Abstract
Interleukin (IL)-6-mediated signaling attenuates the anti-myeloma activity of glucocorticoids (GCs). We therefore sought to evaluate whether CNTO 328, an anti-IL-6 monoclonal antibody in clinical development, could enhance the apoptotic activity of dexamethasone (dex) in pre-clinical models of myeloma. CNTO 328 potently increased the cytotoxicity of dex in IL-6-dependent and –independent human myeloma cell lines (HMCLs), including a bortezomib-resistant HMCL. Isobologram analysis revealed that the CNTO 328/dex combination was highly synergistic. Addition of bortezomib to CNTO 328/dex further enhanced the cytotoxicity of the combination. Experiments with pharmacologic inhibitors revealed a role for the p44/42 mitogen-activated protein kinase pathway in IL-6-mediated GC resistance. Although CNTO 328 alone induced minimal cell death, it potentiated dex-mediated apoptosis, as evidenced by increased activation of caspases-8, -9, and -3, Annexin-V staining, and DNA fragmentation. The ability of CNTO 328 to sensitize HMCLs to dex-mediated apoptosis was preserved in the presence of human bone marrow stromal cells. Importantly, the increased activity of the combination was also seen in plasma cells from patients with GC-resistant myeloma. Taken together, our data provide a strong rationale for the clinical development of the CNTO 328/dex regimen for patients with myeloma.
Keywords: Akt, Bone marrow microenvironment, glucocorticoid resistance, p44/42 mitogen-activated protein kinase, phosphatidylinositol-3 kinase
Introduction
The activity of glucocorticoids (GCs) for the treatment of multiple myeloma is well established (Salmon, et al 1967). Although patient outcomes have improved with the advent of novel agents, such as the immunomodulatory drugs, thalidomide and lenalidomide, and the proteasome inhibitor bortezomib, GCs remain an important component of multi-agent chemotherapy for newly-diagnosed and relapsed/refractory multiple myeloma (Dimopoulos, et al 2007, Harousseau, et al 2006, Jagannath, et al 2005, Rajkumar, et al 2005, Rajkumar, et al 2008b, Weber, et al 2007). However, resistance to GCs is a problem, as evidenced by response rates to single-agent dexamethasone (dex) in clinical trials that ranged from 43% to 46% in newly-diagnosed myeloma, and 18% to 24% in relapsed and/or refractory disease (Alexanian, et al 1992, Dimopoulos, et al 2007, Rajkumar, et al 2008b, Richardson, et al 2005, Weber, et al 2007). Furthermore, higher doses of GCs have been associated with significant toxicity when given in combination with the immunomodulatory agents (Rajkumar, et al 2008a). Development of therapeutics that overcome known mechanisms of GC resistance is therefore a critical step to improving the efficacy of GC-containing myeloma therapy and allowing the use of lower, more tolerable doses of GCs in combination with newer regimens.
Interleukin (IL)-6 has been shown to enhance the proliferation and survival of myeloma cells, both in suspension and in the presence of bone marrow stromal cells (Anderson, et al 1989, Kawano, et al 1988, Klein, et al 1989, Uchiyama, et al 1993), and to protect them from apoptosis induced by GCs (Chauhan, et al 1997, Hardin, et al 1994, Juge-Morineau, et al 1995, Lichtenstein, et al 1995, Rowley, et al 2000). IL-6 abrogated dex-mediated cytotoxicity in part by activating and recruiting the protein tyrosine phosphatase SHP2 to related adhesion focal tyrosine kinase (RAFTK), thereby interfering with dex-induced activation of RAFTK and subsequent apoptosis (Chauhan, et al 1999, Chauhan, et al 2000). Although the phosphatidylinositol (PI)-3 kinase-Akt signaling pathway has been implicated as a crucial mediator of IL-6-induced GC resistance (Hideshima, et al 2001a), others have shown that additional pathways, including the Ras-p44/42 mitogen activated protein kinase (MAPK) pathway, are likely involved as well (Hsu, et al 2002, Ogawa, et al 2000).
Several growth factor pathways have been shown to confer GC resistance, including other gp 130 family cytokines (Juge-Morineau, et al 1995), insulin-like growth factor-1 (Xu, et al 1997), interferon-alpha (Ferlin-Bezombes, et al 1998, Liu, et al 1999), B-cell activating factor, a proliferation-inducing ligand (Moreaux, et al 2004), plasma cell (PC) cell-derived growth factor (Wang, et al 2006), and fibroblast growth factor receptor-3 (Pollett, et al 2002). Mouse xenograft models of human myeloma and myeloma cell line/bone marrow stromal cell (BMSC) co-culture experiments revealed, however, that IL-6 remains an important GC resistance factor within the bone marrow microenvironment (Cheung and Van Ness 2001, Grigorieva, et al 1998, Honemann, et al 2001, Tassone, et al 2005). We therefore sought to determine whether inhibition of IL-6 signaling with CNTO 328, a chimeric monoclonal antibody with high affinity for human IL-6 currently in clinical development, would enhance the activity of GCs in pre-clinical models of myeloma.
Materials and Methods
Materials
Stock solutions of bortezomib (Millennium Pharmaceuticals; Cambridge, MA), LY294002, and U0126 (Sigma-Aldrich; St. Louis, MO) were prepared in DMSO, stored at −20°C, and diluted in culture media immediately before use. Dexamethasone (Sigma-Aldrich) was prepared in 100% ethanol, stored at −20°C, and diluted in culture media immediately before use. F105, an isotype control antibody that recognizes the CD4 binding site of HIV type 1 gp120, and CNTO 328 were provided by Centocor, Inc. (Horsham, PA), and prepared in 0.15 M sodium chloride and 0.01 M sodium phosphate, pH 7.2. Final vehicle concentrations did not exceed 0.5% (v/v).
Human myeloma cell lines, bone marrow stromal cultures, and patient myeloma samples
The human myeloma cell lines (HMCLs), ANBL-6, KAS-6/1, and MM1.S were provided by Dr. Beverly Mitchell (Stanford University, CA), Dr. Diane Jelinek (Mayo Clinic, MN), and Dr. William Dalton (H. Lee Moffitt Cancer Center, FL), respectively. H-929, RPMI 8226, and U266 HMCLs were obtained from the American Type Culture Collection (Rockville, MD). HMCLs were grown in RPMI 1640 media (Gibco BRL; Grand Island, NY) with 10% fetal bovine serum (FBS, Sigma-Aldrich). IL-6-dependent HMCLs and myeloma patient samples were supplemented with 1 ng/ml of recombinant human IL-6 (R & D Systems; Minneapolis, MN). Plasma cells from patients with myeloma and BMSC cultures were established and cultured as previously described (Voorhees, et al 2007). BMSCs and patient-derived myeloma samples were obtained under University of North Carolina at Chapel Hill Institutional Review Board-approved protocols, and informed consent was obtained in accordance with the Declaration of Helsinki.
Cell Viability Assays
Cell viability was measured using the cell proliferation reagent WST-1 per the manufacturer’s protocol (Roche Applied Science; Indianapolis, IN) as previously described (Voorhees, et al 2007). Cell viability was determined using the following equation: (OD of drug-treated cells/OD control cells)*100.
Cell Death Assays
Activation of cell death was assessed via Annexin-V staining and DNA fragmentation as previously described (Voorhees, et al 2007). 2×105 myeloma cells were seeded onto 4×104 BMSCs 24 hours prior to treatment for BMSC/myeloma cell co-culture experiments. Activation of caspases-3, -8, and -9 was evaluated as previously described (Voorhees, et al 2007). Percent drug-specific apoptosis (Annexin-V staining and caspase-3 activation) was calculated using the following formula: [(% apoptotic cells in treated sample–% apoptotic cells in vehicle control treated sample)/(100–% apoptotic cells in vehicle control treated sample) × 100]. Fold increase in DNA fragmentation was calculated using the following equation: OD of drug-treated cells/OD control-treated cells.
Western Blot Analysis
5×106 cells were treated as indicated in the text. Whole cell lysates were prepared, separated, and transferred to nitrocellulose membranes as previously described (Orlowski, et al 2002). Antibodies against Actin, poly(ADP-ribose) polymerase (PARP) and caspase-3 (Santa Cruz Biotechnology, Inc.; Santa Cruz, CA) were used for immunoblotting.
Statistical analysis
Results were tabulated and graphed using Excel (Microsoft; Seattle, WA) and reported as the mean ± the standard deviation (SD) for typical experiments. Each condition was replicated in at least triplicate wells, and experiments were performed at least three times to ensure reproducibility. Differences between experimental conditions were measured using an unpaired student’s t-test and considered statistically significant at a p value of <0.05. Synergistic activity of the dex/CNTO 328 combination was assessed via isobologram analysis using Calcusyn software (Biosoft; Ferguson, MO). Combination indices (CIs) of ≤0.9 and <0.1 were considered synergistic and very strongly synergistic, respectively.
Results
CNTO 328 enhances dexamethasone-mediated cytoxicity in HMCLs
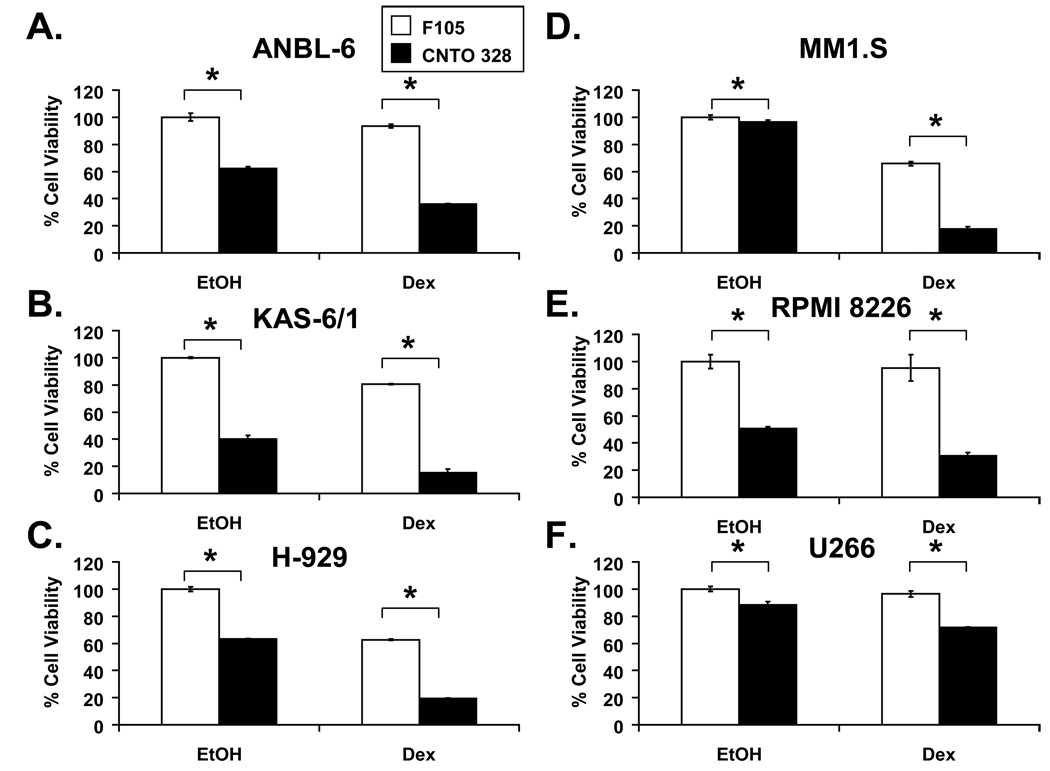
The viability of a panel of IL-6-dependent and –independent HMCLs grown in the presence of 1 ng/mL of IL-6 was assessed after a 48-hour treatment with single-agent CNTO 328, dex, or the combination, and compared to cells treated with a control antibody and vehicle. CNTO 328 exhibited some single-agent growth inhibition in both IL-6-dependent (ANBL-6, KAS-6/1) and –independent (H-929, MM1.S, RPMI 8226, U266) HMCLs. In the presence of 1 ng/ml of IL-6, single-agent dex demonstrated modest activity at best, yielding decrements in cell viability ranging from 5% (RPMI 8226) to 40% (MM1.S). In contrast, inhibition of IL-6 with CNTO 328 sensitized most HMCLs to dex, as demonstrated by decreases in cell viability ranging from 64% (ANBL-6) to 85% (KAS-6/1)(Figure 1A–1E). CNTO 328 potentiated the cytotoxicity of dex to a lesser degree in U266 cells, which have previously been shown to be resistant to GCs (Chauhan, et al 2003)(Figure 1F). To evaluate the possibility that there was synergy between CNTO 328 and dex, isobologram analysis was performed in ANBL-6 cells using escalating doses of each drug at a fixed 1:1 ratio. As shown in Table 1, the combination was very strongly synergistic (CI<0.1) at all dose levels.
Figure 1. The activity of CNTO 328 and dexamethasone in human myeloma cell lines.
ANBL-6 (A), KAS-6/1 (B), H-929 (C), MM1.S (D), RPMI 8226 (E), and U266 (F) HMCLs were treated with 10 µg/ml of the isotype control antibody, F105 (white bars), or CNTO 328 (black bars), and either vehicle control (EtOH) or 1 µM dex for 48 hours. Cytotoxicity was assessed via the WST-1 assay and expressed as percent cell viability relative to vehicle-treated controls. Columns, mean of triplicate cultures; bars, SD. * = p<0.01.
Table I.
Isobologram analysis of the CNTO 328/dexamethasone combination in ANBL-6 cells.
| CNTO 328 (µg/ml) |
Fa (%) |
Dex (µmol/l) |
Fa (%) |
Fa CNTO 328 + Dex (%) |
CI |
|---|---|---|---|---|---|
| 0.1 | 11 | 0.1 | 29 | 59 | <0.1 |
| 0.5 | 20 | 0.5 | 31 | 76 | <0.1 |
| 1 | 28 | 1 | 32 | 82 | <0.1 |
| 5 | 34 | 5 | 32 | 90 | <0.1 |
| 10 | 40 | 10 | 32 | 92 | <0.1 |
CI, combination index; Dex, dexamethasone; Fa, fraction affected.
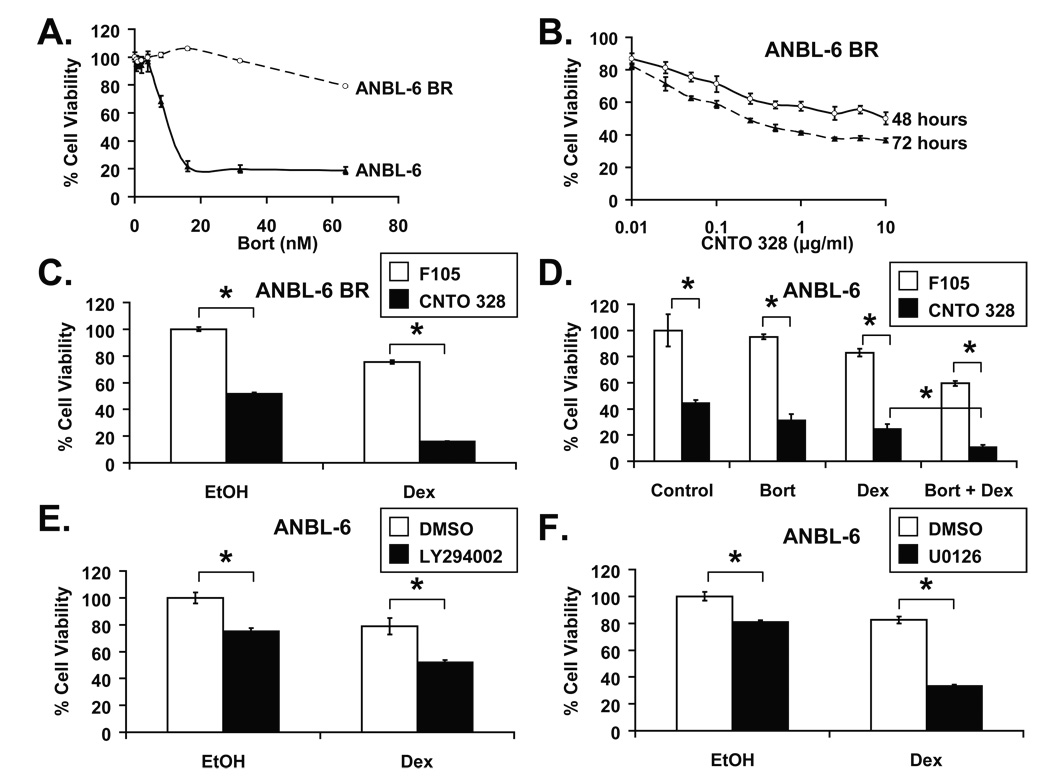
The proteasome inhibitor bortezomib has demonstrated notable activity in HMCLs that are resistant to conventional myeloma therapies, including dex (Hideshima, et al 2001b). Given the increasing use of bortezomib in relapsed/refractory and newly-diagnosed myeloma, it was of interest to determine if the CNTO 328/dex combination was active in bortezomib-resistant myeloma cells. To this end, a bortezomib-resistant ANBL-6 cell line, ANBL-6 BR, was developed by incubating parental ANBL-6 cells in escalating doses of bortezomib. After 24 hours of treatment, ANBL-6 BR cells were fully viable at bortezomib concentrations as high as 32 nM. In contrast, the viability of parental ANBL-6 cells dropped sharply beyond 4 nM of bortezomib (Figure 2A). ANBL-6 BR cells remained IL-6-dependent, as demonstrated by a time- and concentration-dependent reduction in viability with CNTO 328 treatment (Figure 2B). Notably, although single-agent CNTO 328 and dex decreased cell viability by 48% and 24% after 48 hours of treatment, respectively, the combination reduced viability by 84% (Figure 2C), thus demonstrating that ANBL-6 BR cells are not cross-resistant to the CNTO 328/dex combination.
Figure 2. CNTO 328 and dexamethasone in bortezomib-resistant ANBL-6 cells and in combination with bortezomib.
(A) ANBL-6 (open circles) and ANBL-6 BR (closed triangles) HMCLs were treated with escalating doses of bortezomib (bort) for 24 hours. (B) ANBL-6 BR cells were treated with escalating doses of CNTO 328 for 48 (open circles) and 72 hours (closed triangles). (C) ANBL-6 BR cells were treated with 10 µg/ml of F105 (white bars) or CNTO 328 (black bars) and vehicle control (EtOH) or 1 µM of dex for 48 hours. (D) ANBL-6 cells were treated with various combinations of F105 (10 µg/ml), CNTO 328 (10 µg/ml), dex (1 µM), and bortezomib (3 nM) for 48 hours. (E, F) The impact of signaling pathways downstream of IL-6 on glucocorticoid resistance. ANBL-6 cells were treated with vehicle control (DMSO) or 10 µM of the PI-3 kinase inhibitor, LY294002 (D), or 20 µM of the MEK inhibitor, U0126 (E), and EtOH or 1 µM dex for 48 hours. Cell viability for all of the above conditions was assessed using the WST-1 cell proliferation assay. Cytotoxicity was calculated as percent cell viability relative to vehicle-treated controls. Columns, mean of triplicate cultures; bars, SD. * = p<0.01.
The combination of bortezomib and dex has demonstrated additive activity in pre-clinical models of myeloma and has proven to be clinically active in newly-diagnosed and relapsed/refractory patients (Harousseau, et al 2006, Hideshima, et al 2001b, Jagannath, et al 2005, Jagannath, et al 2006). We therefore sought to determine whether CNTO 328 would enhance the anti-myeloma activity of the bortezomib/dex combination. Using the WST-1 proliferation assay in ANBL-6 cells, we noted increased cytotoxicity with the three-drug combination compared with any other one- or two-drug treatment (Figure 2D).
The role of downstream signaling pathways in IL-6-mediated GC resistance
The PI-3 kinase-Akt pathway has been implicated as a central mediator of IL-6-induced GC resistance in the HMCL MM1.S (Hideshima, et al 2001a). To further evaluate the role of the PI-3 kinase-Akt pathway in IL-6-mediated GC resistance, we treated ANBL-6 cells grown in the presence of 1 ng/ml of IL-6 with the PI-3 kinase inhibitor, LY294002, dex, or the combination for 48 hours, and evaluated their viability using the WST-1 assay. In ANBL-6 cells, although dex and LY294002 had minimal cytotoxicity as single agents, as demonstrated by decreases in viability of only 21% and 25%, respectively, the combination led to a 48% reduction in viability (Figure 2E). On the other hand, LY294002 had notable single-agent activity but was not able to sensitize KAS-6/1 cells to dex, suggesting that the PI-3 kinase pathway does not contribute to GC resistance in all cases (Supplementary Figure 1A).
Others have previously shown that IL-6-mediated GC resistance in ANBL-6 cells strongly correlated with the ability of IL-6 to abrogate GC-induced repression of activator protein (AP)-1 activity (Liu, et al 1999). Given the antagonism between AP-1 and GC receptor transcriptional activation (Jonat, et al 1990, Schule, et al 1990, Touray, et al 1991, Yang-Yen, et al 1990), we investigated the role of the p44/42 MAPK pathway, a known modulator of AP-1 transcriptional activity, in IL-6-mediated GC resistance. HMCLs were treated with the MAPK kinase (MEK) inhibitor U0126, dex, or the combination and analyzed using the WST-1 proliferation assay. Notably, treatment of ANBL-6 cells with U0126 or dex decreased cell viability by 19% and 17%, respectively, whereas the combination reduced viability by 66% (Figure 2F). As with LY294002, U0126 did not significantly enhance the cytotoxicity of dex in KAS-6/1 cells (Supplementary Figure 1B).
To gain a better understanding of the relative roles of the PI-3 kinase and p44/42 MAPK pathways in IL-6-mediated GC resistance, ANBL-6 cells were treated with varying combinations of dex, U0126, and LY294002 with or without CNTO 328 for 48 hours and evaluated using the WST-1 cytotoxicity assay. As shown in Supplementary Figure 1C, although the addition of U0126 sensitized ANBL-6 cells to the cytotoxicity of dex in the presence of IL-6, it did not confer additional cytotoxicity beyond that seen with the combination of CNTO 328 and dex. These findings suggested that the p44/42 MAPK pathway was important in mediating IL-6-dependent GC resistance, but did not render the cells more susceptible to GCs in the absence of IL-6 signaling. In contrast, the addition of LY294002 led to further cytoreduction beyond that seen with CNTO 328 and dex. The latter data indicated that inhibition of the PI-3 kinase pathway rendered ANBL-6 cells more innately susceptible to GC-mediated cytotoxicity in a fashion that was not totally dependent on IL-6 signaling.
CNTO 328 potentiates dexamethasone-mediated apoptosis
To evaluate the basis for the enhanced cytotoxicity of the CNTO 328/dex combination, ANBL-6 and KAS-6/1 HMCLs were treated with single-agent CNTO 328, dex, or the combination for 48 hours and analyzed via cell surface Annexin-V staining. Treatment with CNTO 328 or dex alone induced little to modest increases in apoptosis, yielding drug-specific Annexin-V staining of 5.4% and 19.5% with CNTO 328, and 13.6% and −3.8% with dex in ANBL-6 (Figure 3A) and KAS-6/1 cells (Figure 3B), respectively. In contrast, drug-specific Annexin-V staining was seen in 40.2% and 60.8% of KAS-6/1 and ANBL-6 cells treated with the combination. Similarly, DNA fragmentation was enhanced in CNTO 328- and dex-treated HMCLs compared to treatment with either single agent (Figure 3C and 3D). CNTO 328 also potentiated dex-mediated activation of PARP cleavage, another marker of programmed cell death (data not shown).
Figure 3. CNTO 328 potentiates dexamethasone-mediated apoptosis in human myeloma cell lines.
(A, B) ANBL-6 (A) and KAS-6/1 (B) cells were treated with 10 µg/ml of F105 (white bars) or CNTO 328 (black bars) and EtOH or 1 µM of dex for 48 hours, stained with FITC-conjugated Annexin-V and ToPro3, and subjected to flow cytometric analysis. Results are expressed as percent drug-specific apoptosis. (C, D) ANBL-6 (C) and KAS-6/1 (D) cells were treated with 10 µg/ml of F105 (white bars) or CNTO 328 (black bars) and EtOH or 1 µM of dex for 24 hours and evaluated using a DNA fragmentation ELISA. Results are expressed as fold increase in apoptosis over vehicle-treated controls. Columns, mean of triplicate cultures; bars, SD. (E, F) ANBL-6 (E) and KAS-6/1 cells (F) grown in the presence of BMSCs and no supplemental IL-6 were treated with 10 µg/ml F105 (white bars) or CNTO 328 (black bars) and EtOH or 1 µM dex for 48 hours, stained with FITC-conjugated Annexin-V and ToPro3, and subjected to flow cytometric analysis. Percent drug-specific apoptosis is indicated in each of the upper right quadrants of the dot plot. * = p<0.05.
Myeloma cells become IL-6-independent in the presence of BMSCs (Chatterjee, et al 2002). Furthermore, multiple factors present in the bone marrow microenvironment confer myeloma GC resistance. Therefore, we investigated the activity of the CNTO 328/dex combination in ANBL-6 and KAS-6/1 cells grown in the presence of human BMSCs without exogenous IL-6. Importantly, although treatment of ANBL-6 and KAS-6/1 cells with single-agent CNTO 328 or dex for 48 hours led to little or no effect on apoptosis in the presence of BMSCs, CNTO 328 potentiated dex-mediated induction of Annexin-V staining (Figure 3E and 3F). Thus, these results suggested that CNTO 328 enhanced the cytotoxicity of dex by potentiating dex-mediated activation of apoptosis, both in cell suspension and the presence of bone marrow stroma.
To further characterize the basis for enhanced induction of apoptosis with CNTO 328 and dex, ANBL-6 cells were treated with either drug alone, or the combination, incubated with FITC-DEVD-FMK, a fluorescent reagent that specifically binds activated caspase-3, and subjected to flow cytometric analysis. Although treatment with single-agent CNTO 328 and dex led to no or minimally increased drug-specific activation of caspase-3, at 0% and 14%, respectively, the combination dramatically enhanced caspase-3 activation to 69% (Figure 4A). Similarly, CNTO 328 potentiated dexamethasone-mediated cleavage of caspase-3 via Western blot analysis, further confirming that the cytotoxicity of the combination was the result of augmented activation of caspase-dependent cell death (Figure 4B). To determine whether CNTO 328 enhanced dex-mediated induction of the extrinsic or intrinsic apoptotic pathways, treated ANBL-6 cells were incubated with fluorogenic substrates specific for activated caspase-8 and -9, markers of extrinsic and intrinsic pathway activation, respectively, and analyzed by fluorometry. Even though single-agent CNTO 328 did not activate either caspase-8 or -9, it strongly potentiated dex-mediated induction of both caspases, thus demonstrating that CNTO 328 augments dex-mediated cell death through activation of both the extrinsic and intrinsic apoptotic cascades (Figure 4C and 4D).
Figure 4. CNTO 328 potentiates dexamethasone-mediated activation of the intrinsic and extrinsic apoptotic cascades.
(A) ANBL-6 cells were treated with 10 µg/ml F105 (white bars) or CNTO 328 (black bars) and EtOH or 1 µM dex for 48 hours, stained with PE-conjugated Annexin-V and the irreversible caspase-3 inhibitor, FITC-DEVD-FMK, and subjected to flow cytometric analysis. Results are expressed as percent drug-specific induction of caspase-3. (B) Western blot analysis of caspase-3 activation in ANBL-6 cells treated with F105 or CNTO 328 (10 µg/ml) and EtOH or dex (1 µM) for 24 hours. Actin was used as a loading control. FL=full length, CF=cleaved fragment. (C, D) Lysates were prepared from ANBL-6 cells treated with 10 µg/ml F105 (white bars) or CNTO 328 (black bars) and EtOH or 1 µM dex for 48 hours. 30 µg of lysate were incubated with fluorogenic substrate specific for activated caspase-8 (C) or caspase-9 (D). Results are expressed as fold increase in fluorescence units over vehicle-treated control. Columns, mean of triplicate cultures; bars, SD. * = p<0.0001. NS = Not significant.
CNTO 328 potentiates the cytotoxicity of dexamethasone in patient myeloma samples
We further characterized the activity of the CNTO 328/dex combination in CD138+ plasma cells derived from 7 patients with either newly-diagnosed or relapsed/refractory myeloma, or plasma cell leukemia. Of note, all patients from which the samples were collected harbored some level of clinical resistance to GCs, as evidenced by the fact that 7 out of 7 had disease which had previously not responded, or did not subsequently respond, to GC-based therapy (Supplementary Table 1). CD138+ plasma cells were treated with CNTO 328, dex, or the combination for 48 hours and subsequently evaluated using the WST-1 proliferation assay. Single-agent CNTO 328 exhibited varying degrees of cytotoxicity, with reductions in cell viability ranging from −6% to 67%, thus demonstrating that there is considerable variability of in vitro IL-6 dependence between patient samples. The in vitro activity of single-agent dex in the presence of 1 ng/ml of IL-6 was modest in most patient samples, with decreases in cell viability ranging from −11% to 51%. Statistically significant decreases in cell viability with single-agent dex relative to F105/EtOH-treated controls were only seen in 2 cases (samples 001 and 007). The combination of CNTO 328 and dex led to greater cytotoxicity then either agent alone in most samples, with the exception of sample 005, and was significant in four out of seven (p<0.05, Table 2). Importantly, the enhanced activity of the combination was seen in samples obtained from newly-diagnosed and relapsed/refractory myeloma patients, those with high-risk cytogenetic features, and those with GC-resistant disease (Supplementary Table 1).
Table II.
In vitro percent viability of patient myeloma samples after a 48-hour treatment with CNTO 328 and dexamethasone.
| Sample No. | Control (%) | CNTO 328 | Dex (%) | CNTO 328 + Dex (%) |
|---|---|---|---|---|
| 001 | 100 ± 3.5 | 75 ± 1.4 | 72 ± 4.6 | 17 ± 2.8* |
| 002 | 100 ± 13.8 | 106 ± 6.0 | 84 ± 13.2 | 73 ± 4.8 |
| 003 | 100 ± 32.3 | 90 ± 17.6 | 68 ± 18.5 | 47 ± 14.3 |
| 004 | 100 ± 31.3 | 40 ± 13.9 | 103 ± 25.1 | 13 ± 4.1* |
| 005 | 100 ± 15.2 | 106 ± 3.2 | 111 ± 2.5 | 94 ± 0.4 |
| 006 | 100 ± 13.0 | 33 ± 5.6 | 104 ± 11.8 | 11 ± 4.6* |
| 007 | 100 ± 7.2 | 72 ± 4.8 | 49 ± 8.3 | 29 ± 8.2* |
P < 0.05.
Discussion
Although GCs are an effective class of agents for the treatment of myeloma, resistance is a significant problem. The development of therapeutics that target known mechanisms of GC resistance represents an attractive strategy for improving patient outcomes to GC-based combination therapy. Herein, we show that inhibition of IL-6 with CNTO 328, a clinical grade, monoclonal neutralizing anti-IL-6 antibody, potently sensitized HMCLs, both in suspension and in the presence of BMSCs, as well as CD138+ plasma cells derived from patients with myeloma, to dex-mediated cytotoxicity via potentiation of dex-induced activation of the intrinsic and extrinsic apoptotic pathways. CNTO 328/dex was active regardless of HMCL dependence on IL-6 for growth and survival. The combination was also active in CD138+ plasma cells isolated from patients with documented resistance to GC-based therapy. Notably, activity was demonstrated in a sample derived from a plasma cell leukemia patient with documented resistance to bortezomib (sample 007, Supplementary Table 1), as well as a bortezomib-resistant HMCL, thus suggesting that bortezomib resistance does not confer cross-resistance to the CNTO 328/dex combination in vitro. As the CNTO 328/dex regimen moves into the clinic, it will be interesting to see the level of activity in patients with documented resistance to prior GC- and bortezomib-based therapy.
One might predict that inhibition of IL-6 would be an ineffective means of overcoming GC resistance, particularly given the growing list of factors present in the bone marrow microenvironment that confer resistance to this class of agents. However, previous results demonstrated that inhibition of IL-6 signaling with either anti-IL-6 antibodies or Sant7, an antagonist that interferes with IL-6/IL-6 receptor interactions, enhanced the cytotoxicity of dex in ANBL-6, XG-1 and INA-6 HMCLs, as well as in patient myeloma samples, even when grown in the presence of human BMSCs (Cheung and Van Ness 2001, Grigorieva, et al 1998, Honemann, et al 2001). Similarly, although Sant7 or dex alone did not inhibit INA-6 cell growth in the SCID-Hu murine xenograft model of human myeloma, as assessed by measurement of soluble IL-6 receptor levels, the combination synergistically slowed their proliferation (Tassone, et al 2005). In our hands, while single-agent CNTO 328 and dex had minimal apoptotic activity in the presence of stroma, the combination significantly enhanced induction of cell death in ANBL-6 and KAS-6/1 cells. However, it should be noted that induction of apoptosis in ANBL-6 cells treated with anti-IL-6 antibodies and dex was not as robust when the cells were grown in the presence of stroma as opposed to cell suspension with exogenous IL-6 (Cheung and Van Ness 2001). Furthermore, others have shown that bone marrow stroma protected patient myeloma cells from dex-mediated cell death more effectively than 10 ng/ml of IL-6 (Grigorieva, et al 1998). We noted a decrease in the level of apoptosis achieved with CNTO 328/dex when ANBL-6 and KAS-6/1 cells were grown in the presence of stroma compared with 1 ng/ml of exogenous IL-6 in cell suspension. Taken together, the existing data and our current results demonstrate that IL-6 retains a central role in mediating GC resistance within the bone marrow microenvironment despite the presence of other resistance factors. Moving forward, it will be of interest to evaluate the efficacy of dex when combined with anti-IL-6 therapeutics alone and in combination with inhibitors of other GC resistance factors present within the bone marrow microenvironment.
Targeting of crucial signaling pathways downstream of IL-6 and other factors that mediate GC resistance represents an attractive approach to improving the efficacy of GCs within the bone marrow microenvironment. The PI-3 kinase-Akt pathway has been identified as an important component of IL-6-mediated GC resistance in myeloma cells (Hideshima, et al 2001a, Ogawa, et al 2000). In support of this, our results demonstrated that treatment with the PI-3 kinase inhibitor, LY294002, enhanced the cytotoxicity of dex in ANBL-6 cells grown in the presence of IL-6. However, investigators have shown that the PI-3 kinase-Akt pathway was not activated by IL-6 in all HMCLs and patient myeloma samples (Pene, et al 2002, Tu, et al 2000). Furthermore, in our hands, inhibition of PI-3 kinase only partially reversed IL-6-mediated GC resistance in ANBL-6 cells, and was not able to overcome resistance in KAS-6/1 cells at all. One additional pathway that has been implicated in IL-6-mediated GC resistance is the p44/42 MAPK pathway (Ogawa, et al 2000). Our current results also support a role for the p44/42 MAPK pathway, at least in some cases, as treatment with the MEK inhibitor, U0126, significantly enhanced the cytotoxicity of dex in ANBL-6 cells grown in the presence of IL-6. Similarly, others have shown that a MEK inhibitor in clinical development, AZD6244, sensitized the IL-6-dependent HMCL, INA-6, to dex (Tai, et al 2007). These data would suggest that multiple signaling pathways contribute to IL-6-mediated GC resistance, and that there is likely to be significant inter-individual variation.
Interestingly, others have shown that IL-6-mediated suppression of dex-induced apoptosis in ANBL-6 cells was strongly associated with its ability to abrogate inhibition of AP-1 DNA binding by dex (Liu, et al 1999). Similarly, augmentation of T cell receptor signaling via IL-2 or CD28 co-stimulation abrogated dex-induced suppression of CD4+ T cell proliferation, c-Fos expression, and consequent AP-1 activity. Pre-treatment of the CD4+ T cells with U0126 overcame IL-2/CD28-mediated GC resistance (Tsitoura and Rothman 2004). Given these results, as well as a wealth of literature documenting the antagonistic interactions between AP-1 and the GC receptor (Jonat, et al 1990, Schule, et al 1990, Touray, et al 1991, Yang-Yen, et al 1990), modulation of AP-1 activity may be an important mechanism of GC resistance in myeloma. In support of this, transient transfection of a dominant negative construct of c-Jun, a component of AP-1 complexes, into RPMI 8226 cells significantly enhanced dex-mediated cell death in the presence of IL-6 (Xu, et al 1998). Clearly, further investigation into the role of AP-1 and upstream effectors of AP-1 activity in growth factor-mediated GC resistance is warranted.
Numerous factors intrinsic to myeloma cells have been shown to confer GC resistance in vitro, including activating mutations in N-Ras and K-Ras, TNF receptor-associated factor (TRAF)-3 deletions, as well as over-expression of heat shock protein (HSP)-27 (Chauhan, et al 2003, Keats, et al 2007, Rowley, et al 2000). In our hands, CNTO 328 was not able to overcome GC resistance in all patient samples tested, nor in all HMCLs, further supporting the idea that resistance is not mediated by the bone marrow microenvironment in all cases. Furthermore, others have shown that IL-6 could only confer dex resistance in the CD45+, immature fraction of patient malignant plasma cells, which is notable given the important role that CD45 plays in IL-6-mediated proliferation (Fujii, et al 1999, Grigorieva, et al 1998, Mahmoud, et al 1998). Thus, the existing pre-clinical data, as well as ours, would suggest that there is significant inter- and intra-individual variation in GC resistance. Ultimately, it is likely that multiple pathways of GC resistance will need to be targeted, including those derived from the bone marrow microenvironment as well as those intrinsic to the myeloma cells themselves, to optimally improve outcomes to GC-based therapy.
We have previously shown that CNTO 328 enhances the cytotoxicity of bortezomib in pre-clinical models of myeloma by accelerating bortezomib-mediated activation of the extrinsic and intrinsic apoptotic cascades (Voorhees, et al 2007). Given the increasing use of bortezomib/dex combinations in clinical practice, we were interested in determining the activity of bortezomib in combination with CNTO 328 and dex. Importantly, we noted increased activity with the three-drug regimen. As such, one promising avenue for future clinical investigation will be the incorporation of CNTO 328 into bortezomib/GC-based therapy.
Taken together, we have shown that IL-6 is an important GC resistance factor in myeloma that is effectively targeted by CNTO 328. Our data provide a strong rationale for the clinical development of the CNTO 328/dex combination for patients with myeloma. To this end, an on-going phase II clinical study of CNTO 328 and dexamethasone in relapsed/refractory myeloma has demonstrated promising preliminary results (Voorhees, et al 2008).
Supplementary Material
KAS-6/1 cells were treated with vehicle (DMSO) or 10 µM of the PI-3 kinase inhibitor, LY294002 (A), or 20 µM of the MEK inhibitor, U0126 (B), and EtOH or 1 µM dex for 48 hours. (C) ANBL-6 cells were treated with varying combinations of vehicle (veh.), 1 µM dex, 20 µM U0126 (U) and/or 10 µM LY294002 (L) with or without CNTO 328 for 48 hours. P value for CNTO 328 + dex vs. CNTO 328 + dex + U0126: 0.253. P value for CNTO 328 + dex vs. CNTO 328 + dex + LY294002: 0.00378. Cell viability was assessed using the WST-1 cell proliferation assay. Cytotoxicity was calculated as percent cell viability relative to vehicle-treated controls. Columns, mean of triplicate cultures; bars, SD.
Acknowledgements
P.M.V. would like to acknowledge support from the National Institutes of Health and the National Center for Research Resources (K12 RR17667). R.Z.O., a Leukemia & Lymphoma Society Scholar in Clinical Research, would like to acknowledge support from the Leukemia & Lymphoma Society (6096-07), the Multiple Myeloma Research Foundation, and the National Cancer Institute (RO1 CA102278).
Abbreviations
- Ac
acetyl
- AMC
amino-methyl coumarin
- AP
activator protein
- BMSCs
bone marrow stromal cells
- bort
bortezomib
- CI
combination index
- dex
dexamethasone
- DMSO
dimethylsulfoxide
- EtOH
ethanol
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- FMK
fluoromethyl ketone
- GC
glucocorticoid
- HMCLs
human myeloma cell lines
- HSP
heat shock protein
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MEK
MAPK kinase
- PC
plasma cell
- PE
phycoerythrin
- PI-3 kinase
phosphatidylinositol-3 kinase
- PARP
poly(ADP-ribose) polymerase
- RAFTK
related adhesion focal tyrosine kinase
- SCID-Hu
severe combined immunodeficiency-human hybrid
- SD
standard deviation
- TRAF
Tumor Necrosis Factor Receptor-associated factor
Also, standard single letter abbreviations for amino acids are used to indicate the sequences of peptide-based caspase inhibitors.
Footnotes
Conflicts of Interest and Financial Disclosure Statement: P.M.V., G.W.S., D.J.K., Q.C., S.A.H., and R.Z.O. have no financial disclosures or other conflicts of interest to report. J.A.N. is employed by Centocor, Inc., who supplied CNTO 328 for these studies.
References
- Alexanian R, Dimopoulos MA, Delasalle K, Barlogie B. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80:887–890. [PubMed] [Google Scholar]
- Anderson KC, Jones RM, Morimoto C, Leavitt P, Barut BA. Response patterns of purified myeloma cells to hematopoietic growth factors. Blood. 1989;73:1915–1924. [PubMed] [Google Scholar]
- Chatterjee M, Honemann D, Lentzsch S, Bommert K, Sers C, Herrmann P, Mathas S, Dorken B, Bargou RC. In the presence of bone marrow stromal cells human multiple myeloma cells become independent of the IL-6/gp130/STAT3 pathway. Blood. 2002;100:3311–3318. doi: 10.1182/blood-2002-01-0102. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Hideshima T, Pandey P, Treon S, Teoh G, Raje N, Rosen S, Krett N, Husson H, Avraham S, Kharbanda S, Anderson KC. RAFTK/PYK2-dependent and -independent apoptosis in multiple myeloma cells. Oncogene. 1999;18:6733–6740. doi: 10.1038/sj.onc.1203082. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure R, Burger R, Munshi N, Ohtake Y, Saxena S, Anderson KC. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102:3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Pandey P, Hideshima T, Treon S, Raje N, Davies FE, Shima Y, Tai YT, Rosen S, Avraham S, Kharbanda S, Anderson KC. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Pandey P, Ogata A, Teoh G, Treon S, Urashima M, Kharbanda S, Anderson KC. Dexamethasone induces apoptosis of multiple myeloma cells in a JNK/SAP kinase independent mechanism. Oncogene. 1997;15:837–843. doi: 10.1038/sj.onc.1201253. [DOI] [PubMed] [Google Scholar]
- Cheung WC, Van Ness B. The bone marrow stromal microenvironment influences myeloma therapeutic response in vitro. Leukemia. 2001;15:264–271. doi: 10.1038/sj.leu.2402022. [DOI] [PubMed] [Google Scholar]
- Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, San Miguel J, Hellmann A, Facon T, Foa R, Corso A, Masliak Z, Olesnyckyj M, Yu Z, Patin J, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Ferlin-Bezombes M, Jourdan M, Liautard J, Brochier J, Rossi JF, Klein B. IFN-alpha is a survival factor for human myeloma cells and reduces dexamethasone-induced apoptosis. J Immunol. 1998;161:2692–2699. [PubMed] [Google Scholar]
- Fujii R, Ishikawa H, Mahmoud MS, Asaoku H, Kawano MM. MPC-1-CD49e- immature myeloma cells include CD45+ subpopulations that can proliferate in response to IL-6 in human myelomas. Br J Haematol. 1999;105:131–140. [PubMed] [Google Scholar]
- Grigorieva I, Thomas X, Epstein J. The bone marrow stromal environment is a major factor in myeloma cell resistance to dexamethasone. Exp Hematol. 1998;26:597–603. [PubMed] [Google Scholar]
- Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Xiao H, Epstein J. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, Hulin C, Benboubker L, Fuzibet JG, Renaud M, Moreau P, Avet-Loiseau H. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91:1498–1505. [PubMed] [Google Scholar]
- Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001a;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001b;61:3071–3076. [PubMed] [Google Scholar]
- Honemann D, Chatterjee M, Savino R, Bommert K, Burger R, Gramatzki M, Dorken B, Bargou RC. The IL-6 receptor antagonist SANT-7 overcomes bone marrow stromal cell-mediated drug resistance of multiple myeloma cells. Int J Cancer. 2001;93:674–680. doi: 10.1002/ijc.1388. [DOI] [PubMed] [Google Scholar]
- Hsu JH, Shi Y, Hu L, Fisher M, Franke TF, Lichtenstein A. Role of the AKT kinase in expansion of multiple myeloma clones: effects on cytokine-dependent proliferative and survival responses. Oncogene. 2002;21:1391–1400. doi: 10.1038/sj.onc.1205194. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Richardson PG, Barlogie B, Berenson JR, Singhal S, Irwin D, Srkalovic G, Schenkein DP, Esseltine DL, Anderson KC. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica. 2006;91:929–934. [PubMed] [Google Scholar]
- Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Juge-Morineau N, Francois S, Puthier D, Godard A, Bataille R, Amiot M. The gp 130 family cytokines IL-6, LIF and OSM but not IL-11 can reverse the anti-proliferative effect of dexamethasone on human myeloma cells. Br J Haematol. 1995;90:707–710. doi: 10.1111/j.1365-2141.1995.tb05605.x. [DOI] [PubMed] [Google Scholar]
- Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, Tanaka H, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Braggio E, Henry T, Zhu YX, Fogle H, Price-Troska T, Ahmann G, Mancini C, Brents LA, Kumar S, Greipp P, Dispenzieri A, Bryant B, Mulligan G, Bruhn L, Barrett M, Valdez R, Trent J, Stewart AK, Carpten J, Bergsagel PL. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B, Zhang XG, Jourdan M, Content J, Houssiau F, Aarden L, Piechaczyk M, Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989;73:517–526. [PubMed] [Google Scholar]
- Lichtenstein A, Tu Y, Fady C, Vescio R, Berenson J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol. 1995;162:248–255. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- Liu P, Oken M, Van Ness B. Interferon-alpha protects myeloma cell lines from dexamethasone-induced apoptosis. Leukemia. 1999;13:473–480. doi: 10.1038/sj.leu.2401334. [DOI] [PubMed] [Google Scholar]
- Mahmoud MS, Ishikawa H, Fujii R, Kawano MM. Induction of CD45 expression and proliferation in U-266 myeloma cell line by interleukin-6. Blood. 1998;92:3887–3897. [PubMed] [Google Scholar]
- Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C, Moine P, Rossi JF, Klein B, Tarte K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Nishiura T, Oritani K, Yoshida H, Yoshimura M, Okajima Y, Ishikawa J, Hashimoto K, Matsumura I, Tomiyama Y, Matsuzawa Y. Cytokines prevent dexamethasone-induced apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in a new multiple myeloma cell line. Cancer Res. 2000;60:4262–4269. [PubMed] [Google Scholar]
- Orlowski RZ, Small GW, Shi YY. Evidence that inhibition of p44/42 mitogen-activated protein kinase signaling is a factor in proteasome inhibitor-mediated apoptosis. J Biol Chem. 2002;277:27864–27871. doi: 10.1074/jbc.M201519200. [DOI] [PubMed] [Google Scholar]
- Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, Lacombe C, Bouscary D. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–6597. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- Pollett JB, Trudel S, Stern D, Li ZH, Stewart AK. Overexpression of the myeloma-associated oncogene fibroblast growth factor receptor 3 confers dexamethasone resistance. Blood. 2002;100:3819–3821. doi: 10.1182/blood-2002-02-0608. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, Zeldenrust SR, Kumar S, Greipp PR, Fonseca R, Lust JA, Russell SJ, Kyle RA, Witzig TE, Gertz MA. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Jacobus S, Callander N, Fonseca R, Vesole D, Williams MV, Abonour R, Siegel DS, Katz M, Greipp PR. Randomized trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed myeloma (E4A03), a trial coordinated by the Eastern Cooperative Oncology Group: Analysis of response, survival, and outcome wi. J Clin Oncol (Meeting Abstracts) 2008a;26:8504. [Google Scholar]
- Rajkumar SV, Rosinol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, Olesnyckyj M, Yu Z, Knight R, Zeldis JB, Blade J. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008b;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Rowley M, Liu P, Van Ness B. Heterogeneity in therapeutic response of genetically altered myeloma cell lines to interleukin 6, dexamethasone, doxorubicin, and melphalan. Blood. 2000;96:3175–3180. [PubMed] [Google Scholar]
- Salmon SE, Shadduck RK, Schilling A. Intermittent high-dose prednisone (NSC-10023) therapy for multiple myeloma. Cancer Chemother Rep. 1967;51:179–187. [PubMed] [Google Scholar]
- Schule R, Rangarajan P, Kliewer S, Ransone LJ, Bolado J, Yang N, Verma IM, Evans RM. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62:1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Tai YT, Fulciniti M, Hideshima T, Song W, Leiba M, Li XF, Rumizen M, Burger P, Morrison A, Podar K, Chauhan D, Tassone P, Richardson P, Munshi NC, Ghobrial IM, Anderson KC. Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis. Blood. 2007;110:1656–1663. doi: 10.1182/blood-2007-03-081240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tassone P, Neri P, Burger R, Savino R, Shammas M, Catley L, Podar K, Chauhan D, Masciari S, Gozzini A, Tagliaferri P, Venuta S, Munshi NC, Anderson KC. Combination therapy with interleukin-6 receptor superantagonist Sant7 and dexamethasone induces antitumor effects in a novel SCID-hu In vivo model of human multiple myeloma. Clin Cancer Res. 2005;11:4251–4258. doi: 10.1158/1078-0432.CCR-04-2611. [DOI] [PubMed] [Google Scholar]
- Touray M, Ryan F, Jaggi R, Martin F. Characterisation of functional inhibition of the glucocorticoid receptor by Fos/Jun. Oncogene. 1991;6:1227–1234. [PubMed] [Google Scholar]
- Tsitoura DC, Rothman PB. Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells. J Clin Invest. 2004;113:619–627. doi: 10.1172/JCI18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. Cancer Res. 2000;60:6763–6770. [PubMed] [Google Scholar]
- Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–3720. [PubMed] [Google Scholar]
- Voorhees PM, Chen Q, Kuhn DJ, Small GW, Hunsucker SA, Strader JS, Corringham RE, Zaki MH, Nemeth JA, Orlowski RZ. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clin Cancer Res. 2007;13:6469–6478. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- Voorhees PM, Manges RF, Sonneveld P, Somlo G, Jagannath S, Zweegman S, Munteanu M, Vermeulen JT, Xie H, Orlowski RZ. Phase II study evaluating the efficacy and safety of CNTO328 in combination with dexamethasone for patients with relapsed/refractory multiple myeloma (MM) J Clin Oncol (Meeting Abstracts) 2008;26:8593. [Google Scholar]
- Wang W, Hayashi J, Serrero G. PC cell-derived growth factor confers resistance to dexamethasone and promotes tumorigenesis in human multiple myeloma. Clin Cancer Res. 2006;12:49–56. doi: 10.1158/1078-0432.CCR-05-0929. [DOI] [PubMed] [Google Scholar]
- Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, Olesnyckyj M, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- Xu F, Gardner A, Tu Y, Michl P, Prager D, Lichtenstein A. Multiple myeloma cells are protected against dexamethasone-induced apoptosis by insulin-like growth factors. Br J Haematol. 1997;97:429–440. doi: 10.1046/j.1365-2141.1997.592708.x. [DOI] [PubMed] [Google Scholar]
- Xu FH, Sharma S, Gardner A, Tu Y, Raitano A, Sawyers C, Lichtenstein A. Interleukin-6-induced inhibition of multiple myeloma cell apoptosis: support for the hypothesis that protection is mediated via inhibition of the JNK/SAPK pathway. Blood. 1998;92:241–251. [PubMed] [Google Scholar]
- Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
KAS-6/1 cells were treated with vehicle (DMSO) or 10 µM of the PI-3 kinase inhibitor, LY294002 (A), or 20 µM of the MEK inhibitor, U0126 (B), and EtOH or 1 µM dex for 48 hours. (C) ANBL-6 cells were treated with varying combinations of vehicle (veh.), 1 µM dex, 20 µM U0126 (U) and/or 10 µM LY294002 (L) with or without CNTO 328 for 48 hours. P value for CNTO 328 + dex vs. CNTO 328 + dex + U0126: 0.253. P value for CNTO 328 + dex vs. CNTO 328 + dex + LY294002: 0.00378. Cell viability was assessed using the WST-1 cell proliferation assay. Cytotoxicity was calculated as percent cell viability relative to vehicle-treated controls. Columns, mean of triplicate cultures; bars, SD.