Kinetochore–microtubule interactions: steps towards bi-orientation
Our understanding of the intricate molecular events leading to proper chromosome attachments on mitotic spindles and subsequent equal segregation has been greatly advanced in recent years, progress that is summarized in this EMBO Member's Review from Tomoyuki Tanaka.
Keywords: bi-orientation, chromosome segregation, kinetochore, microtubule, mitosis
Abstract
Eukaryotic cells segregate their chromosomes accurately to opposite poles during mitosis, which is necessary for maintenance of their genetic integrity. This process mainly relies on the forces generated by kinetochore–microtubule (KT–MT) attachment. During prometaphase, the KT initially interacts with a single MT extending from a spindle pole and then moves towards a spindle pole. Subsequently, MTs from the other spindle pole also interact with the KT. Eventually, one sister KT becomes attached to MTs from one pole while the other sister to those from the other pole (sister KT bi-orientation). If sister KTs interact with MTs with aberrant orientation, this must be corrected to attain proper bi-orientation (error correction) before the anaphase is initiated. Here, I discuss how KTs initially interact with MTs and how this interaction develops into bi-orientation; both processes are fundamentally crucial for proper chromosome segregation in the subsequent anaphase.
Introduction
The proper segregation of sister chromatids to opposite poles of the cell during mitosis is crucial for cell proliferation. This topic has important medical relevance because chromosome mis-segregation can have causative functions in a variety of human diseases such as cancers and congenital disorders, which are characterized by chromosome instability and aneuploidy (Chandhok and Pellman, 2009; Holland and Cleveland, 2009; Thompson et al, 2010).
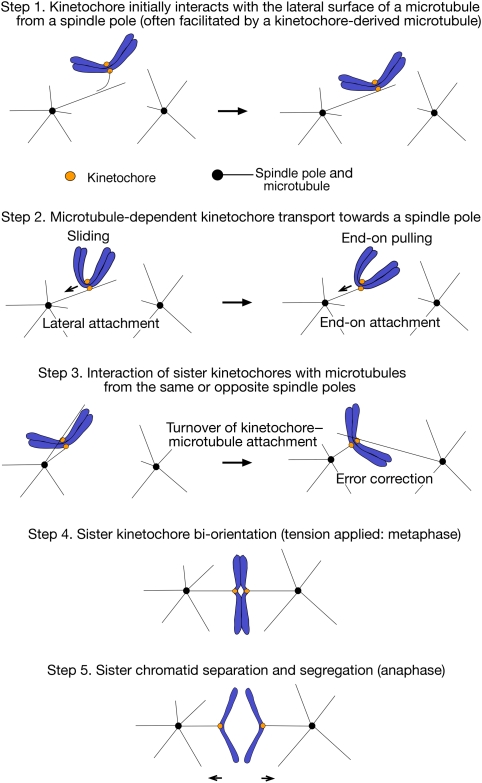
The segregation of sister chromatids during mitosis mainly depends on the forces generated by microtubules (MTs) that attach to kinetochores (KTs) (Tanaka and Desai, 2008). For proper chromosome segregation, KTs must capture spindle MTs and properly align on the mitotic spindle before anaphase onset. Cells undergo these processes in a step-wise manner as follows (Tanaka, 2008):
The KT initially interacts with the lateral surface of a single MT extending from a spindle pole (spindle-pole MT) (Figure 1, step 1, right).
Subsequently, the KT is transported along a spindle-pole MT towards a spindle pole (Figure 1, step 2, left).
The KT is tethered at the plus end of a spindle-pole MT (conversion from the lateral to the end-on attachment) (Figure 1, step 2, right).
Subsequently, sister KTs attach to MTs extending from opposite spindle poles (sister KT bi-orientation). If sister KTs attach to MTs with aberrant orientation, such errors must be corrected by turnover of the KT–MT attachment (error correction), before anaphase onset (Figure 1, steps 3 and 4).
Figure 1.
Step-wise development of kinetochore–microtubule interactions in eukaryotic cells. The figure shows kinetochore–microtubule (KT–MT) interactions during prometaphase (steps 1–3), metaphase (step 4) and anaphase (step 5). Prometaphase in budding yeast is as defined in Kitamura et al (2007). Step 1: The KT initially interacts with the lateral surface of a single MT (lateral attachment), which extends from one spindle pole (spindle-pole MT) (right) (Hayden et al, 1990; Rieder and Alexander, 1990; Tanaka et al, 2005a). This process is often facilitated by the interaction between a KT-derived MT and a spindle-pole MT (left) (see Figure 2) (Khodjakov et al, 2003; Maiato et al, 2004; Kitamura et al, 2010). It is not yet known whether both or only one of the sister KTs attaches to an MT during lateral attachment. Step 2: Once loaded on a spindle-pole MT, the KT is transported along the MT lateral surface towards a spindle pole (sliding; left) (Hayden et al, 1990; Rieder and Alexander, 1990; Tanaka et al, 2005a). Subsequently, at least in yeast, the KT is tethered at the end of a single spindle-pole MT (end-on attachment) and transported further as the MT shrinks (end-on pulling; right) (Kitamura et al, 2007; Tanaka et al, 2007). Step 3: Following the KT transport towards a spindle pole, both sister KTs could attach to MTs. If both sister KTs attach to MTs from the same spindle pole (syntelic attachment; see Figure 4), the KT–MT attachment must be turned over until proper bi-orientation is established (error correction; see Figure 5) (Nicklas, 1997; Tanaka et al, 2002). Step 4: The turnover of the KT–MT attachment stops once tension is generated across sister KTs upon the establishment of bi-orientation (see Figure 5) (Nicklas, 1997; Tanaka et al, 2002; Dewar et al, 2004). The number of MTs whose plus ends attach to a single KT increases as more tension is applied on metazoan KTs (King and Nicklas, 2000), while only a single MT attaches to each sister KT in budding yeast (Winey et al, 1995). Step 5: Once all sister KTs bi-orient on the spindle, cohesion between sister chromatids is removed, causing sister chromatid segregation to opposite spindle poles during anaphase (Nasmyth, 2002). Sister chromatid separation proceeds along a chromosome arm from the centromere to the telomere in budding yeast (Renshaw et al, 2010). The end-on KT–MT attachment is maintained as the MTs depolymerize and sister KTs are pulled towards the opposite poles during anaphase A, which is followed by the pole-to-pole distance being enlarged during anaphase B.
All these steps are crucial to ensure high-fidelity chromosome segregation in the subsequent anaphase (Figure 1, step 5). In this review article, I discuss the mechanisms ensuring the initial KT–MT interaction and subsequent sister KT bi-orientation on the mitotic spindle.
Note that the following topics are touched upon briefly in the context of this article, but are not the main focus of it: KT composition and assembly (Westermann et al, 2007; Cheeseman and Desai, 2008; Santaguida and Musacchio, 2009); MT dynamics on the mitotic spindle (Walczak and Heald, 2008; Dumont and Mitchison, 2009); chromosome congression on the metaphase plate (Kops et al, 2010); the spindle-assembly checkpoint (SAC) (Musacchio and Salmon, 2007; Burke and Stukenberg, 2008; Khodjakov and Rieder, 2009) and KT–MT interactions in meiosis (Brar and Amon, 2008; Sakuno and Watanabe, 2009). These topics have been reviewed in detail in the indicated references.
Initial KT–MT interaction: mechanisms facilitating their first encounter
KTs must secure their first contact with spindle-pole MTs, and this happens with the following timing in the cell cycle. In metazoan cells, because MT-organizing centres (MTOCs), called centrosomes (Azimzadeh and Bornens, 2007), locate outside of the nucleus in interphase, MTs extending from MTOCs can interact with KTs only after the nuclear envelope is broken down at the beginning of mitosis (prometaphase). This is known as ‘open' mitosis (Sazer, 2005). On the other hand, in many single-cell eukaryotes such as budding yeast, the nuclear envelope is not broken down during mitosis (known as ‘closed' mitosis) (Heath, 1980). In budding yeast, KTs are connected to MTOCs (called spindle-pole bodies) by MTs during most of the cell cycle (Winey and O'Toole, 2001). However, KTs are transiently disassembled upon centromere DNA replication. This causes centromere detachment from MTs for a few minutes (Kitamura et al, 2007), which is followed by KT reassembly and re-association with MTs.
How do KTs initially interact with MTs in yeast and metazoan cells? KTs initially attach to the lateral side of a single spindle-pole MT (Hayden et al, 1990; Rieder and Alexander, 1990; Tanaka et al, 2005a) (Figure 1, step 1, right). The lateral MT surface, called the lattice, provides a much larger contact surface compared with MT tips, thus contributing to an efficient first encounter with KTs. The capture of the MT lattice by KTs was initially discovered in newt lung cells (Hayden et al, 1990; Rieder and Alexander, 1990) and subsequently found in budding and fission yeast (Tanaka et al, 2005a; Franco et al, 2007; Gachet et al, 2008); therefore, this is an evolutionarily conserved process among eukaryotic cells.
Before the initial interaction between MTs and KTs, MTs undergo repeated growth and shrinkage in various directions, as if they are searching for KTs (Kirschner and Mitchison, 1986). However, the initial encounter is thought to happen more efficiently than is likely to be expected from a random search-and-capture process (Wollman et al, 2005). What then are the mechanisms that could contribute to the efficient KT–MT encounter? In vertebrate cells that undergo open mitosis, a concentration gradient of Ran-GTP is formed around chromosomes and ‘guide' spindle-pole MTs towards them (Carazo-Salas and Karsenti, 2003; Caudron et al, 2005; Kalab et al, 2006; O'Connell and Khodjakov, 2007; Clarke and Zhang, 2008). Relevant to this process, CDK11 has been identified as a Ran-GTP-dependent MT stabilization factor (Yokoyama et al, 2008).
The mechanism dependent on the Ran-GTP gradient is indeed effective over a long range (∼20 μm) (Athale et al, 2008). However, Ran-GTP cannot make a substantial gradient over a shorter range (e.g. < 5 μm) presumably because of its rapid diffusion. Moreover, in cells that undergo closed mitosis, such as yeast, a Ran-GTP gradient is presumably not formed during mitosis, as its concentration is expected to be uniformly high in the nucleus, similar to the interphase nucleus in metazoan cells (Kalab et al, 2006).
Initial KT interaction with spindle-pole MTs is facilitated by KT-derived MTs
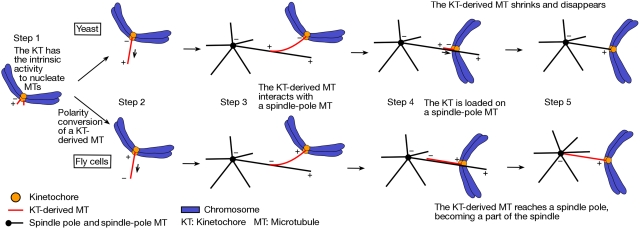
Given the argument above, do any additional mechanisms facilitate the initial KT–MT encounter, in particular over a short distance? In yeast, fly and vertebrate cells, it has been revealed that MTs are generated not only at spindle poles but also at KTs (Khodjakov et al, 2003; Maiato et al, 2004; Kitamura et al, 2010). Such KT-derived MTs subsequently interact with MTs extending from spindle poles (i.e. spindle-pole MTs) along their length, facilitating KT loading onto the lattice of spindle-pole MTs (Figure 1, step 1, left). Notably, KTs generate MTs more often when KT interaction with spindle-pole MTs is delayed (Kitamura et al, 2010); this may occur as KTs become more mature while they remain unattached to the spindle. Thus, the generation of KT-derived MTs seems to help avoid a further delay in the KT encounter with spindle-pole MTs.
Intriguingly, whereas KT-derived MTs have plus ends distal to KTs in yeast cells, their polarity is opposite in Drosophila cells and perhaps also in human cells (Khodjakov et al, 2003; Maiato et al, 2004; Kitamura et al, 2010). This discrepancy is reconciled if, in both organisms, short MTs (perhaps 50–500 nm) are generated initially with plus ends distal to KTs (Figure 2, step 1) and if the MT polarity is converted in Drosophila (and other metazoan) cells, but not in yeast (Figure 2, step 2). Consistent with this notion, the γ-tubulin complex is present at KTs in vertebrate cells (Mishra et al, 2010) and could facilitate MT nucleation at KTs with plus ends distal to KTs. The minus ends of such short MTs are not embedded at KT plates in vertebrate cells (Witt et al, 1980) and their distal plus ends might be subsequently captured by KT plates (Figure 2, step 2, bottom). Such polarity conversion could be promoted by plus end-directed motors on KTs in metazoan cells (e.g. CENP-E). On the other hand, no plus end-directed motors are found at the yeast KTs, before sister KT bi-orientation (Tanaka et al, 2007), consistent with a lack of the polarity conversion of KT-derived MTs in yeast.
Figure 2.
Kinetochore-derived microtubules in yeast and fly cells. The figure shows generation of MTs at KTs and the roles of KT-derived MTs during early mitosis in yeast (budding yeast) and fly (Drosophila) cells. Step 1: In both organisms, the KT can nucleate MTs and generate short MTs with distal plus ends (see discussion in Maiato et al, 2004; Kitamura et al, 2010); ‘+' and ‘−' designate the polarity of relevant MTs. Step 2: In yeast (top), a KT-derived MT extends by tubulin polymerization at the MT plus end, which remains distal to the KT (Kitamura et al, 2010). On the other hand, in fly cells (bottom), the plus end of a KT-derived MT is caught on the KT (polarity conversion) and this MT extends by tubulin polymerization at the KT, with the minus end distal to the KT (Khodjakov et al, 2003; Maiato et al, 2004). Step 3: In both organisms, the KT-derived MT interacts with a spindle-pole MT along their length, which facilitates KT loading onto the lateral surface of the spindle-pole MT (Khodjakov et al, 2003; Kitamura et al, 2010). Steps 4 and 5: Once the KT is loaded onto the spindle-pole MTs in yeast, already-existing KT-derived MTs shrink and disappear, and the KT cannot generate new MTs (Kitamura et al, 2010). On the other hand, in fly cells, KT-derived MTs can extend further and often reach a spindle pole, becoming a part of the spindle (Khodjakov et al, 2003; Maiato et al, 2004).
Irrespective of their polarity, KT-derived MTs often interact with spindle-pole MTs along their length, facilitating KT loading onto the spindle-pole MTs (Khodjakov et al, 2003; Kitamura et al, 2010) (Figure 2, step 3). However, depending on their polarity, the consequences of that interaction differ. When KT-derived MTs have their minus ends distal to KTs, these distal ends often reach spindle poles and become tethered there, thus contributing to the formation of the spindle (Figure 2, steps 4 and 5, bottom) (Khodjakov et al, 2003; O'Connell et al, 2009). In contrast, in budding yeast, KT-derived MTs have a shorter lifetime and disappear soon after KT loading onto spindle-pole MTs (Figure 2, steps 4 and 5, top) (Kitamura et al, 2010). In budding yeast, Stu2 (the orthologue of vertebrate XMAP215/ch-TOG) localizes at KTs and has a central function in generating MTs at KTs (Kitamura et al, 2010).
Conversion from the lateral to end-on KT–MT attachment
Once bound to the MT lattice (lateral attachment), KTs are transported towards a spindle pole along the MT (sliding; Figure 1, step 2). The poleward KT transport is especially crucial when KTs are located far away from the mitotic spindle. KT sliding along an MT is promoted by minus end-directed motor proteins; dynein in vertebrate cells (King et al, 2000; Yang et al, 2007) and Kar3, a kinesin-14 family member, in budding yeast (Tanaka et al, 2005a, 2007).
While the KT is associated with the MT lattice, the plus end of the shrinking MT often catches up with the KT, leading to the KT becoming tethered at MT plus end (end-on attachment) and being pulled further towards a spindle pole as the MT shrinks (Tanaka et al, 2007) (end-on pulling; Figure 1, step 2).
Is the end-on KT–MT attachment simply a lateral attachment close to a tip of the MT? At least in budding yeast, the end-on attachment shows clearly different features from lateral attachment, as follows (Tanaka et al, 2005a, 2007): (1) the speed of poleward KT motion is slower during lateral attachment than during end-on attachment, (2) the speed of MT shrinkage is similar in the presence and absence of the KT on its lateral side, but is slowed down once end-on attachment is established, (3) MT rescue (conversion from MT shrinkage to re-growth) occurs during lateral attachment (KT sliding), but not during end-on attachment (KT end-on pulling) and (4) different regulators are involved during lateral and end-on attachment; that is Kar3 is required for poleward sliding and the Dam1 complex for end-on pulling but not vice versa (see below).
Therefore, the end-on attachment is different from the lateral attachment, not only in the KT position but also in their mechanism. The end-on attachment can sustain KT–MT attachment stably (Grishchuk et al, 2005; Joglekar et al, 2010), presumably more so than lateral attachment; indeed, KT detachment from MTs happens in a small number of cases (∼5%) during lateral attachment, but not during end-on attachment (Tanaka et al, 2007). Thus, end-on attachment might be ideal to sustain KT–MT interaction when bi-orientation is subsequently established and tension is applied across sister KTs (see below).
In the next two sections, I discuss how the Ndc80 complex, the Dam1 complex and its functional equivalents make interface of KT–MT interaction during the lateral and end-on attachment.
Interface of the KT–MT attachment: the Ndc80 complex
KTs are large protein complexes, consisting of dozens of protein components (Westermann et al, 2007; Cheeseman and Desai, 2008; Santaguida and Musacchio, 2009). Among them, the Ndc80 and Dam1 complexes have important functions in making a direct contact with MTs.
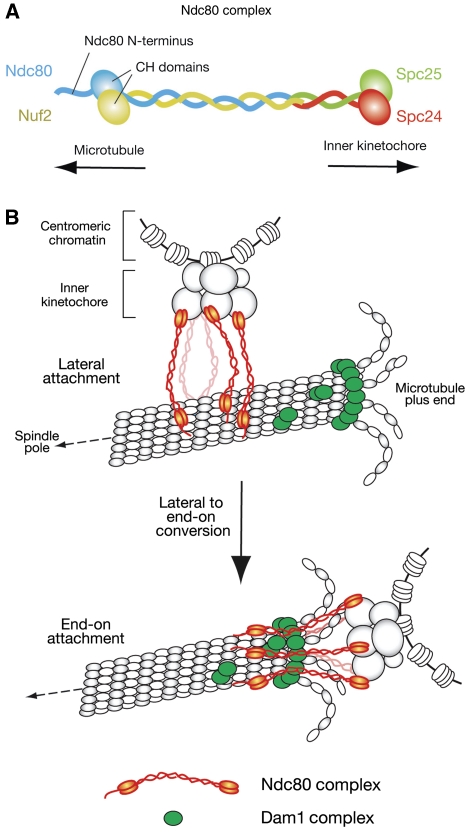
The Ndc80 complex is an outer KT component, structurally conserved from yeast to vertebrates, and defects in it lead to severely impaired KT–MT interaction (reviewed in Cheeseman and Desai, 2008; Tanaka and Desai, 2008; Santaguida and Musacchio, 2009). The Ndc80 complex is composed of four proteins (Figure 3A): Ndc80 (also called Hec1 in mammals), Nuf2, Spc24 and Spc25. Ndc80–Nuf2 and Spc24–Spc25 form heterodimers with a globular domain at the end of a coiled-coil shaft (Ciferri et al, 2005; Wei et al, 2005, 2006). Their coiled-coil shafts interact with each other and hold two heterodimers together, making a heterotetramic rod structure with globular domains at both ends. The Spc24–Spc25 globular domain is oriented towards the inner KT and the rod structure projects the Ndc80–Nuf2 globular domain outwards to MTs (DeLuca et al, 2006; Joglekar et al, 2009; Wan et al, 2009).
Figure 3.
The Ndc80 and Dam1 complexes make interface for kinetochore–microtubule attachment. (A) Structure of the Ndc80 complex, which consists of Ndc80, Nuf2, Spc24 and Spc25 proteins (Wei et al, 2007; Ciferri et al, 2008). The globular domains of Ndc80 and Nuf2 are folded into calponin-homology (CH) domains. (B) The role of the Ndc80 and Dam1 complexes in the conversion of lateral to end-on KT–MT attachment (see Figure 1, step 2). During the lateral attachment, the Ndc80 complex (shown in red) binds an MT at its CH domains and unstructured N-terminus (Cheeseman et al, 2006; Wei et al, 2007; Ciferri et al, 2008). To convert lateral to end-on attachment, it is probably crucial that the Ndc80 complex becomes associated with the Dam1 complex (shown in green) (Lampert et al, 2010; Tien et al, 2010; our unpublished results), which localizes at the MT plus end and forms an oligomer and/or a ring encircling the MT (reviewed in Westermann et al, 2007; Nogales and Ramey, 2009). The Ndc80 N-terminus is suggested to strengthen the MT association (Guimaraes et al, 2008; Miller et al, 2008). This region may be also important for inter-complex association (Ciferri et al, 2008; Alushin et al, 2010). It is not yet clear whether the Ndc80 complexes go through the inside or outside of a Dam1-complex ring, if the ring is formed around an MT.
The Ndc80–Nuf2 globular domain directly interacts with the MT lattice in vitro (Cheeseman et al, 2006; Wei et al, 2007; Wilson-Kubalek et al, 2008). Consistent with this, the Ndc80 complex has a crucial role in KT association with the MT lattice in vivo in budding yeast (Figure 1, step 1, right; Tanaka et al, 2005a). X-ray crystallography revealed that the Ndc80–Nuf2 globular domain is made of two calponin-homology (CH) domains (Wei et al, 2007; Ciferri et al, 2008). Positively charged residues in the CH domains are important for MT lattice binding (Ciferri et al, 2008). Another MT-associated protein EB1 also folds as a CH domain (Hayashi and Ikura, 2003), which is, therefore, a commonly used structure for MT association. In Ndc80, an unstructured N-terminal 80–100 residue basic region, protruding from the CH domain, is suggested to strengthen the MT association (Guimaraes et al, 2008; Miller et al, 2008) and inter-complex association (Ciferri et al, 2008; Alushin et al, 2010).
The Ndc80 complex is linked to the inner KT complex through association between its Spc24–25 globular domain and the Mis12 complex (Cheeseman et al, 2006; Maskell et al, 2010; Petrovic et al, 2010). The Mis12 complex also associates with another KT component KNL1, which is also called Blinkin, Spc105 and Spc7 in vertebrates, budding and fission yeast, respectively. This interacting protein set of Ndc80 complex, Mis12 complex and KNL1, is called the KMN network, which shows higher affinity for MTs than the Ndc80 complex alone (Cheeseman et al, 2006). In particular, KNL1 seems to make an additional direct MT-binding interface (Cheeseman et al, 2006; Kiyomitsu et al, 2007; Pagliuca et al, 2009; Welburn et al, 2010).
As discussed in the previous section, KTs initially interact with the MT lattice and subsequently attach to the plus ends of MTs; that is the lateral attachment is converted to end-on attachment (Figure 1, step 2; Figure 3B). Presumably, the Ndc80 complex is involved not only in the lateral attachment but also in the end-on attachment; consistent with this, an injection of an antibody against the Ndc80 CH domain changed the dynamics of KT–MT interactions in metaphase (DeLuca et al, 2006), moreover the Ndc80 complex can couple a microsphere at the end of a dynamic MT in an in vitro reconstituted system (McIntosh et al, 2008; Powers et al, 2009).
Interface of the KT–MT attachment: the Dam1 complex and its functional equivalents
In yeast cells, the Dam1 complex also has a crucial role in the end-on KT–MT attachment. The Dam1 complex, also called DASH, is composed of 10 proteins and has been identified in budding yeast (reviewed in Westermann et al, 2007; Nogales and Ramey, 2009) and fission yeast (Liu et al, 2005; Sanchez-Perez et al, 2005). The Dam1 complex is not a part of the KT during the lateral KT–MT attachment, and only upon end-on attachment, it is loaded on the KT (Tanaka et al, 2007) (Figure 3B). The Dam1 complex has the ability to track the plus end of a shrinking MT (Westermann et al, 2006; Tanaka et al, 2007) and, once loaded on the KT, it mediates the end-on pulling of the KT by a shrinking MT in budding yeast (Tanaka et al, 2007). A similar role is also suggested for the Dam1 complex in fission yeast (Franco et al, 2007; Gachet et al, 2008). During this process, the Dam1 complexes form oligomers and/or a ring structure encircling an MT (Figure 3B) (Miranda et al, 2005; Westermann et al, 2005; Wang et al, 2007; Gestaut et al, 2008; Grishchuk et al, 2008).
During MT depolymerization, protofilaments splay out at the plus ends. In vitro reconstitution and mathematical models suggest that such protofilament curling produces a force sufficient to move chromosomes towards spindle pole (Grishchuk et al, 2005; Liu and Onuchic, 2006; Efremov et al, 2007). It is suggested that the Dam1 complex is required to convert MT depolymerization to a KT pulling force (Asbury et al, 2006; Westermann et al, 2006; Tanaka et al, 2007).
The KT loading of the Dam1 complex, which occurs when the end-on KT–MT attachment is established (Tanaka et al, 2007), is dependent on the Ndc80 complex (Janke et al, 2002; Shang et al, 2003; Wong et al, 2007). Thus, the Ndc80 and the Dam1 complexes may work together to support the end-on KT–MT attachment (Figure 3B). Consistent with this notion, the Dam1 complex enhances the MT-binding affinity of the Ndc80 complex and facilitates its MT plus-end accumulation in vitro (Lampert et al, 2010; Tien et al, 2010). Furthermore, disrupting the association between the two complexes leads to a defect in the end-on KT–MT attachment, while the lateral attachment is still normal (our unpublished results).
The Dam1 complex has an important function in chromosome motion towards a spindle pole, soon after initial KT–MT interaction and before sister KT bi-orientation (Figure 1, step 2, right; end-on pulling; Kitamura et al, 2007; Tanaka et al, 2007). Presumably, the complex has a similar role in anaphase A, where KTs move towards a spindle pole again by MT end-on pulling (Figure 1, step 5). In addition, the complex may also have a function in tension-coupled chromosome oscillation during metaphase (Franck et al, 2007) (Figure 1, step 4). The important roles of the Dam1 complex in chromosome segregation is also supported by recent finding that Dam1 complexes tethered on a minichromosome lacking a centromere could facilitate its segregation during mitosis (Kiermaier et al, 2009; Lacefield et al, 2009).
Although the Dam1 complex has essential roles in KT association with the end of an MT in budding yeast, convincing orthologues of Dam1 components have not been identified in metazoan cells (Meraldi et al, 2006). However, functional counterparts of the Dam1 complex may be present in metazoa, albeit with little homology in amino-acid sequences. Indeed, the Ska1 complex, consisting of Ska1, Ska2 and Rama1/Ska3, is required for proper chromosome segregation and shows similar functional properties to the Dam1 complex (Hanisch et al, 2006; Daum et al, 2009; Gaitanos et al, 2009; Raaijmakers et al, 2009; Welburn et al, 2009). For example, as with the Dam1 complex, the Ska1 complexes can directly bind MTs, oligomerize into a ring-like structure and facilitate the processive motion of a microsphere along a depolymerizing MT in vitro (Welburn et al, 2009). Moreover, similarly to the Dam1 complex, the KT loading of the Ska1 complex is dependent on Ndc80 (Hanisch et al, 2006).
Furthermore, Cep57 and Bod1 are proposed to be alternative candidates for functional counterparts of the Dam1 complex in vertebrate cells (Emanuele and Stukenberg, 2007; Porter and Swedlow, 2007). From analogy to yeast (as discussed above), the Dam1 functional counterparts are expected to be involved in establishing the end-on KT–MT attachment; in Caenorhabditis elegans, this process seems to rely on interplay between spindly, dynein and the dynein-associated RZZ complex (Gassmann et al, 2008).
So far, the Ndc80 complex, KNL1, the Dam1 complex and its functional equivalents are thought to interact with the MT outer surface. However, electron microscopy identified micro-fibrils in vertebrate cells, which extend from the KT to the inner surface of the MT tip in which protofilaments curl out (McIntosh et al, 2008). Although the identity of such micro-fibrils is not yet known, they may also have an important function in tethering the KT at the dynamic MT plus end.
Mechanisms promoting sister KT bi-orientation: KT geometry and error correction
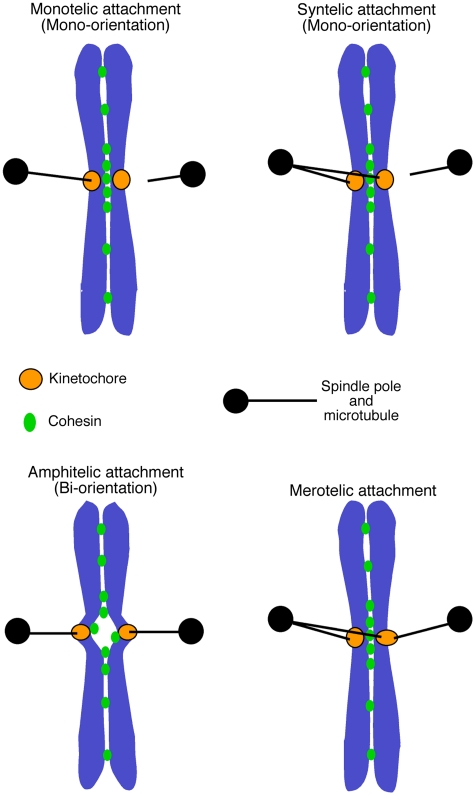
Following the KT transport towards a spindle pole (Figure 1, step 2), one sister KT becomes attached to MTs from one pole while the other sister to those from the other pole (sister KT bi-orientation; Figure 1, step 4). In order to achieve bi-orientation (also called amphitelic attachment; Figure 4), the aberrant KT–MT attachment, for example sister KTs attaching to MTs from the same spindle pole (syntelic attachment; Figure 4), must be either avoided or corrected before anaphase onset. Two kinds of mechanisms could promote this process: the KT geometry (for avoidance) and tension-dependent error correction.
Figure 4.
Modes of kinetochore–microtubule interactions. Monotelic attachment: one of the sister KTs attaches to MTs, whereas the other does not attach to any MTs. Syntelic attachment: both sister KTs attach to MTs extending from one spindle pole. As a result of monotelic or syntelic attachment, sister KTs ‘mono-orient'; that is, they are connected to only one spindle pole directly or indirectly. Amphitelic attachment: one sister kinetochore becomes attached to microtubules from one pole while the other sister to those from the other pole. As a result of amphitelic attachment, sister KTs ‘bi-orient'; that is they are connected to the opposite spindle poles. Merotelic attachment: one sister KT simultaneously attaches to MTs extending from both spindle poles. The figure is adapted from a figure in Tanaka et al (2005b).
The KT geometry relies on a back-to-back position of sister KTs. When one KT attaches to MTs from one spindle pole (monotelic attachment; Figure 4), the constraint in geometry makes the other face the opposite direction, allowing association with MTs only from the opposite pole (Ostergren, 1951). For sister KT geometry, cohesion between sister centromeres is thought to have important functions (Tanaka et al, 1999). More specifically, it was suggested that reduced cohesion at the core centromere, relative to para-centromere regions, facilitates sister KT geometry for bi-orientation in fission yeast (Sakuno et al, 2009). In budding yeast, the core centromeres are looped out from para-centromeres (Yeh et al, 2008; Anderson et al, 2009), which may contribute to this geometry. Thus, an aberrant KT–MT attachment could be avoided by KT geometry.
However, once aberrant attachment is made, the geometry mechanism can no longer correct it. In this circumstance, error correction is important in removal of aberrant KT–MT attachment (Figure 1, step 3). Such error correction was first discovered in meiosis I, where two homologous KTs must bi-orient on the spindle. Here, KTs and spindle poles repeatedly become connected (by MTs) and disconnected until bi-orientation is established (Nicklas, 1997). Using micro-needle manipulation in grasshopper spermatocytes, Nicklas and Koch (1969) showed that tension applied on chromosomes leads to stabilization of KT-spindle-pole connections by MTs.
The importance of tension for KT bi-orientation was also tested in mitosis; an unreplicated circular minichromosome with two centromeres was engineered and its behaviour was observed in budding yeast (Dewar et al, 2004). On this minichromosome, the two centromeres would lack back-to-back geometry, but tension should be generated across them by inter-centromere chromatin, when two centromeres bi-orient on the spindle. Such a minichromosome showed efficient bi-orientation, suggesting that tension across its two KTs is sufficient to promote bi-orientation (Dewar et al, 2004).
The corollary is that, on normal sister KTs, stable KT–MT attachment is established reliant on tension across sister KTs (Figure 1, steps 3 and 4). If KT–MT attachment does not generate tension as happens during syntelic attachment, such attachment would be removed to allow formation of a fresh KT–MT attachment. When bi-orientation is established, tension is generated across sister KTs, leading to stable KT–MT attachment, thus completing the error correction (Nicklas and Koch, 1969; Nicklas, 1997; Dewar et al, 2004).
In this process of turnover of the KT–MT attachment, cohesion between sister centromeres is necessary to generate tension when bi-orientation is established. Thus, cohesion between sister centromeres is important not only for KT geometry (see above), but also for the error correction mechanism. Cohesin proteins are required for sister chromatid cohesion (Nasmyth, 2002) and they are indeed necessary to ensure sister KT bi-orientation (Tanaka et al, 2000; Sonoda et al, 2001; Dewar et al, 2004; Vagnarelli et al, 2004; Eckert et al, 2007).
In vertebrate cells, mono-oriented KTs (with monotelic or syntelic attachment; Figure 4) can move from the vicinity of a spindle pole to the middle of the spindle, along MTs that attach to other already bi-oriented KTs (Kapoor et al, 2006). This motion should enhance the chance for such KTs to interact with MTs from the opposite pole, thus providing an additional mechanism to facilitate turnover of KT–MT attachments, leading to bi-orientation.
Although the tension-dependent error correction seems to suffice for efficient establishment bi-orientation in mitosis (see above), KT geometry may also facilitate bi-orientation as a redundant mechanism (Indjeian and Murray, 2007; Sakuno et al, 2009). Thus, sister KT geometry is generally thought to assist bi-orientation. However, KTs tend to maintain their geometry in mammalian cells and, following syntelic attachment, sister KTs may remain juxtaposed even after aberrant KT–MT attachment is removed (Loncarek et al, 2007). In this circumstance, KT geometry has to be overridden by the error correction for bi-orientation to become established.
In budding yeast, only one MT is thought to attach to a single KT in metaphase (Winey et al, 1995). In contrast, multiple MTs attach to a single KT in fission yeast and metazoan cells (McDonald et al, 1992; Ding et al, 1993). In this situation, errors could occur in such a way that a single KT becomes attached to MTs from opposite spindle poles (merotelic attachment; Figure 4). How do cells avoid or correct this type of error? Merotelic attachments are probably discouraged by the KT geometry. For example, a candidate KT clamp ensuring this geometry has been suggested in fission yeast (Gregan et al, 2007).
However, KT geometry may not be sufficient to avoid merotelic attachment. For example, the presence of an extra spindle pole often causes merotelic attachments, following the fusion of two spindle poles (Ganem et al, 2009); this would be difficult to avoid by KT geometry. In fact, if merotelic attachments are formed, they can still be corrected before anaphase onset (see the next section; Cimini et al, 2003; Cimini et al, 2006). Even if merotelic attachments persist until anaphase, the imbalance between MT forces applied on sister KTs could lead to their proper segregation, albeit with some delay (Cimini et al, 2004).
Regulators for the error correction: Aurora B/Ipl1 kinase
What factors are involved in the error correction to facilitate sister KT bi-orientation? A series of evidence suggests that Aurora B (called Ipl1 in budding yeast), an evolutionarily conserved serine/threonine protein kinase, has a crucial role in this process.
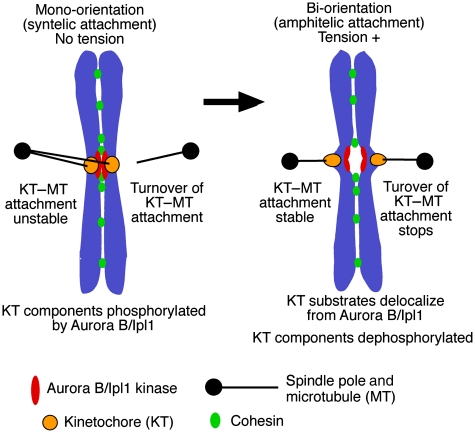
When Ipl1 is defective in yeast cells, KTs are able to interact with MTs; however, sister KT bi-orientation is defective in the majority of cells (Biggins and Murray, 2001; He et al, 2001; Tanaka et al, 2002). In an ipl1 mutant, an unreplicated circular minichromosome with two centromeres (see above) often failed to bi-orient, suggesting that Ipl1 could facilitate bi-orientation, independently of KT geometry (Dewar et al, 2004). Moreover, in this mutant, the KT failed to change its MT association in the absence of tension, in contrast to wild-type cells (Tanaka et al, 2002; Dewar et al, 2004). It was, therefore, suggested that Ipl1 kinase promotes turnover of the KT–MT interaction to eliminate KT–MT interactions that do not generate tension across sister KTs (Tanaka et al, 2002) (Figure 5). Aurora B has a similar role in mammalian cells, as they accumulated syntelic KT–MT attachments when this kinase was defective (Hauf et al, 2003; Lampson et al, 2004).
Figure 5.
How Aurora B/Ipl1 kinase promotes the error correction of kinetochore–microtubule attachment. The Aurora B/Ipl1 kinase facilitates sister KT bi-orientation by promoting the turnover of KT–MT attachment in a tension-dependent manner; this process is thought to take place as follows (Tanaka et al, 2002; Dewar et al, 2004; Liu et al, 2009). While syntelic attachment does not generate tension across sister KTs (left), the Aurora B/Ipl1 kinase phosphorylates KT components such as Ndc80 and Dam1 (see references in text), which promotes turnover of the KT–MT attachment. When bi-orientation is established as a result of this turnover (right), tension is applied across sister KTs, causing delocalization of the KT substrates from Aurora B/Ipl1, which leads to their de-phosphorylation. This in turn makes the KT–MT attachment more stable and stops the turnover of this attachment. Thus, sister KT bi-orientation is stably maintained and the error correction is completed for the KT–MT attachment.
Aurora B/Ipl1 kinase localizes at centromeres in prometaphase and metaphase and it can phosphorylate KT components, which is thought to weaken the KT–MT attachment and facilitate its turnover (Figure 5, left). Crucial substrates of this kinase include the Dam1-complex components in budding yeast (Cheeseman et al, 2002; Zhang et al, 2005), and Ndc80 (Cheeseman et al, 2006; DeLuca et al, 2006; Ciferri et al, 2008; Akiyoshi et al, 2009a) and KNL1 (Liu et al, 2010; Welburn et al, 2010) in yeast and mammalian cells. Phosphorylation of Dam1 is clustered at its C-terminus and reduces interaction with the Ndc80 complex (see the section above) (Cheeseman et al, 2002; Shang et al, 2003; Lampert et al, 2010; Tien et al, 2010). On the other hand, phosphorylation of Ndc80 is clustered at its N-terminal basic region and reduces the affinity of the Ndc80 complex for MTs (Cheeseman et al, 2006; Ciferri et al, 2008) and inter-complex association (Ciferri et al, 2008; Alushin et al, 2010). Moreover, dam1, ndc80 and knl1 mutants mimicking constitutive de-phosphorylation show defects in bi-orientation (Cheeseman et al, 2002; DeLuca et al, 2006; Welburn et al, 2010), suggesting that their phosphorylation by Aurora B/Ipl1 is crucial for bi-orientation.
In metazoan cells, multiple MTs attach to a single KT, where Aurora B may have a more complex function in promoting bi-orientation. For example, inhibition of Aurora B leads to not only syntelic attachment but also merotelic attachment (Cimini et al, 2006; Knowlton et al, 2006; see section above; Figure 4). In vertebrate cells, the MT-depolymerizing activity of MCAK is regulated by Aurora B, which could be important in removing syntelic and merotelic attachments (Ohi et al, 2003; Andrews et al, 2004; Lan et al, 2004; Ohi et al, 2004; Knowlton et al, 2006; Zhang et al, 2007).
Meanwhile, once bi-orientation is established and tension is applied on KTs, cells must stop the turnover of the KT–MT attachment (Figure 5, right) (Tanaka et al, 2002; Pearson et al, 2004); otherwise, bi-orientation would not be stably maintained. It was demonstrated that Aurora B/Ipl1 substrates Dam1 and KNL1, whose phosphorylation is important for bi-orientation, are de-phosphorylated when sister KTs bi-orient and tension is applied across sister KTs (Keating et al, 2009; Welburn et al, 2010).
How does this de-phosphorylation occur when tension is applied on sister KTs? Evidence suggests that when tension is applied, Aurora B/Ipl1 substrates at the KTs are pulled out due to spindle MT forces and, therefore, delocalize from the Aurora B/Ipl1 kinase that is enriched at inner centromere regions (Figure 5, right). This was first suggested in budding yeast (Tanaka et al, 2002; Shimogawa et al, 2009) and subsequently demonstrated using a FRET sensor in mammalians cells (Liu et al, 2009; Welburn et al, 2010). This notion is consistent with the reports that intra-KT stretching becomes prominent upon sister KT bi-orientation (Maresca and Salmon, 2009; Uchida et al, 2009), which will facilitate delocalization of outer KTs (including Aurora B/Ipl1 substrates) from this kinase.
As an alternative mechanism, it is proposed that the kinase activity of Aurora B/Ipl1 might be regulated by tension. For example, Bir1 and Sli15 (Survivin and INCENP in metazoa), binding partners of Ipl1 (reviewed in Ruchaud et al, 2007; Carmena et al, 2009), are proposed to act as a tension sensor (Sandall et al, 2006). Another model proposes that MTs, aberrantly associated with KTs, may enhance the activity of Aurora B kinase (Rosasco-Nitcher et al, 2008).
Upon anaphase onset, Aurora B/Ipl1 kinases relocate from KTs to the spindle, thus they are also known as ‘chromosome passenger complex (CPC)', together with binding partners Survivin/Bir1 and INCENP/Sli15 (reviewed in Ruchaud et al, 2007). This relocation is regulated by Cdc14 phosphatase (Pereira and Schiebel, 2003; Mirchenko and Uhlmann, 2010), cyclin B degradation (Parry et al, 2003; Oliveira et al, 2010) and a kinesin-6 member Mklp2 (Gruneberg et al, 2004; Hummer and Mayer, 2009; Vazquez-Novelle and Petronczki, 2010), as shown in yeast, fly and mammalian cells, respectively. The Aurora B/Ipl1 relocation from the KT is presumably important to stop turnover of KT–MT attachment during anaphase, in which tension on the KT is considerably reduced. In fact, when this relocation of Aurora B/Ipl1 is inhibited, KTs show continuous back-and-forth motion on the anaphase spindle (Parry et al, 2003; Oliveira et al, 2010) and the SAC is re-engaged (Mirchenko and Uhlmann, 2010; Oliveira et al, 2010); these events probably reflect the resumed turnover of the KT–MT attachment in anaphase.
More regulators for the error correction: Bub1, Sgo, Haspin, PP1 and Mps1
Several regulators are involved in targeting the CPC (including Aurora B/Ipl1) at centromeres in early mitosis, thus ensuring the error correction in KT–MT attachment. Recent studies have revealed that two pathways ensure this process. In the first pathway, Bub1 kinase phosphorylates histone H2A at centromere regions (Kawashima et al, 2010), which recruits Sgo proteins (Sgo1 in budding yeast and mammals; Sgo2 in fission yeast) that in turn bind Bir1 (fission yeast) and another CPC component Borealin (mammals) when they are phosphorylated by cyclinB/CDK (Tsukahara et al, 2010). In the second pathway, Haspin kinases phosphorylate histone H3 at threonine 3 (Dai et al, 2005), which binds Survivin/Bir1 (Kelly et al, 2010; Wang et al, 2010; Yamagishi et al, 2010).
While the two pathways work partially redundantly, Aurora B is enriched at inner centromere regions where both pathways are active (Yamagishi et al, 2010). Indeed, sister KT bi-orientation and resultant chromosome congression become partially defective in yeast and mammalian cells with mutation or depletion of Bub1 (Asakawa et al, 2005; Meraldi and Sorger, 2005; Windecker et al, 2009), Sgo (Fernius and Hardwick, 2007; Kawashima et al, 2007; Vanoosthuyse et al, 2007; Kiburz et al, 2008) or Haspin (Dai et al, 2005). Notably, defects in both pathways cause more extensive chromosome mis-segregation (Yamagishi et al, 2010).
Meanwhile, PP1 (called Glc7 in budding yeast) is a phosphatase, which is thought to implement de-phosphorylation of Aurora B/Ipl1 substrates (reviewed in De Wulf et al, 2009). Consistent with this notion, Glc7 mutants show a defect in the KT–MT interaction (Bloecher and Tatchell, 1999; Sassoon et al, 1999). It was recently suggested that Fin1 and KNL1 are associated with Glc7 and PP1, respectively, to facilitate their recruitment to the KT (Akiyoshi et al, 2009b; Liu et al, 2010). In particular, the KNL1-PP1 association is enhanced when Aurora B-dependent KNL1 phosphorylation is reduced (Liu et al, 2010), constituting a possible positive feedback loop for rapid de-phosphorylation of Aurora B substrates at the KT when tension is applied across sister KTs upon bi-orientation.
Mps1 is an evolutionarily conserved protein kinase, which is required for the SAC and, in some organisms, for duplication of MTOCs of the mitotic spindle (reviewed in Winey and Huneycutt, 2002). On the other hand, independent of these functions, Mps1 has an important role in chromosome segregation (Jones et al, 2005), especially in sister KT bi-orientation (Maure et al, 2007; Araki et al, 2010). Similarly to Aurora B/Ipl1, Mps1 promotes turnover of any KT–MT attachment that does not generate tension in budding yeast (Maure et al, 2007). Similarly, in mammalian cells, Mps1 is required for the error correction of the KT–MT attachment (Jelluma et al, 2008). It is currently a matter of debate whether Mps1 regulates the Aurora B kinase (Jelluma et al, 2008; Sliedrecht et al, 2010), or vice versa (Hewitt et al, 2010; Santaguida et al, 2010), or they work in parallel pathways (Maure et al, 2007; Maciejowski et al, 2010) to promote sister KT bi-orientation.
Error correction and SAC: intertwined relationship
The spindle-assembly checkpoint (SAC) is a surveillance mechanism that delays anaphase onset if sister KTs fail to bi-orient, or if any KTs fail to attach to MTs (reviewed in Musacchio and Salmon, 2007; Burke and Stukenberg, 2008; Khodjakov and Rieder, 2009). The SAC and error correction are often argued in the same context, but they are distinct mechanisms having different effects. While the error correction selectively promotes bi-orientation, the SAC does not promote bi-orientation by itself, but can buy the time for the error correction to establish bi-orientation. Although the error correction is an essential mechanism for cell growth in any eukaryote so far studied, the SAC is not essential for cell proliferation as long as cells have sufficient time to establish bi-orientation, as is the case in budding and fission yeast (reviewed in Lew and Burke, 2003) and Drosophila (Buffin et al, 2007).
Nonetheless, the SAC often remains unsatisfied in situations where the error correction must operate to promote bi-orientation. For example, in both the SAC and error correction, a lack of tension is somehow ‘sensed' on KTs, engaging cells to take required actions. Therefore, it may not be surprising that common regulators are used at the KT both to signal the SAC and to facilitate the error correction. Aurora B/Ipl1, Mps1 (reviewed in Nezi and Musacchio, 2009), Sgo (Indjeian et al, 2005) and Bub1 (Asakawa et al, 2005; Windecker et al, 2009) are the examples of such common regulators. Given that SAC remains unsatisfied when KTs lack MT attachment, the involvement of Aurora B/Ipl1 and Mps1 (and Sgo and Bub1 as they recruit Aurora B/Ipl1 at the KT; see above) in both pathways may be explained as follows: these kinases may transiently create the MT-free KTs by facilitating the turnover of the KT–MT attachment, thus keeping the SAC unsatisfied (Tanaka et al, 2002; Ditchfield et al, 2003; Pinsky et al, 2006).
However, the actual relationship between the SAC and error correction might be more complex. For example, the following results cannot be explained by the above ‘simple' explanation: (1) Mps1 is required for the SAC in nocodazole-treated cells, where KTs lack MT attachment (Winey and Huneycutt, 2002), (2) Aurora B is also required for the SAC in the same circumstance, at least in some organisms (Kallio et al, 2002; Petersen and Hagan, 2003), (3) Mad3 phosphorylation by Ipl1 is required for the SAC, but not for the error correction, in the absence of tension (King et al, 2007) and (4) phosphorylation of Ndc80 N-terminus by Mps1 is required for the SAC, but not for establishing sister KT bi-orientation (Kemmler et al, 2009). Thus, phosphorylation of the KT components and SAC components by Aurora B/Ipl1 and Mps1 kinases may signal the SAC, in part, independently of the turnover of KT–MT attachment.
Conclusions and perspectives
Establishing sister KT bi-orientation is a pivotal process ensuring equal segregation of the genetic information into daughter cells upon cell division. In particular, the tension-dependent error correction is a crucial mechanism to secure sister KT bi-orientation. Sister KT bi-orientation and the error correction are not only fundamental for normal cell growth, but might also be important to prevent generation of cancers. Indeed, a recent report suggested that KT–MT attachment is more stable in cancer cells than in normal cells (Bakhoum et al, 2009), arguing that persistence of aberrant KT–MT attachment underlies the chromosome instability found in cancer cells. Thus, a defect in the error correction may have a causative function in generation of some type of cancers.
To understand the steps towards sister KT bi-orientation, many pertinent questions remain to be answered. For example, how do the Ndc80 complex, Dam1 complex (or a functional equivalent) and KNL1 cooperate to convert the lateral KT–MT attachment to the end-on attachment? What is the relative contribution of the error correction and KT geometry towards sister KT bi-orientation? What processes do cells undergo during turnover of the KT–MT attachment leading to the error correction; for example how is the old attachment removed and the new one established? How does Aurora B/Ipl1 promote turnover of the KT–MT attachment through phosphorylation of KT substrates? How does Mps1 contribute to the error correction; if Aurora B/Ipl1 is not the only target of Mps1, what are crucial substrates of Mps1 for the error correction? The combined efforts in biochemistry, structural analyses, biophysics, genetics, cell biology and in silico study will advance research in this field.
Acknowledgments
I thank Lesley Clayton for helpful comments on the manuscript, and Yusuke Oku for making Figure 3. I apologize to all the authors whose work could not be cited due to space limitation. Research in the author's lab has been supported by Cancer Research UK, Medical Research Council, Wellcome Trust, Human Frontier Science Program, Lister Research Institute Prize and Association for International Cancer Research. The author is a Senior Research Fellow of Cancer Research UK.
Footnotes
The author declares that he has no conflict of interest.
References
- Akiyoshi B, Nelson CR, Ranish JA, Biggins S (2009a) Analysis of Ipl1-mediated phosphorylation of the Ndc80 kinetochore protein in Saccharomyces cerevisiae. Genetics 183: 1591–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi B, Nelson CR, Ranish JA, Biggins S (2009b) Quantitative proteomic analysis of purified yeast kinetochores identifies a PP1 regulatory subunit. Genes Dev 23: 2887–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alushin GM, Ramey VH, Pasqualato S, Ball DA, Grigorieff N, Musacchio A, Nogales E (2010) The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature 467: 805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M, Haase J, Yeh E, Bloom K (2009) Function and assembly of DNA looping, clustering, and microtubule attachment complexes within a eukaryotic kinetochore. Mol Biol Cell 20: 4131–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004) Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6: 253–268 [DOI] [PubMed] [Google Scholar]
- Araki Y, Gombos L, Migueleti SP, Sivashanmugam L, Antony C, Schiebel E (2010) N-terminal regions of Mps1 kinase determine functional bifurcation. J Cell Biol 189: 41–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa K, Toya M, Sato M, Kanai M, Kume K, Goshima T, Garcia MA, Hirata D, Toda T (2005) Mal3, the fission yeast EB1 homologue, cooperates with Bub1 spindle checkpoint to prevent monopolar attachment. EMBO Rep 6: 1194–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbury CL, Gestaut DR, Powers AF, Franck AD, Davis TN (2006) The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proc Natl Acad Sci USA 103: 9873–9878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athale CA, Dinarina A, Mora-Coral M, Pugieux C, Nedelec F, Karsenti E (2008) Regulation of microtubule dynamics by reaction cascades around chromosomes. Science 322: 1243–1247 [DOI] [PubMed] [Google Scholar]
- Azimzadeh J, Bornens M (2007) Structure and duplication of the centrosome. J Cell Sci 120(Part 13): 2139–2142 [DOI] [PubMed] [Google Scholar]
- Bakhoum SF, Genovese G, Compton DA (2009) Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr Biol 19: 1937–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S, Murray AW (2001) The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev 15: 3118–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloecher A, Tatchell K (1999) Defects in Saccharomyces cerevisiae protein phosphatase type I activate the spindle/kinetochore checkpoint. Genes Dev 13: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar GA, Amon A (2008) Emerging roles for centromeres in meiosis I chromosome segregation. Nat Rev Genet 9: 899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffin E, Emre D, Karess RE (2007) Flies without a spindle checkpoint. Nat Cell Biol 9: 565–572 [DOI] [PubMed] [Google Scholar]
- Burke DJ, Stukenberg PT (2008) Linking kinetochore-microtubule binding to the spindle checkpoint. Dev Cell 14: 474–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas RE, Karsenti E (2003) Long-range communication between chromatin and microtubules in Xenopus egg extracts. Curr Biol 13: 1728–1733 [DOI] [PubMed] [Google Scholar]
- Carmena M, Ruchaud S, Earnshaw WC (2009) Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol 21: 796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudron M, Bunt G, Bastiaens P, Karsenti E (2005) Spatial coordination of spindle assembly by chromosome-mediated signaling gradients. Science 309: 1373–1376 [DOI] [PubMed] [Google Scholar]
- Chandhok NS, Pellman D (2009) A little CIN may cost a lot: revisiting aneuploidy and cancer. Curr Opin Genet Dev 19: 74–81 [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR III, Chan CS, Drubin DG, Barnes G (2002) Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111: 163–172 [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127: 983–997 [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A (2008) Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 9: 33–46 [DOI] [PubMed] [Google Scholar]
- Ciferri C, De Luca J, Monzani S, Ferrari KJ, Ristic D, Wyman C, Stark H, Kilmartin J, Salmon ED, Musacchio A (2005) Architecture of the human ndc80-hec1 complex, a critical constituent of the outer kinetochore. J Biol Chem 280: 29088–29095 [DOI] [PubMed] [Google Scholar]
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, Salek M, Rappsilber J, Moores CA, Salmon ED, Musacchio A (2008) Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Cameron LA, Salmon ED (2004) Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr Biol 14: 2149–2155 [DOI] [PubMed] [Google Scholar]
- Cimini D, Moree B, Canman JC, Salmon ED (2003) Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci 116(Part 20): 4213–4225 [DOI] [PubMed] [Google Scholar]
- Cimini D, Wan X, Hirel CB, Salmon ED (2006) Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol 16: 1711–1718 [DOI] [PubMed] [Google Scholar]
- Clarke PR, Zhang C (2008) Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol 9: 464–477 [DOI] [PubMed] [Google Scholar]
- Dai J, Sultan S, Taylor SS, Higgins JM (2005) The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev 19: 472–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum JR, Wren JD, Daniel JJ, Sivakumar S, McAvoy JN, Potapova TA, Gorbsky GJ (2009) Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr Biol 19: 1467–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wulf P, Montani F, Visintin R (2009) Protein phosphatases take the mitotic stage. Curr Opin Cell Biol 21: 806–815 [DOI] [PubMed] [Google Scholar]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED (2006) Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127: 969–982 [DOI] [PubMed] [Google Scholar]
- Dewar H, Tanaka K, Nasmyth K, Tanaka TU (2004) Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature 428: 93–97 [DOI] [PubMed] [Google Scholar]
- Ding R, McDonald KL, McIntosh JR (1993) Three-dimensional reconstruction and analysis of mitotic spindles from the yeast, Schizosaccharomyces pombe. J Cell Biol 120: 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS (2003) Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol 161: 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont S, Mitchison TJ (2009) Force and length in the mitotic spindle. Curr Biol 19: R749–R761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert CA, Gravdahl DJ, Megee PC (2007) The enhancement of pericentromeric cohesin association by conserved kinetochore components promotes high-fidelity chromosome segregation and is sensitive to microtubule-based tension. Genes Dev 21: 278–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremov A, Grishchuk EL, McIntosh JR, Ataullakhanov FI (2007) In search of an optimal ring to couple microtubule depolymerization to processive chromosome motions. Proc Natl Acad Sci USA 104: 19017–19022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MJ, Stukenberg PT (2007) Xenopus Cep57 is a novel kinetochore component involved in microtubule attachment. Cell 130: 893–905 [DOI] [PubMed] [Google Scholar]
- Fernius J, Hardwick KG (2007) Bub1 kinase targets Sgo1 to ensure efficient chromosome biorientation in budding yeast mitosis. PLoS Genet 3: e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck AD, Powers AF, Gestaut DR, Gonen T, Davis TN, Asbury CL (2007) Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nat Cell Biol 9: 832–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Meadows JC, Millar JB (2007) The Dam1/DASH complex is required for the retrieval of unclustered kinetochores in fission yeast. J Cell Sci 120 (Part 19): 3345–3351 [DOI] [PubMed] [Google Scholar]
- Gachet Y, Reyes C, Courtheoux T, Goldstone S, Gay G, Serrurier C, Tournier S (2008) Sister kinetochore recapture in fission yeast occurs by two distinct mechanisms, both requiring dam1 and klp2. Mol Biol Cell 19: 1646–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanos TN, Santamaria A, Jeyaprakash AA, Wang B, Conti E, Nigg EA (2009) Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J 28: 1442–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Godinho SA, Pellman D (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460: 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Essex A, Hu JS, Maddox PS, Motegi F, Sugimoto A, O'Rourke SM, Bowerman B, McLeod I, Yates JR III, Oegema K, Cheeseman IM, Desai A (2008) A new mechanism controlling kinetochore-microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev 22: 2385–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestaut DR, Graczyk B, Cooper J, Widlund PO, Zelter A, Wordeman L, Asbury CL, Davis TN (2008) Phosphoregulation and depolymerization-driven movement of the Dam1 complex do not require ring formation. Nat Cell Biol 10: 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregan J, Riedel CG, Pidoux AL, Katou Y, Rumpf C, Schleiffer A, Kearsey SE, Shirahige K, Allshire RC, Nasmyth K (2007) The kinetochore proteins Pcs1 and Mde4 and heterochromatin are required to prevent merotelic orientation. Curr Biol 17: 1190–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishchuk EL, Molodtsov MI, Ataullakhanov FI, McIntosh JR (2005) Force production by disassembling microtubules. Nature 438: 384–388 [DOI] [PubMed] [Google Scholar]
- Grishchuk EL, Spiridonov IS, Volkov VA, Efremov A, Westermann S, Drubin D, Barnes G, Ataullakhanov FI, McIntosh JR (2008) Different assemblies of the DAM1 complex follow shortening microtubules by distinct mechanisms. Proc Natl Acad Sci USA 105: 6918–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA (2004) Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol 166: 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes GJ, Dong Y, McEwen BF, Deluca JG (2008) Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol 18: 1778–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch A, Sillje HH, Nigg EA (2006) Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J 25: 5504–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, Van Meel J, Rieder CL, Peters JM (2003) The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol 161: 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Ikura M (2003) Crystal structure of the amino-terminal microtubule-binding domain of end-binding protein 1 (EB1). J Biol Chem 278: 36430–36434 [DOI] [PubMed] [Google Scholar]
- Hayden JH, Bowser SS, Rieder CL (1990) Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol 111: 1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Rines DR, Espelin CW, Sorger PK (2001) Molecular analysis of kinetochore-microtubule attachment in budding yeast. Cell 106: 195–206 [DOI] [PubMed] [Google Scholar]
- Heath IB (1980) Variant mitoses in lower eukaryotes: indication of the evolution of mitosis? Int Rev Cytol 64: 1–80 [DOI] [PubMed] [Google Scholar]
- Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, Green S, Taylor SS (2010) Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J Cell Biol 190: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AJ, Cleveland DW (2009) Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat Rev Mol Cell Biol 10: 478–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer S, Mayer TU (2009) Cdk1 negatively regulates midzone localization of the mitotic kinesin Mklp2 and the chromosomal passenger complex. Curr Biol 19: 607–612 [DOI] [PubMed] [Google Scholar]
- Indjeian VB, Murray AW (2007) Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Curr Biol 17: 1837–1846 [DOI] [PubMed] [Google Scholar]
- Indjeian VB, Stern BM, Murray AW (2005) The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307: 130–133 [DOI] [PubMed] [Google Scholar]
- Janke C, Ortiz J, Tanaka TU, Lechner J, Schiebel E (2002) Four new subunits of the Dam1-Duo1 complex reveal novel functions in sister kinetochore biorientation. EMBO J 21: 181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelluma N, Brenkman AB, van den Broek NJ, Cruijsen CW, van Osch MH, Lens SM, Medema RH, Kops GJ (2008) Mps1 phophorylates Borealin to control Aurora B activity and chromosome alignment. Cell 132: 233–246 [DOI] [PubMed] [Google Scholar]
- Joglekar AP, Bloom K, Salmon ED (2009) In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr Biol 19: 694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar AP, Bloom KS, Salmon ED (2010) Mechanisms of force generation by end-on kinetochore-microtubule attachments. Curr Opin Cell Biol 22: 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MH, Huneycutt BJ, Pearson CG, Zhang C, Morgan G, Shokat K, Bloom K, Winey M (2005) Chemical genetics reveals a role for Mps1 kinase in kinetochore attachment during mitosis. Curr Biol 15: 160–165 [DOI] [PubMed] [Google Scholar]
- Kalab P, Pralle A, Isacoff EY, Heald R, Weis K (2006) Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature 440: 697–701 [DOI] [PubMed] [Google Scholar]
- Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ (2002) Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol 12: 900–905 [DOI] [PubMed] [Google Scholar]
- Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, McEwen BF, Khodjakov A (2006) Chromosomes can congress to the metaphase plate before biorientation. Science 311: 388–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS, Watanabe Y (2007) Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev 21: 420–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y (2010) Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327: 172–177 [DOI] [PubMed] [Google Scholar]
- Keating P, Rachidi N, Tanaka TU, Stark MJ (2009) Ipl1-dependent phosphorylation of Dam1 is reduced by tension applied on kinetochores. J Cell Sci 122(Part 23): 4375–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H (2010) Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330: 235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmler S, Stach M, Knapp M, Ortiz J, Pfannstiel J, Ruppert T, Lechner J (2009) Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J 28: 1099–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM (2003) Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J Cell Biol 160: 671–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL (2009) The nature of cell-cycle checkpoints: facts and fallacies. J Biol 8: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiburz BM, Amon A, Marston AL (2008) Shugoshin promotes sister kinetochore biorientation in Saccharomyces cerevisiae. Mol Biol Cell 19: 1199–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiermaier E, Woehrer S, Peng Y, Mechtler K, Westermann S (2009) A Dam1-based artificial kinetochore is sufficient to promote chromosome segregation in budding yeast. Nat Cell Biol 11: 1109–1115 [DOI] [PubMed] [Google Scholar]
- King EM, Rachidi N, Morrice N, Hardwick KG, Stark MJ (2007) Ipl1p-dependent phosphorylation of Mad3p is required for the spindle checkpoint response to lack of tension at kinetochores. Genes Dev 21: 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JM, Hays TS, Nicklas RB (2000) Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J Cell Biol 151: 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JM, Nicklas RB (2000) Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J Cell Sci 113(Part 21): 3815–3823 [DOI] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T (1986) Beyond self-assembly: from microtubules to morphogenesis. Cell 45: 329–342 [DOI] [PubMed] [Google Scholar]
- Kitamura E, Tanaka K, Kitamura Y, Tanaka TU (2007) Kinetochore microtubule interaction during S phase in Saccharomyces cerevisiae. Genes Dev 21: 3319–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura E, Tanaka K, Komoto S, Kitamura Y, Antony C, Tanaka TU (2010) Kinetochores generate microtubules with distal plus ends: their roles and limited lifetime in mitosis. Dev Cell 18: 248–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomitsu T, Obuse C, Yanagida M (2007) Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell 13: 663–676 [DOI] [PubMed] [Google Scholar]
- Knowlton AL, Lan W, Stukenberg PT (2006) Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol 16: 1705–1710 [DOI] [PubMed] [Google Scholar]
- Kops GJ, Saurin AT, Meraldi P (2010) Finding the middle ground: how kinetochores power chromosome congression. Cell Mol Life Sci 67: 2145–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield S, Lau DT, Murray AW (2009) Recruiting a microtubule-binding complex to DNA directs chromosome segregation in budding yeast. Nat Cell Biol 11: 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert F, Hornung P, Westermann S (2010) The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J Cell Biol 189: 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM (2004) Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol 6: 232–237 [DOI] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT (2004) Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol 14: 273–286 [DOI] [PubMed] [Google Scholar]
- Lew DJ, Burke DJ (2003) The spindle assembly and spindle position checkpoints. Annu Rev Genet 37: 251–282 [DOI] [PubMed] [Google Scholar]
- Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM (2009) Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 323: 1350–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Vleugel M, Backer CB, Hori T, Fukagawa T, Cheeseman IM, Lampson MA (2010) Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol 188: 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Onuchic JN (2006) A driving and coupling ‘Pac-Man' mechanism for chromosome poleward translocation in anaphase A. Proc Natl Acad Sci USA 103 (49): 18432–18437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, McLeod I, Anderson S, Yates JR, He X (2005) Molecular analysis of kinetochore architecture in fission yeast. EMBO J 24: 2919–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncarek J, Kisurina-Evgenieva O, Vinogradova T, Hergert P, La Terra S, Kapoor TM, Khodjakov A (2007) The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature 450: 745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J, George KA, Terret ME, Zhang C, Shokat KM, Jallepalli PV (2010) Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol 190: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H, Rieder CL, Khodjakov A (2004) Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J Cell Biol 167: 831–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca TJ, Salmon ED (2009) Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol 184: 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell DP, Hu XW, Singleton MR (2010) Molecular architecture and assembly of the yeast kinetochore MIND complex. J Cell Biol 190: 823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maure JF, Kitamura E, Tanaka TU (2007) Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism. Curr Biol 17: 2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KL, O′Toole ET, Mastronarde DN, McIntosh JR (1992) Kinetochore microtubules in PTK cells. J Cell Biol 118: 369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JR, Grishchuk EL, Morphew MK, Efremov AK, Zhudenkov K, Volkov VA, Cheeseman IM, Desai A, Mastronarde DN, Ataullakhanov FI (2008) Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell 135: 322–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, McAinsh AD, Rheinbay E, Sorger PK (2006) Phylogenetic and structural analysis of centromeric DNA and kinetochore proteins. Genome Biol 7: R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Sorger PK (2005) A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J 24: 1621–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, Johnson ML, Stukenberg PT (2008) Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80(Hec1). Curr Biol 18: 1785–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JJ, De Wulf P, Sorger PK, Harrison SC (2005) The yeast DASH complex forms closed rings on microtubules. Nat Struct Mol Biol 12: 138–143 [DOI] [PubMed] [Google Scholar]
- Mirchenko L, Uhlmann F (2010) Sli15(INCENP) dephosphorylation prevents mitotic checkpoint reengagement due to loss of tension at anaphase onset. Curr Biol 20: 1396–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra RK, Chakraborty P, Arnaoutov A, Fontoura BM, Dasso M (2010) The Nup107-160 complex and gamma-TuRC regulate microtubule polymerization at kinetochores. Nat Cell Biol 12: 164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8: 379–393 [DOI] [PubMed] [Google Scholar]
- Nasmyth K (2002) Segregating sister genomes: the molecular biology of chromosome separation. Science 297: 559–565 [DOI] [PubMed] [Google Scholar]
- Nezi L, Musacchio A (2009) Sister chromatid tension and the spindle assembly checkpoint. Curr Opin Cell Biol 21: 785–795 [DOI] [PubMed] [Google Scholar]
- Nicklas RB (1997) How cells get the right chromosomes. Science 275: 632–637 [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Koch CA (1969) Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J Cell Biol 43: 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E, Ramey VH (2009) Structure-function insights into the yeast Dam1 kinetochore complex. J Cell Sci 122(Part 21): 3831–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell CB, Khodjakov AL (2007) Cooperative mechanisms of mitotic spindle formation. J Cell Sci 120(Part 10): 1717–1722 [DOI] [PubMed] [Google Scholar]
- O'Connell CB, Loncarek J, Kalab P, Khodjakov A (2009) Relative contributions of chromatin and kinetochores to mitotic spindle assembly. J Cell Biol 187: 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi R, Coughlin ML, Lane WS, Mitchison TJ (2003) An inner centromere protein that stimulates the microtubule depolymerizing activity of a KinI kinesin. Dev Cell 5: 309–321 [DOI] [PubMed] [Google Scholar]
- Ohi R, Sapra T, Howard J, Mitchison TJ (2004) Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell 15: 2895–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RA, Hamilton RS, Pauli A, Davis I, Nasmyth K (2010) Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei. Nat Cell Biol 12: 185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergren G (1951) The mechanisms of co-orientation in bivalents and mutivalents. Hereditas 37: 85–156 [Google Scholar]
- Pagliuca C, Draviam VM, Marco E, Sorger PK, De Wulf P (2009) Roles for the conserved spc105p/kre28p complex in kinetochore-microtubule binding and the spindle assembly checkpoint. PLoS One 4: e7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DH, Hickson GR, O′Farrell PH (2003) Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr Biol 13: 647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Yeh E, Gardner M, Odde D, Salmon ED, Bloom K (2004) Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase. Curr Biol 14: 1962–1967 [DOI] [PubMed] [Google Scholar]
- Pereira G, Schiebel E (2003) Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science 302: 2120–2124 [DOI] [PubMed] [Google Scholar]
- Petersen J, Hagan IM (2003) S. pombe Aurora kinase/survivin is required for chromosome condensation and the spindle checkpoint attachment response. Curr Biol 13: 590–597 [DOI] [PubMed] [Google Scholar]
- Petrovic A, Pasqualato S, Dube P, Krenn V, Santaguida S, Cittaro D, Monzani S, Massimiliano L, Keller J, Tarricone A, Maiolica A, Stark H, Musacchio A (2010) The MIS12 complex is a protein interaction hub for outer kinetochore assembly. J Cell Biol 190: 835–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky BA, Kung C, Shokat KM, Biggins S (2006) The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol 8: 78–83 [DOI] [PubMed] [Google Scholar]
- Porter IM, Swedlow JR (2007) Bod1, a novel kinetochore protein required for chromosome biorientation. J Cell Biol 179: 187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AF, Franck AD, Gestaut DR, Cooper J, Gracyzk B, Wei RR, Wordeman L, Davis TN, Asbury CL (2009) The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 136: 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers JA, Tanenbaum ME, Maia AF, Medema RH (2009) RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J Cell Sci 122(Part 14): 2436–2445 [DOI] [PubMed] [Google Scholar]
- Renshaw MJ, Ward JJ, Kanemaki M, Natsume K, Nedelec FJ, Tanaka TU (2010) Condensins promote chromosome recoiling during early anaphase to complete sister chromatid separation. Dev Cell 19: 232–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Alexander SP (1990) Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol 110: 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT (2008) Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science 319: 469–472 [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC (2007) Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol 8: 798–812 [DOI] [PubMed] [Google Scholar]
- Sakuno T, Tada K, Watanabe Y (2009) Kinetochore geometry defined by cohesion within the centromere. Nature 458: 852–858 [DOI] [PubMed] [Google Scholar]
- Sakuno T, Watanabe Y (2009) Studies of meiosis disclose distinct roles of cohesion in the core centromere and pericentromeric regions. Chromosome Res 17: 239–249 [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez I, Renwick SJ, Crawley K, Karig I, Buck V, Meadows JC, Franco-Sanchez A, Fleig U, Toda T, Millar JB (2005) The DASH complex and Klp5/Klp6 kinesin coordinate bipolar chromosome attachment in fission yeast. EMBO J 24: 2931–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandall S, Severin F, McLeod IX, Yates JR III, Oegema K, Hyman A, Desai A (2006) A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell 127: 1179–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Musacchio A (2009) The life and miracles of kinetochores. EMBO J 28: 2511–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Tighe A, D'Alise AM, Taylor SS, Musacchio A (2010) Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J Cell Biol 190: 73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon I, Severin FF, Andrews PD, Taba MR, Kaplan KB, Ashford AJ, Stark MJ, Sorger PK, Hyman AA (1999) Regulation of Saccharomyces cerevisiae kinetochores by the type 1 phosphatase Glc7p. Genes Dev 13: 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazer S (2005) Nuclear envelope: nuclear pore complexity. Curr Biol 15: R23–R26 [DOI] [PubMed] [Google Scholar]
- Shang C, Hazbun TR, Cheeseman IM, Aranda J, Fields S, Drubin DG, Barnes G (2003) Kinetochore protein interactions and their regulation by the Aurora kinase Ipl1p. Mol Biol Cell 14: 3342–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa MM, Widlund PO, Riffle M, Ess M, Davis TN (2009) Bir1 is required for the tension checkpoint. Mol Biol Cell 20: 915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliedrecht T, Zhang C, Shokat KM, Kops GJ (2010) Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PLoS One 5: e10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E, Matsusaka T, Morrison C, Vagnarelli P, Hoshi O, Ushiki T, Nojima K, Fukagawa T, Waizenegger IC, Peters JM, Earnshaw WC, Takeda S (2001) Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells. Dev Cell 1: 759–770 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kitamura E, Kitamura Y, Tanaka TU (2007) Molecular mechanisms of microtubule-dependent kinetochore transport toward spindle poles. J Cell Biol 178: 269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, Prescott AR, Antony C, Tanaka TU (2005a) Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434: 987–994 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Cosma MP, Wirth K, Nasmyth K (1999) Identification of cohesin association sites at centromeres and along chromosome arms. Cell 98: 847–858 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Fuchs J, Loidl J, Nasmyth K (2000) Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nat Cell Biol 2: 492–499 [DOI] [PubMed] [Google Scholar]
- Tanaka TU (2008) Bi-orienting chromosomes: acrobatics on the mitotic spindle. Chromosoma 117: 521–533 [DOI] [PubMed] [Google Scholar]
- Tanaka TU, Desai A (2008) Kinetochore-microtubule interactions: the means to the end. Curr Opin Cell Biol 20: 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K (2002) Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108: 317–329 [DOI] [PubMed] [Google Scholar]
- Tanaka TU, Stark MJ, Tanaka K (2005b) Kinetochore capture and bi-orientation on the mitotic spindle. Nat Rev Mol Cell Biol 6: 929–942 [DOI] [PubMed] [Google Scholar]
- Thompson SL, Bakhoum SF, Compton DA (2010) Mechanisms of chromosomal instability. Curr Biol 20: R285–R295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien JF, Umbreit NT, Gestaut DR, Franck AD, Cooper J, Wordeman L, Gonen T, Asbury CL, Davis TN (2010) Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J Cell Biol 189: 713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Tanno Y, Watanabe Y (2010) Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467: 719–723 [DOI] [PubMed] [Google Scholar]
- Uchida KS, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T (2009) Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol 184: 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnarelli P, Morrison C, Dodson H, Sonoda E, Takeda S, Earnshaw WC (2004) Analysis of Scc1-deficient cells defines a key metaphase role of vertebrate cohesin in linking sister kinetochores. EMBO Rep 5: 167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoosthuyse V, Prykhozhij S, Hardwick KG (2007) Shugoshin 2 regulates localization of the chromosomal passenger proteins in fission yeast mitosis. Mol Biol Cell 18: 1657–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Novelle MD, Petronczki M (2010) Relocation of the chromosomal passenger complex prevents mitotic checkpoint engagement at anaphase. Curr Biol 20: 1402–1407 [DOI] [PubMed] [Google Scholar]
- Walczak CE, Heald R (2008) Mechanisms of mitotic spindle assembly and function. Int Rev Cytol 265: 111–158 [DOI] [PubMed] [Google Scholar]
- Wan X, O′Quinn RP, Pierce HL, Joglekar AP, Gall WE, DeLuca JG, Carroll CW, Liu ST, Yen TJ, McEwen BF, Stukenberg PT, Desai A, Salmon ED (2009) Protein architecture of the human kinetochore microtubule attachment site. Cell 137: 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JM (2010) Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 330: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Ramey VH, Westermann S, Leschziner AE, Welburn JP, Nakajima Y, Drubin DG, Barnes G, Nogales E (2007) Architecture of the Dam1 kinetochore ring complex and implications for microtubule-driven assembly and force-coupling mechanisms. Nat Struct Mol Biol 14: 721–726 [DOI] [PubMed] [Google Scholar]
- Wei RR, Al-Bassam J, Harrison SC (2007) The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat Struct Mol Biol 14: 54–59 [DOI] [PubMed] [Google Scholar]
- Wei RR, Schnell JR, Larsen NA, Sorger PK, Chou JJ, Harrison SC (2006) Structure of a central component of the yeast kinetochore: the Spc24p/Spc25p globular domain. Structure 14: 1003–1009 [DOI] [PubMed] [Google Scholar]
- Wei RR, Sorger PK, Harrison SC (2005) Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc Natl Acad Sci USA 102: 5363–5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR III, Cheeseman IM (2009) The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell 16: 374–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Vleugel M, Liu D, Yates JR III, Lampson MA, Fukagawa T, Cheeseman IM (2010) Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell 38: 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S, Avila-Sakar A, Wang HW, Niederstrasser H, Wong J, Drubin DG, Nogales E, Barnes G (2005) Formation of a dynamic kinetochore-microtubule interface through assembly of the Dam1 ring complex. Mol Cell 17: 277–290 [DOI] [PubMed] [Google Scholar]
- Westermann S, Drubin DG, Barnes G (2007) Structures and functions of yeast kinetochore complexes. Annu Rev Biochem 76: 563–591 [DOI] [PubMed] [Google Scholar]
- Westermann S, Wang HW, Avila-Sakar A, Drubin DG, Nogales E, Barnes G (2006) The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature 440: 565–569 [DOI] [PubMed] [Google Scholar]
- Wilson-Kubalek EM, Cheeseman IM, Yoshioka C, Desai A, Milligan RA (2008) Orientation and structure of the Ndc80 complex on the microtubule lattice. J Cell Biol 182: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windecker H, Langegger M, Heinrich S, Hauf S (2009) Bub1 and Bub3 promote the conversion from monopolar to bipolar chromosome attachment independently of shugoshin. EMBO Rep 10: 1022–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Huneycutt BJ (2002) Centrosomes and checkpoints: the MPS1 family of kinases. Oncogene 21: 6161–6169 [DOI] [PubMed] [Google Scholar]
- Winey M, Mamay CL, O'Toole ET, Mastronarde DN, Giddings TH Jr, McDonald KL, McIntosh JR (1995) Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J Cell Biol 129: 1601–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, O'Toole ET (2001) The spindle cycle in budding yeast. Nat Cell Biol 3: E23–E27 [DOI] [PubMed] [Google Scholar]
- Witt PL, Ris H, Borisy GG (1980) Origin of kinetochore microtubules in Chinese hamster ovary cells. Chromosoma 81: 483–505 [DOI] [PubMed] [Google Scholar]
- Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A (2005) Efficient chromosome capture requires a bias in the ‘search-and-capture' process during mitotic-spindle assembly. Curr Biol 15: 828–832 [DOI] [PubMed] [Google Scholar]
- Wong J, Nakajima Y, Westermann S, Shang C, Kang JS, Goodner C, Houshmand P, Fields S, Chan CS, Drubin D, Barnes G, Hazbun T (2007) A protein interaction map of the mitotic spindle. Mol Biol Cell 18: 3800–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y (2010) Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330: 239–243 [DOI] [PubMed] [Google Scholar]
- Yang Z, Tulu US, Wadsworth P, Rieder CL (2007) Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr Biol 17: 973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Haase J, Paliulis LV, Joglekar A, Bond L, Bouck D, Salmon ED, Bloom KS (2008) Pericentric chromatin is organized into an intramolecular loop in mitosis. Curr Biol 18: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Gruss OJ, Rybina S, Caudron M, Schelder M, Wilm M, Mattaj IW, Karsenti E (2008) Cdk11 is a RanGTP-dependent microtubule stabilization factor that regulates spindle assembly rate. J Cell Biol 180: 867–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Lin W, Latham JA, Riefler GM, Schumacher JM, Chan C, Tatchell K, Hawke DH, Kobayashi R, Dent SY (2005) The Set1 methyltransferase opposes Ipl1 Aurora kinase functions in chromosome segregation. Cell 122: 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lan W, Ems-McClung SC, Stukenberg PT, Walczak CE (2007) Aurora B phosphorylates multiple sites on mitotic centromere-associated kinesin to spatially and temporally regulate its function. Mol Biol Cell 18: 3264–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]