Two striking features of the eyeball are first that it is more or less spherical and second that some parts of it are transparent. If you examine the eye of a cat, for example, it is possible to see how the transparent cornea is an inverted dome positioned above the iris and pupil. The shape and transparency of this “window” onto the visual system are both extremely important. Although there is a lens inside the eye, hidden behind the pupil, it turns out that refraction of light crossing the curved cornea plays a dominant role in the focusing of incoming light onto the retina at the back of the globe. The lens acts only as a fine focus mechanism because its shape can be changed and this alters the effective focal length of the optical system in order for us to switch our gaze between near and distant objects without losing sharpness of the image. Looking again at the cat’s eye, one can see that the cornea more or less resembles an optical lens of the type normally made from glass. Now elementary physics tells us that the curvature of the cornea dictates its optical characteristics. How does the cornea come to have its unique shape? Although many different factors are involved, one of the governing issues is the existence of a hydrostatic pressure within the eye. It is this hydrostatic pressure which keeps the eyeball in a roughly spherical shape and keeps the walls of the eyeball taut.
The pressure is approximately 15mm Hg higher inside the human eye than outside. The globe is pressurized with fluid in much the same way as a basketball is pressurized with air. In the eye, the pressure is called the intraocular pressure (IOP). The pressure is the direct result of a continual secretion of fluid into the interior cavity of the eyeball. The fluid is watery and transparent – it is aqueous humor. The aqueous humor is produced by the ciliary body.
Structure of the Ciliary Body
Because the ciliary body is hidden behind the iris, it is impossible to see it no matter how deeply you gaze into someone’s eyes. It is a ring of tissue on the inner wall of the eyeball, positioned just behind the rear-facing (posterior) surface of the iris. The base of the ciliary body is home to the ciliary muscle, the contraction of which causes the lens to assume a more rounded shape. This is because the lens is suspended by fine ligaments, called zonules, which attach to the ciliary body. When the ciliary muscle contracts, the anchoring point of the zonules moves slightly inward, relaxing tension on the zonules and the natural elasticity of the lens causes it to take on a more spherical shape. This is how our focus shifts. It is the process of accommodation (see Kaufman, 1992). When the ciliary muscle relaxes, there is a slight outward motion that tightens the zonules and flattens the lens.
The surface of the ciliary body is elaborated into a series of ridges named ciliary processes. These ridges on the surface of the ciliary body look rather similar to the fins on a motorcycle engine and in a way they serve a similar purpose – to increase surface area. On a motorcycle engine, the ridges provide a large surface area to dissipate heat. On the ciliary body, the purpose is to increase the surface area available for fluid secretion.
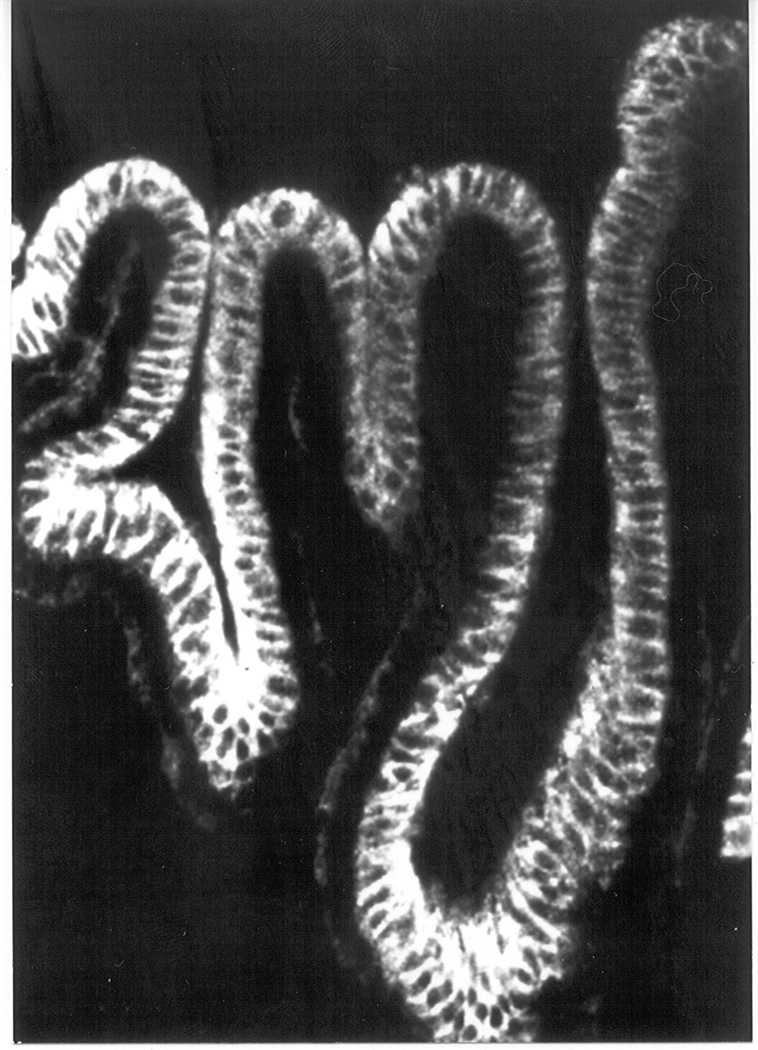
The ciliary processes have a radial orientation, each ridge pointing toward the pupil (Fig 1). In some species, the ridges extend part way across the posterior surface of the iris. Ciliary body anatomy has been described in great detail by Tamm and Lutjen-Drecoll (1996). The entire surface of the ciliary body, the ridges as well as the valleys in between, is covered with a specialized epithelium – ciliary epithelium (Fig 2). What is unique, and not very well understood, is the fact that this covering is a bilayer made of two different epithelial cell types. Other epithelial cell barriers in the body are either monolayers such as lung airway epithelium, or have multiple layers made up of a single cell type, such as corneal epithelium. In the ciliary epithelium bilayer, the two cell layers have different developmental origins. The cell layer farthest from the interior of the eyeball is developmentally related to the retinal pigment epithelium. True to their origin, these cells contain black pigment granules and, not very creatively, this half of the ciliary epithelium bilayer has been termed the pigmented cell layer. The other half of the bilayer, the nonpigmented layer, is developmentally related to the neural retina. The pigmented and nonpigmented ciliary epithelial cells (PE and NPE) have numerous differences other than the presence or absence of pigment. Nonpigmented cells are much larger. Nonpigmented cells also contain many more mitochondria than pigmented cells and this probably signifies a higher degree metabolic activity in the nonpigmented cell layer. One remarkable feature of the nonpigmented ciliary epithelial cells is the high degree infolding on that part of their surface, which faces aqueous humor. This invagination of what is the basolateral membrane of the nonpigmented ciliary epithelium, appears to be another anatomical feature providing the ciliary body with an enormous surface area available for fluid secretion: not only are there the foldings of the tissue into the ridges and valleys of the ciliary processes, but there are also tiny invaginations on the surface of each nonpigmented ciliary epithelial cell.
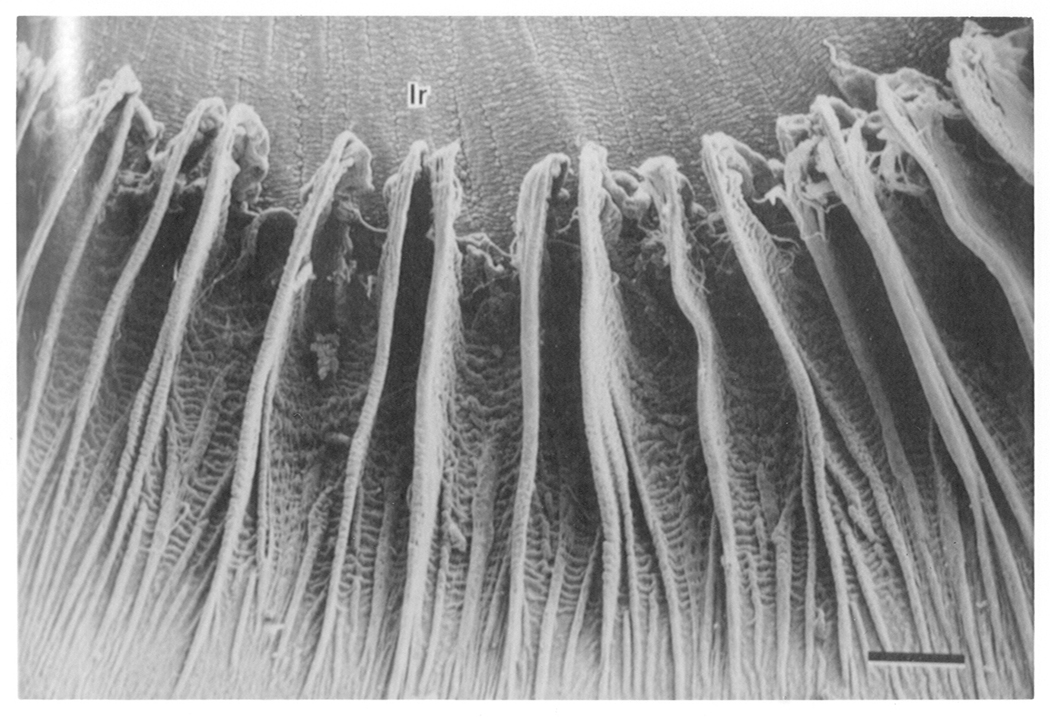
Figure 1. Topography of dog ciliary processes.
Shown in this scanning electromicrograph, the ciliary processes resemble fins that are arranged radially at the root of the iris on its posterior surface. Ir = iris. Bar = 0.5mm. Taken from Morrison et al. Invest Opthalmol Vis Sci. 28, 1325–1340, 1987, J.B. Lippincott Company. Used with permission.
Figure 2.
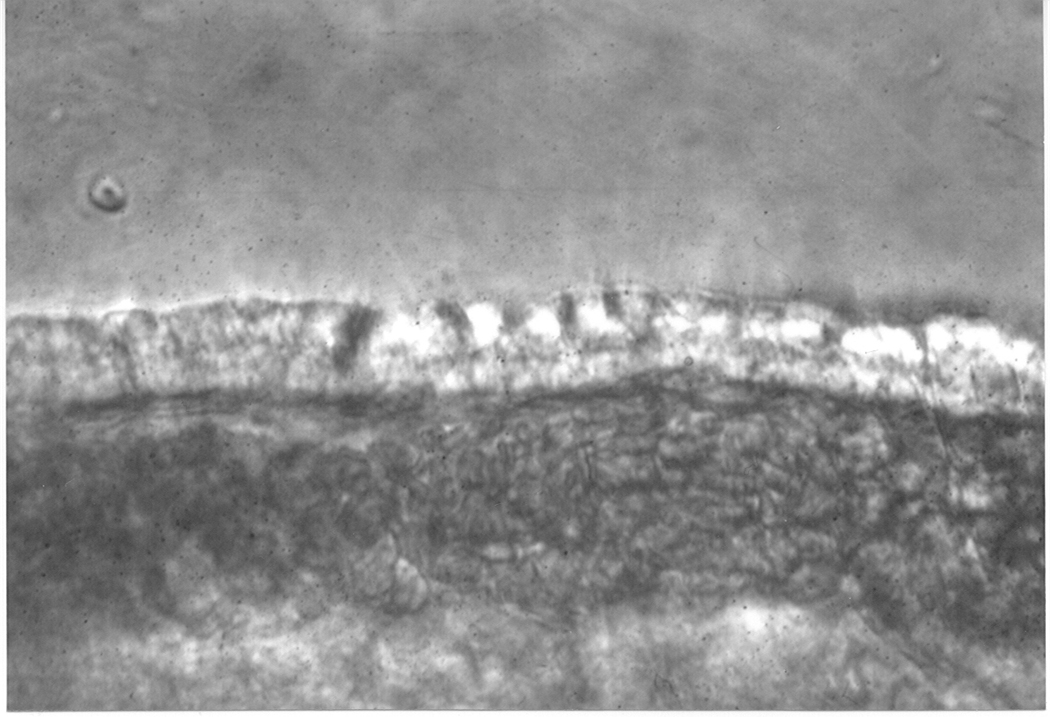
Figure 2a. Light photomicrograph showing the edge of a ciliary process from an albino rabbit. The bilayer of epithelial cells (indicated by an arrow) can be seen covering the blood capillaries within the ciliary process.
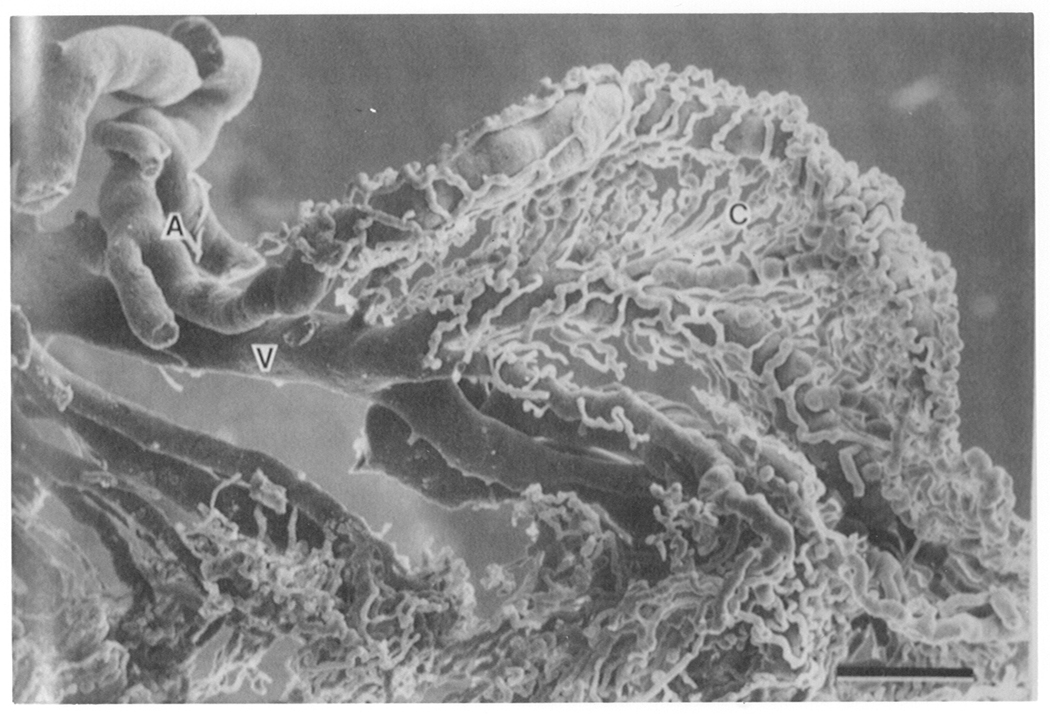
Figure 2b. Scanning electromicrograph of arterioles (A) and veins (V) entering and exiting the goat ciliary process. These blood vessels serve the very elaborate capillary bed (C). Bar = 0.15mm. From Morrison et al. Invest Opthalmol Vis Sci. 28,1325–1340, 1987, J.B. Lippincott Company. Used with permission.
Blood Capillaries in the Ciliary Processes
The interior of each ciliary process is filled with a complicated pipework of blood vessels (Fig 2). From one standpoint, it could be said that the elaboration of the ciliary body into the ridges of the ciliary processes might be a strategy to pack more capillaries into the tissue. Why would this be advantageous? Well, as we shall see later, the ciliary epithelium requires the constituents of blood plasma in order to produce aqueous humor. Also, it is probably fair to assume that in order to fuel their secretory machinery, the ciliary epithelial cells themselves have a high demand for nutrients which must be delivered by the blood. Thinking along these lines, one can easily see that if the rate of blood flow to the ciliary body were to be reduced sufficiently, then the rate of aqueous humor secretion will be slowed. Importantly, the body itself is very well equipped to regulate blood flow. In many parts of the body, factors such as norepinephrine and some prostaglandins constrict blood vessels while other factors such as nitric oxide cause relaxation. At any one moment, blood vessel diameter in a tissue is governed by an equilibrium between endogenous relaxing factors and constricting factors. Almost certainly, the body is able to control what goes on in the ciliary process (i.e. the rate of aqueous humor secretion) by controlling the delivery of blood to the ciliary process. Can drugs be applied to the eye to do the same thing? Probably so. Using an ingenious microcasting technique, investigators have been able to make plastic replicas of the ciliary body vasculature. When blood vessel replicas from the ciliary processes of normal eyes are compared with replicas from eyes treated with catecholamines such as epinephrine, there is clear evidence of vasoconstriction (Funk & Rohen, 1987).
Barrier function of the ciliary epithelium
For the most part, epithelial cells separate different compartments in the body. In the eye, the ciliary epithelium bilayer forms a barrier between a part of the body that is one of the most densely vascularized and a part of the body that has no blood vessels at all. One of the unique features of the eyeball is the absence of blood in the optical pathway. Neither the cornea, anterior chamber, lens or vitreous body contain blood. This makes sense. Blood is very messy stuff and not at all transparent. Hold a test tube filled with blood up to the light and simply nothing shines through. The situation is improved if the blood cells are removed, for example by placing the test tube in a centrifuge and spinning the cells to the bottom to leave just the blood plasma on top. However, the optical transparency through the tube of blood plasma is still poor. Blood plasma contains such a high concentration of large protein molecules that it scatters light. Such an optical disturbance is known as Rayleigh scatter, an effect demonstrated very nicely by shining a flashlight across a smoke-filled room. In these health conscious days when smoke- filled rooms are hard to find, a room with dusty air will do! The more you can see the glow of the light beam as it crosses the air in the room, the greater is the fraction of light being scattered off-track by the suspended particles instead of continuing in a straight path to shine on the opposite wall. A similar kind of light scatter would interfere with our vision if blood plasma macromolecules were permitted to get into the aqueous humor of the eye. What stops blood proteins getting into the eye? The barrier seems to be the nonpigmented ciliary epithelium of the ciliary body.
In the tangled network of blood vessels inside each ciliary process, the sheath of vascular endothelium surrounding each capillary is rather leaky. Although blood cells are generally constrained inside the capillaries, plasma constituents leak out. This has been shown very nicely by electron microscopic studies in which a chemical marker such as horseradish peroxidase (HRP, an enzyme) introduced into the systemic blood supply soon was found to appear in the stroma of the ciliary processes, outside the blood vessels (see Raviola, 1977). Since HRP is a protein with a rather high molecular weight (approximately 44kDa), we are comfortable in assuming it is unable to cross the plasma membrane to get inside a cell. Therefore, one can conclude that in the ciliary process, this large molecule is able to pass fairly easily between the endothelial cells on the capillary walls. Now, what is important is the fact that Raviola observed HRP did not pass into the interior of the eye. It did penetrate between the pigmented ciliary epithelial cells. It also filled up the space between the pigmented cell layer and the nonpigmented cell layer. However, it did not penetrate between the nonpigmented ciliary epithelial cells. This is an important clue – it tells us the site of the diffusion barrier between blood and aqueous humor.
Physiologists coined the term “blood-aqueous barrier” to describe the group of structures that separate the two fluids. The nonpigmented ciliary epithelium layer is one part of this system; the other part of the blood-aqueous barrier is the tight endothelium layer surrounding the blood capillaries in the iris. The tightness of these cell layers effectively isolates the anterior of the eyeball from the blood circulation, leaving easy access only for very small molecules which are highly diffusible. From one standpoint, the system can be said to behave like a molecular sieve. To examine this feature experimentally, a researcher would typically introduce a tracer solute into the bloodstream, generally a molecule “labeled” with a radioactive isotope, then measure its rate of appearance in samples of aqueous humor taken from the eye. Size is a big factor; ions and small solutes such as sucrose penetrate into the eye quite quickly while large molecules and proteins hardly penetrate at all (see Davson, 1990). It is important to note that lipid soluble substances do not conform to this pattern. They penetrate much more rapidly than one might predict from their molecular weight. The reason for this is simple; their lipophilic nature enables them to pass through cellular barriers. The phospholipid composition of the cell membrane provides little in the way of a diffusion obstacle to a lipophilic molecule. The characteristics of the blood-aqueous barrier are particularly important when it comes to the development of pharmacological agents. If one’s goal is to create an oral anti-inflammatory agent to cure intraocular inflammation, one of the big hurdles is finding a compound which gets into the eye in an effective concentration. It is difficult to establish therapeutic levels of many drugs inside the eye simply because the compounds do not readily cross the blood-aqueous barrier.
The absence of blood in the interior of the eye means that a lot of generally useful things are missing. Components of the immune system for example. The result is a situation where the interior of the eye has rather unusual immunological characteristics. However, this can be changed – in response to injury, the blood-aqueous barrier “breaks down” and plasma constituents are allowed to flood into the aqueous humor. Thus, the appearance of protein in aqueous humor is one of the aftermaths of ocular injury and also a telltale sign of ocular inflammation. Such protein entry is detected as “flare”, or light scatter (Rayleigh scatter), when a focused beam of light is shone through the aqueous humor compartment. It is easy to see how temporarily sacrificing visual acuity is well worth the advantage of getting clotting factors inside the eye to seal a penetrating corneal wound and the benefits of permitting the entry of immune factors and leukocytes to deal with invading microorganisms. But how does breakdown of the blood-aqueous barrier occur? The molecular mechanism remains slightly mysterious but the site of breakdown seems to lie within the nonpigmented ciliary epithelium cell layer. It appears that the cell-cell junctions in this layer, the junctions that constitute the diffusion barrier, can be “broken” in response to external stimuli.
Tight junctions between NPE cells
Adjacent cells in the nonpigmented ciliary epithelium layer are joined by protein structures called tight junctions (Fig. 3). In a typical tight junction, each cell expresses an assembly of several different protein molecules including and ZO-1 and occludin. Part of the tight junction protrudes from the plasma membrane and the junctional proteins form a bridge to similar molecules protruding from the neighboring cell. Inside the cell, the tight junction assembly is connected to the actin cytoskeleton. Viewed by scanning electron microscopy, the tight junction is a tangled web of protein fibrils, which forms a band or collar that wraps around one part of the lateral surface of each cell. To one side of this boundary is the cell’s apical surface; to the other side is the basolateral surface. Importantly, the junctional complex prevents the diffusion of large molecules in the extracellular space between the cells. The collar of occludin protein threads acts as a seal that large molecules in the extracellular fluid are unable to cross. Thus it is the tight junctions between cells of the nonpigmented ciliary epithelium which constitute the barrier to the passage of plasma proteins from the stroma of the ciliary process into the aqueous humor. Importantly, cell biologists have found that the architecture of the tight junction can change very quickly (Schneeberger and Lynch, 1992). In some tissues, there is good evidence that certain stimuli cause the occludin threads to untangle sufficiently for diffusion pathways to open up between the cells. Most probably, this is what takes place in the nonpigmented ciliary epithelium cell layer when the blood-aqueous barrier breaks down.
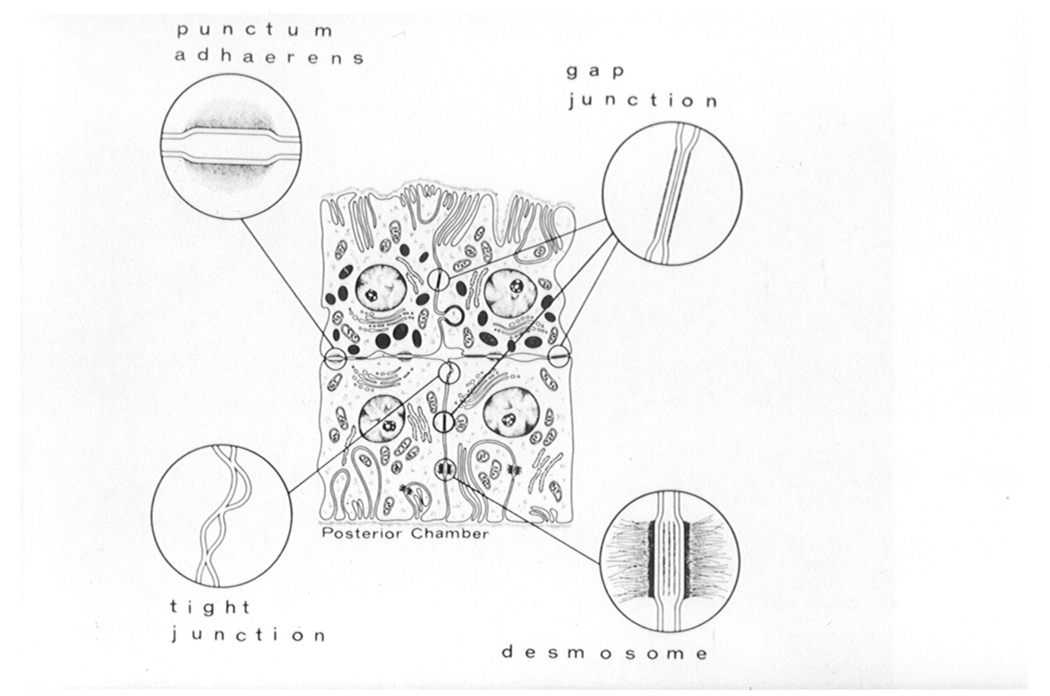
Figure 3. Diagram showing the location of cell-cell junctions in the ciliary epithelium.
The pigmented ciliary epithelium is shown at the top with the nonpigmented ciliary epithelium beneath. Note that tight junctions are located in the nonpigmented ciliary epithelium cell layer, the layer which faces the posterior chamber of the eye. From Raviola, 1977, Exp. Eye Res., 25(Suppl), 25–63, Academic Press, Inc. Used with permission.
Polarized distribution of ion transporters
In addition to restricting solute diffusion in the extracellular space between adjacent nonpigmented ciliary epithelium cells, the tight junction complexes constitute a line of demarcation between two very different parts of the nonpigmented ciliary epithelium cell surface. To the one side of the tight junction is the basolateral surface which, as described earlier, is the highly invaginated part of the cell that faces the interior of the eye. To the other side of the tight junction is the apical surface, which faces the apical surface of cells in the pigmented ciliary epithelium layer. In most types of epithelial cell, the ion transport mechanisms (ion channels, pumps, exchangers, and so forth) are not distributed evenly across the cell surface. Instead, some ion transporters are located on the apical surface but not the basolateral surface and vice versa. The molecules are said to be distributed in a polarized fashion. In simple terms, this polarized distribution of ion transport molecules permits the cell to preferentially import certain solutes across one surface and then export them across the other. In this way, epithelial sheets are able to support a net flux of solute across the cell layer. Often, this is accompanied by a flux of water which, due to osmotic forces, is obliged to follow the solute flux. Is this how the ciliary epithelium produces aqueous humor? Probably so, but the situation is likely to be more complicated. Other factors such as hydrostatic pressure might also contribute to fluid flow via a process of ultrafiltration across the ciliary epithelium along a paracellular (between the cells) pathway. Some aspects of the process remain somewhat mysterious because in the ciliary body, the epithelial sheet comprises two distinct cell layers.
Nonpigmented ciliary epithelium
Focusing first on the nonpigmented ciliary epithelium, immunocytochemical studies have shown very clearly that Na,K-ATPase, the active sodium-potassium transport mechanism, is expressed much more densely on the basolateral surface of the cell than the apical surface (Fig 4). Here, the cells express several different isoforms (molecular variants) of Na,K-ATPase, a feature, which suggests the cells are specialized for active sodium-potassium transport (Coca-Prados & Sanchez-Torres, 1998). As in all other cells, the Na,K-ATPase hydrolyzes ATP to produce ADP and uses the energy from each ATP molecule to go through a series of conformational shifts which permit the molecule to translocate three sodium ions outward and two potassium ions inward. Among other things, this active sodium-potassium transport mechanism makes for a relatively low concentration of sodium (<15 mM) in the cytoplasm of the nonpigmented ciliary epithelium. For comparison, the concentration of sodium in the extracellular fluid (blood plasma on one side, aqueous humor on the other side) is approximately 145 mM. As a result, there is a driving force, which encourages sodium in the extracellular fluid to cross the plasma membrane and enter the nonpigmented epithelium cell. It seems quite possible that sodium entry could occur across the apical cell surface (the side opposite the Na,K-ATPase), possibly via ion exchange mechanisms such as Na-H exchange, possibly via cotransporter mechanisms such as Na/K/2Cl cotransporter and importantly via gap junctions which form a conduit to the PE cell (see below). In this way, sodium effectively goes into the NPE cell on one side and out the other. Chloride also crosses the cell, entering apically via gap junctions, and possibly also by an apically located chloride-bicarbonate exchanger or Na/K/2Cl cotransporter (which use the inward sodium gradient as a driving force) and leaving via chloride channels which have been proposed to be polarized, like Na,K-ATPase, on the basolateral membrane. Transepithelial ion movement might be expected to set up a small electrical voltage across the nonpigmented ciliary epithelium layer and this would tend to energize the movement of additional sodium and chloride ions through the extracellular space between the cells. Because of their size, diffusion of sodium and chloride ions is not likely to be hindered by the tight junctions.
Figure 4. Distribution of Na,K-ATPase in the ciliary epithelium of the chick.
The confocal microscopic image shows the result of an immunolocalization study carried out using an antibody directed against the beta-1 subunit of Na,K-ATPase. As judged by fluorescence intensity, Na,K-ATPase beta-1 polypeptide is abundant in the nonpigmented ciliary epithelium layer, particularly at the basal and lateral surfaces (the NPE layer points upward in this photograph). The Na,K-ATPase beta-1 signal is much more faint in the pigmented cell layer (downward-facing cells in this picture) where it is seen at the basal surface. Taken from an unpublished collaborative study by the author, Amy E. Moseley (University of Cincinnati, Cincinnati, OH), and Steven Bassnett (Washington University, St. Louis, MO).
If a model along the lines described above (but perhaps more complex) is true to life, the net transepithelial shift of ions across the nonpigmented ciliary epithelium could feasibly drive the movement of water. Specialized water channels, termed aquaporins, may facilitate the passage of water through the plasma membrane of the cell (see Verkman, 2003). Water movement may also be made more efficient by the invaginations on the basolateral surface of the nonpigmented ciliary epithelium. Because these clefts are long and narrow, sodium and chloride ions shifted into the extracellular fluid at the closed end of the invaginations could create localized “pockets” of hyperosmotic solution. Water would tend to move out of the nonpigmented ciliary epithelium cytoplasm to progressively dilute the hyperosmotic solution in the cleft, so promoting the bulk movement of near-isosmotic fluid out of the open end of the invagination (for a theoretical explanation of this process, see Reuss, 1977). This begins to give insight into the way ciliary epithelium forms aqueous humor. Recent studies indicate cultured nonpigmented ciliary epithelium monolayers are indeed capable of transporting fluid in an apical to basal direction and that ouabain, an Na,K-ATPase inhibitor, abolishes the process (Patil et al., 2001). However, the nonpigmented cells represent just half the ciliary epithelium bilayer. There is the role of the pigmented cell layer to be considered.
Cooperation between pigmented and nonpigmented cell layers
It seems likely that the two cell types in the ciliary epithelium bilayer work together as something of a functional syncitium. The basis for thinking there could be such a cooperative arrangement was the discovery of gap junctions between adjacent nonpigmented and pigmented ciliary epithelium cells (Raviola and Raviola, 1978). Gap junctions are a type of cell-cell contact that form a conduit from one cell to the other, often allowing the exchange of fairly large molecules. Gap junctions have a very characteristic appearance when tissues are studied by electron microscopy. The molecular structure of a gap junction is fairly well understood. Membrane-spanning proteins called connexins are expressed on the cell surface and connexins from one cell reach out across the extracellular space to link with connexins on the neighboring cell. This protein bridge seems to form a cell-cell pathway that in some respects resembles a giant ion channel (for a detailed review, see Saez et al., 2003). Because charged ions penetrate the connexin channel rather easily, the electrical resistance of the pathway is low and cells joined by gap junctions are electrically coupled. Some researchers have taken advantage of this electrical coupling to study gap junction characteristics by measuring electrical resistance; the resistance between non-coupled cells is large whereas it is remarkably low when the cells are coupled via gap junctions. Electrophysiological studies on the ciliary epithelium have confirmed gap junction-mediated coupling of the nonpigmented and pigmented ciliary epithelium. Importantly, electrophysiological studies have also led to the demonstration that, as in other tissues, the gap junctions between the nonpigmented and pigmented ciliary epithelium can switch from an “open” to a “closed” conformation (Shi et al., 1996). Adjacent cells might be able to choose whether or not to open their gap junctions and cooperate with their neighbors. Another useful way for researchers to study gap junctions is to use microinjection techniques to deposit fluorescent dye into one cell then record the movement of the dye to adjacent cells as it passes through gap junctions (Oh et al., 1994). The rate of diffusion can be quite rapid.
Gap junctions seem to constitute a quick route for solutes to pass between cells. By means of gap junctions, it is possible to see how the nonpigmented and pigmented ciliary epithelium cells may share cytoplasmic signalling molecules to coordinate cell function. Activation of receptors on the surface of the pigmented ciliary epithelium could potentially cause an increase of cytoplasmic cAMP or cytoplasmic calcium which diffuses to the nonpigmented cells to modulate the activity of ion transport mechanisms on the opposite side of the ciliary epithelium bilayer. However, bearing in mind that the ciliary epithelium bilayer is essentially a secretory epithelium, it is possible that gap junctions between the nonpigmented and pigmented ciliary epithelium serve a more fundamental purpose. Sodium and chloride ions are probably able to enter the ciliary epithelium bilayer across the basolateral surface of the pigmented epithelium, then diffuse via apically-located gap junctions to the nonpigmented epithelium in order to exit through the Na,K-ATPase, ion channels and cotransporters on the nonpigmented cell’s basolateral surface.
Bilayer model of ion transport
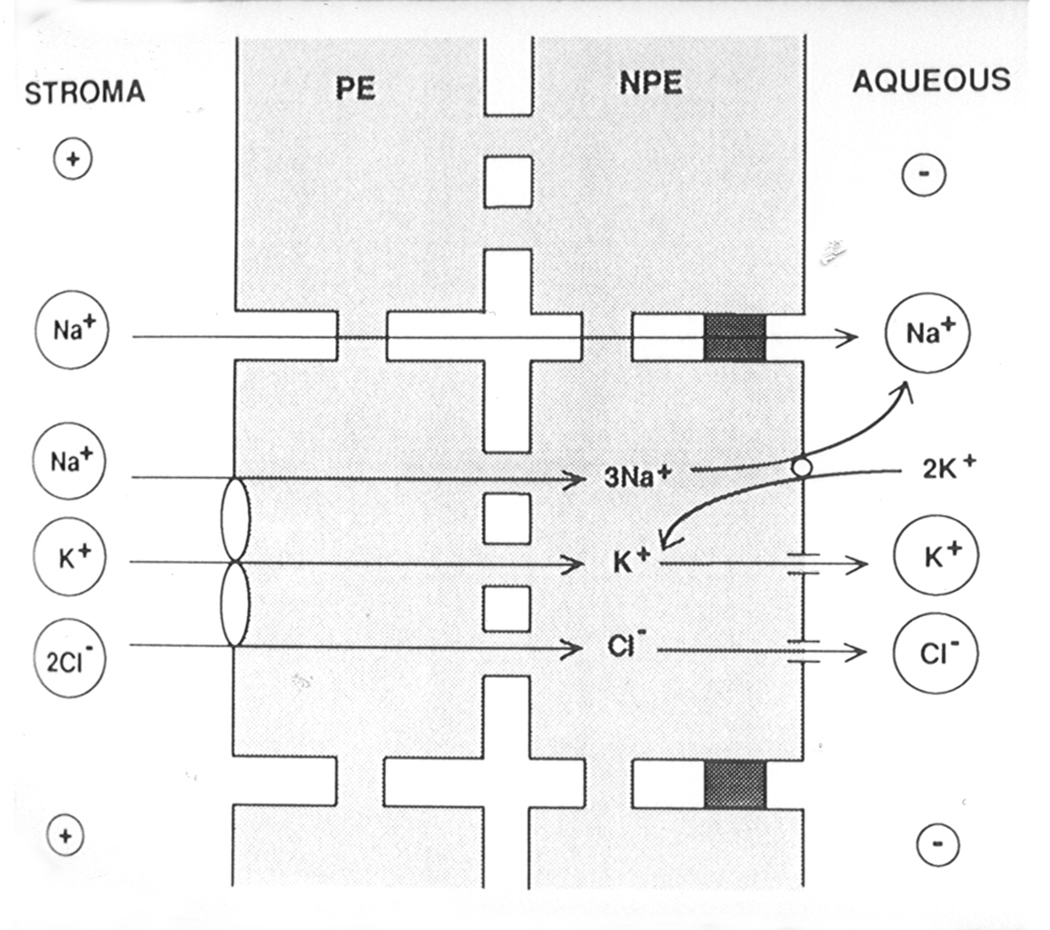
Today, most researchers have adopted the idea that ion (and water) transport across the ciliary epithelium adheres to some sort of syncitial, or cooperative, scheme which involves coupling of the nonpigmented and pigmented cell layers (Jacob and Civan, 1996). This type of model generally depicts an arrangement of some ion transporters at the basolateral surface of the nonpigmented cells, other ion transporters at the basolateral surface of the pigmented cells (Fig 5). In some respects, the pigmented ciliary epithelium is acting rather like the “apical” side of the nonpigmented cell.
Figure 5. A theoretical model depicting the principal ion transport mechanisms thought to underlie the secretion of aqueous humor by the ciliary epithelium.
Nonpigmented cells (NPE) and pigmented cells (PE) communicate via gap junctions. Densely shaded areas between NPE cells represent tight junctions. The model shows sodium, potassium and chloride entering the PE via a Na/K/2Cl cotransporter and passing via gap junctions to the NPE. There, sodium is exported via Na,K-ATPase (the sodium pump). In the NPE, potassium imported by Na,K-ATPase is recycled out of the cell via potassium channels. Chloride exits the NPE via chloride channels. Taken from Coca-Prados et al. 1995, Am. J. Physiol. 268, C572–C579, The American Physiological Society. Used with permission.
In most schemes, entry of sodium and chloride ions is proposed to occur via Na-H exchange, chloride-bicarbonate exchange and Na|K|2Cl cotransport at the basolateral surface of the pigmented cell layer. Na,K-ATPase and chloride channels on the basolateral side of the nonpigmented cell shift the sodium and chloride across the opposite side of the bilayer. Potassium channels on the basolateral surface of the nonpigmented cell permit recirculation of potassium ions brought into the cell by the Na,K-ATPase. Recent analysis of ion concentrations in the two cells of the bilayer using an electron microscopic x-ray microanalysis technique suggests chloride and potassium concentrations within the cytoplasm of the nonpigmented ciliary epithelium might also perhaps be sufficient to drive outward ion flow via a Na|K|2Cl exchanger (McLaughlin et al., 2001).
What biological advantage the bilayer arrangement has over a system based on transport across a simple single layer of nonpigmented ciliary epithelium (with apical solute entry) is open to debate. There are puzzling questions still to be answered regarding the role of the two ciliary epithelium cell layers. We do know that the ciliary body shifts some solutes (prostaglandins for example) outward from the eye. Possibly the pigmented cell layer enables solute transport to occur in this reverse direction. It is also possible that an important purpose of the pigmented ciliary epithelium is not for solute transport but to absorb stray light – the interior surface of a good optical device is almost always coated with a matte black finish. It needs to be stressed, that theoretical models of ion transport by the ciliary epithelium bilayer are just that – theoretical models. They are “best guesses” based on the data in hand but there is no certainty that they are correct. In actuality, it turns out to be very difficult to prove how the ciliary epithelium works because it is a tricky tissue on which to do definitive experiments.
Divided chamber studies
If physiologists had their way, the ciliary epithelium bilayer would be a flat sheet that could be placed with ease and precision in an Ussing chamber. This device, named after the Danish physiologist, H. H. Ussing, is a divided chamber in which an epithelium is arranged between two separate fluid baths. In a typical Ussing chamber experiment, electrodes in each bath are used to measure transepithelial resistance, voltage and current. Under short circuit conditions, the current is a measure of the rate of net ion transport across the epithelium. Using the same apparatus, actual ion fluxes can be measured by depositing radioisotopes of sodium, chloride or potassium in the fluid bath on one side of the epithelium and recording the rate of appearance of the isotope in the fluid bath on the opposite side of the cell sheet. To make experiments more informative still, transport inhibitors (e.g. ouabain, amiloride or bumetanide) or ion channel blockers can be added to one side or the other in order to track down the location of specific transport mechanisms to a particular face of the epithelium.
Unfortunately, the ciliary epithelium is not an ideal flat sheet. Until recently, the most common experimental approach has been to mount the entire iris and ciliary body in a divided chamber, using plastic disc to occlude the iris and pupil. Because the ciliary body is elaborated into ridges (the ciliary processes) it is hard to know the precise value for the surface area of ciliary epithelium interposed between the two fluid baths. Without a measurement of actual surface area, it is difficult to calculate with certainty the specific electrical resistance of the epithelium. Too, the tissues which remain on the stromal side of the preparation could influence the way the epithelium bilayer works in vitro. In spite of these hindrances to interpretation, the iris-ciliary body Ussing chamber preparation has been used with considerable success. The tissue does indeed generate a short circuit current that is sensitive to transport inhibitors like ouabain (Krupin et al., 1984). Under certain conditions, a net flux of 14C-labeled ascorbic acid can be demonstrated in blood-to-aqueous direction (Chu and Candia, 1987). This correlates nicely with the long known fact that the ascorbic acid concentration in aqueous humor is some twenty times higher than the concentration in blood plasma. The ciliary epithelium actively transports ascorbic acid into the aqueous humor and the mechanism still works well in the divided chamber preparation. Other studies with the iris-ciliary body Ussing chamber preparation illustrate the presence of bicarbonate exchange mechanisms in the ciliary epithelium and this fits with the well-documented slowing in the rate of aqueous humor production elicited by carbonic anhydrase inhibitors (To et al., 2001). Carbonic anhydrase inhibitors are sulfonamide compounds which reduce the supply of cytoplasmic bicarbonate by inhibiting the reversible, enzyme-catalyzed, reaction which generates bicarbonate from carbon dioxide that diffuses into the cell.
In an effort to improve the Ussing chamber preparation, some investigators have successfully developed a way to use enzymic digestion to gently free the ciliary epithelium from the ciliary body (for details on the technique see Chen and Sears, 1997). The cell bilayer, which appears to remain viable for some time, can be mounted in a divided chamber and used for studies of transepithelial ion transport. Promising results have been obtained. However, in spite of the advances that have been made, there are still some important questions yet to be answered. One rather fundamental issue is the relatively small magnitude of the short circuit current measured across either the iris ciliary body or the ciliary epithelium bilayer Ussing chamber preparation. The short circuit current tallies with only a modest net transepithelial ion flow and this would be a bit too small to provide the osmotic drag needed to account for the rate of fluid secretion which we know takes place in vivo. There are many possible explanations for this. For example, it would not be surprising if some cellular functions simply do not survive the dissection of the tissue. Some cellular functions may not work when the blood supply ceases (as it does when the iris-ciliary body is isolated and placed in the divided chamber). It is also fair to say that in vivo, the production of aqueous humor may not be linked entirely to active solute transport. Another mechanism might contribute; ultrafiltration.
Hydrostatic pressure and oncotic pressure
Blood capillaries are pressurized. It has been estimated that in vivo, there is a capillary hydrostatic pressure gradient across the ciliary epithelium bilayer; the pressure is generally estimated at ≥15mm Hg higher on the ciliary process stromal side. Undoubtedly, this pressure would tend to force fluid between the nonpigmented ciliary epithelium cells. As described earlier, tight junctions between adjacent cells restrict the passage of large molecules so what emerges on the other side of the epithelium should be a solution lacking macromolecules but with an ionic composition rather similar to that of plasma. Put simply, the epithelial barrier could act rather like a filter through which fluid is pushed by the force of a pressure gradient. This process of fluid formation is termed ultrafiltration. In actuality, it has been something of a challenge to sort out the extent to which ultrafiltration might contribute to the overall process of aqueous humor production. As yet, there is no clear answer. A third and still different factor to be considered is the oncotic pressure gradient across the ciliary epithelium. There is a tendency for water to move across a semipermeable barrier (in this case, the ciliary epithelium) from a fluid compartment which has a low concentration of dissolved macromolecules (such as the aqueous humor) to a fluid compartment which has a high concentration of dissolved macromolecules (blood plasma). It is important to note that the oncotic pressure gradient (estimated at ~ 14mm Hg) and hydrostatic pressure gradient are in opposite directions. The oncotic pressure gradient will tend to cause absorption of water from aqueous humor back into the ciliary process stroma, more or less balancing out the inwardly – directed flow of fluid caused by the hydrostatic pressure gradient.
The argument for the contribution of active ion secretion as a driving force for aqueous humor production originates from compositional studies of aqueous humor. Chemical analysis of newly formed aqueous humor revealed that the concentration of some solutes such as sodium and bicarbonate is slightly but significantly different from that expected in an ultrafiltrate of blood plasma (Davson, 1990). In addition, physiologists confirmed the need for active sodium transport by demonstrating that it is possible to slow the rate of aqueous humor production in anesthetized animals by introducing ouabain (a specific inhibitor of Na,K-ATPase) either into the blood or into the interior cavity of the eyeball (see Davson, 1990).
Volume regulation and water movement
In the human eye, aqueous humor is produced at a rate of 1–2 µl/min. At first glance, this rate seems rather slow. However, it has been calculated that with this rate of flow, the throughput of fluid for each nonpigmented ciliary epithelium cell is equivalent to changing the cell’s own volume every 3 minutes. It is easy to see how small mismatches of water entry and exit could cause swelling or shrinkage. To survive, the ciliary epithelial cells must be good at volume regulation.
The mechanisms which cells use to regulate their volume have been studied in a number of tissues. First, we should understand that eukaryotic cells are at the mercy of osmotic forces since they have little in the way of solid architecture to prevent shape changes. Cells shrink if subjected to a hyperosmotic external solution and swell if subjected to hypoosmotic external solution. The rate at which this occurs is a function of the permeability of the cell membrane to water molecules. Some cells are more water permeable than others and recently researchers have come up with an explanation of why this might be so. Some cells express water channels. Termed aquaporins, water channels are plasma membrane-spanning proteins that seem to provide a conduit for water molecules to enter or exit the cell. Other membrane proteins such as glucose transporters also are capable of conducting the passage of water across the plasma membrane but with a much slower throughput (for review, see Fischbarg and Vera, 1995). As might be expected from its role in shifting water, the nonpigmented ciliary epithelium cell layer expresses aquaporin molecules (see Verkman, 2003). The cells are probably highly permeable to water. Consistent with this notion, microscopic studies with intact, living, ciliary processes have shown the NPE respond with rapid volume changes when external osmolarity is changed (Farahbahksh and Fain, 1987). Now cells tend to dislike volume changes – in many tissues swelling or shrinkage triggers a volume recovery response during which the cells attempt to return to their original size. There is still debate as to how a cell senses its own volume; perhaps it is able to detect a change in the concentration of cytoplasmic macromolecules or possibly it is able to sense changes in the cytoskeleton caused by swelling or shrinkage. In any case, cell shrinkage often causes a compensatory episode of swelling driven by the activation of solute entry mechanisms such as the import of sodium chloride via the coupled action of a Na-H exchanger and chloride-bicarbonate exchanger; this response is called a Regulatory Volume Increase (RVI). Cell swelling causes a different response, generally a compensatory episode of shrinkage driven by the loss of solute via ion channels which are allowed to open; this response is termed a Regulatory Volume Decrease (RVD).
Several researchers have directed their attention to the study of volume regulatory responses to seek answers to questions regarding the function of ion channel and ion exchange mechanisms in nonpigmented and pigmented ciliary epithelial cells. When responses in the two different cell types where compared, something rather interesting became apparent. Pigmented ciliary epithelium cells appear to have a good RVI response but weak RVD response - they seem to be adapted for solute and water uptake (Edelman et al., 1994). Just the opposite, nonpigmented ciliary epithelium cells seem better at RVD than RVI - they appear to be adapted for solute and water loss. These characteristics match up nicely with the bilayer cooperativity models of ion transport across the ciliary epithelium which, in simple terms, are based on solute and water entry through the pigmented cell layer, exit via the nonpigmented cell layer.
Controlling the rate of aqueous humor formation
The human ciliary body continually produces 1–2 µl of fluid every minute, day in, day out, as long as we live. Is the rate of aqueous humor production always the same? The answer is no. This explains why textbooks generally quote the rate as a range instead of a single value (e.g. 1.5 µl/min). It turns out that human aqueous humor formation rate follows a circadian rhythm, higher in the daytime hours and lower during the nighttime. Certain species like the rabbit have the opposite pattern - the rate of production is highest at nighttime. The existence of a circadian rhythm tells us there are endogenous pathways, which control the rate of aqueous humor production. How does this occur? Well, the ciliary body receives both sympathetic and parasympathetic innervation and there is evidence that aqueous humor production rate is subject to neural control. It has been shown, for example, that the circadian pattern of aqueous humor flow is changed considerably in experimental animals that have been subjected to ganglionectomy (removal of the cervical ganglion) (Gregory et al., 1985). Some mechanistic questions regarding circadian control of aqueous humor flow are still unanswered. While we know that the rate of aqueous humor production can be 40% lower at night in humans, it has proved tricky to define what aspect of ciliary body function is actually responsible slowing fluid production during the dark hours. Aqueous humor formation could be slowed as a consequence of reducing blood flow in the ciliary process capillaries. But equally, the rate of formation could be altered as a result of changes in the function in the ion transport machinery (ion channels, co-transporters or the Na,K-ATPase) in the ciliary epithelium bilayer or even by opening or closure of gap junctions between the pigmented and nonpigmented cells. Some responses are difficult to interpret because it turns out that peptides such as endothelin-1 and neurotransmitters such as norepinephrine probably elicit changes in both ciliary epithelium function as well as in blood flow to the ciliary processes (Pang and Yorio, 1997). Moreover, the ciliary body itself appears capable of synthesizing then releasing regulatory peptides and neuropeptides that may act in an autocrine manner to alter aqueous humor production (Coca-Prados et al., 1999).
Working on the rationale that most neurotransmitters, neuropeptides and hormones work via cell surface receptors, researchers have sought to identify the types of receptors expressed in the ciliary body. There is good evidence that adrenergic alpha and beta receptors, dopamine receptors, prostaglandin receptors as well as peptide receptors are localized on the ciliary epithelium. Since most cell surface receptors bring about changes inside the cell by causing the generation of cytoplasmic second messengers, some research groups have sought to determine how the appearance of second messengers such as cAMP tally with changes in aqueous humor formation. It turns out that cAMP levels in the ciliary body and aqueous humor change with a circadian rhythm (Yoshitomi et al., 1991). Moreover, the rate of aqueous humor production can be lowered dramatically by cholera toxin and forskolin, two compounds which cause elevation of cAMP in the ciliary body (see Sears, 1984). On the surface, this suggests that the machinery of aqueous humor formation is controlled by receptors which elevate cAMP in the ciliary epithelium. This could be so, but the system is tantalizingly complex. While some agents that elevate ciliary body cAMP slow aqueous humor production, one of the most widely used clinical strategies to reduce aqueous humor flow in humans is to use beta-adrenergic antagonists (Zimmerman, 1997) and these are drugs which tend to decrease cAMP in the ciliary body. It turns out that the rate of aqueous humor production can be reduced both by beta-adrenergic agonists and alpha-adrenergic agonists and this goes against the normal situation where activation of alpha and beta receptors triggers opposing physiological responses. There are other pharmacological agents that slow aqueous inflow (for review, see Zimmerman, 1996). A well known class of compounds used clinically to reduce aqueous humor production is the carbonic anhydrase inhibitor family of drugs. These compounds are thought to reduce the availability of cytoplasmic bicarbonate ions in the ciliary epithelium bilayer with the result that bicarbonate-dependent ion transport mechanisms are inhibited (To et al., 2001). Still in the experimental stage, researchers are considering whether aqueous humor formation can be suppressed using drugs that inhibit specific ion transport mechanisms such as the Na-H exchanger (Avila et al., 2002). Some clinically proven drugs, like pilocarpine and latanoprost, act on the ciliary body but target the ciliary muscle, the contraction of which can cause mechanical changes that increase the ability of aqueous humor to exit the eye. These compounds lower IOP even though they do not slow the rate of aqueous humor formation.
Why is there a need for pharmacological tools to slow aqueous humor production? Such agents can be used in the therapy of glaucoma. Glaucoma is a blinding disease which causes vision loss as the result of premature death of ganglion cells in the retina. Persons with glaucoma often have high intraocular pressure. There is plenty of evidence to suggest that in many (but perhaps not all) instances, retinal ganglion cell death could be the result of tissue damage caused at the optic nerve head by abnormally high pressure within the eye. Lowering intraocular pressure by slowing the production of aqueous humor is often an effective means of retarding progression of the disease. The quest for new and improved glaucoma therapies creates a pressing need for more research to explain how the various different cells in the ciliary body do their job and to determine how we might target the development of new drugs that trick the ciliary body into changing the rate of aqueous humor secretion.
Acknowledgement
USPS Research Grant #EY06915, the Kentucky Lions Eye Foundation and an unrestricted grant from Research to Prevent Blindness, Inc.
REFERENCES
- Avila MY, Seidler RW, Stone RA, Civan MM. Inhibitors of NHE-1 Na+/H+ exchange reduce mouse intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2002;43:1897–1902. [PubMed] [Google Scholar]
- Chen S, Sears M. A low conductance chloride channel in the basolateral membranes of the non-pigmented ciliary epithelium of the rabbit eye. Curr. Eye Res. 1997;16:710–718. doi: 10.1076/ceyr.16.7.710.5064. [DOI] [PubMed] [Google Scholar]
- Chu TC, Candia OA. Active transport of ascorbate across the isolated rabbit ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 1988;29:594–599. [PubMed] [Google Scholar]
- Coca-Prados M, Anguita J, Chalfant MS, Civan MM. PKC-sensitive Cl− channels associated with ciliary epithelial homologue of pICln−. Am. J. Physiol. 1995;268:C572–C579. doi: 10.1152/ajpcell.1995.268.3.C572. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Escribano J, Ortego J. Differential gene expression in the human ciliary epithelium. Progress. Ret. Eye Res. 1999;18:403–4729. doi: 10.1016/s1350-9462(98)00026-3. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Sanchez-Torres J. Molecular approaches to the study of the Na+,K+-ATPase and chloride channels in the ocular ciliary epithelium. In: Civan MM, editor. The Eye’s Aqueous Humor: from Secretion to Glaucoma. San Diego: Academic Press; 1998. pp. 25–53. [Google Scholar]
- Davson H. Aqueous humor and the intraocular pressure. In: Davson H, editor. Physiology of the Eye. New York: Academic Press; 1990. pp. 9–81. [Google Scholar]
- Edelman JL, Sachs G, Adorante JD. Ion transport asymmetry and functional coupling in bovine pigmented and nonpigmented ciliary epithelium cells. Am. J. Physiol. 1994;266:C1210–C1221. doi: 10.1152/ajpcell.1994.266.5.C1210. [DOI] [PubMed] [Google Scholar]
- Farahbakhsh NA, Fain GL. Volume regulation of non-pigmented cells from ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 1987;28:934–944. [PubMed] [Google Scholar]
- Fischbarg J, Vera JC. Multifunctional transporter models: lessons from the transport of water, sugars, and ring compounds by GLUTs. Am. J. Physiol. 1995;268:C1077–C1089. doi: 10.1152/ajpcell.1995.268.5.C1077. [DOI] [PubMed] [Google Scholar]
- Funk R, Rohen JW. SEM studies on the functional morphology of the rabbit ciliary process vasculature. Exp. Eye Res. 1987;45:579–595. doi: 10.1016/s0014-4835(87)80068-4. [DOI] [PubMed] [Google Scholar]
- Gregory DS, Aviado DG, Sears ML. Cervical ganglionectomy alters the circadian rhythm of intraocular pressure in New Zealand white rabbits. Curr. Eye Res. 1985;4:1273–1279. doi: 10.3109/02713688509017687. [DOI] [PubMed] [Google Scholar]
- Jacob TJC, Civan MM. Role of ion channels in aqueous humor formation. Am. J. Physiol. 1996;271:C703–C720. doi: 10.1152/ajpcell.1996.271.3.C703. [DOI] [PubMed] [Google Scholar]
- Kaufman PL. Accommodation and Presbyopia: Neuromuscular and Biophysical Aspects. In: Hart WM Jr, editor. Adler’s Physiology of the Eye. St. Louis: Mosby; 1992. pp. 391–411. [Google Scholar]
- Krupin T, Reinach PS, Candia OA, Podos SM. Transepithelial electrical measurements on the isolated rabbit iris-ciliary body. Exp. Eye Res. 1984;38:115–123. doi: 10.1016/0014-4835(84)90096-4. [DOI] [PubMed] [Google Scholar]
- McMaughlin CW, Zellhuber-McMillan S, Peart D, Purves RD, Macknight AD, Civan MM. Regional differences in ciliary epithelial cell transport properties. J. Memb. Biol. 2001;182:213–222. doi: 10.1007/s00232-001-0045-x. [DOI] [PubMed] [Google Scholar]
- Morrison JC, DeFrank MP, Van Buskirk EM. Comparative microvascular anatomy of mammalian ciliary processes. Invest. Ophthalmol. Vis. Sci. 1987;28:1325–1340. [PubMed] [Google Scholar]
- Oh J, Krupini T, Tang LQ, Sveen J, Lahlum RA. Dye coupling of rabbit ciliary epithelial cells in vitro. Invest. Ophthalmol. Vis. Sci. 1994;17:2509–2514. [PubMed] [Google Scholar]
- Pang IH, Yorio T. Ocular actions of endothelins. Proc. Soc. Exp. Biol. Med. 1997;215:21–34. doi: 10.3181/00379727-215-44110. [DOI] [PubMed] [Google Scholar]
- Patil RV, Han Z, Yiming M, Yang J, Iserovich P, Wax MB, Fischbarg J. Fluid transport by human nonpigmented ciliary epithelial layers in culture: a homeostatic role for aquaporin 1. Am. J. Physiol. (Cell) 2001;281:C1139–C1145. doi: 10.1152/ajpcell.2001.281.4.C1139. [DOI] [PubMed] [Google Scholar]
- Raviola G. The structural basis of the blood-ocular barriers. Exp. Eye Res. 1977;25 Supp:27–63. doi: 10.1016/s0014-4835(77)80009-2. (Review) [DOI] [PubMed] [Google Scholar]
- Raviola G, Raviola E. Intercellular junctions in the ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 1978;17:958–981. [PubMed] [Google Scholar]
- Reuss L. Epithelial Transport. In: Hoffman JF, editor. Handbook of Physiology. Oxford, U.K: Oxford University Press; 1997. pp. 309–388. [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. Structure, function and regulation of cellular tight junctions. Am J Physiol. 1992;252:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Sears ML. Autonomic Nervous System: adrenergic Agonists. In: Sears ML, editor. Handbook of Experimental Pharmacology, Vol. 69, Pharmacology of the Eye. Berlin: Springer-Verlag; 1984. pp. 193–248. [Google Scholar]
- Shi XP, Zamudo AC, Candia OA, Wolosin JM. Adrenocholinergic modulation of junctional communications between the pigmented and nonpigmented layers of the ciliary body epithelium. Invest. Ophthalmol. Vis. Sci. 1996;37:1037–1046. [PubMed] [Google Scholar]
- Tamm ER, Lutjen-Drecoll E. Ciliary body. Microsc. Res. Tech. 1996;33:390–439. doi: 10.1002/(SICI)1097-0029(19960401)33:5<390::AID-JEMT2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- To CH, Do CW, Zamudio AC, Candia OA. Model of ionic transport for bovine ciliary epithelium: effects of acetazolamide and HCO3. Am. J. Physiol. (Cell) 2001;280:C1521–C1530. doi: 10.1152/ajpcell.2001.280.6.C1521. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Role of aquaporin water channels in eye function. Exp. Eye. Res. 2003;76:137–143. doi: 10.1016/s0014-4835(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Yoshitomi T, Horio B, Gregory DS. Changes in aqueous norepinephrine and cyclic adenosine monophosphate during the circadian cycle in rabbits. Invest. Ophthalmol. Vis. Sci. 1991;32:1609–1613. [PubMed] [Google Scholar]
- Zimmerman TJ. In: Textbook of ocular pharmacology. Zimmerman TJ, editor. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]