mTOR signalling pathway leads to myeloproliferative syndrome in Foxo3−/− mice
This work reports a novel myeloid progenitor phenotype in Foxo3a-deficient mice that exhibit increased reactive oxygen species signalling and reduced expression of Lnk, a negative regulator of mitogenic cytokine signalling. Thus, the paper unveils a molecular link from Foxo3a deficiency to malignant progression.
Keywords: FoxO, Lnk, mTOR, myeloproliferation, ROS
Abstract
Reactive oxygen species (ROS) participate in normal intracellular signalling and in many diseases including cancer and aging, although the associated mechanisms are not fully understood. Forkhead Box O (FoxO) 3 transcription factor regulates levels of ROS concentrations, and is essential for maintenance of hematopoietic stem cells. Here, we show that loss of Foxo3 causes a myeloproliferative syndrome with splenomegaly and increased hematopoietic progenitors (HPs) that are hypersensitive to cytokines. These mutant HPs contain increased ROS, overactive intracellular signalling through the AKT/mammalian target of rapamycin signalling pathway and relative deficiency of Lnk, a negative regulator of cytokine receptor signalling. In vivo treatment with ROS scavenger N-acetyl-cysteine corrects these biochemical abnormalities and relieves the myeloproliferation. Moreover, enforced expression of Lnk by retroviral transfer corrects the abnormal expansion of Foxo3−/− HPs in vivo. Our combined results show that loss of Foxo3 causes increased ROS accumulation in HPs. In turn, this inhibits Lnk expression that contributes to exaggerated cytokine responses that lead to myeloproliferation. Our findings could explain the mechanisms by which mutations that alter Foxo3 function induce malignancy. More generally, the work illustrates how deregulated ROS may contribute to malignant progression.
Introduction
Oxidative stress, broadly defined as an imbalance between generation and detoxification of reactive oxygen species (ROS), is deleterious to cells and implicated in a number of degenerative diseases and malignancies (reviewed in Beckman and Ames, 1998). In addition, excess accumulation of ROS impacts cellular aging, whereas the ability to resist oxidative stress is associated with evolutionary conserved enhanced longevity (Beckman and Ames, 1998). Although ROS are considered to be toxic byproducts of cellular metabolism, increasing evidence support the notion that ROS have a critical role in normal cellular signalling. In particular, ROS are generated by cytokine signalling and impact the function of a rapidly expanding list of numerous effectors (reviewed in Thannickal and Fanburg, 2000). How these activities affect normal and pathological physiology is not fully understood.
ROS are particularly deleterious to hematopoietic stem cells, specifically as they age (Ito et al, 2004; Miyamoto et al, 2007; Tothova et al, 2007; Yalcin et al, 2008; and reviewed in Ghaffari, 2008). A tightly controlled balance between hematopoietic stem and progenitor cell compartments maintains normal blood cell homeostasis throughout life. Alterations of this balance result in various disorders including leukaemias or bone marrow failure. For instance, myeloproliferative disorders are a group of hematopoietic malignancies whose incidence increase with age, exhibit enhanced proliferation and survival of one or more myeloid lineage cells that arises from an unbalanced expansion of hematopoietic myeloid progenitor cells (Tefferi and Gilliland, 2007).
The Forkhead FoxO family of transcription factors are critical regulators of oxidative stress and exert this function at least partly by upregulating the expression of several anti-oxidant enzymes (Kops et al, 2002; Nemoto and Finkel, 2002; Murphy et al, 2003; Marinkovic et al, 2007; Tothova et al, 2007; Yalcin et al, 2008). FoxO1, FoxO3 and FoxO4 are wildly expressed while FoxO6 is predominantly expressed in neuronal tissues. Loss of a single FoxO leads to distinct phenotypes in mice, underscoring their diverse non-redundant functions in vivo (Castrillon et al, 2003; Hosaka et al, 2004). In particular, female Foxo3-deficient mice exhibit a premature infertility associated with ovarian follicle depletion early on in life (Castrillon et al, 2003; Hosaka et al, 2004). In addition, both Foxo3−/− hematopoietic stem and erythroid cell compartments exhibit enhanced susceptibility to oxidative stress (Marinkovic et al, 2007; Miyamoto et al, 2007; Yalcin et al, 2008).
FoxOs also regulate cellular responses to genotoxic stress, consistent with a tumour suppressor function (Paik et al, 2007). In response to stress such as DNA damage or oxidative stress, FoxOs induce cell cycle arrest, repair damaged DNA or initiate apoptosis by modulating genes that control these processes (Brunet et al, 1999; Dijkers et al, 2000; Medema et al, 2000; Nakamura et al, 2000; Tran et al, 2002; Alvarez et al, 2003; Ghaffari et al, 2003; Marinkovic et al, 2007; Yalcin et al, 2008). FoxO genes are also found at chromosomal breakpoints in certain cancers, including acute myeloid leukaemias (FoxO3 and FoxO4) (Borkhardt et al, 1997; Hillion et al, 1997). Moreover, FoxO3 regulates the expression and activity of ataxia telangiectasia-mutated protein kinase, suggesting an important role in the maintenance of genomic stability (Tsai et al, 2008; Yalcin et al, 2008).
Function of FoxO is restrained primarily by the phosphoinositide-3-kinase (PI3-kinase)/AKT signalling pathway (Biggs et al, 1999; Brunet et al, 1999; Dijkers et al, 2000; Kashii et al, 2000; Nakae et al, 1999; Rena et al, 1999; Tang et al, 1999; and reviewed in Greer and Brunet, 2008). The AKT serine threonine protein kinase regulates a wide range of metabolic processes through phosphorylation of numerous effectors, including FoxO and mammalian target of rapamycin (mTOR) (Gingras et al, 1998; Brunet et al, 1999; Inoki et al, 2002; Manning et al, 2002) a kinase that stimulates cell growth and proliferation through multiple effectors including ribosomal S6 kinase (S6K1) and the eukaryotic initiation factor 4E-binding protein.
In response to cytokines, growth factors or oncoproteins, activated AKT kinase directly phosphorylates FoxO on three conserved residues, resulting in their nuclear exclusion and subsequent degradation (Biggs et al, 1999; Brunet et al, 1999; Matsuzaki et al, 2003; Plas and Thompson, 2003; Hu et al, 2004). In contrast, stress stimuli, or inhibition of PI3-kinase/AKT signalling pathway by growth factor/cytokine withdrawal, induce FoxO's nuclear localization, thereby enhancing their transcriptional activity (Essers et al, 2004; Lehtinen et al, 2006; van der Horst et al, 2006). The PI3-kinase/AKT signalling pathway is activated in numerous human and animal malignancies, although how this contributes to the pathogenesis of these diseases is not entirely clear (Ugo et al, 2004; Bellacosa et al, 2005; Dai et al, 2005; Yilmaz et al, 2006; Zhang et al, 2006). In addition to AKT, a number of kinases regulate the activity of FoxO both positively and negatively. In addition to phosphorylation, FoxO proteins are subject of several other post-translational modifications such as acetylation, methylation and ubiquitination whose combined integrated signals determine the activity of FoxOs.
Recent findings have established a critical function for FoxO family members in the regulation of normal and malignant hematopoietic stem cell activity (Miyamoto et al, 2007; Tothova et al, 2007; Yalcin et al, 2008; Naka et al, 2010). In particular, Foxo3's suppression of ROS is essential for the maintenance of hematopoietic stem cell quiescence and homeostasis (Miyamoto et al, 2007; Yalcin et al, 2008). In addition, abnormal repression of Foxo3 has been implicated in the pathogenesis of myeloproliferative disorders and other haematological malignancies (Ghaffari et al, 2003; Komatsu et al, 2003; Fernandez de Mattos et al, 2004; Essafi et al, 2005). Despite these findings, of the entire scope of Foxo3 functions, regulation of hematopoietic progenitors (HPs) is not fully defined (Miyamoto et al, 2007; Yalcin et al, 2008).
Here, we show that loss of Foxo3 results in a myeloproliferative syndrome in mice. We further demonstrate that increased ROS accumulation in Foxo3−/− primitive myeloid progenitors activates the cytokine-induced AKT/mTOR signalling pathway and expands Foxo3-deficient primitive myeloid progenitors. Accordingly, this myeloproliferative syndrome is ameliorated by systemic administration of ROS scavengers. Moreover, Lnk (SH2B3), a negative regulator of cytokine signalling, is directly implicated in this process. Our combined data indicate that Foxo3 modulates HP homeostasis by controlling cytokine-dependent production of, and response to, ROS. These cumulative findings illustrate new mechanisms through which deregulated ROS could contribute to the development of malignancies.
Results
Foxo3−/− mice exhibit a myeloproliferative syndrome
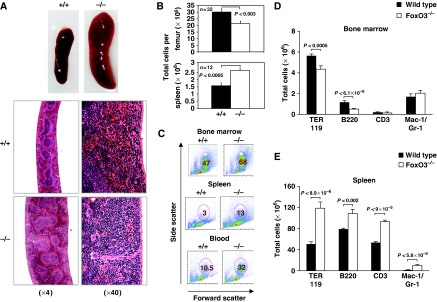
Foxo3−/− mice display increased white blood cell counts, with an increased circulating neutrophils (P<0.03) and monocytes (P<0.01), and a concomitant reduction of circulating lymphocytes (P<0.005) and red blood cells (Table I; Marinkovic et al, 2007). These anomalies of the peripheral blood are associated with myeloproliferative syndrome (Figure 1). Foxo3-deficient mice exhibit an enlarged spleen (Figure 1A), increased number of splenocytes (Figure 1B) and extramedullary hematopoiesis (Figure 1C, D and E, Supplementary Figure 1A), with increased frequency of erythrocytic and granulocytic lineages (Supplementary Figure 1). Concomitantly, the bone marrow is hypocellular (Figure 1B) with decreased production of mature B and erythroid cells (Figure 1D; Supplementary Figure 1; Marinkovic et al, 2007). Histopathology analysis corroborated these findings, showing increased extramedullary hematopoiesis containing erythroid and myeloid cells in the spleen and liver of Foxo3−/− mice (Figure 1A and Supplementary Figure 2).
Table 1. Comparison of blood parameters of Foxo3+/+ and Foxo3−/− mice.
| Parameters | Foxo3+/+ | Foxo3−/− | P-value |
|---|---|---|---|
| WBC (× 1000/μl) | 9.45±1.13 | 13.48±1.16 | 0.01 |
| Neutrophils (%) | 8.98±1.66 | 13.80±1.76 | 0.03 |
| Lymphocytes (%) | 83.69±1.98 | 75.55±2.09 | 0.005 |
| Monocytes (%) | 2.98±0.58 | 6.87±1.59 | 0.01 |
| Eosinophils (%) | 2.70±0.25 | 2.33±0,39 | 0.21 |
| Basophils (%) | 1.34±0.32 | 0.95±0.09 | 0.14 |
| Results for wild type (n=10) and Foxo3−/− (n=21) blood are shown as mean±s.e.m. The analyses are from at least three independent experiments. | |||
Figure 1.
Myeloproliferative-like syndrome in Foxo3−/− mice. (A) Representative whole-mount (upper panels) and histological sections (lower panels) of spleens from 11-week-old wild-type (+/+) and Foxo3−/− (−/−) mice. Increased extramedullary myeloid hematopoiesis in the red pulp and minimal depletion of marginal zone lymphocytes with the retention of the T-cell regions in Foxo3−/− spleen, as compared with the wild-type are shown. (B) Total number of bone marrow (n=32) and spleen cells (n=12) is shown, Student's t-test. (C) Representative FACS plots of FSC versus SSC of bone marrow, spleen and blood of wild-type and Foxo3−/− mice are shown. Percentages of FSChighSSChigh (granulocytic) cells are marked. (D) Total number of bone marrow cells in each lineage is plotted. Total number of bone marrow erythroid (TER 119, n=11), B (B220, n=14) and T cells (CD3, n=11) and neutrophils (Gr-1/Mac-1, n=11) is shown. (E) Total number of cells in each lineage of the spleen, TER 119 (n=8), B220 (n=8), CD3 (n=11) and Gr-1/Mac-1 (n=11) (Student's t-test). The analyses are from at least four independent experiments.
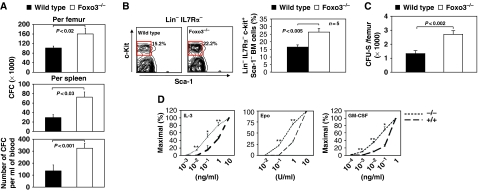
In agreement with a myeloproliferative syndrome, the myeloid progenitor compartment is significantly enhanced in the bone marrow, spleen and peripheral blood of Foxo3-deficient mice (Figure 2A and Supplementary Figure 3). In particular, myeloid colony-forming unit-granulocyte-macrophage-derived colonies are increased in numbers and size (Supplementary Figure 3 and data not shown).
Figure 2.
Enhanced hematopoietic progenitor activity in Foxo3−/− mice. (A) Progenitor-derived colonies were measured in the bone marrow, spleen and blood of wild-type and Foxo3−/− mice. The analyses are from four independent experiments in each of which two to three animals were either pooled or analysed independently. (B) Frequency (left panel) of highly enriched myeloid progenitor Lin− IL7Rα− Sca-1− c-Kit+ compartment (right panel) in wild-type and Foxo3−/− bone marrow is shown (n=5 in each group, Student's t-test). The frequency of Sca-1− c-Kit+ cells within Lin− IL7Rα−-gated cells is shown (the frequency of Lin− IL7Rα− Sca-1− c-Kit+ cells within bone marrow is 1.3±0.11% for wild type and 2.2±0.2% for Foxo3−/−). One representative of three independent experiments is shown. (C) CFU-S-derived colonies formed in the spleen were measured 12 days after in vivo injection of 105 wild-type or Foxo3−/− bone marrow cells into lethally irradiated hosts; representative of two independent experiments, n=5 in each group is shown, Student's t-test. (D) Colony-forming cell ability of wild-type and Foxo3−/− progenitors was measured after plating 105 cells in semi-solid methylcellulose cultures in three replicates in the presence of limiting doses of the indicated cytokines (colonies of 20 or more cells were counted after 8 days and the numbers of colonies present at each cytokine concentration were calculated as percentages of the number formed in the highest concentration of the indicated cytokine). Mean+s.e.m. of three independent experiments, each pool of two to three mice. Student's t-test; *P<0.05, **P<0.01.
Similarly, the size of primitive myeloid progenitor pool was enhanced in Foxo3−/− bone marrow (Figure 2B). The compartment of colony-forming-spleen day 12 (CFU-Sd12)-derived colonies was also increased (P<0.002; Figure 2C), further supporting an expansion of the early myeloid progenitors. In addition, Foxo3−/− HP cells were overly sensitive to cytokines (Figure 2D) and generated significantly larger-size colonies in vitro (Supplementary Figure 3 and data not shown), which are the hallmarks of myeloproliferative disorders (Ghaffari et al, 1999; Levine and Gilliland, 2008). These data suggest that Foxo3 suppresses HP production and proliferation by inhibiting cytokine signalling. These findings were surprising, as Foxo3−/− hematopoietic stem cells are not highly cycling in vivo and do not generate excessive number of HPs in culture (Yalcin et al, 2008), suggesting that these observations were not simply the result of a highly proliferative hematopoietic stem cell compartment in Foxo3−/− mice.
ROS-mediated amplification of AKT/mTOR signalling pathway enhances primitive HP compartment in Foxo3−/− mice
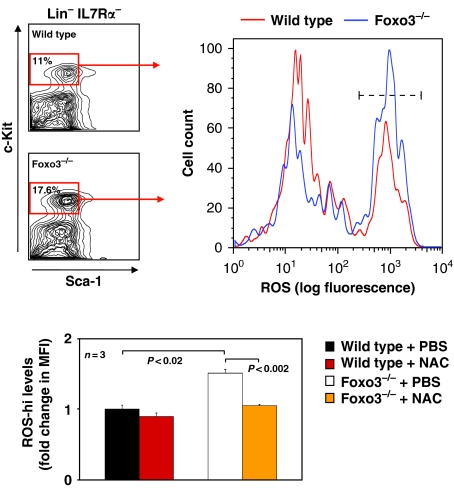
Foxo3 suppresses ROS in many cell types, including in hematopoietic cells, by regulating a programme of anti-oxidant gene expression (Kops et al, 1999; Nemoto and Finkel, 2002; Marinkovic et al, 2007; Miyamoto et al, 2007; Yalcin et al, 2008). Accordingly, Foxo3-mutant lineage-negative bone marrow cells exhibited reduced expression of several anti-oxidant enzyme genes (Supplementary Figure 4). In addition, ROS were significantly overaccumulated in different Foxo3−/− subpopulations of lineage-negative cells enriched for myeloid progenitors (Figure 3 and Supplementary Figure 5). ROS concentrations were highly enhanced (approximately 1.6-fold, P<0.02; Figure 3) in Foxo3−/− Lin− IL7Rα− Sca-1− c-Kit+ cells, a population that encompasses all myeloid progenitors (Akashi et al, 2000), and increased significantly in freshly isolated Foxo3−/− common myeloid progenitors (CMP), as compared with their wild-type counterparts (approximately 1.2-fold, P<0.03; Supplementary Figure 5). CMP is a highly pure population of hematopoietic cells giving rise to megakaryocyte/erythrocyte and granulocyte/monocyte progenitors (Akashi et al, 2000). Similar results were obtained from analysis of ROS accumulation in total Foxo3-deficient bone marrow depleted from lineage-restricted cells (data not shown). In vivo treatment of mice with ROS scavenger N-acetyl-cysteine (NAC, 100 mg/kg), normalized the levels of ROS in Foxo3−/− Lin− IL7Rα− Sca-1− c-Kit+ cell population (Figure 3), supporting the specificity of ROS measurement. Interestingly, these experiments revealed two distinct populations of ROS-containing cells (ROS-high or ROS-hi and ROS-low) in primitive myeloid progenitors of both wild-type and Foxo3−/− origin. The significant increase of ROS observed in Foxo3−/− Lin− IL7Rα− Sca-1− c-Kit+ cell population was entirely in ROS-hi fraction (Figure 3). This ROS-hi subpopulation may be the one that elicits cellular responses, such as increased cell cycle or apoptosis, to oxidative stress in primitive myeloid progenitors.
Figure 3.
Enhanced ROS accumulation in Foxo3−/− primitive myeloid progenitors. Representative FACS profile of bone marrow Lin− IL7Rα− Sca-1− c-Kit+ that contain 98% of all myeloid progenitor cells (Akashi et al, 2000) (left panel). Frequency of c-Kit+ Sca1− cells within Lin− IL7Rα− cells is shown. Endogenous ROS concentrations were measured in freshly isolated Lin− IL7Rα− Sca-1− c-Kit+ cells (right panel) from wild-type or Foxo3−/− mice treated daily in vivo with NAC (100 mg/kg) or PBS for 15 days; fold change in mean fluorescence intensity (MFI) of ROS in gated subpopulations (ROS-hi using - - - - - - - gate), as compared with control wild-type cells treated with PBS is shown as mean±s.e.m., n=3; Student's t-test. One of two independent experiments is shown.
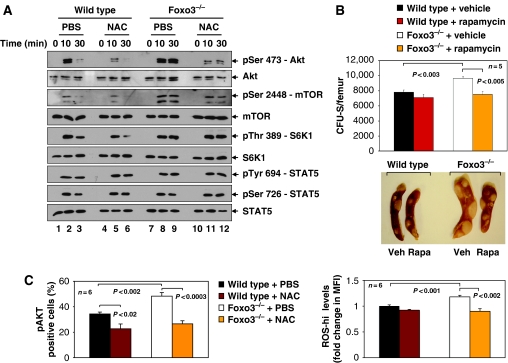
To investigate the mechanisms of enhanced myeloproliferation caused by loss of Foxo3, we interrogated cytokine-mediated activation of principal signalling pathways in bone-marrow-derived HPs. Freshly isolated bone marrow cells were depleted of mature lineages by immunoselection (Lin− cells), and subjected to cytokine starvation followed by stimulation with interleukin-3 (IL-3). Myeloid progenitors constitute a significant majority of Lin− cells expressing IL-3 receptor at the steady state. To our surprise, IL-3 stimulation of Foxo3−/− Lin− cells led to hyperphosphorylation of AKT, mTOR and mTOR substrate S6K1 (Figure 4A). In contrast, STAT5 proteins, another effector of IL3 signalling, were not affected in these cells (Figure 4A). Similar results showing specific hyperactivation of the AKT/mTOR pathway in Foxo3−/− cells were obtained with other cytokines such as erythropoietin (Epo, data not shown). In agreement with in vivo overactivation of AKT/mTOR signalling pathway mediating enhanced generation of early myeloid progenitors in Foxo3−/− mice, in vivo administration of the mTOR inhibitor rapamycin resulted in significant reduction of Foxo3−/−-derived CFU-Sd12, as compared with controls in lethally irradiated hosts (Figure 4B).
Figure 4.
mTOR mediates the enhancement of Foxo3−/− hematopoietic progenitor cell compartment. (A) Western blot analysis of phosphorylation of signalling proteins in lineage-negative bone marrow cells isolated from wild-type and Foxo3−/− mice (n=4). Mice were administered daily with NAC (100 mg/kg) or PBS in vivo for 3 days, after which lineage-negative cells were isolated, serum- and cytokine starved for 2 h and stimulated with IL-3 (20 ng/ml) for the indicated time points (0, 10 and 30 min) in vitro in the absence or presence of NAC (100 μM) before preparing the whole cell extract; representative immunoblot of three independent experiments is shown. (B) Number of CFU-Sd12-derived colonies formed in the spleens of lethally irradiated mice reconstituted with 105 wild-type or Foxo3−/− bone marrow cells detected after 12 days during which mice were administered either rapamycin (Rapa; 4 mg/kg) or vehicle (Veh) intraperitoneally for 5 days a week. Results shown are mean±s.e.m. (n=5 in each group, Student's t-test). One representative of three independent experiments is shown. Representative spleen from each group is shown in the lower panel. (C) ROS levels (right panel) and phosphorylated AKT protein kinase (left panel) were measured by FACS in Lin− IL7Rα− Sca-1− c-Kit+ bone marrow cells of wild-type and Foxo3−/− mice treated daily with NAC or PBS for 2 weeks in vivo, after which isolated cells were serum- and cytokine starved for 2 h and stimulated with IL-3 (20 ng/ml) in vitro for 10 min. Results are mean±s.e.m. of percentage of Lin− IL7Rα− Sca-1− c-Kit+ bone marrow cells that express phosphoAKT (pAKT), as detected by FACS (n=6 in each group, Student's t-test). Endogenous ROS-hi levels were measured in Lin− IL7Rα− Sca-1− c-Kit+ bone marrow cells of the same mice as in the left panel, at the end of the 2 week in vivo treatment (right panel), and shown as fold change in MFI of experimental as compared with control wild-type cells (n=6 in each group, Student's t-test). Animals were analysed individually. One of two independent experiments is shown.
Normal cytokine signalling, including signalling by IL-3 (Sattler et al, 1999), is mediated in part by ROS in in vitro cultured cells (Thannickal and Fanburg, 2000; Finkel, 2003). Thus, we investigated whether abnormal increase of ROS contributes to the hyperactivation of AKT/mTOR signalling pathway caused by loss of Foxo3 in primary cells in vivo. We treated mice with the ROS scavenger NAC (100 mg/kg), and tested IL-3 signalling responses by examining phosphorylation of downstream targets AKT, mTOR and S6K1 (Figure 4A). In vivo treatment with NAC specifically reduced the intensity of IL-3-mediated phosphorylation of AKT and mTOR in Foxo3-mutant cells enriched for HPs. Surprisingly, reduction in phospho-mTOR in response to NAC did not impact phosphorylation of the mTOR target S6K1 in Foxo3 mutants, as compared with normal hematopoietic cells (Figure 4A). Although the mechanism of lack of pS6K1 response to NAC in Foxo3-mutant cells is not clear, it is possible that the residual pmTOR kinase activity, despite the presence of NAC (Figure 4A, lanes 11 and 12), is sufficient for phosphorylation of S6K1 to the same extent as controls, especially if the activity of a phospho-S6K1-specific phosphatase is reduced in Foxo3−/− cells. NAC treatment did not alter STAT5 activity, as determined by phosphorylation on tyrosine 694 or serine 726 (Figure 4A).
Interestingly, NAC treatment specifically attenuated IL-3-mediated phosphorylation of AKT and S6K1 in wild-type HPs (Figure 4A), suggesting that ROS participate in specific cytokine signalling pathways in primary bone marrow cells in vivo. Next, we asked whether this data remains valid in populations of lineage-negative bone marrow cells that contain all myeloid but not lymphoid progenitors. We found that AKT was hyperphosphorylated in response to IL-3 in Foxo3−/− myeloid (Lin− IL7Rα− Sca-1− c-Kit+) progenitors, as measured by flow cytometric analysis of intracellular pAKT (Figure 4C). NAC treatment reduced pAKT significantly in this highly enriched population of myeloid progenitors, in both wild-type and Foxo3−/− mice (Figure 4C, Supplementary Figure 6). As anticipated, in vivo treatment with NAC reduced ROS concentrations significantly in Foxo3−/− Lin− IL7Rα− Sca-1− c-Kit+ bone marrow cells (Figure 4C, right panel).
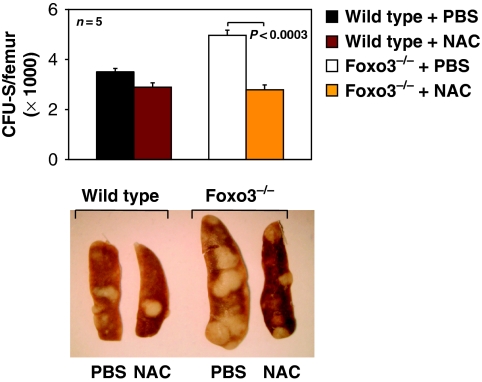
Importantly, and in agreement with the results above, in vivo administration of NAC normalized the number and the size of multipotential Foxo3−/−-derived CFU-Sd12 in lethally irradiated hosts without any significant impact on wild-type CFU-Sd12 (P<0.0003, n=5; Figure 5). Taken together, these results indicate that ROS specifically amplify cytokine-mediated AKT/mTOR signalling pathway to stimulate the expansion of HPs in Foxo3−/− mice.
Figure 5.
NAC treatment corrects the expansion of Foxo3−/− primitive multipotential hematopoietic progenitor compartment in vivo. CFU-S-derived colonies were measured 12 days after injection of 105 wild-type or Foxo3−/− bone marrow cells into lethally irradiated hosts and treated daily with NAC (100 mg/kg) or PBS. One representative of three independent experiments is shown (n=5 in each group, Student's t-test). Representative spleen of each group is shown in the bottom panel.
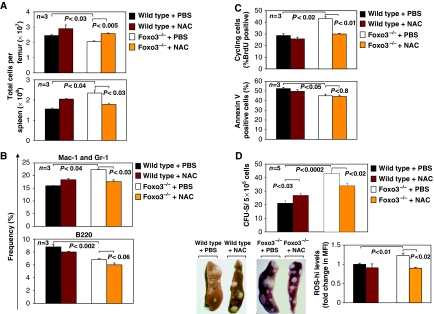
To investigate whether accumulation of ROS in myeloid progenitors contributes to the pathogenesis of the myeloproliferative syndrome, we subjected wild-type and Foxo3-deficient mice to a short, 15-day treatment with NAC. Interestingly, in vivo NAC administration treated some of the myeloproliferative symptoms in Foxo3-deficient mice (Figure 6, Supplementary Figure 6). The in vivo treatment with NAC normalized the total number of bone marrow and spleen cells in Foxo3−/− mice (Figure 6A). This treatment also had a specific and significant effect on the frequency and total number of immature myeloid (Mac-1/Gr-1 positive) cells in the bone marrow without any impact on B cells (Figure 6B and Supplementary Figure 6).
Figure 6.
NAC treatment ameliorates myeloproliferative syndrome in Foxo3−/− mice in vivo. (A) Mice were treated daily with NAC (100 mg/kg) or PBS and their total number in the bone marrow and spleen was measured after 15 days (n=3 in each group). (B) Frequency of myeloid (Mac-1 and Gr-1 positive) and B (B220 positive) cells in the bone marrow of mice from A (n=3 in each group). (C) Percentage of BrdU- (upper) and annexin-V-binding positive (lower) cells in wild-type and Foxo3−/− Lin− IL7Rα− Sca-1− c-Kit+ cells was analysed by flow cytometry after 15 days in vivo of NAC (100 mg/kg) or PBS treatment of mice from A (n=3 in each group). (D) Wild-type or Foxo3−/− mice were treated daily with NAC (100 mg/kg) or PBS for 2 weeks after which bone marrow cells were isolated and injected (5 × 105 cells) into lethally irradiated hosts in the absence of any further treatment. CFU-S-derived colonies were counted in the hosts 12 days after cell injection, n=5 in each group; Student's t-test. Representative spleen from each group is shown in the lower left panel. ROS-hi concentrations were measured in an aliquot of Lin− IL7Rα− Sca-1− c-Kit+ cells isolated from each mouse 15 days after in vivo NAC or PBS treatment. ROS levels are shown as fold change in MFI as compared to control wild type cells (bottom right panel). One representative of two independent experiments is shown.
To identify the mechanism by which ROS regulate the myeloproliferative syndrome, we asked whether ROS impact the proliferation or apoptosis rate of Foxo3−/− myeloid progenitor cells. Thus, mice were treated in vivo with NAC and the rate of 5-bromo-2-deoxyuridine (BrdU) incorporation in Lin− IL7Rα− Sca-1− c-Kit+ cells was measured. We found that Foxo3−/− myeloid progenitors exhibit a highly and significantly increased proliferation, as compared with their wild-type counterparts (Figure 6C, top panel). This was not accompanied by any modification of their apoptotic rate (Figure 6C, bottom panel). NAC treatment significantly reduced the percentage of cycling Foxo3-deficient myeloid progenitor cells (Figure 6C, top panel), without having any effect on their apoptotic rate (Figure 6C, bottom panel). ROS regulation of proliferation of Foxo3−/− primitive myeloid progenitor cells is in accord with our earlier findings of ROS-induced amplification of AKT/mTOR signalling pathway in these cells (Figure 4). We further asked whether pretreatment with NAC is sufficient to normalize the activity of Foxo3−/− primitive HPs in transplanted hosts in vivo. As shown in Figure 6D, pretreatment of Foxo3−/− mice with NAC significantly decreased the number of multipotential CFU-Sd12-derived colonies formed in the spleen of lethally irradiated hosts (upper panel and lower left panel), likely through reduction of the levels of ROS in primitive myeloid progenitors (Figure 6D, bottom right panel). These results strongly suggest that the myeloproliferation in Foxo3−/− mice is mediated by ROS and is sensitive to NAC. Altogether, these results indicate that the treatment with ROS scavenger NAC improves, at least partially, the myeloproliferative phenotype of Foxo3−/− mice.
Lnk, a negative regulator of cytokine signalling, is directly implicated in enhanced Foxo3−/− primitive HP cell activity
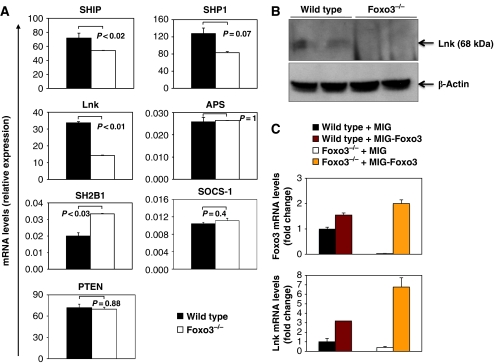
To further examine how Foxo3 regulates HP expansion, we used real-time QRT–PCR to compare the expression of numerous regulators of cytokine signalling in lineage-depleted (Lin−) cells from WT and Foxo3−/− bone marrow. The function of some of negative regulators is known to be modulated by ROS (Thannickal and Fanburg, 2000); thus, we initially focused on negative regulators. The expression of most negative regulators of cytokine signalling was not significantly affected by loss of Foxo3. These include protein tyrosine phosphatase (SHP1, PTPN6) a negative regulator of JAK2 signalling, suppressor of cytokine signalling 1, which negatively regulates STATs, and PTEN, a negative regulator of PI3-kinase/AKT signalling (Figure 7A). However, the expression of SH2-containing inositol-5-phosphatase (SHIP), another negative regulator of PI3-kinase signalling, was significantly reduced. In addition, the expression of the adaptor protein Lnk (SH2B3), a negative regulator of cytokine signalling (Takaki et al, 2002; Velazquez et al, 2002; Tong and Lodish, 2004; Tong et al, 2005), was significantly downregulated in Foxo3−/− lineage-negative progenitors, as determined by QRT–PCR (Figure 7A) and confirmed by western blot analysis (Figure 7B). Altered Lnk expression is due to loss of Foxo3, as overexpression of Foxo3 in primitive bone marrow cells significantly enhanced Lnk expression (Figure 7C). Lnk (SH2B3) belongs to a family of SH2-containing adaptor proteins, which also includes SH2-B1 and APS (SH2B2) (Rudd, 2001). Expression of SH2-B1, which is a positive regulator of cytokine signalling, was significantly increased in Foxo3-mutant cells (Figure 7A). Thus, deregulation of Lnk and SH2-B1 could contribute to the enhanced cytokine responses observed in Foxo3−/− progenitors.
Figure 7.
Deregulated expression of modulators of cytokine signalling in Foxo3−/− hematopoietic progenitor cells. (A) QRT–PCR expression analysis of cytokine receptor signalling regulatory genes in lineage-negative wild-type and Foxo3−/− cells. Quantification of target genes is relative to β-actin. Results are mean±s.e.m. of duplicate analysis of at least three cDNAs, each generated from a pool of two to three wild-type or Foxo3−/− mice; Student's t-test. (B) Western blot analysis of endogenous Lnk protein in total bone marrow cells isolated from two wild-type and two Foxo3−/− mice; representative immunoblot of three independent experiments is shown. (C) QRT–PCR analysis of Foxo3 (upper panel) and Lnk (lower panel) expression in mononuclear cells derived from 5-FU-treated wild-type and Foxo3−/− mice, transduced with MSCV-IRES-GFP (MIG) vector control or MIG-Foxo3; results are mean±s.e.m. of three independent experiments.
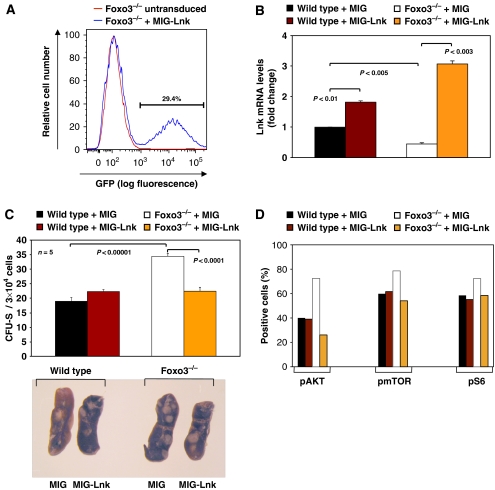
Lnk-deficient mice exhibit a myeloproliferative-like disorder similar to what we observe here with loss of Foxo3 (Takaki et al, 2002; Velazquez et al, 2002). Thus, we asked whether decreased expression of Lnk contributes to the myeloproliferation observed in Foxo3-mutant mice. To address this, mice were treated with high-dose 5-fluorouracil (5-FU), which ablates proliferating hematopoietic cells (Suda et al, 1983; Lemieux et al, 1995), and populations of bone marrow mononuclear cells highly enriched for primitive hematopoietic stem and progenitors were isolated and transduced with the bicistronic retroviral vector MSCV-IRES-GFP (MIG) alone or with MIG encoding for Lnk (MIG-Lnk). Forced expression of Lnk (Figure 8A) was confirmed in an aliquot of transduced GFP-positive wild-type or Foxo3−/− primitive hematopoietic cells (Figure 8B). Retrovirally transduced cells from Figure 8A and B were injected into lethally irradiated hosts and CFU-Sd12 were measured after 12 days. Ectopic expression of Lnk in contrast to vector control normalized the number of CFU-Sd12 derived from Foxo3-mutant mice detected in lethally irradiated hosts (Figure 8C), indicating that the relative loss of Lnk contributes to the overactivation of the CFU-Sd12 compartment in Foxo3−/− mice. This is likely by constraining cytokine-mediated hyperactivation of AKT/mTOR signalling pathway, as ectopic expression of Lnk, but not of vector control, in primitive Foxo3−/− hematopoietic cells reduced the phosphorylation of AKT, mTOR and the S6K1-substrate S6 ribosomal protein in these cells in response to IL-3 (Figure 8D, Supplementary Figure 7).
Figure 8.
Decreased expression of Lnk is critical for increased activity of primitive hematopoietic progenitor cell compartment in Foxo3-deficient mice. (A) Flow cytometry profile of GFP expression in wild-type or Foxo3−/− bone marrow lineage-negative cells derived from 5-FU-treated mice and transduced with MIG encoding for Lnk (MIG-Lnk; FACS profile was almost identical with MIG vector control). (B) QRT–PCR analysis of Lnk expression in FACS-sorted retrovirally transduced GFP+ gated cells from A; results are mean±s.e.m. of three independent experiments; Student's t-test (C) Number of CFU-S-derived colonies formed in the spleens of lethally irradiated hosts (n=5 in each group) reconstituted with 5-FU-treated bone marrow mononuclear cells transduced with retroviral vector MIG or MIG-Lnk. FACS-sorted GFP-positive (3 × 104)-transduced primitive hematopoietic wild-type or Foxo3−/− cells (from A, B) were injected into lethally irradiated hosts and spleen colonies were counted 12 days later. Results shown as mean±s.e.m. (n=5 mice); Student's t-test. Representative spleens are shown in the lower panel. One representative of three independent experiments is shown. (D) Quantification of phosphorylated AKT, mTOR and S6 proteins in mononuclear cells derived from 5-FU-treated wild-type or Foxo3−/− mice transduced with MIG vector control or MIG-Lnk in response to IL-3. The percentage of cells with phoshorylated AKT, mTOR or S6 proteins was measured in GFP+ gated cells. Results shown are mean of two independent experiments.
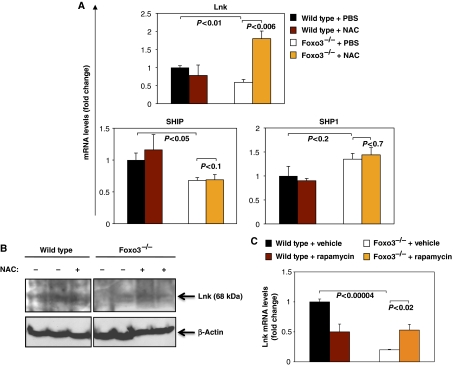
Foxo3 could regulate Lnk expression through a number of different mechanisms. We could not detect any consensus FoxO binding site in regulatory regions of mouse Lnk gene that is conserved among mammals, suggesting the absence of direct transcriptional effects. Therefore, we investigated whether elevated ROS concentrations inhibit Lnk gene expression. Thus, mice were treated with NAC (100 mg/kg) or PBS for 3 days in vivo, and bone marrow myeloid progenitors (Lin− IL7Rα− Sca-1− c-Kit+) were analysed for Lnk expression. NAC treatment significantly increased the expression of Lnk mRNA in Foxo3−/− myeloid progenitors, without having any impact on the expression levels of SHIP or SHP1 (Figure 9A). In addition treatment with NAC did not affect the expression of Lnk in wild-type cells. This was confirmed by western blot analysis of Lnk (Figure 9B). Consistent with these results, in vitro treatment of bone marrow cells with hydrogen peroxide reduced Lnk expression, although this effect was dependent on H2O2 concentration (Supplementary Figure 8). Given ROS-mediated activation of mTOR signalling (Figure 4), the effect of ROS on Lnk (Figure 9A, B and Supplementary Figure 8) and the impact of Lnk on AKT/mTOR signalling (Figure 8D and Supplementary Figure 7), we asked whether mTOR signalling has any impact on the expression of Lnk. As shown in Figure 9C, rapamycin treatment increased Lnk expression in bone marrow cells to some extent, suggesting that mTOR signalling participates in the regulation of Lnk expression. The mTOR regulation of Lnk expression might be through its control of ROS (Supplementary Figure 9). These results suggest that intracellular ROS concentrations are critical for the regulation of expression of the negative regulator of cytokine signalling Lnk.
Figure 9.
Regulation of Lnk expression is mediated by ROS in Foxo3−/− primitive myeloid progenitor cells. (A) QRT–PCR gene expression analysis of regulators of cytokine signalling in Lin− IL7Rα− Sca-1− c-Kit+ bone marrow cells from mice (n=3) treated in vivo for 3 consecutive days with NAC (100 mg/kg) or PBS. One representative of three independent experiments is shown. (B) Western blot analysis of endogenous Lnk protein in total bone marrow cells isolated from wild-type and Foxo3−/− mice daily treated with NAC (100 mg/kg) or PBS in vivo for 3 days. A representative immunoblot of two independent experiments is shown. (C) QRT–PCR analysis of Lnk expression in total bone marrow cells isolated from wild-type and Foxo3−/− mice and treated in vitro with rapamycin (2 μM) or vehicle for 24 h (n=3).
Discussion
Excessive ROS concentrations lead to abnormal proliferation, growth and malignancies (Neumann et al, 2003; Ito et al, 2004). Here, we have identified deregulation of sensitivity of cellular signalling to physiological ROS as an additional mechanism by which ROS compromise normal growth and lead to malignancies. Our key finding is that overaccumulated ROS in Foxo3−/− mice exert a critical role in expansion of primitive HP cell compartment and the regulation of myeloproliferation in these mice. We have shown that Foxo3−/− mice display a myeloproliferative syndrome characterized by splenomegaly, abnormal enhanced production of primitive HPs in hematopoietic organs, extramedullary hematopoiesis, high sensitivity of HPs to cytokines and significant increase in the production of white blood cells. This phenotype is reminiscent of myeloproliferative disorders and suggests that Foxo3 has a role in these diseases. In addition, sensitivity of the Foxo3−/− myeloproliferative phenotype to ROS scavenger NAC suggests that this compound may have some effect in myeloproliferative diseases in general.
Overaccumulation of ROS is the principal mediator of the amplification of Foxo3−/− primitive HP pool, as ROS scavenger NAC normalized the number of Foxo3−/−-derived CFU-Sd12 and the cycling of Foxo3−/− primitive myeloid progenitors, and ameliorated the myeloproliferative syndrome phenotype of Foxo3−/− mice (Figures 3, 4, 5, 6). One could hypothesize that potential DNA damage in ROS-hi subpopulation (Figure 3) of Foxo3−/− Lin− IL7Rα− Sca-1− c-Kit+ cells may contribute to acquisition of growth-enhancing mutations in this cell population, leading to malignant progression.
Our results suggest that ROS regulation of Lnk is an integral part of balancing cytokine receptor signalling by regulating their sensitivity to various modulations of physiological ROS. Relative decrease of Lnk expression as a result of loss of Foxo3 leads to enhanced AKT/mTOR signalling pathway, increased cycling and expansion of Foxo3-mutant HP cells (Figure 8D and Supplementary Figure 7). Several recent reports establish a strong link between alteration of Lnk expression and myeloproliferative syndromes in mouse and man (Baran-Marszak et al, 2010; Bersenev et al, 2010; Oh et al, 2010), further highlighting the potential importance of ROS regulation of Lnk signalling pathway in understanding the underlying mechanism of myeloproliferative syndromes.
ROS are generated by oncoproteins and several cytokine and growth factor stimuli (Sundaresan et al, 1995; Thannickal and Fanburg, 1995; Irani et al, 1997; Sattler et al, 1999, 2000; Vafa et al, 2002; Zhu et al, 2006). Although the exact mechanism of generation of ROS by cytokine receptor signalling in non-phagocytic cells is not known, it is believed that by modifying the function of many signalling proteins, ROS participate in normal cellular signalling and regulate cell proliferation (reviewed in Thannickal and Fanburg, 2000; Ghaffari, 2008). Here, we have shown that, in addition to its known targets such as tyrosine phosphatase 1B (PTPB1, PTPN1) and PTEN (Hecht and Zick, 1992; Barford et al, 1994; Barrett et al, 1999a, 1999b; Meng et al, 2002, 2004; Savitsky and Finkel, 2002; Finkel, 2003; Seo et al, 2005), ROS regulate the expression of the adaptor protein Lnk. While ROS regulated the activity of these phosphatases, ROS control Lnk through modulation of expression of its transcript. Although the exact mechanism of this control is not known, ROS are known to modulate the expression and activity of several transcription factors including the AP1 complex (Thannickal and Fanburg, 2000). Lnk regulatory regions contain several evolutionarily conserved AP1 sites, suggesting that ROS might regulate Lnk expression through modulation of AP1 activity.
Interestingly, it was reported recently, while this paper was under preparation, that in Drosophila, Lnk negatively regulates lifespan via control of oxidative stress (Slack et al, 2010). In addition, these studies suggested that dFoxo represses Lnk expression directly. These findings are consistent with our results here, given that Lnk functions as a positive regulator of insulin receptor signalling and PI3-kinase in Drosophila, in contrast to its roles in mammals (Werz et al, 2009).
The high sensitivity of AKT and mTOR phosphorylation (Figure 4) in Foxo3−/− myeloid progenitor compartment to the administration of anti-oxidants (Figures 5, 6) suggests that the expansion of this population is by AKT/mTOR amplification and is mediated by ROS. ROS-mediated amplification of mTOR signalling pathway is consistent with the redox modulation of the interaction of mTOR with its regulator raptor (Sarbassov and Sabatini, 2005) and its repression of oxidative stress in hematopoietic cells (Chen et al, 2008). Collectively, these findings strongly suggest that loss of Foxo3 amplifies ROS-mediated cytokine signalling, in particular AKT/mTOR signalling pathway, in primitive myeloid progenitor cells in vivo. Importantly, the short period of anti-oxidant treatments in all these experiments (Figures 4, 5, 6, 8) strongly suggests that a hematopoietic stem cell effect is unlikely.
AKT protein kinases are frequently activated in a broad array of tumours, including haematological malignancies (reviewed in Bellacosa et al, 2005; Bhaskar and Hay, 2007; Manning and Cantley, 2007). Here, we found a specific overactivation of AKT/mTOR signalling in response to cytokines that contributes significantly to the enhanced generation of primitive myeloid progenitors in Foxo3-deficient mice (Figures 3, 4, 5, 6). In contrast to the overactivation of AKT/mTOR signalling, we did not detect any significant increase in phosphorylation of STAT5 (Ser 726 or Tyr 694), another major target of cytokine signalling, in response to cytokines in Foxo3-mutant cells (Figure 4A). One potential explanation might be that STAT5 follows a distinct phosphorylation kinetic in wild-type lineage-negative bone marrow cells, as compared with AKT or mTOR.
The phenotype in Foxo3−/− mice resembles human myeloproliferative disorders that include chronic myeloid leukemia (CML), polycythemia vera, essential thrombocythemia and primary myelofibrosis. Constitutive suppression of Foxo3 is critical for growth and survival of cells transformed by BCR-ABL, the key mediator of CML pathogenesis (Ghaffari et al, 2003; Komatsu et al, 2003). Our present findings support the notion that constitutive suppression of Foxo3 activity by oncoproteins in myeloproliferative disorders such as by BCR-ABL in CML (Ghaffari et al, 2003; Komatsu et al, 2003; Naka et al, 2010) or, potentially, by JAK2V617F in polycythemia vera, may be a significant contributor to the pathogenesis of these disorders. In addition, our results in Foxo3-deficient mice recapitulate the myeloproliferative phenotype of the triple Foxo-deficient mice (Tothova et al, 2007). Together, these findings indicate that Foxo3 is the principal FoxO operating in hematopoietic stem, as we and others have previously reported (Miyamoto et al, 2007, 2008; Yalcin et al, 2008), and in progenitor cells, as shown in this study, and have an essential non-redundant function in hematopoietic homoeostasis. In agreement with this, Foxo3 was recently shown to be critical for the maintenance of leukaemic stem cells in CML (Naka et al, 2010). Despite similarities in our findings, there are few distinctions between our results and that of Miyamoto et al (2007) on Foxo3 regulation of hematopoietic stem and progenitor cells, including lack of Foxo3−/− myeloproliferation in Miyamoto et al's report. These discrepancies are likely due to distinct mouse strains used. As previously reported (Dejean et al, 2009), loss of Foxo3 did not lead to lymphoproliferation at the steady state (Table I, Figure 1 and Supplementary Figures 1 and 10), suggesting that the lymphoproliferative phenotype observed in Foxo3 gene-trap mice (Lin et al, 2004) might be due to the strategy or, as has been previously suggested (Dejean et al, 2009) to the mixed strain used to generate these mice.
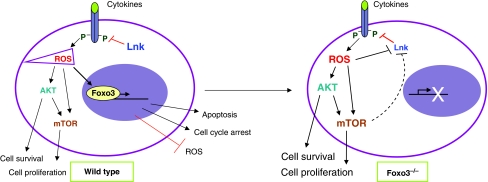
On the basis of these results, we propose a working model that postulates that, in contrast to hematopoietic stem cells in which Foxo3 is constitutively active (Yamazaki et al, 2006; Yalcin et al, 2008), in HPs transcriptional activity and nuclear localization of Foxo3 are modulated by cytokine signalling and ROS. In turn, Foxo3 balances the regulation of cytokine signalling and ROS (see Figure 10). This model projects that ROS amplification of cytokine signalling participates in the activation of HP cells, producing more ROS that will mobilize Foxo3 to the nucleus once accumulated above a certain threshold. Indeed, it is known that ROS accumulation stimulates nuclear localization of FoxO via activation of multiple kinases (Greer and Brunet, 2008). Thus, according to this model, nuclear Foxo3 will in turn suppress ROS and/or induce apoptosis/cell cycle arrest in the progenitor population to maintain hematopoietic homeostasis.
Figure 10.
Model for Foxo3 regulation of hematopoietic progenitor cell activity. In hematopoietic progenitors, cytokine receptor signalling generates ROS that further activate receptor signalling (AKT/mTOR), leading to cell proliferation, increased production of ROS and ultimate induction of Foxo3 nuclear localization once ROS are accumulated above certain threshold. Signalling is modulated by cytokine receptor regulators including Lnk, a negative regulator. In Foxo3−/− hematopoietic progenitors, decreased expression of Lnk, associated with significant increase in ROS accumulation, enhance cytokine-mediated signalling, leading to myeloproliferation.
Our combined results indicate that abnormal expression of Lnk coupled with accumulation of ROS leads to amplified cytokine signalling, in particular AKT/mTOR signalling pathway, and altered hematopoietic homeostasis in Foxo3−/− mice. Thus, modulations of ROS accumulation significantly amplify alterations of cytokine signalling and may lead to malignancies. These findings may be critical in understanding the role of anti-oxidant pathways in promoting malignancies and cellular aging.
Materials and methods
Mice
The generation and genotyping of mice were performed as previously described (Castrillon et al, 2003). Progenies, aged 8–12 weeks, of Foxo3+/− mice (129 × FBV/n) backcrossed to FVB/n were intercrossed with littermates to generate the experimental cohort (F6). In transplantation experiments, Foxo3+/− mice backcrossed 10 generations onto C57BL6 were used. Wild-type littermates were used as controls in all experiments. Protocols were approved by the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine.
Cells
Blood samples were collected and lineage-negative cells were separated using a mouse progenitor cell enrichment kit (StemCell Technologies, Canada), as previously described (Marinkovic et al, 2007; Yalcin et al, 2008). Bone marrow mononuclear cells were isolated using lympholyte M density separation medium according to manufacturer's protocol (Cederlane, Hornby, Ontario, Canada).
In vitro clonogenic progenitor assay
Myeloid clonogenic assays were performed as previously described (Ghaffari et al, 2006; Zhao et al, 2006; Yalcin et al, 2008). Cells (5 × 106 peripheral blood, 5 × 105 spleen and 3 × 104 bone marrow cells) were cultured in MethoCult 3234 (StemCell Technologies) containing 50 ng/ml rat stem cell factor (SCF), 10 ng/ml IL6, 10 ng/ml IL3 and 3 U/ml Epo (all from PeproTech EC, Rocky Hill, NJ, USA). Colonies were counted after 8–10 days.
Colony-forming unit spleen assay day 12
Bone marrow (1 × 105) cells from 8 to 10-week-old wild-type or Foxo3−/− littermates or GFP-positive transduced cells were isolated and intravenously injected into recipient C57BL6 mice (Charles River Laboratory) previously subjected to 10 Gy irradiation. Recipient spleens were excised 12 days later, fixed in Telleyesniczky's solution and macroscopic spleen colonies were counted as described (Till and Mc, 1961).
Flow cytometry and cell sorting
Antibody staining and flow cytometry analysis were previously described (Marinkovic et al, 2007; Yalcin et al, 2008). For CMP isolation (Lin− IL-7Rα− Sca-1− c-Kit+ FcγRlowCD34+) (Akashi et al, 2000), cells were stained with anti-c-Kit (BD Biosciences), anti-FcR (eBioscience) and anti-CD34 (eBioscience) antibodies directly conjugated with fluorescein isothiocyanate, phycoerythrin or allophycocyanin, and with biotinylated multi-lineage monoclonal antibody cocktail (StemCell Technologies) as well as biotinylated antibodies against IL-7R (eBioscience) and Sca-1 (BD Biosciences) visualized with PECyc7-Streptavidin or Pacific Blue-Streptavidin.
To measure intracellular AKT phosphorylation, Lin− IL7-Rα− Sca-1− c-Kit+ cells were fixed with fix/permeabilization buffer (BD Biosciences) and incubated with 1:50 dilution of anti-pSer473 AKT antibody (Cell Signaling Technology). To measure protein phosphorylation in response to Lnk expression, transduced cells were starved in vitro in Iscove's modified Dulbecco's medium (IMDM) with 0.1% FCS for 2 h, stimulated with IL-3 (10 ng/ml) and fixed, and optimum phosphorylation was detected by flow cytometry (15 and 45 min for pmTOR/pS6 and pAKT, respectively). Cells were incubated with 1:100 dilution of anti-pSer473 AKT, pSer2448 mTOR and pSer235/236 S6 antibodies (Cell Signaling Technology). Samples were washed and protein phosphorylation was analysed in GFP+-gated cells by flow cytometry.
Cell proliferation and apoptosis assays
Mice were injected intravenously with 2 mg of BrdU. At 19 h post injection, bone marrow Lin− IL7Rα− Sca-1− c-Kit+ cells were fixed and stained with anti-BrdU antibody (BD Biosciences) for flow cytometric analysis of cell proliferation.
Freshly isolated Lin− IL7Rα− Sca-1− c-Kit+ cells were assayed for annexin-V (BD Biosciences) binding to measure apoptotic cells.
Measurement of intracellular ROS
ROS were measured as previously described (Marinkovic et al, 2007; Yalcin et al, 2008).
RNA isolation and QRT–PCR
Total RNA was isolated using RNeasy Mini Kit (Qiagen). First-strand cDNA was synthesized using SuperScript (Invitrogen). Quantitative RT–PCR was performed using SYBR Green JumpStart Taq ReadyMix (Takara) in duplicates using ABI Prism 7900 HT Cycler (Applied Biosystems). Gene-specific primers spanning intron–exon boundary were designed by Primer Express 2.0 (ABI) and subjected to BLAST analysis to ensure the primer specificity. The PCR cycle parameters were as follows: 95°C for 10″ followed by 45 cycles at 95°C for 5″, 60°C for 34″ and 72°C for 30″. Relative quantification was achieved using the sequence detection system software (Applied Biosystems) and a fractional cycle number at which threshold fluorescence was obtained (threshold cycle, CT); for each analysis, comparative CT method for quantification of the target genes relative to β-actin as the reference was used. Results shown as fold change are relative to wild type controls. Primer sequences are listed in Supplementary Table 1.
Western blot analysis
Freshly isolated lineage-negative bone marrow cells were starved in vitro for 2 h in IMDM with 0.1% FCS and then stimulated with IL-3 (20 ng/ml) for the indicated time points. Cells were harvested and lysates were prepared in 1 × RIPA lysis buffer (20 mM sodium phosphate, 300 mM sodium chloride, 4 mM EDTA) containing 2% sodium deoxycholate, 2% NP-40, 0.2% SDS, 400 μM sodium orthovanadate, 0.2% β-mercaptoethanol, 2 mM PMSF and 100 mM sodium fluoride and protease cocktail inhibitors (Roche). Samples were run on SDS–PAGE, blotted and probed with following antibodies at 1:1000 dilutions: anti-pSer473 AKT, anti-AKT, anti-pThr389 S6Kinase, anti-S6Kinase, anti-pSer2448 mTOR, anti-mTOR and anti-pTyr694 STAT5 (all from Cell Signaling Technology) and anti-Lnk (M-20; Santa Cruz). Anti-pSer726 STAT5 (Upstate Biotechnology) and anti-STAT5 (BD Biosciences) were used at final concentrations of 1:500 and 1:200, respectively.
Histology
Spleen and liver tissues from 11-week-old wild-type or FoxO3−/− littermates were collected and paraffin embedded after 10% formalin fixation. Sections (8–10 μm) were stained with hematoxylin and eosin for histology analysis.
In vivo treatment with N-acetyl-L-cysteine or rapamycin
Mice received intraperitoneal administration of 100 mg/kg of body weight NAC (Sigma, St. Louis, MO, USA) in PBS (pH 7.4) for the indicated time period, as previously described (Yalcin et al, 2008). Cultured cells were incubated with NAC (100 μM) for the indicated time period. Rapamycin (Sigma) was administrated intraperitoneally (4 mg/kg in PBS containing 5% Tween 80, 5% PEG 400 and 4% ethanol) for 5 days a week (Yilmaz et al, 2006).
For in vitro rapamycin treatment, total bone marrow cells isolated from wild-type and Foxo3−/− mice were cultured with rapamycin (final concentration 2 μM) or vehicle for 24 h after which mRNA was isolated for QRT–PCR analysis.
Retroviral production and transduction of 5-FU-treated bone marrow mononuclear cells
Retroviral supernatants were produced as previously described (Zhao et al, 2006). Bone marrow mononuclear cells isolated from mice treated 4 days previously with 5-FU (150 mg/kg, Sigma) were pre-stimulated for 2 days in IMDM containing 15% heat-inactivated FCS supplemented with IL-6 (10 ng/ml), IL-3 (6 ng/ml) and SCF (100 ng/ml; PeproTech EC), after which cells were resuspended in retroviral supernatants (multiplicity of infection of 10) for 2 consecutive days and plated on retronectin-coated dishes in IMDM–15% FCS containing the same factors. At 48 h after initiation, live GFP-positive cells were FACS sorted and used for experiments.
Statistical analysis
The unpaired two-tail Student's t-test was used for all experiments. A P-value of <0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank Ron DePinho (Dana Farber Cancer Institute) for kindly providing Foxo3+/− mice and Italas George and Marcos Grisotto for FACS sorting. We also thank Carlo Brugnara (Children's Hospital, Boston) for blood cell analysis, Nai-Wen Chi (UCSD) for discussions and Mitch Weiss (University of Pennsylvania) for critical reading of this manuscript. Cell sorting was performed at the Flow Cytometry Shared Research Facility of Mount Sinai School of Medicine. This work was supported, in part, by National Institutes of Health Grant RO1 DK077174, an American Cancer Society Research Scholarship (RSG LIB-110480), a Black Family Stem Cell Institute Exploratory Research Award and a Roche Foundation for Anemia Research (RoFAR) Award to SG.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akashi K, Traver D, Miyamoto T, Weissman IL (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197 [DOI] [PubMed] [Google Scholar]
- Alvarez B, Garrido E, Garcia-Sanz JA, Carrera AC (2003) Phosphoinositide 3-kinase activation regulates cell division time by coordinated control of cell mass and cell cycle progression rate. J Biol Chem 278: 26466–26473 [DOI] [PubMed] [Google Scholar]
- Baran-Marszak F, Magdoud H, Desterke C, Alvarado A, Roger C, Harel S, Mazoyer E, Cassinat B, Chevret S, Tonetti C, Giraudier S, Fenaux P, Cymbalista F, Varin-Blank N, Le Bousse-Kerdiles MC, Kiladjian JJ, Velazquez L (2010) Expression level and differential JAK2-V617F-binding of the adaptor protein Lnk regulates JAK2-mediated signals in myeloproliferative neoplasms. Blood (advance online publication) [DOI] [PubMed] [Google Scholar]
- Barford D, Flint AJ, Tonks NK (1994) Crystal structure of human protein tyrosine phosphatase 1B. Science 263: 1397–1404 [PubMed] [Google Scholar]
- Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, Chock PB (1999a) Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem 274: 34543–34546 [DOI] [PubMed] [Google Scholar]
- Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB (1999b) Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38: 6699–6705 [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78: 547–581 [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR (2005) Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res 94: 29–86 [DOI] [PubMed] [Google Scholar]
- Bersenev A, Wu C, Balcerek J, Jing J, Kundu M, Blobel GA, Chikwava KR, Tong W (2010) Lnk constrains myeloproliferative diseases in mice. J Clin Invest 120: 2058–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N (2007) The two TORCs and Akt. Dev Cell 12: 487–502 [DOI] [PubMed] [Google Scholar]
- Biggs WH III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96: 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkhardt A, Repp R, Haas OA, Leis T, Harbott J, Kreuder J, Hammermann J, Henn T, Lampert F (1997) Cloning and characterization of AFX, the gene that fuses to MLL in acute leukemias with a t(X;11)(q13;q23). Oncogene 14: 195–202 [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868 [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA (2003) Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301: 215–218 [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P (2008) TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 205: 2397–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Chung IJ, Krantz SB (2005) Increased erythropoiesis in polycythemia vera is associated with increased erythroid progenitor proliferation and increased phosphorylation of Akt/PKB. Exp Hematol 33: 152–158 [DOI] [PubMed] [Google Scholar]
- Dejean AS, Beisner DR, Ch'en IL, Kerdiles YM, Babour A, Arden KC, Castrillon DH, DePinho RA, Hedrick SM (2009) Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol 10: 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ (2000) Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol 20: 9138–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH, Lam EW (2005) Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene 24: 2317–2329 [DOI] [PubMed] [Google Scholar]
- Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23: 4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez de Mattos S, Essafi A, Soeiro I, Pietersen AM, Birkenkamp KU, Edwards CS, Martino A, Nelson BH, Francis JM, Jones MC, Brosens JJ, Coffer PJ, Lam EW (2004) FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol Cell Biol 24: 10058–10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T (2003) Oxidant signals and oxidative stress. Curr Opin Cell Biol 15: 247–254 [DOI] [PubMed] [Google Scholar]
- Ghaffari S (2008) Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 10: 1923–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari S, Daley GQ, Lodish HF (1999) Growth factor independence and BCR/ABL transformation: promise and pitfalls of murine model systems and assays. Leukemia 13: 1200–1206 [DOI] [PubMed] [Google Scholar]
- Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R (2003) Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proc Natl Acad Sci USA 100: 6523–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari S, Kitidis C, Zhao W, Marinkovic D, Fleming MD, Luo B, Marszalek J, Lodish HF (2006) AKT induces erythroid-cell maturation of JAK2-deficient fetal liver progenitor cells and is required for Epo regulation of erythroid-cell differentiation. Blood 107: 1888–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N (1998) 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev 12: 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A (2008) FOXO transcription factors in ageing and cancer. Acta Physiol (Oxf) 192: 19–28 [DOI] [PubMed] [Google Scholar]
- Hecht D, Zick Y (1992) Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate in vitro. Biochem Biophys Res Commun 188: 773–779 [DOI] [PubMed] [Google Scholar]
- Hillion J, Le Coniat M, Jonveaux P, Berger R, Bernard OA (1997) AF6q21, a novel partner of the MLL gene in t(6;11)(q21;q23), defines a forkhead transcriptional factor subfamily. Blood 90: 3714–3719 [PubMed] [Google Scholar]
- Hosaka T, Biggs WH III, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC (2004) Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA 101: 2975–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC (2004) IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117: 225–237 [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657 [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ (1997) Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 275: 1649–1652 [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T (2004) Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431: 997–1002 [DOI] [PubMed] [Google Scholar]
- Kashii Y, Uchida M, Kirito K, Tanaka M, Nishijima K, Toshima M, Ando T, Koizumi K, Endoh T, Sawada K, Momoi M, Miura Y, Ozawa K, Komatsu N (2000) A member of Forkhead family transcription factor, FKHRL1, is one of the downstream molecules of phosphatidylinositol 3-kinase-Akt activation pathway in erythropoietin signal transduction. Blood 96: 941–949 [PubMed] [Google Scholar]
- Komatsu N, Watanabe T, Uchida M, Mori M, Kirito K, Kikuchi S, Liu Q, Tauchi T, Miyazawa K, Endo H, Nagai T, Ozawa K (2003) A member of forkhead transcription factor FKHRL1 is a downstream effector of STI571-induced cell cycle arrest in BCR-ABL-expressing cells. J Biol Chem 278: 6411–6419 [DOI] [PubMed] [Google Scholar]
- Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM (1999) Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398: 630–634 [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419: 316–321 [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A (2006) A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125: 987–1001 [DOI] [PubMed] [Google Scholar]
- Lemieux ME, Rebel VI, Lansdorp PM, Eaves CJ (1995) Characterization and purification of a primitive hematopoietic cell type in adult mouse marrow capable of lymphomyeloid differentiation in long-term marrow ″switch″ cultures. Blood 86: 1339–1347 [PubMed] [Google Scholar]
- Levine RL, Gilliland DG (2008) Myeloproliferative disorders. Blood 112: 2190–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Hron JD, Peng SL (2004) Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 21: 203–213 [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC (2002) Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell 10: 151–162 [DOI] [PubMed] [Google Scholar]
- Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S (2007) Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117: 2133–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A, (2003) Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 100: 11285–11290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM (2000) AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404: 782–787 [DOI] [PubMed] [Google Scholar]
- Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK (2004) Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem 279: 37716–37725 [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK (2002) Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9: 387–399 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Tabuko K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motayama N, Suda T, Hirao A (2007) Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1: 101–112 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, Suda T (2008) FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood 112: 4485–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–283 [DOI] [PubMed] [Google Scholar]
- Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A (2010) TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 463: 676–680 [DOI] [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D (1999) Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem 274: 15982–15985 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR (2000) Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol 20: 8969–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Finkel T (2002) Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295: 2450–2452 [DOI] [PubMed] [Google Scholar]
- Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA (2003) Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424: 561–565 [DOI] [PubMed] [Google Scholar]
- Oh ST, Simonds EF, Jones C, Hale MB, Goltsev Y, Gibbs KD Jr, Merker JD, Zehnder JL, Nolan GP, Gotlib J (2010) Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood 116: 988–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA (2007) FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128: 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DR, Thompson CB (2003) Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem 278: 12361–12366 [DOI] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P (1999) Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274: 17179–17183 [DOI] [PubMed] [Google Scholar]
- Rudd CE (2001) Lnk adaptor: novel negative regulator of B cell lymphopoiesis. Sci STKE 2001: PE1. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Sabatini DM (2005) Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem 280: 39505–39509 [DOI] [PubMed] [Google Scholar]
- Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, Greenfield EA, Salgia R, Griffin JD (2000) The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem 275: 24273–24278 [DOI] [PubMed] [Google Scholar]
- Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, Griffin JD (1999) Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood 93: 2928–2935 [PubMed] [Google Scholar]
- Savitsky PA, Finkel T (2002) Redox regulation of Cdc25C. J Biol Chem 277: 20535–20540 [DOI] [PubMed] [Google Scholar]
- Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K (2005) The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell 16: 348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C, Werz C, Wieser D, Alic N, Foley A, Stocker H, Withers DJ, Thornton JM, Hafen E, Partridge L (2010) Regulation of lifespan, metabolism, and stress responses by the Drosophila SH2B protein, Lnk. PLoS Genet 6: e1000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Suda J, Ogawa M (1983) Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol 117: 308–318 [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299 [DOI] [PubMed] [Google Scholar]
- Takaki S, Morita H, Tezuka Y, Takatsu K (2002) Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, Lnk. J Exp Med 195: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ED, Nunez G, Barr FG, Guan KL (1999) Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem 274: 16741–16746 [DOI] [PubMed] [Google Scholar]
- Tefferi A, Gilliland DG (2007) Oncogenes in myeloproliferative disorders. Cell Cycle 6: 550–566 [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL (1995) Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334–30338 [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028 [DOI] [PubMed] [Google Scholar]
- Till JE, Mc CE (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14: 213–222 [PubMed] [Google Scholar]
- Tong W, Lodish HF (2004) Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis. J Exp Med 200: 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Zhang J, Lodish HF (2005) Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood 105: 4604–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, Depinho RA, Gilliland DG (2007) FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339 [DOI] [PubMed] [Google Scholar]
- Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ Jr, DiStefano PS, Chiang LW, Greenberg ME (2002) DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296: 530–534 [DOI] [PubMed] [Google Scholar]
- Tsai WB, Chung YM, Takahashi Y, Xu Z, Hu MC (2008) Functional interaction between FOXO3a and ATM regulates DNA damage response. Nat Cell Biol 10: 460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugo V, Marzac C, Teyssandier I, Larbret F, Lecluse Y, Debili N, Vainchenker W, Casadevall N (2004) Multiple signaling pathways are involved in erythropoietin-independent differentiation of erythroid progenitors in polycythemia vera. Exp Hematol 32: 179–187 [DOI] [PubMed] [Google Scholar]
- Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM (2002) c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell 9: 1031–1044 [DOI] [PubMed] [Google Scholar]
- van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burgering BM (2006) FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol 8: 1064–1073 [DOI] [PubMed] [Google Scholar]
- Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A, Paige CJ, Pawson T (2002) Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med 195: 1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz C, Kohler K, Hafen E, Stocker H (2009) The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genet 5: e1000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, Sarkar A, Grisotto M, Taneja R, Ghaffari S (2008) Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem 283: 25692–25705 [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Iwama A, Takayanagi S, Morita Y, Eto K, Ema H, Nakauchi H (2006) Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J 25: 3515–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ (2006) Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441: 475–482 [DOI] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, Wu H, Li L (2006) PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441: 518–522 [DOI] [PubMed] [Google Scholar]
- Zhao W, Kitidis C, Fleming MD, Lodish HF, Ghaffari S (2006) Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood 107: 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QS, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ (2006) G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood 107: 1847–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.