Abstract
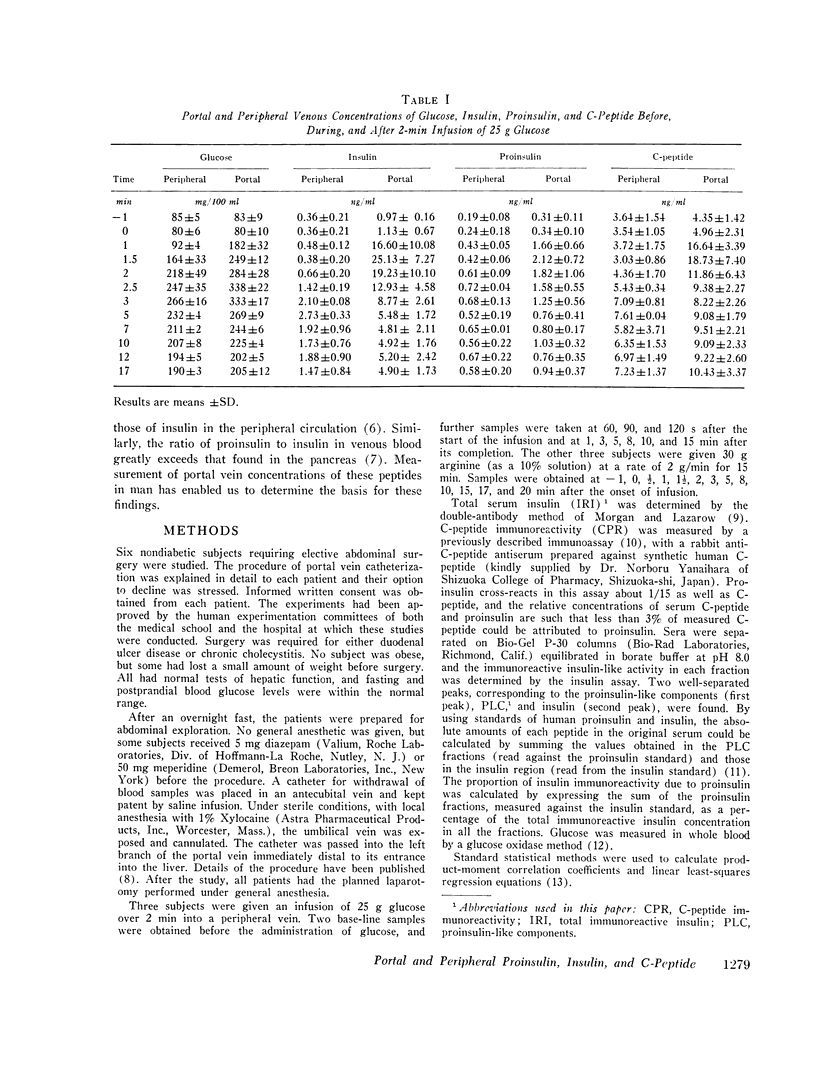
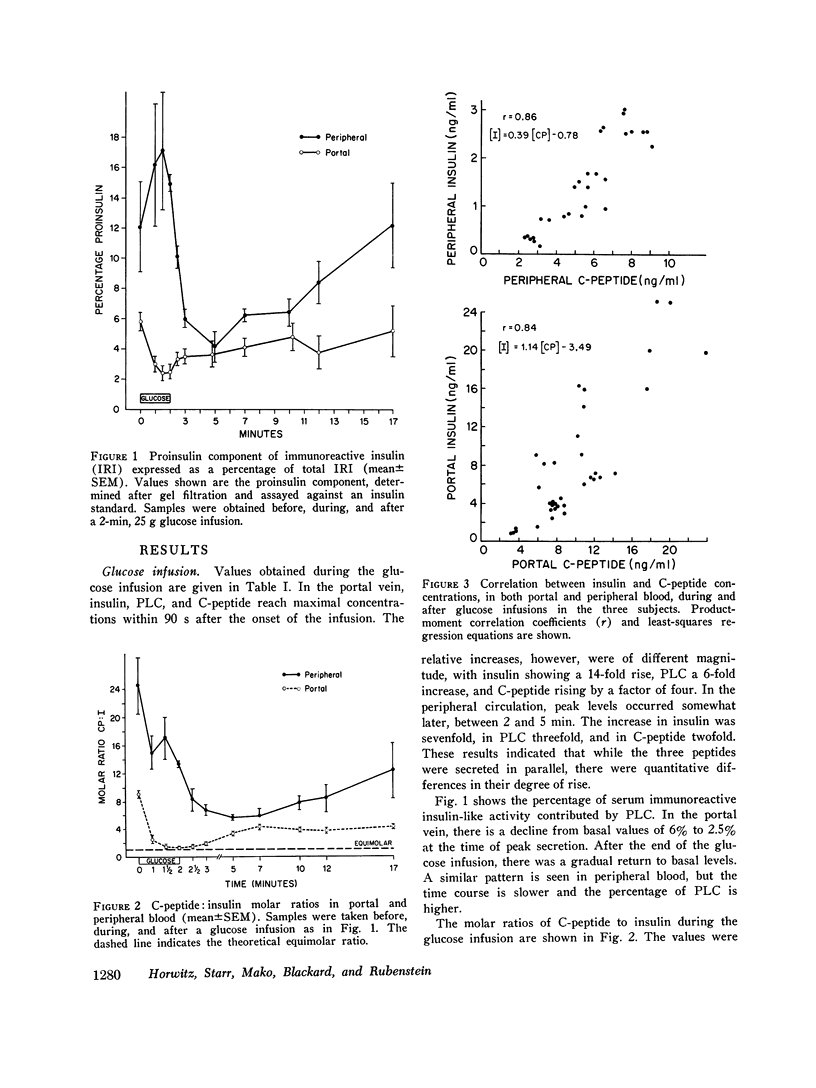
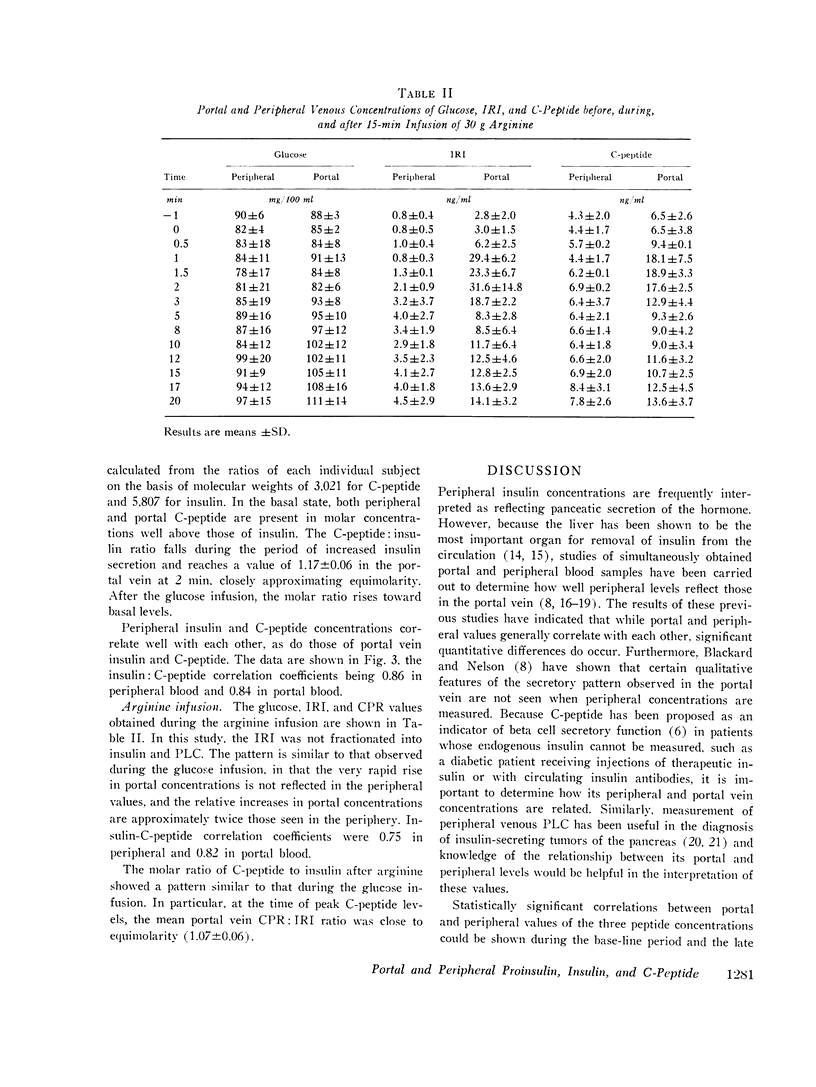
Concentrations of insulin, proinsulin, and C-peptide were measured in portal and peripheral venous blood in six nondiabetic, nonobese subjects. Portal vein samples were obtained by umbilical vein catheterization. Three subjects were studied with intravenous infusion of 25 g glucose, and three with 30 g arginine. Insulin and proinsulin were determined in the insulin immunoassay after separation by gel filtration, and C-peptide was measured by direct immunoassay. With both glucose and arginine stimulation, portal vein levels of all three peptides peaked at 90-120 s after the onset of the stimulus. Relative increases in insulin concentration were greater than those of proinsulin or C-peptide. In peripheral venous blood, maximal levels of the three peptides were observed later (2-5 min), and the increase in insulin relative toproinsulin and C-peptide was not as great. At the time of peak secretion, portal vein insulin and C-peptide approached equimolar concentrations, and proinsulin, as measured against an insulin standard, comprised approximately 2.5% of the total immunoreactive insulin. After stimulation by glucose or arginine, portal insulin, proinsulin and C-peptide levels were not correlated with the concentrations measured in simultaneously drawn peripheral samples. At all sampling times, however, significant correlation was found between insulin and C-peptide in both peripheral and portal blood. The results indicate that under the conditions studied, insulin and C-peptide are secreted in equimolar concentrations in man, and that proinsulin is secreted in the same proportion to insulin as found in the pancreas. Consideration of the relative secretory and metabolic rates of the three beta cell peptides explains their peripheral concentrations. The data further support the use of plasma C-peptide as an indicator of beta cell secretory function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger W., Göschke H., Moppert J., Künzli H. Insulin concentrations in portal venous and peripheral venous blood in man following administration of glucose, galactose, xylitol and tobutamide. Horm Metab Res. 1973 Jan;5(1):4–8. doi: 10.1055/s-0028-1093991. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes. 1970 May;19(5):302–306. doi: 10.2337/diab.19.5.302. [DOI] [PubMed] [Google Scholar]
- Block M. B., Mako M. E., Steiner D. F., Rubenstein A. H. Circulating C-peptide immunoreactivity. Studies in normals and diabetic patients. Diabetes. 1972 Oct;21(10):1013–1026. doi: 10.2337/diab.21.10.1013. [DOI] [PubMed] [Google Scholar]
- Burr I. M., Balant L., Stauffacher W., Renold A. E., Grodsky G. Dynamic aspects of proinsulin release from perifused rat pancreas. Lancet. 1969 Oct 25;2(7626):882–883. doi: 10.1016/s0140-6736(69)92333-2. [DOI] [PubMed] [Google Scholar]
- Chance R. E., Ellis R. M. Proinsulin. Single-chain precursor of insulin. Arch Intern Med. 1969 Mar;123(3):229–236. doi: 10.1001/archinte.123.3.229. [DOI] [PubMed] [Google Scholar]
- Erwald R., Hed R., Nygren A., Röjdmark S., Sundblad L., Wiechel K. L. Immunoreactive insulin in the portal and the peripheral venous blood after intravenous tolbutamide administration. Diabetes. 1971 Oct;20(10):686–690. doi: 10.2337/diab.20.10.686. [DOI] [PubMed] [Google Scholar]
- Erwald R., Hed R., Nygren A., Röjdmark S., Sundblad L., Wiechel K. L. Insulin concentration in portal and peripheral venous blood after oral glucose in human pancreatitis. Acta Med Scand. 1973 Jul-Aug;1-2(1):103–109. doi: 10.1111/j.0954-6820.1973.tb19413.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith S. J., Yalow R. S., Berson S. A. Significance of human plasma insulin Sephadex fractions. Diabetes. 1969 Dec;18(12):834–839. doi: 10.2337/diab.18.12.834. [DOI] [PubMed] [Google Scholar]
- Gorden P., Sherman B., Roth J. Proinsulin-like component of circulating insulin in the basal state and in patients and hamsters with islet cell tumors. J Clin Invest. 1971 Oct;50(10):2113–2122. doi: 10.1172/JCI106705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman R. A., Fink G., Shapiro J. R., Selawry H., Recant L. Proinsulin and insulin release with a human insulinoma and adjacent nonadenomatous pancreas. J Clin Endocrinol Metab. 1973 May;36(5):978–987. doi: 10.1210/jcem-36-5-978. [DOI] [PubMed] [Google Scholar]
- Gutman R. A., Lazarus N. R., Penhos J. C., Fajans S., Recant L. Circulating proinsulin-like material in patients with functioning insulinomas. N Engl J Med. 1971 May 6;284(18):1003–1008. doi: 10.1056/NEJM197105062841803. [DOI] [PubMed] [Google Scholar]
- Kaden M., Harding P., Field J. B. Effect of intraduodenal glucose administration on hepatic extraction of insulin in the anesthetized dog. J Clin Invest. 1973 Aug;52(8):2016–2028. doi: 10.1172/JCI107386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa Y., Kuzuya T., Ide T., Kosaka K. Plasma insulin responses to glucose in femoral, hepatic, and pancreatic veins in dogs. Am J Physiol. 1966 Aug;211(2):442–448. doi: 10.1152/ajplegacy.1966.211.2.442. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Rubenstein A. H. Metabolism of proinsulin, insulin, and C-peptide in the rat. J Clin Invest. 1973 May;52(5):1113–1121. doi: 10.1172/JCI107277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabchi A. E. Clinical significance of proinsulin in human plasma. South Med J. 1972 Jul;65(7):833–838. doi: 10.1097/00007611-197207000-00013. [DOI] [PubMed] [Google Scholar]
- Lockwood D. H., Misbin R. I. Proinsulin content of adult and fetal pancreatic tissue extracted after rapid freezing in situ. Horm Metab Res. 1972 Jul;4(4):232–234. doi: 10.1055/s-0028-1094055. [DOI] [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Oyer P. E., Steiner D. F. Identification of proinsulin and C-peptide in human serum by a specific immunoassay. Proc Natl Acad Sci U S A. 1970 Sep;67(1):148–155. doi: 10.1073/pnas.67.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Steiner D. F. Human serum proinsulin. J Clin Invest. 1970 Mar;49(3):497–507. doi: 10.1172/JCI106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi G. K., Letarte J., Fraser T. R. Proinsulin content of pancreas in human fetuses of healthy mothers. Lancet. 1970 Jan 3;1(7636):7–9. doi: 10.1016/s0140-6736(70)90522-2. [DOI] [PubMed] [Google Scholar]
- Rastogi G. K., Sinha M. K., Dash R. J. Insulin and proinsulin content of pancreases from diabetic and nondiabetic subjects. Diabetes. 1973 Nov;22(11):804–807. doi: 10.2337/diab.22.11.804. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Block M. B., Starr J., Melani F., Steiner D. F. Proinsulin and C-peptide in blood. Diabetes. 1972;21(2 Suppl):661–672. doi: 10.2337/diab.21.2.s661. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Pottenger L. A., Mako M., Getz G. S., Steiner D. F. The metabolism of proinsulin and insulin by the liver. J Clin Invest. 1972 Apr;51(4):912–921. doi: 10.1172/JCI106886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFER A., GERSTENFELD S. The photometric microdetermination of blood glucose with glucose oxidase. J Lab Clin Med. 1958 Mar;51(3):448–460. [PubMed] [Google Scholar]
- SAMOLS E., RYDER J. A. Studies on tissue uptake of insulin in man using a differential immunoassay for endogenous and exogenous insulin. J Clin Invest. 1961 Nov;40:2092–2102. doi: 10.1172/JCI104435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMOLS E., RYDER J. A. Studies on tissue uptake of insulin in man using a differential immunoassay for endogenous and exogenous insulin. J Clin Invest. 1961 Nov;40:2092–2102. doi: 10.1172/JCI104435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando H., Borg J., Steiner D. F. Studies on the secretion of newly synthesized proinsulin and insulin from isolated rat islets of Langerhans. J Clin Invest. 1972 Jun;51(6):1476–1485. doi: 10.1172/JCI106944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein P. S. Islet cell tumors: current concepts and management. Ann Intern Med. 1973 Aug;79(2):239–257. doi: 10.7326/0003-4819-79-2-239. [DOI] [PubMed] [Google Scholar]
- Sherman B. M., Pek S., Fajans S. S., Floyd J. C., Jr, Conn J. W. Plasma proinsulin in patients with functioning pancreatic islet cell tumors. J Clin Endocrinol Metab. 1972 Aug;35(2):271–280. doi: 10.1210/jcem-35-2-271. [DOI] [PubMed] [Google Scholar]
- Starr J. I., Rubenstein A. H. Metabolism of endogenous proinsulin and insulin in man. J Clin Endocrinol Metab. 1974 Feb;38(2):305–308. doi: 10.1210/jcem-38-2-305. [DOI] [PubMed] [Google Scholar]
- Waddell W. R., Sussman K. E. Plasma insulin after diversion of portal and pancreatic venous blood to vena cava. J Appl Physiol. 1967 Apr;22(4):808–812. doi: 10.1152/jappl.1967.22.4.808. [DOI] [PubMed] [Google Scholar]



