Abstract
Objective
Examine relationships of diffusion tensor imaging (DTI) fractional anisotropy (FA) to executive function (EF) and attention measures following early childhood (3–7 years) traumatic brain injury (TBI).
Design
Exploratory correlation and comparison study.
Setting
Children’s hospital outpatient facilities.
Participants
9 children with a history of TBI (age = 7.89 ± 1.00 years; Glasgow Coma Scale (GCS) = 10.11 ± 4.68) were compared to 12 children with OI (age = 7.51 ± 0.95). All children were at least 12 months post injury at time of evaluation.
Main Outcome Measures
FA in various regions of interest (ROI), EF and attention measures.
Results
FA values primarily in the frontal white matter tracks correlated with EF measures. Separate tasks of inhibition and switching correlated significantly with FA in bilateral frontal lobes. Tasks combining both inhibition and switching correlated significantly with FA values in the left frontal lobe. Tasks of attention negatively correlated with FA values in the right frontal white matter and the superior longitudinal fasciculus.
Conclusions
Associations between late measurement of FA and EF measures following early childhood TBI suggest that persistent white matter changes, especially in the frontal white matter, may provide an index of EF deficits.
Keywords: Brain injuries, child, diffusion magnetic resonance imaging, neuropsychology
1. Introduction
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality in the pediatric population. According to the Centers for Disease Control more than one million brain injuries occur per year [1]. In children, TBI results in 2,685 deaths, 37,000 hospitalizations, and 435,000 emergency visits yearly [1]. Additionally, because these injuries occur at a young age, they have a life-long impact. Neurocognitive deficits frequently occur after TBI and significantly contribute to the persistent morbidity.
Diffusion Tensor Imaging (DTI) has been used recently to assess white matter structure after TBI [2,3,12, 32]. DTI indirectly measures white matter organization via measurements of water diffusion with the primary metric known as fractional anisoptropy (FA). FA measures are from 0–1 with scores of zero indicating free diffusion while a score of one indicates movement of water in one direction only. In general, decreased FA is thought to be correlated with less structure; thus, indicating decreased white matter organization. In normal development, the majority of myelination occurs by age five years, but maturation of white matter is ongoing and continues through the third decade of life [5,6,13, 24,25,27]. FA correlates with this maturation process. FA increases throughout normal development during adolescence and young adulthood [27]. Characterization of white matter structure after TBI and its relationship to neurocognitive maturation and development may provide an important avenue for understanding recovery, especially since the majority of deficits noted after TBI are cognitive in nature.
Because neurodevelopment is occurring at such a rapid rate throughout childhood and young adulthood, it is essential to evaluate the effects TBI has during different developmental stages. Because the peak incidence of TBI in children occurs during two vastly different periods of development, i.e., early childhood and adolescence, it may be important to examine the relationships between neural and cognitive outcomes in these groups separately. Additionally, since executive function (EF) development coincides with normal white matter maturation and development during late childhood and adolescence, examining the effects TBI has on this process is necessary. Previous research in the early childhood TBI population has shown that FA measured a minimum of twelve months post TBI correlated with injury severity as defined by Glasgow Coma Scale (GCS) scores [33]; however, the relationship with neuropsychological outcomes, specifically EF, was not examined. The goal of the present study is to begin to define the relationship of FA measured during the chronic stage of recovery following early childhood TBI with neuropsychological outcomes, specifically within EF domains. We hypothesize that late measurements (more than 12 months post injury) of FA in regions of interest (ROI) located in the corpus callosum, frontal white matter tracts, long white matter tracts, and internal capsule will correlate with EF measures in individuals who sustained an early childhood TBI.
2. Materials and methods
2.1. Participants
Children in this study were recruited from a larger, prospective behavioral study that involved the prospective examination of social environmental influences on child and family outcomes of TBI or Orthopedic injury (OI) in early childhood. Outcomes were measured soon after injury, and at 6, 12, and 18 months post-injury. Inclusion criteria for both groups included age between 36 and 84 months at the time of injury, an overnight stay in the hospital, English as the primary spoken language, no documentation of child abuse as the cause of injury, and no reported history of developmental disability or medical condition associated with neuropsychological deficits (e.g. seizure disorder). Additional inclusion criteria for the TBI included a group score of less than 15 or a GCS of 15 with associated neuroimaging findings of a brain injury on CT or MRI, and TBI due to blunt external trauma. Additional inclusion criteria for the OI group included documented bone fracture not involving the head and no documented loss of consciousness or symptoms of brain injury at any time. Parents or guardians of children previously enrolled in the original study who were older than 6 years of age and at least 12 months post injury were contacted for participation in the neuroimaging study. Fourteen children with TBI and 17 children with OI were eligible to participate in the imaging study. Ten children with TBI and 13 children with OI agreed to participate. Usable imaging was obtained on 9 TBI and 12 OI participants. OI participants were matched on time since injury, age, sex, ethnicity, and handedness with the TBI group and recruited for the imaging study as control subjects. See Table 1 for clinical and demographic information on the participants. In the TBI group, two children had a severe TBI, and 7 had a moderate TBI. The average GCS score was 10.22. A severe TBI was defined as a GCS score of 8 or less; a moderate TBI was defined as a GCS score of 9–12 or a score of 13–15 associated with neuroimaging findings on CT or MRI; and a mild TBI was defined as a GCS score of 13–15 with no imaging abnormalities (None of the TBI subjects were classified as mild TBI). The TBI and OI groups did not differ significantly in age, sex ratio, verbal intelligence quotient, or maternal education. Two of the TBI group and one of the OI group were left-handed; one in the OI group was ambidextrous. Institutional Review Board approval and parental or guardian consent were obtained prior to initiating the study.
Table 1.
Participant clinical and demographic information
| Group | Age (yrs) | Time since injury (yrs) | Sex | Injury mechanism | GCS |
|---|---|---|---|---|---|
| TBI | 9.04 | 3.33 | F | Fall | 15 |
| TBI | 6.72 | 3.08 | M | Ped vs. MVC | 9 |
| TBI | 9.01 | 2.83 | M | Bicycle | 13 |
| TBI | 9.10 | 2.75 | M | Fall | 15 |
| TBI | 6.93 | 2.50 | M | Fall | 14 |
| TBI | 7.82 | 2.17 | M | Ped vs. MVC | 10 |
| TBI | 8.33 | 1.75 | F | Fall | 9 |
| TBI | 6.90 | 1.25 | F | Fall | 3 |
| TBI | 7.17 | 1.25 | M | MVC | 3 |
| OI control | 7.34 | 3.00 | M | Fall | NA |
| OI control | 6.55 | 2.75 | M | Rough housing | NA |
| OI control | 8.72 | 2.75 | M | Fall | NA |
| OI control | 7.08 | 2.67 | M | Playground | NA |
| OI control | 6.47 | 2.50 | M | Fall | NA |
| OI control | 9.10 | 2.33 | F | Sledding | NA |
| OI control | 7.68 | 2.00 | M | Bicycle | NA |
| OI control | 8.62 | 2.00 | F | Fall | NA |
| OI control | 6.63 | 1.92 | F | Playground | NA |
| OI control | 8.19 | 1.75 | F | Trampoline | NA |
| OI control | 6.46 | 1.00 | M | Ped vs. MVC | NA |
| OI control | 7.32 | 1.46 | F | Furniture fell on leg | NA |
TBI = traumatic brain injury; OI = orthopedic injury; MVC = motor vehicle collision.
3. MR imaging evaluations
Diffusion tensor imaging acquisition and data processing were completed as described in Yuan et al. [33]. All images were acquired without the use of sedation and were obtained at least twelve months after injury because this is commonly considered to represent the chronic stage of recovery after TBI. All participants underwent MRI/DTI examination on a 3T MRI scanner (Trio, Siemens, Erlangen, Germany). DTI (single shot spin-echo echo-planar imaging) was acquired with an acquisition matrix = 128 × 128, FOV = 25.6 cm × 25.6 cm, TR = 6000 ms, TE = 87 ms, 46 contiguous axial slices, slice thickness = 2 mm, voxel size = 2 × 2 × 2 mm3. During the scan, diffusion gradients were applied along 12 non-collinear directions with a b-value of 1000 s/mm2. An additional set of images without diffusion weighting (b0 = 0 s/mm2) were also acquired. The scan was repeated four times to improve signal to noise ratio. The acquisition time for DTI images was 5:48 minutes.
A T1 weighted sagittal 3D MP-RAGE (magnetization-prepared rapid acquisition of gradient echo) was acquired. This was a high-resolution imaging covering the whole brain to provide anatomic reference (TR=2000 ms, TE=2.93 ms, FOV=21.9 × 21.9 cm2, acquisition matrix = 256 × 205, slice thickness = 1 mm, voxel size = 0.86×1.07×1mm3, NEX=1, acquisition time = 3:50 minutes). A fall-back sequence was prepared and used for two participants who were found to have excessive motion. This was a similar 3D MG-RAGE sequence at a lower resolution (0.86 × 1.71 × 2 mm3) and thus required shorter acquisition time (2:30 minutes).
The structural images were evaluated by a board-certified pediatric neuroradiologist for structural abnormalities in the brain, including evidence of volume loss, abnormal signal intensity, or evidence of hemorrhage. Four of the nine TBI participants demonstrated findings consistent with evidence of chronic brain injury, including small bilateral frontal cysts, volume loss with mild, moderate and severe encephalomalacia, and prominent perivascular and subarachnoid spaces. Three individuals in the OI group demonstrated imaging findings, including a Chiari I malformation and small syrinx, supravermian cyst, and abnormal signal in the right parietal white matter with mildly prominent perivascular spaces. The MR abnormalities in the OI group were incidental findings not related to their injury. The findings were not localized within the ROI or within white matter pathways interconnected to the ROI. All imaging studies were performed without the use of sedation.
4. Neurobehavioral assessments
As part of the initial study all children participated in a comprehensive neurobehavioral evaluation at approximately 12 and 18 months post injury. Neurobehavioral data from the 18 month time point were used in this study. However, when 18 month data was unavailable, testing done at approximately 12 months was used. Primary care giver ratings of EF were assessed using the Global Executive Composite (GEC) score from the Behavior Rating Inventory of Executive Function (BRIEF) [10]. Higher scores on the BRIEF are associated with poorer ratings. The Shape School is a modified Stroop test developed to evaluate domains of EF including selective attention, cognitive flexibility, and processing speed in young children [7]. In this test, the child names cartoon-like “faces” differing in color and shape according to specified conditions or rules. In the first condition, the child is asked to name the color of the faces (Simple Naming), in the second to name the colors of happy faces while ignoring sad ones (Inhibition), and in the third to name the color of some faces but the shapes of others depending on a stimulus cue (Switching). Finally, in the fourth or Both condition, the child is asked to both inhibit naming of some faces and to switch between naming colors and shapes depending on the stimulus cue (Both). An efficiency score is calculated by subtracting the number of incorrect answers from the correct answers and then dividing by the time it took to complete the task (# correct responses – # incorrect responses/ time) for each subtest. A higher efficiency score corresponds to better performance on each of these conditions. Additionally, two subtests from the Test of Everyday Attention for Children (TEA-Ch) were used to assess attentional capacity [22]. Specifically, the Sky Search: selective/focused attention (Attn scale) and Score! sustained attention (Score scale) subtests were used. There are two parts to the Sky Search test. In the first part, children are asked to circle targets on a large plastic sheet that are interspersed with distractors. The number of correctly identified targets is recorded. In the second part, children are asked to circle targets on a large sheet without distracters present. This part of the test is designed to correct for motor speed. The raw scores on the two parts of the test are combined and then used to calculate one standardized score. On the Score! subtest children are asked to count sounds that they heard over ten separate trials. The sounds are separated by differing time intervals to assess sustained attention. Again, raw scores are used to calculate a standardized score as per the TEA-Ch manual instructions. Higher standardized scores on both subtests correlate with better attentional capacity.
5. Statistical analysis
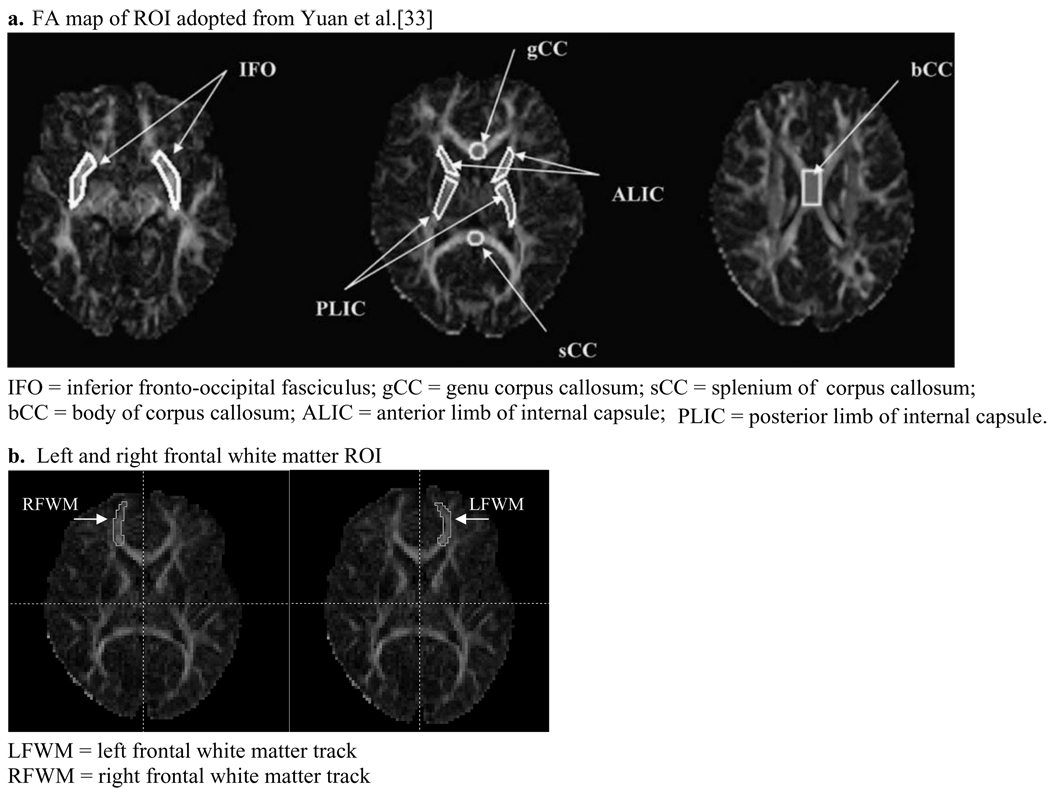
Various white matter regions have been reported to be susceptible to damage after TBI, including the corpus callosum, frontal regions, internal capsule, superior longitudinal fasciculus, and inferior fronto-occipital fasciculus [2,4,11,12,17,18,20,23,26,28,29,33]. In this study we used the same ROI as described in Yuan et al. [33], including the splenium of the corpus callosum (sCC), genu of the corpus callosum (gCC), body of the corpus callosum (bCC), anterior limb of the internal capsule (ALIC), posterior limb of the internal capsule (PLIC), inferior fronto-occipital fasciculus (IFO), and the superior longitudinal fasciculus (SLF). Because EF has been correlated with anatomical findings in the frontal lobes [15], we also added two additional ROI in the left (LFWM) and right (RWFM) frontal white matter tracks. The ROI delineation for LFWM and RFWM was performed by one rater (A.R.) for all the subjects. The delineation for the rest of the ROI was performed by another rater (W.Y.) as described elsewhere [33]. The intra-rater reliability was assessed with a paired t-test by comparing the results from two repetitions. No statistical difference was found [33]. See Fig. 1a and b for sample ROI maps. A Mann-Whitney U test was used to evaluate for significant differences in FA values in ROI between the TBI and OI groups with a p-value of 0.05 as the threshold. A Mann-Whitney U test was also used to compare the neurocognitive assessment scores in each group. Spearman correlations between neurocognitive assessment scores and FA values in ROI for all subjects and within each group were performed. Spearman correlation values in the OI and TBI groups were then compared using a Fischer r to z transformation to determine significance. VassarStats: Website for Statistical Computation [21] (http://faculty.vassar.edu/lowry/VassarStats.html) was used to calculate p-values. P-value threshold of 0.05 was used to define significance. Spearman correlations between GCS scores and FA values in ROI were also performed.
Fig. 1.
FA map of ROI.
6. Results
6.1. Comparison of FA and neurobehavioral/executive function measure scores between groups
Mean FA values in each of the ROI were compared between the TBI and OI groups (Table 2). Significantly (p ≤ 0.05) lower FA values were found in the TBI group compared to the OI group in the gCC (p = 0.039), ALIC (p = 0.031), and PLIC (p = 0.008). EF scores on neurobehavioral testing did not differ significantly between the OI and TBI groups (Table 3). Group differences on parent-rated executive dysfunction (BRIEF) approached significance (p = 0.087).
Table 2.
Comparison of mean and standard deviation of ROI FA values between the OI (n =12) and TBI (n =9) groups using the Mann-Whitney U test
| ROI | FA OI | FA TBI | p-value |
|---|---|---|---|
| RFWM | 0.463 ± 0.048 | 0.438 ± 0.075 | 0.153 |
| LFWM | 0.465 ± 0.035 | 0.440 ± 0.045 | 0.185 |
| sCC | 0.771 ± 0.058 | 0.692 ± 0.112 | 0.059 |
| gCC | 0.770 ± 0.039 | 0.730 ± 0.042 | 0.039 |
| bCC | 0.746 ± 0.055 | 0.687 ± 0.116 | 0.198 |
| ALIC | 0.579 ± 0.037 | 0.539 ± 0.042 | 0.031 |
| PLIC | 0.628 ± 0.041 | 0.576 ± 0.027 | 0.008 |
| IFO | 0.454 ± 0.052 | 0.453 ± 0.063 | 0.746 |
| SLF | 0.500 ± 0.053 | 0.486 ± 0.047 | 0.432 |
ROI = region of interest; FA = fractional anisotropy; OI = orthopedic injury; TBI = traumatic brain injury; RFWM = right frontal white matter track; LFWM = left frontal white matter track; sCC = splenium corpus callosum; gCC = genu corpus callosum; bCC = body corpus callosum; ALIC = anterior limb internal capsule; PLIC = posterior limb internal capsule; IFO = inferior fronto-occipital fasciculus; SLF=superior longitudinal fasciculus.
Table 3.
Comparison of mean and standard deviation of executive function and attention measure scores between the OI (n = 12) and TBI (n = 9) groups using the Mann-Whitney U test
| Neurocognitive measure | OI | TBI | p-value |
|---|---|---|---|
| Shape School Inhibit | 1.130 ± 0.578 | 1.240 ± 0.452 | 0.776 |
| Shape School Switch | 0.407 ± 0.430 | 0.449 ± 0.303 | 0.938 |
| Shape School Both | 0.641 ± 0.207 | 0.641 ± 0.312 | 0.899 |
| BRIEF | 51.417 ± 11.024 | 60.111 ± 9.090 | 0.087 |
| ATTN scale | 7.750 ± 2.005 | 7.556 ± 4.613 | 0.857 |
| Score scale | 9.833 ± 3.904 | 11.556 ± 3.244 | 0.542 |
OI = orthopedic injury; TBI = traumatic brain injury; RFWM = right frontal white matter track; LFWM = left frontal white matter track; BRIEF = Behavior rating inventory of executive function; ATTN Scale = Sky Search: selective/focused attention; Score scale = Score!: sustained attention; sCC = splenium corpus callosum; gCC = genu corpus callosum; bCC = body corpus callosum; ALIC = anterior limb internal capsule; PLIC = posterior limb internal capsule; IFO = inferior fronto-occipital fasciculus; SLF = superior longitudinal fasciculus.
6.2. Correlation of FA values in all subjects
Correlation of FA values in ROI with EF measures in all subjects was significant in several areas (Table 4). Better performance on specific tasks of inhibition significantly correlated with higher FA in the RFWM (p = 0.015), LFWM (p = 0.000), bCC (p = 0.026), and the SLF (p = 0.038). Better performance on specific tasks of switching also significantly correlated with higher FA in the RFWM (p = 0.003), LFWM (p = 0.000), bCC (p =0.012), and the SLF (p =0.048). When combining tasks of inhibition and switching, better performance significantly correlated with higher FA in only the RFWM (p = 0.039) and LFWM (p = 0.001). Superior parent rating of executive dysfunction (BRIEF) did not correlate significantly with higher FA values in any of the ROI, but approached significance (p < 0.1) in the RFWM (p = 0.091), sCC (p = 0.065), gCC (p = 0.058), bCC (p = 0.054), and PLIC (p = 0.077) Poorer performance on tasks of attention correlated significantly with higher FA values in the RFWM (p = 0.008). FA values in the sCC, gCC, ALIC, PLIC, and IFO did not correlate significantly with any of the EF or attention measures in the sample as a whole.
Table 4.
Spearman correlations of executive function and attention measures with FA in ROI for all subjects (n = 21). The TBI (n = 9) and OI (n = 12) groups were combined
| ROI | SS INH | SS SWT | SS BOTH | BRIEF | ATTN scale | Score scale |
|---|---|---|---|---|---|---|
| RFWM | 0.525* | 0.633** | 0.476* | −0.379 | −0.565** | −0.206 |
| LFWM | 0.809** | 0.771** | 0.692** | −0.357 | −0.155 | −0.127 |
| sCC | 0.184 | 0.269 | 0.364 | −0.410 | −0.170 | −0.324 |
| gCC | −0.216 | 0.122 | 0.066 | −0.421 | 0.052 | 0.260 |
| bCC | 0.483* | 0.550* | 0.349 | −0.426 | −0.361 | −0.012 |
| ALIC | 0.196 | 0.195 | −0.057 | −0.253 | −0.135 | 0.130 |
| PLIC | 0.082 | 0.181 | −0.059 | −0.394 | −0.098 | −0.025 |
| IFO | −0.018 | 0.030 | 0.161 | −0.307 | −0.183 | −0.300 |
| SLF | 0.455* | 0.447* | 0.307 | −0.341 | −0.204 | −0.094 |
ROI = region of interest; OI = orthopedic injury; TBI = traumatic brain injury; SS INH = Shape School Inhibit task; SS SWT=Shape School Switching task; SS BOTH=Shape School Combination task; BRIEF = Behavior rating inventory of executive function; ATTN Scale = Sky Search: selective/focused attention; Score scale = Score!: sustained attention; RFWM = right frontal white matter track; LFWM = left frontal white matter track; sCC = splenium corpus callosum; gCC=genu corpus callosum; bCC=body corpus callosum; ALIC=anterior limb internal capsule; PLIC = posterior limb internal capsule; IFO = inferior fronto-occipital fasciculus; SLF = superior longitudinal fasciculus.
= significance < 0.01.
= significance < 0.05.
6.3. Correlation of FA values in groups separately
Correlation of FA values in ROI with EF and attention measures was also performed for the groups separately (Table 5a and 5b). In the TBI group, better performance on tasks of inhibition correlated significantly with higher FA in the LFWM (p = 0.001). Better performance on tasks of switching correlated significantly with higher FA values in both the LFWM (p = 0.005) and RFWM (p =0.004). Better performance on tasks combining both inhibition and switching correlated significantly with FA in the LFWM (p = 0.006). Poorer performance on attention tasks (Attn scale and Score scale) correlated significantly with higher FA in the RFWM (p = 0.004 on the Attn scale and p = 0.047 on the Score scale) and SLF (p = 0.037 on the Attn scale). In the OI group, better performance on tasks of inhibition correlated significantly with higher FA in the RFWM (p = 0.047), LFWM (p = 0.000), bCC (p = 0.047), and SLF (p = 0.047). Better performance on tasks of switching correlated significantly with higher FA in the LFWM (p = 0.011). Better performance on tasks combining both inhibition and switching correlated significantly with higher FA in the LFWM (p = 0.034). Parent rating of EF correlated significantly with FA in the RFWM (p = 0.043). FA values in the sCC, gCC, ALIC, PLIC, and IFO were not significantly correlated in the individual groups with any of the neurobehavioral measures used.
Table 5.
Spearman correlation of executive function measures and FA values within each group (TBI [n = 9] or OI [n = 12]) and comparisons of correlations between groups (p-value)
| A. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Shape school inhibit | Shape school switch | Shape school both | |||||||
| ROI | OI | TBI | p-value | OI | TBI | p-value | OI | TBI | p-value |
| RFWM | 0.583* | 0.598 | 0.968 | 0.554 | 0.878** | 0.184 | 0.539 | 0.704 | 0.653 |
| LFWM | 0.901** | 0.898** | 0.976 | 0.700* | 0.872** | 0.395 | 0.613* | 0.898** | 0.215 |
| sCC | 0.183 | 0.435 | 0.596 | 0.148 | 0.405 | 0.617 | 0.389 | 0.429 | 0.936 |
| gCC | −0.072 | −0.318 | 0.624 | 0.465 | −0.429 | 0.936 | 0.432 | −0.464 | 0.107 |
| bCC | 0.582* | 0.555 | 0.936 | 0.529 | 0.599 | 0.857 | 0.358 | 0.523 | 0.734 |
| ALIC | 0.426 | −0.066 | 0.322 | 0.323 | −0.086 | 0.447 | −0.138 | 0.150 | 0.631 |
| PLIC | 0.062 | 0.456 | 0.412 | 0.122 | 0.410 | 0.576 | 0.067 | 0.000 | 0.912 |
| IFO | −0.260 | 0.291 | 0.952 | −0.143 | 0.229 | 0.497 | −0.077 | 0.600 | 0.200 |
| SLF | 0.583* | 0.443 | 0.719 | 0.388 | 0.578 | 0.653 | 0.223 | 0.582 | 0.465 |
| B. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BRIEF | Attn scale | Score scale | |||||||
| ROI | OI | TBI | p-value | OI | TBI | p-value | OI | TBI | p-value |
| RFWM | −0.591* | 0.000 | 0.197 | −0.291 | −0.849** | 0.070 | 0.129 | −0.672* | 0.074 |
| LFWM | −0.194 | −0.408 | 0.653 | 0.139 | −0.349 | 0.337 | −0.005 | −0.319 | 0.535 |
| sCC | −0.481 | −0.303 | 0.689 | −0.073 | −0.152 | 0.881 | 0.012 | −0.625 | 0.159 |
| gCC | −0.351 | 0.092 | 0.384 | 0.205 | 0.093 | 0.826 | 0.395 | 0.456 | 0.889 |
| bCC | −0.401 | −0.439 | 0.928 | −0.062 | −0.466 | 0.401 | 0.331 | −0.275 | 0.234 |
| ALIC | 0.113 | −0.265 | 0.465 | −0.015 | 0.000 | 0.976 | 0.405 | 0.151 | 0.596 |
| PLIC | −0.282 | 0.246 | 0.303 | −0.078 | −0.255 | 0.726 | 0.522 | −0.655 | 0.010 |
| IFO | −0.288 | −0.377 | 0.849 | −0.120 | −0.311 | 0.704 | −0.399 | −0.349 | 0.912 |
| SLF | −0.302 | −0.229 | 0.881 | 0.392 | −0.698* | 0.016 | 0.160 | −0.332 | 0.337 |
ROI = region of interest; OI = orthopedic injury; TBI = traumatic brain injury; SS INH = Shape School Inhibit task; SS SWT = Shape School Switching task; SS BOTH = Shape School Combination task; BRIEF = Behavior rating inventory of executive function; ATTN Scale = Sky Search: selective/focused attention scale; Score scale = Score!: sustained attention scale; RFWM = right frontal white matter track; LFWM = left frontal white matter track; sCC = splenium corpus callosum; gCC = genu corpus callosum; bCC = body corpus callosum; ALIC = anterior limb internal capsule; PLIC = posterior limb internal capsule; IFO = inferior fronto-occipital fasciculus; SLF = superior longitudinal fasciculus.
significant correlation within each group (OI or TBI) of p < 0.01.
significant correlation within each group (OI or TBI) of p < 0.05.
p-values represent comparison of the correlations between the groups. Bold values represent significance of < 0.05 and italicized values represent values of < 0.1.
Comparisons of correlations between the OI and TBI groups are shown in Tables 5a and 5b. Significant (p < 0.05) differences between the TBI and OI group correlations on measures of attention were seen in the PLIC (p = 0.010 on the Score scale) and SLF (p = 0.016 on the Attn scale). Comparison of correlations were approaching significance in the RFWM with attention tasks (p = 0.070 on the Attn scale and p = 0.074 on the Score scale). No other correlations were significant or approached significance in the remaining ROI.
6.4. FA correlation with GCS scores
GCS scores correlated significantly with FA values in the bCC (correlation coefficient = 0.698; p-value = 0.037). GCS did not correlate with any of the remaining ROI with correlation coefficients ranging from 0.153–0.537 (p-values 0.136–0.695).
7. Discussion
The present study builds upon previous research by examining the relationship of white matter integrity to EF skills following pediatric TBI in a relatively homogenous sample of children who sustained a traumatic injury in early childhood. Findings of significant correlations between FA values in the frontal white matter and laboratory-based measures of EF provide partial support for our hypotheses.
Previous studies have examined the relationship of DTI findings after pediatric TBI to various global outcome measures (i.e., Glasgow Outcome Scale), symptom reports, and neuropsychological measures, including IQ, EF domains, memory, and others. In adults, FA measured in various brain regions after TBI has been correlated with a range of global and neuropsychological outcomes. FA measured acutely (1–10 days) after mild TBI is decreased compared to controls in areas commonly involved in diffuse axonal injury (central semiovale, corpus callosum, and internal capsule) [14, 23]. The decreased FA appears to persist years after injury [14]. Late measurements of FA after TBI demonstrated decreased anisotropy in the major white matter tracts in the temporal, frontal, parietal, and occipital lobes and correlated with learning and memory indices [26]. Other studies of chronic TBI showed that decreased FA in various areas of the brain was associated with poorer EF, attention, and memory [16, 17]. FA also changes over time after TBI in adults [4, 28]. Sidaros et al. showed FA was decreased approximately 5 weeks after TBI in multiple ROI and demonstrated that it may increase, remain unchanged, or become further depressed when measured at 12 months after TBI [28]. When FA reached normal or supra-normal levels 12 months after injury in certain ROI, it was associated with favorable outcomes [28]. Alternatively, when FA remained depressed, it was associated with unfavorable outcomes [28]. Overall, in adults, FA measured both acutely and chronically after TBI correlates with neurocognitive outcomes, and in general, decreased FA is associated with poorer outcomes.
The relationship of FA with cognitive and neuropsychological outcomes following pediatric TBI has not been examined as extensively as in the adult population. However, recent studies are beginning to elucidate this relationship [8,19,29–31]. Wilde et al. demonstrated that FA was significantly lower in the genu, body, and splenium of the corpus callosum in children (mean age = 12.9 years) with moderate to severe TBI (mean time since TBI = 3.1 years) [29]. They also showed that higher FA was related to increased processing speed, improved interference resolution, and better functional outcome as measured by a dichotomized Glasgow Outcome Scale (GOS). Wozniak et al. found that children sustaining mild and moderate TBIs between the ages of 10–18 years had lower FA in the inferior frontal, superior frontal, and supracallosal areas compared to controls [31]. FA in the frontal and supracallosal regions was also correlated with EF [31]. Levin et al. demonstrated that a composite score measurement of white matter integrity using fiber tracking analysis correlated with global outcome measures in moderate to severe pediatric TBI three months after injury [19]. Ewing-Cobbs et al. correlated FA measurements in the isthmus and splenium of the corpus callosum with neuropsychological outcomes (IQ, working memory, motor, and academic skills) in pediatric (average age 9 years) TBI at least three months after injury (mean = 39.1 ± 46.4 months) [8]. In general, these studies have demonstrated that decreased FA after pediatric TBI is associated with poorer neurocognitive outcomes. However, contrary to the general trend of higher FA correlating with better outcomes, one study demonstrated that FA measured acutely, within six days post injury, in the corpus callosum was increased in adolescents with mild TBI, and correlated with increased post-concussive symptoms [30]. Age, injury severity, and outcomes of interest varied widely across these studies making generalization difficult. Additionally, many of these studies used convenience control samples and did not necessarily match subjects for potential confounding variables, including age, baseline education, parental education, and the potential psychosocial effects of the injury itself. Thus, the present study represents a next step in elucidating the relationship of white matter integrity to recovery from pediatric TBI.
We examined the relationship of FA obtained late (> 12 months) after early childhood TBI in multiple ROI with EF and attention measures. Late measurements of FA primarily in both frontal lobes correlated significantly with EF measures in the TBI and OI comparison groups. FA in the bCC and SLF also correlated with EF measures in the comparison group. These findings may indicate that FA measured in the frontal white matter tracks could potentially be used as an index of EF or dysfunction. Additionally, since FA is a measure of white matter organization, white matter disorganization in the frontal white matter tracks could possibly be the neuroanatomical explanation for executive dysfunction that occurs after early childhood TBI. Better characterization of the effect early childhood TBI has on the development and maturation of white matter tracks in relation to the development of EF will be an important future research direction, especially since EF is known to develop and mature in late childhood through adolescence.
Correlation of parent ratings of EF (BRIEF) with FA findings in the RFWM, sCC, gCC, bCC, and PLIC approached significance when all subjects were evaluated. However, the correlations did not reach significance when the TBI subjects were evaluated separately. We are likely seeing greater significance of correlations when the subjects are combined because of the increased power afforded by an increase in the number of subjects. These findings may indicate that FA may be a marker of subjective ratings of EF.
The negative correlation between performance on attention tasks from the TEA-Ch and FA values was counter to our hypotheses and prior research. There are several possible explanations for these findings. First, individual measures of EF, including alerting, orienting, and executive control have been shown not to be correlated when tested separately [9]. This is in agreement with our study where TEA-Ch measures of attention were not correlated with Shape School measures of EF. Second, some evidence suggests that sustained attention as assessed by the TEA-Ch is less affected by pediatric TBI than attention and EF more broadly. Third, the scaling of standardized scores on the TEA-Ch for younger children may also afford a potential explanation. The age-based norms for the TEA-CH mean that relatively low raw scores convert to relatively high standard scores for children at the youngest ages. Because FA values also increase with age, younger children may have both higher attention scores coupled with lower FA values. This negative association may have been intensified in the TBI group because sustained attention may not have been adversely affected by the TBI despite changes in white matter integrity. Taken together, these findings suggest that EF skills may be more sensitive than attention to white matter alterations, particularly in the frontal lobes, following early childhood TBI.
FA was significantly different between the groups in multiple ROI, including the gCC, ALIC, and PLIC (Table 2). FA was lower in the TBI group in these ROI; however, these values did not correlate with EF or attention measures in the TBI group. Because examination of these subjects was done late (> 12 months) after TBI, reorganization of white matter tracts to compensate for these deficits could be a potential explanation. Additionally, because these subjects are at a stage when their white matter tracts are undergoing active development and maturation, the dynamic nature of this process could limit the ability to detect correlations between FA and neurocognitive measures. Serial evaluation of the relationship of FA with EF measures and attention measures throughout development might allow identification of potential correlations.
GCS in our study only correlated with FA measured late after early pediatric TBI in the bCC. This is in contrast to previous work where FA measured late in multiple ROI correlated with GCS [33]. However, the method used to calculate FA in this study was a voxel-wise calculation method instead of ROI that we used. Further work should be done to better define the relationship of GCS and FA changes after pediatric TBI.
7.1. Limitations
The main limitation in our study is its small sample size. Larger numbers of study participants could provide the power to detect more subtle relationships, especially since there were multiple correlations that were nearing significance. We also performed multiple correlations in multiple ROI, thus leading to numerous (possibly 7 or 8) expected false positive results. However, we were limited in terms of statistical approaches due to our small sample size. Overall, the present study is exploratory in nature. We have identified potential trends, but larger studies will need to be performed to examine these relationships more thoroughly. Additionally, the TBI group in this study predominantly included those with moderate TBI. This is a potential strength and weakness. It is a strength because we were able to examine a relatively homogenous sample; however, the relationship of FA to recovery after differing severities of TBI is also important to elucidate.
7.2. Conclusions
Our study suggests that DTI-measured FA in the frontal lobes late after early childhood TBI is related to neurocognitive recovery, specifically EF. Unlike previous studies that have examined the relationship of FA to recovery after pediatric TBI, this study specifically examined FA measured late in a relatively homogenous sample of children that sustained an early TBI. It is important to look at this subset of patients separately because neurodevelopment is rapidly occurring during this time period and recovery after TBI is likely to be significantly effected by this developmental stage. Future studies should be performed to better define the relationship of FA to neurocognitive recovery in children that sustained an early TBI. Performing DTI serially would allow tracking of white matter changes over time and could provide insight into how these tracks reorganize after TBI and how such reorganization relates to recovery of EF skills. Examining the effect TBI has on subsequent development of white matter tracks in late childhood and early adolescence may shed light on the late effects of TBI. Our study provides some insight to these questions, but larger studies and studies focused specifically on these questions are warranted. Better understanding of the relationship of white matter track changes or reorganization that occurs after pediatric TBI could allow better prediction of the expected deficits and potentially lead to the development of improved interventions to address these deficits.
Acknowledgements
This work was supported in part by 1) NIH grant RO1-HD044279 from the National Council on Medical Rehabilitation Research in the National Institute of Child Health and Human Development; 2) Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 1UL1RR026314 (The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH); and 3) NIH grant RO1-HD38578 from the US National Institute of Child Health and Human Development. Funding for the fMRI scans was provided by the Association of Volunteers of the Convalescent Hospital for Children, Cincinnati Children’s Hospital Medical Center.
References
- 1.Traumatic Brain Injury. 2009 http://www.cdc.gov/ncipc/factsheets/tbi.htm Vol.
- 2.Akpinar E, Koroglu M, Ptak T. Diffusion tensor MR imaging in pediatric head trauma. J Comput Assist Tomogr. 2007;31(5):657–661. doi: 10.1097/RCT.0b013e318033df1a. [DOI] [PubMed] [Google Scholar]
- 3.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 4.Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, Sherman JE, Johnson SC. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42(2):503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey BJ, Giedd JN, Thomas KM. Biol Psychol. 1–3. Vol. 54. 2000. Structural and functional brain development and its relation to cognitive development; pp. 241–257. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 7.Espy KA, Bull R, Martin J, Stroup W. Measuring the development of executive control with the shape school. Psychol Assess. 2006;18(4):373–381. doi: 10.1037/1040-3590.18.4.373. [DOI] [PubMed] [Google Scholar]
- 8.Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Jr, Fletcher JM, Barnes M, Zhang X, Hasan KM. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage. 2008;42(4):1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 10.Gioia G, Isquith P, Guy SC, Lauren K. Behavior Rating Inventory of Executive Function. Lutz, FL: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- 11.Greenberg G, Mikulis DJ, Ng K, DeSouza D, Green RE. Use of diffusion tensor imaging to examine subacute white matter injury progression in moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2008;89(12 Suppl):S45–S50. doi: 10.1016/j.apmr.2008.08.211. [DOI] [PubMed] [Google Scholar]
- 12.Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, Wu O, Sorensen AG. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25(3):370–376. [PMC free article] [PubMed] [Google Scholar]
- 13.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, Grossman RI. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 15.Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17(3):213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy MR, Wozniak JR, Muetzel RL, Mueller BA, Chiou HH, Pantekoek K, Lim KO. White matter and neurocognitive changes in adults with chronic traumatic brain injury. J Int Neuropsychol Soc. 2009;15(1):130–136. doi: 10.1017/S1355617708090024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, Pandey CM, Narayana PA. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neurocognitive function. J Neurotrauma. 2009;26(4):481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- 19.Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, Chia J, Vasquez AC, Hunter JV. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo C, Shifteh K, Gold T, Bello JA, Lipton ML. Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J Comput Assist Tomogr. 2009;33(2):293–297. doi: 10.1097/RCT.0b013e31817579d1. [DOI] [PubMed] [Google Scholar]
- 21.Lowry R. VassarStats: Web Site for Statistical Computation. Vol. 2009 [Google Scholar]
- 22.Manly T, Robertson IH, Anderson V, Nimmo-Smith I. The Test of Everyday Attention for Children (TEA-Ch) London: Harcourt Assessment; 1999. [DOI] [PubMed] [Google Scholar]
- 23.Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22(2):115–122. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa H, Iwasaki S, Kichikawa K, Fukusumi A, Taoka T, Ohishi H, Uchida H. Normal myelination of anatomic nerve fiber bundles: MR analysis. AJNR Am J Neuroradiol. 1998;19(6):1129–1136. [PMC free article] [PubMed] [Google Scholar]
- 25.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 26.Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, Pickard JD. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29(1):117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, Paulson OB, Jernigan TL, Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131(Pt 2):559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 29.Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- 30.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, Chia J, Levin HS. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 31.Wozniak JR, Krach L, Ward E, Mueller BA, Muetzel R, Schnoebelen S, Kiragu A, Lim KO. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22(5):555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Rasmussen IA, Lagopoulos J, Haberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J Neurotrauma. 2007;24(5):753–765. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- 33.Yuan W, Holland SK, Schmithorst VJ, Walz NC, Cecil KM, Jones BV, Karunanayaka P, Michaud L, Wade SL. Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR Am J Neuroradiol. 2007;28(10):1919–1925. doi: 10.3174/ajnr.A0698. [DOI] [PMC free article] [PubMed] [Google Scholar]