Abstract
This work evaluated gelatin microparticles and biodegradable composite scaffolds for the controlled release of bone morphogenetic protein-2 (BMP-2) in vitro and in vivo. Gelatin crosslinking (10 and 40 mM glutaraldehyde), BMP-2 dose (6 and 60 ng BMP-2 / mg dry microparticles), buffer type (phosphate buffered saline (PBS) and collagenase-containing PBS), and gelatin type (acidic and basic) were investigated for their effects on BMP-2 release. Release profiles were also observed using poly(lactic-co-glycolic acid) (PLGA) microparticles with varying molecular weights (8,300 and 57,500). In vitro and in vivo studies were conducted using radiolabeled BMP-2; the chloramine-T method was preferred over Bolton-Hunter reagent for radioiodination with this system. BMP-2 release from PLGA microparticles resulted in a moderate burst release followed by minimal cumulative release, while BMP-2 release from gelatin microparticles exhibited minimal burst release followed by linear release kinetics in vitro. Growth factor dose had a small effect on its normalized release kinetics probably because of an equilibrium between gelatin-bound and unbound BMP-2. Differences in release from acidic and basic gelatin microparticles may result from the different pretreatment conditions used for gelatin synthesis. The in vitro release kinetics for both gelatin microparticles alone and within composite scaffolds were dependent largely on the extent of gelatin crosslinking; varying buffer type served to confirm that controlled release relies on enzymatic degradation of the gelatin for controlled release. Finally, in vivo studies with composite scaffolds exhibited minimal burst and linear release up to 28 days. In summary, dose effects on BMP-2 release were found to be minimal while varying gelatin type and release medium can alter release kinetics. These results demonstrate a systematic control of BMP-2 delivery from gelatin microparticles can be achieved by altering the extent of basic gelatin crosslinking.
Keywords: Controlled release, Growth factor delivery, Bone morphogenetic protein, Gelatin microparticles, Bone tissue engineering
Introduction
Because of its potency as an osteogenic growth factor, bone morphogenetic protein-2 (BMP-2) may be particularly useful in healing critical size defects (CSDs) in which bone cannot regenerate sufficiently to bridge the length of the defect. Every year, several hundreds of thousands of cases of these large defects will occur that result in impaired, improper, or no healing of the bone. Many different reasons can account for this inability to heal, including inadequate vascularity, infection, micromotion, and instability at the site of the defect [1]. In all such cases, surgical intervention is often required to achieve union of the defect. These harsh surgical techniques rely on autografts, allografts, and bone cements, but all of these come with their own drawbacks [2–4].
In the case of BMP-2, it has been shown to be present in the various stages of bone healing, from the initial phases of fracture repair [5] as well as during later stages involving chondrogenesis and osteogenesis [6]. However, delivery of BMP-2 alone to a defect results in its quick clearance from the site since it has a very short half-life [7]. Therefore, biomaterials are necessary as carriers to maintain BMP-2 retention at the defect site; additionally, they may be used to provide controlled and sustained delivery of the growth factor to mimic its temporal profile during natural bone healing in vivo.
The most common group of synthetic biomaterials consists of poly(glycolic acid) (PGA), poly(l-lactic acid) (PLLA), and copolymers of lactic and glycolic acid (PLGA) [8]. They have been utilized in biomedical applications for several decades, and also recently for bone tissue engineering [9–11]. PLGA beads have been mixed with BMPs to produce accelerated bone regeneration in a bone defect [12]. BMP-2 has also been delivered from PLGA bioerodible particles [13] mixed with either a carboxymethylcellulose or a methylcellulose carrier. This matrix was used in a rabbit cranial defect to provide bony bridging [14]. Porous PLG matrices [15] and PLG-poly(ethylene glycol) (PEG) matrices [16, 17] have also been used for BMP-2 delivery.
For bone tissue engineering, many studies have utilized a collagen sponge [18, 19]. It is commonly used to deliver BMPs [20]. Release of BMP-2 from collagen has been extensively characterized and results have demonstrated that its interaction with the growth factor is based on electrostatic attraction. BMP-2 release from collagen sponges was also evaluated in a rat ectopic assay; changes in the isoelectric points of BMP-2 via succinylation resulted in almost 100% release within 24 hours [21]. Additionally, collagen is naturally found in bone, so it can act as a physical substrate for bone regeneration as well [12].
Our laboratory is developing a system of novel biomaterials for the controlled delivery of bioactive molecules. This system is based on gelatin microparticles which serve as carriers for growth factors. Gelatin has shown utility as a biomaterial for tissue engineering, having previously been used as disks for the delivery of several growth factors, including basic fibroblast growth factor (bFGF), transforming growth factor-beta1 (TGF-β1), and BMP-2 [22]. More recently, a delivery system based on gelatin microparticles was used to provide controlled release of TGF-β1 and insulin-like growth factor [23, 24]. The controlled release of growth factors from gelatin is due to the enzymatic degradation of gelatin, which occurs by matrix metalloproteinases such as collagenase, and the extent of gelatin crosslinking can affect its degradation [25].
The association between the growth factor and gelatin is largely based on electrostatic interactions that mimic in vivo sequestering [26]. Acidic and basic gelatin types have isoelectric points of 5 and 9, respectively. Depending on the isoelectric points of the growth factor, the appropriate gelatin type can be chosen for delivery. For example, bFGF with an isoelectric point (IEP) of 9.6 is positively charged at physiological pH, while acidic gelatin is negatively charged (IEP = 5). Using this combination, sustained release of bFGF from acidic gelatin disks has been demonstrated [26].
Gelatin microparticles can also be easily synthesized and loaded with the growth factor after synthesis, thus protecting the growth factor from any harsh processing conditions required for microparticle generation. Poly(lactic-co-glycolic acid) (PLGA) microparticles have been commonly used for controlled delivery of BMP-2, but in general, they are prepared with the growth factor incorporated within [27, 28]. This may expose the protein to various organic solvents and temperature variations which may denature the growth factor completely or significantly reduce its bioactivity in vivo. Few studies have evaluated BMP-2 release from PLGA microparticles when the growth factor is loaded by adsorption after the preparation of microparticles [13, 29, 30].
The choice of microparticles over disks allows for incorporation of the microparticles within other scaffold carriers that can provide different material properties. In our delivery system, composites scaffolds are generated which consist of gelatin microparticles incorporated within the porous network of a polymer scaffold. The polymer scaffold, made of poly(propylene fumarate) (PPF), is biodegradable [31] and its biocompatibility in terms of soft and hard tissue response has also been demonstrated [32]. Additionally, PLGA microparticles have been embedded within porous PPF scaffolds for the purposes of bone repair in segmental defects [33]. In this study, the porous PPF scaffold serves as a carrier for growth factor-loaded microparticles and can guide the development and growth of new tissue.
The goal of this study was to evaluate gelatin microparticles for the controlled release of BMP-2. First, we investigated the effects of gelatin crosslinking, growth factor dose, release medium, and gelatin type on BMP-2 release kinetics. We hypothesized that decreasing gelatin crosslinking would result in higher BMP-2 release. Similarly, we also hypothesized that growth factor release in collagenase-containing PBS would be higher due to faster degradation of the gelatin. Based on previous work discussed above, we hypothesized that BMP-2, with an IEP of 8.5 would have lower release from acidic gelatin (IEP = 5) compared to basic gelatin (IEP = 9) due to stronger electrostatic interactions. Finally, we hypothesized that higher doses of BMP-2 loaded in the gelatin microparticles would result in higher burst release of the growth factor after 24 hours.
In vitro release was evaluated by radiolabeling of BMP-2 with the commonly used chloramine-T method, and gelatin crosslinking, growth factor dose, buffer type, and gelatin type were varied as parameters. Additionally, the Bolton-Hunter radiolabeling method, which has been shown to cause some reduction of BMP-2 bioactivity [34, 35] and possibly affect its release, was also used to label a subset of samples to evaluate its effect on BMP-2 release profiles in vitro.
We also chose to compare gelatin microparticles with PLGA microparticles for BMP-2 release. In order to compare efficacy, both types were loaded by diffusion/adsorption in this study. PLGA molecular weight and growth factor dose were varied and their effects on BMP-2 release in vitro were evaluated.
After identifying which parameters affected BMP-2 release from microparticles alone, we tested these parameters for in vitro release from composite scaffolds of gelatin microparticles within a porous PPF scaffold. An in vivo study was also conducted with optimized parameters to evaluate the release profile of BMP-2 from the composite scaffolds in a subcutaneous mouse model.
Materials and Methods
Experimental Design
Both acidic (IEP = 5) and basic (IEP = 9) gelatin were evaluated for controlled release of BMP-2 (IEP = 8.5). Gelatin microparticles were crosslinked with two concentrations of glutaraldehyde (10 and 40 mM). PLGA microparticles were prepared using two weight average molecular weights (8,300 and 57,500). A low and high dose of growth factor (6 and 60 ng of BMP-2 per mg of dry microparticles) was used for loading. These doses of BMP-2 were identical to those used for VEGF [36] and TGF- β1 [24] studies previously conducted in our laboratory, allowing for comparisons between the growth factor release kinetics.
Samples were incubated in two buffer types: phosphate buffered saline (PBS) and collagenase-containing PBS (Coll) with 400 ng/mL of bacterial collagenase 1A – a collagenase which recognizes and digests part of gelatin’s amino acid sequence [37]. In vitro studies were conducted to evaluate BMP-2 release kinetics from gelatin and PLGA microparticles. Gelatin microparticles were also incorporated within a porous PPF scaffold and BMP-2 release kinetics were evaluated in vitro and in vivo in a mouse subcutaneous model.
Radioiodination of BMP-2
Human recombinant BMP-2 (Astellas Pharma Inc., Tokyo, Japan) was radioiodinated using the chloramine-T method of Greenwood et al. [38]. First, 4 mL of NaI125 was added to a solution of 40 mL of BMP-2 (3 mg/mL) in 5 mM glutamic acid, 2.5 wt% glycine, 0.5 wt% sucrose, and 0.01 wt% Tween 80 (pH 4.5). This was followed by the addition of 100 µL of chloramine-T (0.2 mg/mL) (Nacalai Tesque, Inc., Kyoto, Japan) in 0.5 M potassium phosphate-buffered solution (pH 7.5) containing 0.5 M NaCl. The mixture was agitated for 2 min at room temperature and the reaction was terminated with 100 µL PBS (pH 7.5) containing 0.4 mg of sodium metabisulfate. Purification was achieved by gel filtration through a Sephadex PD-10 column (Amersham Biosciences, Pittsburg, PA) to remove any free I125 from the solution. A bicinchoninic acid (BCA) protein assay (Pierce Biotechnologies, Rockford, IL) was used to determine the concentration of BMP-2.
To evaluate the effect of different types of radioiodination methods on the release of BMP-2, the Bolton-Hunter method [39] was also used to radiolabel BMP-2 for a subset of the samples (40 mM glutaraldehyde, acidic gelatin, high dose, in both PBS and collagenase-containing PBS). Following evaporation of the benzene carrier, the Bolton-Hunter reagent, an iodinated 3-(4-hydroxyphenyl) propionic acid N-hydroxysuccinimide ester (Perkin Elmer, Waltham, MA), was incubated with BMP-2 on ice with gentle agitation for 2 hours. The reaction was terminated with the addition of 100 µL of glycine (1 mg/mL in water). This was followed by filtration through the Sephadex PD-10 column and quantification with the BCA protein assay.
Gelatin Microparticle Preparation
5 g of gelatin (Nitta Gelatin Co., Osaka, Japan) were dissolved in 45 mL of water and added dropwise to 200 mL olive oil to create a water-in-oil emulsion [23]. The solution was stirred at 500 rpm and chilled to 10°C for 1.5 hours; microparticles were then collected by washing with acetone and vacuum filtration. They were crosslinked overnight in a glutaraldehyde solution and the reaction was terminated by the addition of glycine (25 mg/mL) to block residual aldehyde groups. The microparticles were again washed in acetone and collected by filtration, lyophilized, and then sieved to obtain particles ranging in diameter from 50–100 µm.
BMP-2 incorporation was achieved by diffusional loading; a solution of growth factor in PBS was dripped onto the microparticles at a volume of 5 µL per mg of dry microparticles. Following vortexing, the loaded microparticles were incubated at 4°C for 20 hours.
PLGA Microparticle Preparation
PLGA (50:50 DL, methyl ester endcaps) was obtained from Lakeshore Biomaterials (Birmingham, AL). The two types had weight average molecular weights of 8,300 (8.3K) and 57,500 (57.5K) and polydispersity indices of 7.2 and 33.4, as determined by gel permeation chromatography (GPC) using polystyrene standards for the construction of a calibration curve. As described before, a double emulsion [(water-in-oil)-in water], solvent extraction technique was used for the preparation of PLGA microparticles [28]. In brief, 0.25 g of PLGA was dissolved in 1 mL of methylene chloride. 125 µL of PBS (with 0.1% bovine serum albumin) was added to create the first emulsion and the solution was vortexed for exactly 60 sec; this was followed immediately by addition of 1.5 mL of 0.3% aqueous poly(vinyl alcohol) (PVA) solution (second emulsion) and another 60 secs of vortexing. This was added to 98.5 mL of 0.3% PVA and 100 mL of 0.2% isopropyl alcohol solution and stirred for 1 hour at room temperature. The microparticles were then collected by centrifugation and washed with water three times and then lyophilized. The average diameter of these PLGA microparticles was determined on a Multisizer3 Coulter Counter (Beckman Coulter, Fullerton, CA). BMP-2 incorporation was achieved by dripping a solution of growth factor (1.9 µL/mg of dry microparticles) so that all the growth factor would be adsorbed. Following vortexing, the loaded microparticles were incubated at 4°C for 20 hours.
PPF Synthesis
PPF synthesis involved the generation of a diester intermediate followed by polymerization [40]. First, diethyl fumarate, propylene glycol, hydroquinone, and zinc chloride were combined in a 1:3:0.003:0.01 molar ratio and stirred at 300 rpm and heated to 130°C under a nitrogen purge. Ethanol was distilled out and the reaction was stopped when 90% of the theoretical yield of ethanol was removed. The temperature was then set to 100°C and vacuum (<1 mmHg) was applied. Every 30 min, the temperature was raised 10°C to 130°C and maintained while propylene glycol was removed as a distillate. Samples were collected every hour for GPC analysis and the reaction was terminated once the desired molecular weight was reached. Purification was achieved through a 1.85% hydrochloric acid wash followed by a series of aqueous washes to remove zinc chloride and an ether wash to remove hydroquinone. The purified polymer was then vacuum dried to eliminate any residual solvent and evaluated for final molecular weight by GPC.
Porous PPF Scaffold Fabrication
To generate porous polymer scaffolds, PPF and N-vinyl pyrrolidone were mixed together in a 1:1 mass ratio; this was followed by addition of 0.5 wt% benzoyl peroxide (0.1 mg/mL in acetone) and 80 wt% NaCl (300–500 µm crystals) [41]. This paste was packed into molds (7.5 mm diameter, 1 mm height) and crosslinked overnight at 60°C. The scaffolds were leached in water for 3 days to remove the salt, resulting in a porous structure. These porous PPF scaffolds were then lyophilized overnight, and the surface areas were sanded down to achieve a height of 1 mm. Following flushing with 70% ethanol, the scaffolds were again lyophilized overnight.
Composite Scaffold Generation
Composite scaffolds consisted of gelatin microparticles incorporated within the pores of the PPF scaffolds. 2.5 mg each of loaded and unloaded gelatin microparticles were mixed together in 30 µL of a 24% (w/v) solution of Pluronic F-127 (Sigma Aldrich, St. Louis, MO) in water, injected into a porous PPF scaffold, allowed to gel at room temperature for 10 min [33]. Depending on the experimental group, the loaded microparticles consisted of either the 10 mM or 40 mM type swollen with BMP-2. The unloaded microparticles were 10 mM acidic gelatin microparticles (swollen with PBS alone) that were used to simulate vascular endothelial growth factor (VEGF) loading for use of the scaffolds as dual growth factor delivery systems [42]. For control scaffolds, no gelatin microparticles were used and the growth factor solution was mixed with the Pluronic solution and then injected into a porous PPF scaffold.
Microcomputed Tomography (microCT)
MicroCT analysis provided a means of quantitatively measuring the 3D porosity and porous interconnectivity of the PPF scaffolds in a nondestructive manner. Six 7.5mm × 1mm cylindrical scaffolds were scanned with a SkyScan 1172 high-resolution microCT imaging system (Aartselaar, Belgium) at a 7 µm resolution with a voltage of 40 kV and current of 240 µA. Volumetric reconstruction and analysis were conducted using Nrecon and CT-analyser software provided by SkyScan. A global threshold of 50–255 was used for all analyses.
Porosity was calculated by drawing a cylindrical volume of interest (VOI) within the scaffold and measuring the percent of binarized object volume (scaffold material) within this VOI. The percent porosity was calculated as 100% - % binarized object volume.
Interconnectivity within the scaffold was defined as the percentage of porous volume (VP, volume of void space within the scaffold) that is accessible by a sphere with a given diameter (set to range as multiples of the resolution size from 28 µm – 196 µm). A VOI larger than the scaffold was drawn and a sphere diameter was set. A shrinkwrap function was used to shrink this VOI through any openings which the sphere could pass, and a measurement of the VOI (V) and volume of the binarized object (scaffold) (VS) were taken. If 100% of the porosity was accessible to the sphere, then V = VS; otherwise, VS < V because the volume of the VOI includes the volume of the scaffold plus any void space that is not accessible. Interconnectivity was calculated as follows:
The porous volume can be calculated for each scaffold from the VS and the percent binarized object volume obtained from the previous porosity measurements:
Scanning Electron Microscopy
Samples were freeze-dried and mounted on aluminum stages, sputter-coated with gold for 1 min, and observed by scanning electron microscopy (SEM, FEI Quanta 400 Environmental, Hillsboro, OR) at an accelerating voltage of 20 kV. Microparticles were observed as is, while scaffolds were sliced in half in order to view both surface areas and cross-sections.
In Vitro Release
Growth factor loading of the microparticles and generation of the composite scaffolds was achieved as described above. Scaffolds or microparticles alone (5 mg per sample) were incubated in buffer at 37°C and agitated at 70 rpm. At each time point, the buffer was removed and replaced with fresh buffer. For microparticle release studies, the samples were centrifuged at 3000 rpm for 5 min before each time point to reduce the loss of microparticles during buffer removal. Standards with known amounts of radiolabeled growth factor were used to account for radioactive decay. Release was quantified by monitoring the radioactivity in the removed buffer using a gamma counter (ARC-301B, Aloka Co., Japan), and the results were correlated to a standard curve. For microparticle studies, n = 4–6; for composite scaffolds, n = 5–6 (some samples were lost during buffer removal).
Percent cumulative release was determined by normalizing total BMP-2 released by each time point with the total amount incorporated within the scaffolds or microparticles (this being the sum of BMP-2 released over the study with the amount remaining in the scaffolds or microparticles at the last time point). Release rates were calculated as the slope of % cumulative release over the stated time period and are given as change in % cumulative release per day.
In Vivo Release
This work was in compliance with the appropriate institutional animal care and use committee at Kyoto University. Microparticles, PPF scaffolds, and dry Pluronic were sterilized by ethylene oxide. Water and PBS were syringe-filtered with 0.22 µm filters. Composite scaffolds were measured for initial radioactivity and then implanted into the dorsal subcutis of 6 week old female ddY mice (Shimizu Laboratory Supply Inc., Japan) along the median, approximately 15 mm away from their tail root. At each time point (n = 3), the skin around the implanted site was excised and the underlying fascia was thoroughly wiped to absorb radiolabeled BMP-2, followed by retrieval of the composite scaffold. The radioactivity remaining within scaffold, the removed skin, and the filter paper were measured on the gamma counter. Data are shown as % of radioactivity released as determined by subtracting initial radioactivity by that remaining at each time point (accounting for decay). Time points were at 1, 3, 7, 14, and 28 days.
Statistics
Main effects of gelatin crosslinking, growth factor dose, buffer type, and gelatin type were evaluated using a regression model with release as a response-dependent variable (p<0.05) on SAS statistical software (Cary, NC). Release rates were analyzed using a multi-factor analysis of variance followed by a Tukey-Kramer multiple comparisons test (p<0.05) to determine statistical significance. The results are reported as mean ± standard deviation for n = 4–6 for the microparticle studies, n = 5–6 for the composite scaffolds in vitro, and n = 3 for the in vivo studies.
Results
SEM Analysis
Both gelatin and PLGA microparticles were observed via SEM, confirming their spherical shapes and sizes. The low and high molecular weight PLGA microparticles had an average diameter of 25 ± 1 µm and 42 ± 3 µm respectively, while the gelatin microparticles were sieved for a diameter range of 50 – 100 µm.
Porous PPF scaffolds alone and as a part of composite scaffolds were also observed. Figure 1 shows the porous structure of the PPF scaffolds and the incorporation of the microparticles within the pores of the composite scaffolds. A magnified view of the pores reveals the microparticles embedded within the Pluronic gel in the pores of the scaffolds (Figure 1c).
Figure 1. SEM analysis of PPF and composite scaffolds.
a) Cross-section of a PPF scaffold, b) cross-section of a composite scaffold, and c) magnified view of gelatin microparticles embedded in a Pluronic gel within the porous structure of a PPF scaffold. Bar represents 500 µm.
Porous PPF Scaffolds
GPC analysis determined the final number average molecular weight of the PPF to be 1770 with a polydispersity index of 1.7. The porosity of the PPF scaffolds as measured by microCT analysis averaged 70.9 ± 1.1%. The interconnectivity, given as the % of porosity which is accessible by a sphere of a given diameter, was evaluated over a range of diameters (28, 56, 84, 112, 140, 168, 196 µm). The accessible porosity of the scaffolds was consistently at 98% for diameters up to 140 µm and then dropped drastically to 75% at 168 µm, indicating that the size of the interconnections between pores lies between 140 – 168 µm.
In Vitro Release from Microparticles
The main effects of gelatin crosslinking (10 mM and 40 mM), growth factor dose (6 and 60 ng per mg of dry microparticles as low and high doses), release medium (PBS and collagenase-containing PBS (Coll)), and gelatin type (acidic and basic) on overall BMP-2 release are given in Table 1. Increasing crosslinking extent, dose, and using basic gelatin resulted in decreased release, while the addition of collagenase to the buffer caused increased BMP-2 release.
Table 1.
Main effects of gelatin crosslinking, buffer type, growth factor dose, and gelatin type on overall release.
| Crosslinking | Buffer | Dose | Gelatin | |
|---|---|---|---|---|
| Gelatin MPs | − | + | − | − |
| Composites - In Vitro | − | + | n/a | n/a |
| Composites - In Vivo | − | n/a | n/a | n/a |
(+) and (−) denote increased and decreased effect as gelatin crosslinking was increased, collagenase was added to the buffer, dose was increased, or gelatin was switched from acidic to basic (p<0.05); otherwise n/a if the parameter was not tested.
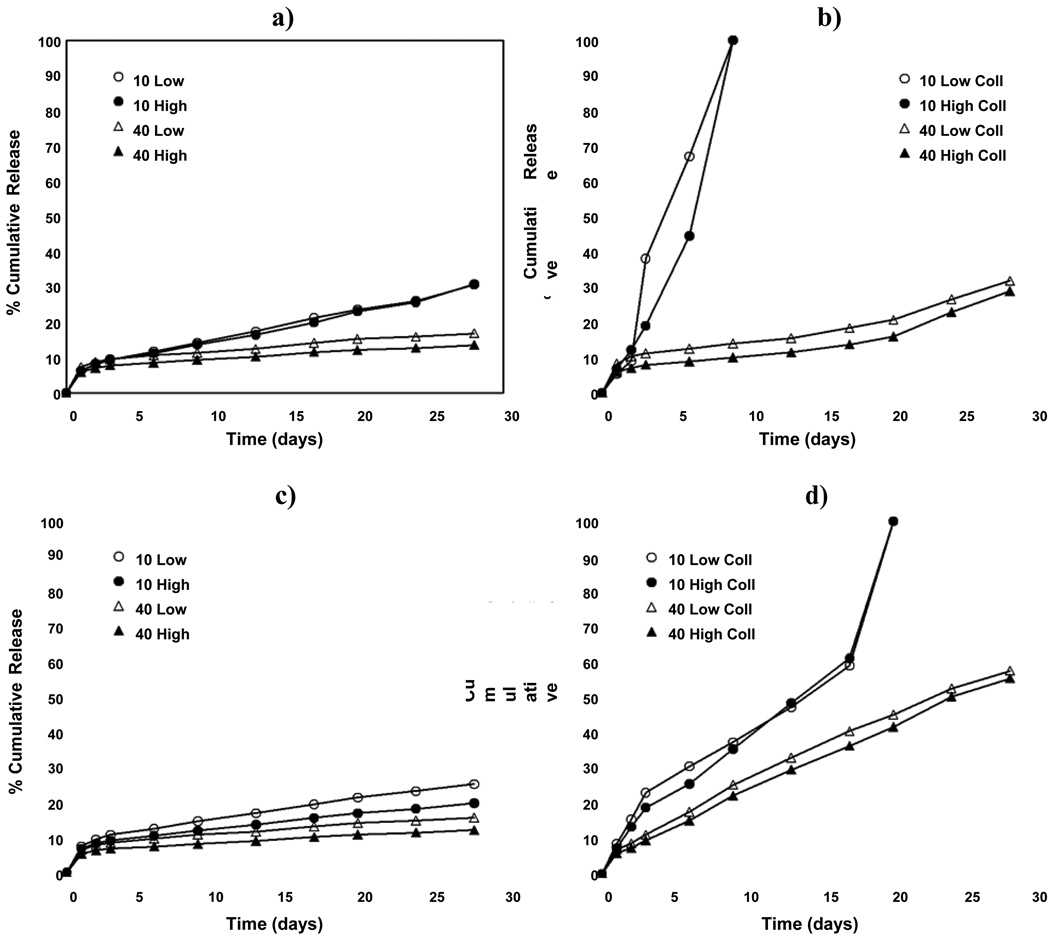
Cumulative release profiles of BMP-2 from gelatin microparticles are shown in Figure 2. To quantify effects between different groups, the release profiles were partitioned into four phases in accordance with previous investigations [23, 43]. A burst release at 24 hours (Phase 1) was observed in all cases, and was generally followed by moderate or slow release. To describe this period, release rates for Phase 2 (days 1–3), Phase 3 (days 3–17), and Phase 4 (days 17–28) were calculated for all treatments (Table 2).
Figure 2. In vitro release of BMP-2 from acidic and basic gelatin microparticles.
Average percent cumulative BMP-2 release from acidic gelatin microparticles in a) PBS and b) collagenase-containing PBS (Coll), and from basic gelatin microparticles in c) PBS and d) collagenase-containing PBS (Coll) as a function of the gelatin crosslinking (10 mM vs. 40 mM) and BMP-2 dose (Low vs. High). Error bars represent means ± standard deviation for n = 4–6.
Table 2.
Burst and cumulative release of BMP-2 from gelatin and PLGA microparticles.
| Phase 1 (%/day) | Phase 2 (%/day) | Phase 3 (%/day) | Phase 4 (%/day) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PBS | Coll | PBS | Coll | PBS | Coll | PBS | Coll | ||
| Acidic Gelatin | ^ | ^ | ^ | ^, * | |||||
| 10 mM Low | 6.3 ± 0.4 | 6.8 ± 0.6 #,^ | 1.6 ± 0.3 | 16.3 ± 2.3*,# | 0.8 ± 0.1 | 10.3 ± 0.7*,# | 0.8 ± 0.3 | ||
| 10 mM High | 6.3 ± 0.5 | 5.3 ± 0.6 | 1.6 ± 0.4 | 6.1 ± 2.0* | 0.8 ± 0.1 | 13.5 ± 0.6* | 1.0 ± 0.3 | ||
| 40 mM Low | 7.3 ± 0.4# | 8.3 ± 0.9 | 1.1 ± 0.1 | 1.5 ± 0.7 | 0.3 ± 0.0 | 0.5 ± 0.1 | 0.2 ± 0.0 | 1.2 ± 0.3 | |
| 40 mM High | 5.9 ± 0.4 | 5.9 ± 0.3 | 0.9 ± 0.1 | 1.0 ± 0.5 | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.2 ± 0.0 | 1.4 ± 0.3 | |
| Basic Gelatin | ^ | ^, *, $ | ^, * | ||||||
| 10 mM Low | 7.4 ± 0.4$ | 8.5 ± 0.6 #,$ | 1.6 ± 0.3 | 7.2 ± 0.1*,$ | 0.6 ± 0.1 | 2.5 ± 0.2# | 0.5 ± 0.2 | 13.7 ± 0.4 | |
| 10 mM High | 6.7 ± 0.3^ | 7.3 ± 0.3^ | 1.2 ± 0.2 | 5.8 ± 0.4* | 0.5 ± 0.1 | 3.1 ± 0.3 | 0.4 ± 0.1 | 13.7 ± 1.3 | |
| 40 mM Low | 6.5 ± 0.6# | 6.9 ± 0.6 | 1.0 ± 0.1 | 2.1 ± 0.2 | 0.3 ± 0.0 | 2.1 ± 0.1 | 0.2 ± 0.0 | 1.6 ± 0.1 | |
| 40 mM High | 5.3 ± 0.3 | 5.9 ± 0.3 | 0.7 ± 0.1 | 1.8 ± 0.1 | 0.2 ± 0.0 | 1.9 ± 0.1 | 0.2 ± 0.0 | 1.8 ± 0.2 | |
| PLGA | ^ | ^ | ^ | ||||||
| 8.3K Low | 47.5 ± 2.9 | 2.8 ± 0.3 | 0.3 ± 0.0 | 0.3 ± 0.1 | |||||
| 8.3K High | 43.4 ± 3.1 | 3.0 ± 0.5 | 0.4 ± 0.0 | 0.4 ± 0.0 | |||||
| 57.5K Low | 38.1 ± 2.0# | 4.2 ± 0.6 | 0.8 ± 0.0# | 0.4 ± 0.1 | |||||
| 57.5K High | 29.5 ± 2.2 | 3.8 ± 0.2 | 1.1 ± 0.1 | 0.4 ± 0.0 | |||||
Average percent values (% release per day) are given with standard deviations for an n = 4–6. Statistical significance (p<0.05) between relevant groups for gelatin crosslinking (10 mM vs. 40 mM) or PLGA molecular weight (8.3K vs. 57.5K) is denoted by (^), for buffer type (PBS vs. Coll) by (*), for growth factor dose (Low vs. High) by (#), and for gelatin type (Acidic vs. Basic) by ($).
As can be seen in Figure 2, release from both acidic and basic gelatin microparticles in PBS exhibited minimal burst release followed by slow to moderate release over the subsequent phases (<2% per day for Phase 2 and <1% per day for Phases 3 and 4, Table 2). In contrast, we observed very different release profiles in collagenase-containing PBS, with higher release rates and BMP-2 release for both gelatin types. However, none of the tested parameters played a large role in affecting burst release (within 24 hours); for all groups, burst release was <10% (Table 2). Release rates for Phase 2 exhibited significant effects of buffer type and gelatin crosslinking. For 10 mM microparticles, we noted significantly higher release rates in collagenase-containing PBS than in PBS (16.3 ± 2.3% vs. 1.6 ± 0.3% per day for the 10 mM Low dose group, p<0.05) and increased degradation of 10 mM acidic microparticles as observed by visual inspection. In addition, the higher crosslinked (40 mM) microparticles in collagenase-containing PBS exhibited lower release rates compared to the 10 mM groups (Table 2) for Phase 2.
In Phase 3, we noted the same effects of buffer and crosslinking as with Phase 2. However, we also observed total degradation of the 10 mM acidic microparticles by day 9 in the collagenase-containing PBS, resulting in 100% release of the BMP-2 (Figure 2b). Additionally, 40 mM basic microparticles show a buffer effect, resulting in higher release rates than the equivalent acidic microparticles (Table 2). In Phase 4, the effect of buffer type was noted for all groups, particularly for 10 mM basic microparticles, which showed total degradation by day 20 and 100% release of BMP-2 (Figure 2d). Thus, the effect of crosslinking was also noted.
With cumulative release at 28 days, both gelatin crosslinking and buffer type affected release in all groups. Microparticles crosslinked with 40 mM glutaraldehyde showed significantly lower cumulative release than 10 mM microparticles, and cumulative release in PBS was also significantly lower than in collagenase-containing PBS. There were also some effects of gelatin type, with 40 mM basic microparticles showing higher release than their acidic counterparts.
As a comparison, a subset of samples (40 mM, acidic gelatin, high dose group) was evaluated for release kinetics when BMP-2 was radioiodinated with Bolton-Hunter Reagent instead of by the chloramine-T method (Figure 3a). It is clear that radiolabeling with Bolton-Hunter reagent had an obvious and substantial effect on the release kinetics; a very large burst release and >80% total cumulative release at 28 days in both PBS and collagenase-containing PBS were observed in this case. This is compared to the corresponding groups labeled with chloramine-T that displayed approximately 10% release in PBS and 30% release in collagenase-containing PBS after 28 days.
Figure 3. In vitro release of BMP-2 after radioiodination with Bolton-Hunter reagent, and release of BMP-2 from PLGA microparticles.
Average percent cumulative BMP-2 release from a) 40 mM acidic gelatin microparticles loaded with a high dose of growth factor in PBS and collagenase-containing PBS (Coll) after radiolabeling with Bolton-Hunter reagent and b) PLGA microparticles as a function of the PLGA molecular weight (8.3K vs. 57.5K) and the growth factor dose (Low vs. High) when radiolabeled with chloramine-T. Error bars represent means ± standard deviation for n = 6.
PLGA microparticles were also evaluated for BMP-2 release (Figure 3b). Low and high molecular weights (8.3K and 57.5K) of PLGA were used, and the same low and high doses of BMP-2 were used again. Both types of microparticles showed a moderate burst release followed by some sustained release over 28 days, but higher molecular weights did result in a significantly lower burst release (Table 2). However, even the smallest burst release (high molecular weight PLGA, ~30%) was substantially higher than from any of the gelatin microparticle groups (Table 2). There was no effect of dose or molecular weight on the cumulative release at 28 days.
In Vitro Release from Composite Scaffolds
In addition to microparticles, composite scaffolds of porous PPF with gelatin microparticles were tested in vitro (Figure 4). Basic gelatin microparticles were used for BMP-2 loading because of the desired linear and sustained release profiles of the growth factor over 28 days. Since the microparticle studies also showed dose playing only a minimal role on normalized release kinetics, a high dose was used for all groups and only gelatin crosslinking and buffer type were varied with the composite scaffolds. As a negative control, a porous PPF scaffold was loaded without any gelatin microparticles, with an equivalent amount of BMP-2 solution mixed in Pluronic gel and injected into the void volume of the scaffold.
Figure 4. In vitro release of BMP-2 from composite scaffolds.
Average percent cumulative BMP-2 release from composite scaffolds with basic gelatin microparticles loaded with a high dose of growth factor in a) PBS and b) collagenase-containing PBS (Coll) as a function of gelatin crosslinking (10 mM, 40 mM, or PPF control scaffolds). Error bars represent means ± standard deviation for n = 5–6.
These PPF controls showed a significantly larger burst release (>55%) than the composites and this was followed by minimal release over 28 days (Table 3) with no effect of buffer. After burst release, the PPF controls exhibited significantly lower release rates from both the 10 and 40 mM composite scaffolds in collagenase-containing PBS (p<0.05). For the composites, we saw the same main effects of gelatin crosslinking and buffer type on overall release of BMP-2 as was observed from the gelatin microparticles alone (Table 1, p<0.05). For burst release, only the 10 mM composites exhibited significantly higher release in collagenase-containing PBS (13.7 ± 3.6% in PBS vs. 26.9 ± 6.7% in collagenase-containing PBS). Buffer effects were also seen for both 10 mM and 40 mM composites on Phase 2 and Phase 3 release rates (Table 3), as well as for cumulative release at 28 days (34.3 ± 10.2% in PBS vs. 75.6 ± 6.2% in collagenase-containing PBS for the 10 mM composites, and 23.0 ± 6.2 % in PBS vs. 56.2 ± 4.8% in collagenase-containing PBS for the 40 mM composites).
Table 3.
Burst and cumulative release of BMP-2 from composite scaffolds in vitro and in vivo
| Phase 1 (%/day) | Phase 2 (%/day) | Phase 3 (%/day) | Phase 4 (%/day) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PBS | Coll | PBS | Coll | PBS | Coll | PBS | Coll | ||
| In Vitro | ^ | ^ | |||||||
| 10 mM High | 13.7 ± 3.6 | 26.9 ± 6.7* | 3.6 ± 1.9 | 11.6 ± 1.7* | 0.4 ± 0.0 | 1.2 ± 0.5* | 0.7 ± 0.2 | 0.8 ± 0.4 | |
| 40 mM High | 11.9 ± 4.9 | 17.8 ± 6.1 | 1.8 ± 0.6 | 7.4 ± 1.2* | 0.3 ± 0.0 | 1.3 ± 0.2* | 0.3 ± 0.1 | 0.5 ± 0.1 | |
| PPF | 56.3 ± 2.3^ | 56.7 ± 2.9 | 4.6 ± 0.7 | 4.9 ± 0.6 | 0.3 ± 0.0 | 0.3 ± 0.0^ | 0.2 ± 0.0 | 0.2 ± 0.0^ | |
| In Vivo | |||||||||
| 10 mM High | 21.2 ± 2.7 | 4.0 ± 2.4 | 1.2 ± 0.1 | 2.2 ± 0.1 | |||||
| 40 mM High | 14.7 ± 0.7 | 5.0 ± 3.5 | 1.4 ± 0.8 | 1.8 ± 0.3 | |||||
| PPF | 55.1 ± 4.1^ | 8.2 ± 2.9 | 2.1 ± 0.5 | 0.3 ± 0.1^ | |||||
Average percent values (% release per day) are given with standard deviations for n = 5–6 for in vitro groups and n = 3 for in vivo groups. Statistical significance (p<0.05) for gelatin crosslinking (10 mM, 40 mM, or PPF control scaffolds) is denoted by (^) and indicates significance for both comparisons with the other two groups. For buffer type (PBS vs. Coll), significance is noted by (*) (p<0.05).
Crosslinking effects between the two composites were also significant, but only within the context of collagenase-containing buffers. For burst and Phase 2 release rates, as well as for cumulative release at 28 days, the 40 mM composites with the higher crosslinking exhibited lower release than 10 mM composites (p<0.05).
A few differences were observed in the release profiles of BMP-2 from composites vs. microparticles alone in collagenase-containing PBS. The 10 mM composites showed a cumulative release of ~75% at 28 days, while the 10 mM microparticles alone exhibited total degradation and 100% release by day 20. Additionally, while the 40 mM composites showed similar cumulative release at 28 days with microparticles (~55%), the release rates in the different phases after burst release were more consistent from microparticles alone (~2% per day, Table 2).
In Vivo Release from Composite Scaffolds
Finally, composite scaffolds with 10 mM and 40 mM gelatin microparticles, along with PPF controls, were also tested in vivo in a subcutaneous mouse model. Upon retrieval of the scaffolds at each time point, a fibrous capsule with extensive neovascularization was observed surrounding the composites. However, no ectopic bone formation was observed. Release kinetics of BMP-2 from the scaffolds is shown in Figure 5. Unlike in vitro results, the PPF controls showed almost 100% cumulative release within 28 days, although the burst releases were similar (~55%, Table 3). Again, both the 10 mM and 40 mM composite groups showed significantly lower burst and cumulative release from the PPF controls. Between each other, the 10 and 40 mM composites did not show significant differences; nevertheless, a main effect of crosslinking (higher crosslinking results in lower release) on overall BMP-2 release was statistically significant (Table 1, p<0.05).
Figure 5. In vivo release of BMP-2 from composite scaffolds.
Average percent cumulative BMP-2 release from composite scaffolds with basic gelatin microparticles loaded with a high dose of growth factor as a function of gelatin crosslinking (10 mM, 40 mM, or PPF control scaffolds). Error bars represent means ± standard deviation for n = 3.
Discussion
This study focused on the evaluation of gelatin microparticles for the controlled release of BMP-2. The growth factor radiolabeling methods of chloramine-T vs. Bolton-Hunter reagent were compared for their effect on BMP-2 release from the gelatin microparticles. Results showed that the method of radioiodination had a significant effect on the BMP-2 release. With Bolton-Hunter radiolabeling, almost 70% of the BMP-2 was released within 24 hours (compared to <10% with the chloramine-T method), indicating that this method disrupts the interaction between the growth factor and gelatin. This may be explained by considering the mechanism of radioiodination. The BH reagent is iodinated 3-(4-hydroxyphenyl) propionic acid N-hydroxysuccinimide ester; in an aqueous solution, it reacts with proteins containing N-terminal amino groups (and lysine residues) and produces the ester as a byproduct. Chloramine-T, on the other hand, modifies existing tyrosine residues with only iodine. Considering that BMP-2 has about twice the number of lysine residues as tyrosine residues, it may be that the addition of the large molecule at each lysine (from the Bolton-Hunter reaction) affects the folding of BMP-2. In fact, the bioactivity of BMP-2 has been shown to be reduced after radiolabeling with Bolton-Hunter reagent [34]. Therefore, in addition to reduced bioactivity, any surface charges or groups that would normally interact with gelatin may be masked because of the different folding of the BMP-2.
For the remainder of the groups, the chloramine-T method was used for radiolabeling. For BMP-2 release from gelatin microparticles, the effects of gelatin crosslinking, growth factor dose, buffer type, and gelatin type on growth factor release profiles were examined in vitro. BMP-2 was loaded at a low and high dose into acidic and basic gelatin microparticles, with 10 mM and 40 mM crosslinking extents, and incubated in PBS and collagenase-containing PBS. In all groups, a minimal burst release (<10% was observed) at 24 hours. While this is different from the very large burst release of BMP-2 (~80%) observed within 1 hour from acidic gelatin disks [26], no direct comparisons can be made due to differences in carrier morphology and crosslinking extents.
We correctly hypothesized that decreasing gelatin crosslinking and addition of collagenase to the release medium would result in higher BMP-2 release. In PBS buffers, the minimal burst release was followed by fairly slow release of BMP-2 over 28 days, similar to in vitro release profiles that have been examined with gelatin microparticles loaded with TGF-β1 in PBS by Holland et al. [43]. Since gelatin does not degrade via hydrolysis, the release of BMP-2 in PBS was due to diffusion initially of any gelatin-unbound BMP-2 and later of any dissociating growth factor out of the microparticles. However, there was a significant effect of collagenase-containing buffer, illustrating that the addition of collagenase resulted in increased release of BMP-2 and confirming that gelatin undergoes enzymatic degradation as previously described [44]. Also in the presence of collagenase, the crosslinking extent played a large role in determining release - more tightly crosslinked microparticles degraded significantly slower than less crosslinked microparticles. For example, in collagenase-containing PBS, 10 mM acidic gelatin microparticles were completely degraded by day 9, exhibiting 100% release of the BMP-2. However, acidic gelatin microparticles crosslinked with 40 mM glutaraldehyde did not show any visible degradation and also had less than 32% cumulative release over 28 days in collagenase-containing PBS.
In addition to buffer type and gelatin crosslinking, the effect of growth factor dose was evaluated on BMP-2 release. We hypothesized that higher doses of BMP-2 loaded in the gelatin microparticles would result in higher burst release of the growth factor after 24 hours. However, we observed that the main effect was decreased release with a higher dose. Still, the differences in burst release between groups of high and low doses were relatively small and not what one might have expected with a high dose being ten times larger than the low dose. Presumably, the ratio of free vs. bound growth factor is similar for both amounts and therefore, the effect on BMP-2 release was minimal for the investigated doses. A similar effect was observed with the release of VEGF from acidic gelatin microparticles in vitro [36]. The amounts of growth factor needed to saturate binding sites are not known at this time.
The role of gelatin type on BMP-2 release was less clear. We hypothesized that BMP-2 would have lower release from acidic gelatin compared to basic gelatin due to stronger electrostatic attractions. However, we observed that the main effect of gelatin type was decreased overall BMP-2 release with the use of basic gelatin. With the case of 10 mM microparticles, acidic gelatin microparticles showed fast degradation and thus 100% release by 9 days, while basic microparticles degraded only after 20 days. The opposite was true with 40 mM microparticles, with acidic microparticles showing slower release than the basic ones. However, both gelatin types at 40 mM crosslinking showed an almost linear release, suggesting zero-order kinetics of release. One can consider the nature of the difference between acidic and basic gelatin, which depends on the method of collagen pretreatment. The alkaline process used for acidic gelatin targets asparagine and glutamine, converting many to aspartate and glutamate and resulting in many more negatively charged amino acids [44]. The pretreatment for basic gelatin does not affect asparagine and glutamine to the same extent. Taking into consideration that collagenase 1A digests gelatin by recognizing the –Pro–X–Gly–Pro– sequence, where X is a neutral amino acid [37], it is likely that the presence of a large number of negatively charged amino acids in acidic gelatin results in the differences between the degradation of acidic vs. basic gelatin.
As a comparison with gelatin microparticles, in vitro BMP-2 release from PLGA microparticles was also observed while varying PLGA molecular weight and growth factor dose. Release profiles of adsorbed BMP-2 from PLGA microparticles showed a moderate burst release followed by minimal release over 28 days, similar to previous work by Schrier et al. [30]. However, there was substantially higher burst and cumulative release than any of the gelatin microparticle groups in PBS. Additionally, there was no effect of PLGA molecular weight or dose on cumulative release at 28 days. Therefore, for the purposes of achieving the desired sustained release of BMP-2 with minimal burst release, the rate of release with 40 mM acidic gelatin microparticles may be too slow over 28 days, and the burst release from PLGA microparticles may be too large. Thus, 40 mM basic gelatin microparticles were used for the generation of the composite scaffolds and subsequent release studies in vitro and in vivo because their release profile showed nominal burst effect and moderate linear release over 28 days.
For in vitro release from composite scaffolds, 10 mM and 40 mM basic gelatin microparticles were loaded with a high dose of BMP-2 and incorporated within a porous PPF scaffold. A negative control of BMP-2 loaded within the porous PPF scaffold without any microparticles was also evaluated. These composites were placed in PBS and collagenase-containing PBS. MicroCT analysis of these scaffolds showed approximately 70% porosity, with almost 99% of that porous volume accessible to spheres under 140 µm in diameter. SEM evaluation of the microparticles alone and within the scaffold confirms that the microparticles were able to infiltrate into the pores of the scaffold.
The main effects for gelatin crosslinking and buffer type were the same as with gelatin microparticles alone. The control scaffolds without any microparticles showed a large burst release followed by minimal release over 28 days. This large burst release of the BMP-2 was likely due to diffusion of gelatin-unbound growth factor through the Pluronic gel, and the minimal release over 28 days, in both PBS and collagenase-containing PBS, suggests adsorption of any growth factor released from gelatin microparticles onto the hydrophobic PPF polymer. As for the 10 mM and 40 mM composite scaffolds, BMP-2 release from them showed similar profiles to release from gelatin microparticles, in both PBS and collagenase-containing PBS. However, a larger burst release and different release kinetics are observed from the composites, compared to the almost linear release seen from microparticles alone. This effect has also been observed with the release of VEGF from acidic gelatin microparticles and their PPF composite scaffolds [36]. It is expected that the initial presence of a Pluronic gel within the pores of the polymer scaffold may affect overall release kinetics; however, since the total amount of loaded gelatin microparticles in the composite scaffolds compared to the microparticles alone was different, no direct comparisons can be made.
In vivo release from composite scaffolds was also evaluated using a mouse subcutaneous model and again the same main effect of gelatin crosslinking on overall release was observed. Interestingly, the PPF control in this case demonstrated similar burst release to the in vitro study but a substantially larger cumulative release over 28 days (of almost 100%). This may be a result of the Vroman effect, with BMP-2 adsorbed to the porous polymer being displaced over time in vivo by other proteins with greater affinity for the polymer. Results also showed that release from the 10 mM and 40 mM composites were similar to in vitro release profiles, but crosslinking effect was not significant in vivo, demonstrating the complexity of translating in vitro results in vivo. One needs to recognize that an in vitro setting is a mere approximation of the intricate protease environment resulting from trauma and during wound healing [45]. This also points to a limitation of this study which utilized a subcutaneous model; future work would involve implantation of the composite scaffolds in an orthotopic site.
Additionally, previous work by Yamamoto et al. [7] has shown that varying crosslinking extent for 6 mm basic gelatin disks can result in varied release profiles of BMP-2 for a subcutaneous mouse model. This serves to emphasize the importance of the form of the delivery vehicle; the degradation of spherical gelatin particles, less than a hundred microns in diameter, is likely to be different than that of gelatin disks millimeters in diameter. However, both the 10 and 40 mM composite scaffolds in this study showed minimal burst release followed by sustained release over 28 days, indicating that this system can be used for the controlled delivery of BMP-2.
Conclusions
This research demonstrates the efficacy of gelatin microparticles for achieving varied release profiles of BMP-2 over 4 weeks. The method of growth factor radiolabeling can significantly affect its release kinetics for gelatin microparticles; with this system, the chloramine-T method was preferred over Bolton-Hunter reagent for radioiodination. BMP-2 release from PLGA microparticles resulted in a moderate burst release followed by minimal cumulative release, while BMP-2 release from gelatin microparticles exhibited minimal burst release followed by linear release kinetics. The relative amount of growth factor associated with gelatin achieved an equilibrium value with no strong dependence on its dose. Differences in release from acidic and basic gelatin microparticles may result from the different pretreatment conditions used for gelatin synthesis; the main effect of basic gelatin was a decrease in the overall release of BMP-2. The addition of collagenase to the release medium resulted in increased release of BMP-2, confirming that gelatin undergoes enzymatic degradation at a rate dependent on the extent of its crosslinking. Accordingly, a systematic control of BMP-2 delivery from gelatin microparticles can be achieved both in vitro and in vivo by altering the extent of basic gelatin crosslinking.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01-DE15164) (AGM) and by a National Science Foundation Graduate Research Fellowship (ZSP).
Abbreviations
- bFGF
basic fibroblast growth factor
- BCA
bicinchoninic acid
- BH reagent
Bolton-Hunter reagent
- BMP-2
bone morphogenetic protein-2
- Coll
collagenase-containing phosphate buffered saline
- CSD
critical size defect
- GPC
gel permeation chromatography
- IEP
isoelectric point
- microCT
microcomputed tomography
- PBS
phosphate buffered saline
- PLGA
poly(lactic-co-glycolic acid)
- PPF
poly(propylene fumarate)
- PVA
poly(vinyl alcohol)
- SEM
scanning electron microscopy
- TGF-β1
transforming growth factor-β1
- VEGF
vascular endothelial growth factor
- VOI
volume of interest
References
- 1.Kirker-Head C, Karageorgiou V, Hofmann S, Fajardo R, Betz O, Merkle HP, Hilbe M, von Rechenberg B, McCool J, Abrahamsen L, Nazarian A, Cory E, Curtis M, Kaplan D, Meinel L. BMP-silk composite matrices heal critically sized femoral defects. Bone. 2007;41:247. doi: 10.1016/j.bone.2007.04.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burchardt H. The biology of bone graft repair. Clin Orthop. 1983:28. [PubMed] [Google Scholar]
- 3.Kirker-Head CA. Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Deliv Rev. 2000;43:65. doi: 10.1016/s0169-409x(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 4.Lewis G. Alternative acrylic bone cement formulations for cemented arthroplasties: present status, key issues, and future prospects. J Biomed Mater Res B Appl Biomater. 2008;84:301. doi: 10.1002/jbm.b.30873. [DOI] [PubMed] [Google Scholar]
- 5.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 6.Bostrom MP. Expression of bone morphogenetic proteins in fracture healing. Clin Orthop. 1998:S116. doi: 10.1097/00003086-199810001-00013. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Takahashi Y, Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue ngineering. Trends Biotechnol. 1998;16:224. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 9.Whang K, Healy KE, Elenz DR, Nam EK, Tsai DC, Thomas CH, Nuber GW, Glorieux FH, Travers R, Sprague SM. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999;5:35. doi: 10.1089/ten.1999.5.35. [DOI] [PubMed] [Google Scholar]
- 10.Kellomaki M, Niiranen H, Puumanen K, Ashammakhi N, Waris T, Tormala P. Bioabsorbable scaffolds for guided bone regeneration and generation. Biomaterials. 2000;29(21):2495. doi: 10.1016/s0142-9612(00)00117-4. [DOI] [PubMed] [Google Scholar]
- 11.Wallkamm B, Schmid J, Hammerle CH, Gogolewski S, Lang NP. Effect of bioresorbable fibres (Polyfibre) and a bioresorbable foam (Polyfoam) on new bone formation. A short term experimental study on the rabbit skull. Clin Oral Implants Res. 2003;14:734. doi: 10.1046/j.0905-7161.2003.00930.x. [DOI] [PubMed] [Google Scholar]
- 12.Cornell CN, Lane JM. Current understanding of osteoconduction in bone regeneration. Clin Orthop. 1998:S267. doi: 10.1097/00003086-199810001-00027. [DOI] [PubMed] [Google Scholar]
- 13.Duggirala SS, Mehta RC, DeLuca PP. Interaction of recombinant human bone morphogenetic protein-2 with poly(d,l lactide-co-glycolide) microspheres. Pharm Dev Technol. 1996;1:11. doi: 10.3109/10837459609031413. [DOI] [PubMed] [Google Scholar]
- 14.Schrier JA, Fink BF, Rodgers JB, Vasconez HC, DeLuca PP. Effect of a freeze-dried CMC/PLGA microsphere matrix of rhBMP-2 on bone healing. AAPS PharmSciTech. 2001;2:E18. doi: 10.1208/pt020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whang K, Tsai DC, Nam EK, Aitken M, Sprague SM, Patel PK, Healy KE. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J Biomed Mater Res. 1998;42:491. doi: 10.1002/(sici)1097-4636(19981215)42:4<491::aid-jbm3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Saito N, Okada T, Horiuchi H, Murakami N, Takahashi J, Nawata M, Ota H, Nozaki K, Takaoka K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol. 2001;19:332. doi: 10.1038/86715. [DOI] [PubMed] [Google Scholar]
- 17.Murakami N, Saito N, Takahashi J, Ota H, Horiuchi H, Nawata M, Okada T, Nozaki K, Takaoka K. Repair of a proximal femoral bone defect in dogs using a porous surfaced prosthesis in combination with recombinant BMP-2 and a synthetic polymer carrier. Biomaterials. 2003;24:2153. doi: 10.1016/s0142-9612(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 18.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Ueda H, Nakamura T, Yamamoto M, Nagata N, Fukuda S, Tabata Y, Shimizu Y. Repairing of rabbit skull defect by dehydrothermally crosslinked collagen sponges incorporating transforming growth factor beta1. J Control Release. 2003;88:55. doi: 10.1016/s0168-3659(02)00481-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang YJ, Lin FH, Sun JS, Huang YC, Chueh SC, Hsu FY. Collagen-hydroxyapatite microspheres as carriers for bone morphogenic protein-4. Artif Organs. 2003;27:162. doi: 10.1046/j.1525-1594.2003.06953.x. [DOI] [PubMed] [Google Scholar]
- 21.Uludag H, Friess W, Williams D, Porter T, Timony G, D'Augusta D, Blake C, Palmer R, Biron B, Wozney J. rhBMP-collagen sponges as osteoinductive devices: effects of in vitro sponge characteristics and protein pI on in vivo rhBMP pharmacokinetics. Ann N Y Acad Sci. 1999;875:369. doi: 10.1111/j.1749-6632.1999.tb08519.x. [DOI] [PubMed] [Google Scholar]
- 22.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91:299. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 24.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Tabata Y, Nagano A, Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng. 1999;5:127. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J. Biomater. Sci. Polym. Ed. 2001;12:77. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 27.Isobe M, Yamazaki Y, Oida S, Ishihara K, Nakabayashi N, Amagasa T. Bone morphogenetic protein encapsulated with a biodegradable and biocompatible polymer. J Biomed Mater Res. 1996;32:433. doi: 10.1002/(SICI)1097-4636(199611)32:3<433::AID-JBM17>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 28.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85-A Suppl 3:75. doi: 10.2106/00004623-200300003-00013. [DOI] [PubMed] [Google Scholar]
- 29.Schrier JA, DeLuca PP. Recombinant human bone morphogenetic protein-2 binding and incorporation in PLGA microsphere delivery systems. Pharm Dev Technol. 1999;4:611. doi: 10.1081/pdt-100101400. [DOI] [PubMed] [Google Scholar]
- 30.Schrier JA, DeLuca PP. Porous bone morphogenetic protein-2 microspheres: polymer binding and in vitro release. AAPS PharmSciTech. 2001;2:E17. doi: 10.1208/pt020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedberg EL, Kroese-Deutman HC, Shih CK, Crowther RS, Carney DH, Mikos AG, Jansen JA. In vivo degradation of porous poly(propylene fumarate)/poly(DL-lactic-co-glycolic acid) composite scaffolds. Biomaterials. 2005;26:4616. doi: 10.1016/j.biomaterials.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Fisher JP, Vehof JW, Dean D, van der Waerden JP, Holland TA, Mikos AG, Jansen JA. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J Biomed Mater Res. 2002;59:547. doi: 10.1002/jbm.1268. [DOI] [PubMed] [Google Scholar]
- 33.Hedberg EL, Kroese-Deutman HC, Shih CK, Crowther RS, Carney DH, Mikos AG, Jansen JA. Effect of varied release kinetics of the osteogenic thrombin peptide TP508 from biodegradable, polymeric scaffolds on bone formation in vivo. J Biomed Mater Res A. 2005;72:343. doi: 10.1002/jbm.a.30265. [DOI] [PubMed] [Google Scholar]
- 34.Wiemann M, Rumpf HM, Bingmann D, Jennissen HP. The binding of rhBMP-2 to the receptors of viable MC3T3-E1 cells and the question of cooperativity. Materialwissenschaft und Werkstofftechnik. 2001;32:931. [Google Scholar]
- 35.Iwasaki S, Tsuruoka N, Hattori A, Sato M, Tsujimoto M, Kohno M. Distribution and characterization of specific cellular binding proteins for bone morphogenetic protein-2. J. Biol. Chem. 1995;270:5476. doi: 10.1074/jbc.270.10.5476. [DOI] [PubMed] [Google Scholar]
- 36.Patel ZS, Ueda H, Yamamoto M, Tabata Y, Mikos AG. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharmaceutical Research. 2008 doi: 10.1007/s11095-008-9685-1. (in press) [DOI] [PubMed] [Google Scholar]
- 37.Haralson MA, Hassell JR. Extracellular matrix : A practical approach. Oxford; New York: IRL Press; 1995. [Google Scholar]
- 38.Greenwood FC, Hunter WM, Glover JS. The Preparation of I-131-Labelled Human Growth Hormone of High Specific Radioactivity. Biochem J. 1963;89:114. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolton AE, Hunter WM. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973;133:529. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shung AK, Behravesh E, Jo S, Mikos AG. Crosslinking characteristics of and cell adhesion to an injectable poly(propylene fumarate-co-ethylene glycol) hydrogel using a water-soluble crosslinking system. Tissue Eng. 2003;9:243. doi: 10.1089/107632703764664710. [DOI] [PubMed] [Google Scholar]
- 41.Porter BD, Oldham JB, He SL, Zobitz ME, Payne RG, An KN, Currier BL, Mikos AG, Yaszemski MJ. Mechanical properties of a biodegradable bone regeneration scaffold. J Biomech Eng. 2000;122:286. doi: 10.1115/1.429659. [DOI] [PubMed] [Google Scholar]
- 42.Patel ZS. Controlled delivery of angiogenic and osteogenic growth factors for bone regeneration. Houston: Rice University; 2008. [Google Scholar]
- 43.Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta 1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release. 2004;94:101. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Ikada Y, Tabata Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31:287. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 45.Parks WC. Matrix metalloproteinase in repair. Wound Repair Regen. 1999;7:423. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]